Abstract
Diverse inhibitory pathways shape cortical information processing; however the relevant interneurons recruited by sensory stimuli and how they impact principal cells are unclear. Here we show that two major interneuron circuits govern dynamic inhibition in space and time within the olfactory cortex. Dendritic-targeting layer 1 interneurons receive strong input from the olfactory bulb and govern early-onset feedforward inhibition. However, this circuit is only transiently engaged during bursts of olfactory bulb input. In contrast, somatic-targeting layer 3 interneurons, recruited exclusively by recurrent excitation from pyramidal cells, produce late-onset feedback inhibition. Our results reveal two complementary interneuron circuits enforcing widespread inhibition, which shifts from the apical dendrites to somata of pyramidal cells during bursts of sensory input.
Introduction
Local inhibitory circuits shape the responses of cortical pyramidal cells to excitatory sensory input (Ferster and Jagadeesh, 1992; Gabernet et al., 2005; Poo and Isaacson, 2009; Wehr and Zador, 2003; Wilent and Contreras, 2005). Interneurons governing cortical inhibition are anatomically and functionally highly diverse and often specialized to target inhibition to different subcellular compartments of principal cells (Markram et al., 2004; Silberberg, 2008; Somogyi et al., 1998). Furthermore, the activation of distinct populations of interneurons is a dynamic process that varies with the strength and timing of excitation (Markram et al., 2004; Silberberg, 2008). For example, trains of excitatory stimuli can produce a progressive shift in inhibition from the soma to the dendrites of principal cells in hippocampus and somatosensory neocortex (Pouille and Scanziani, 2004; Tan et al., 2008). However, in many cortical regions the functional properties of interneuron circuits and how they shape the integration and transmission of sensory information remain unclear.
The primary olfactory (piriform) cortex is a three-layered cortical region that plays an important role in odor discrimination, recognition, and memory (Neville and Haberly, 2004; Wilson et al., 2006). In vivo studies have found that odor-evoked activity is sparse and distributed across the population of layer 2/3 principal cells in piriform cortex (Poo and Isaacson, 2009; Stettler and Axel, 2009). Odors evoke inhibition that is widespread and broadly tuned (Poo and Isaacson, 2009), in contrast to other primary sensory cortices where stimuli elicit balanced excitation and inhibition (Anderson et al., 2000; Wehr and Zador, 2003; Wilent and Contreras, 2005). Local inhibitory pathways are likely to be critical for sparse odor representations by principal cells. However, the interneuron circuits governing sensory-evoked inhibition in piriform cortex are not well established.
Olfactory information is first encoded in the olfactory bulb, where mitral and tufted (M/T) cells belonging to unique glomeruli are activated by particular molecular features of individual odorants (Rubin and Katz, 1999; Uchida et al., 2000; Wachowiak and Cohen, 2001). A fundamental feature of M/T cell activity is that odor-evoked responses are tightly coupled to respiration (Bathellier et al., 2008; Margrie and Schaefer, 2003; Rinberg et al., 2006; Soucy et al., 2009; Spors and Grinvald, 2002). During a single respiratory cycle, activated M/T cells typically fire short bursts of action potentials (APs) at a frequency of 10-50 Hz (Cang and Isaacson, 2003; Margrie and Schaefer, 2003) and the number of APs is correlated with the strength of input from olfactory receptor neurons. This sensory information is relayed via M/T cell axons within the lateral olfactory tract (LOT) directly to the piriform cortex. M/T cell axons in the LOT make collateral projections only within the most superficial layer of piriform cortex (layer 1a) and form excitatory synaptic contacts onto the distal apical dendrites of layer 2/3 principal cells (Neville and Haberly, 2004). Given the characteristic temporal structure of odor-evoked M/T cell activity, inhibitory circuits in olfactory cortex may have features that optimize the processing of bursting sensory input.
Here we show that bursts of M/T cell activity drive a progressive shift in inhibition from the distal apical dendrite to soma of pyramidal cells in piriform cortex and we reveal two complementary interneuron circuits that govern the spatio-temporal dynamics of this inhibition. Dendritic-targeting interneurons in layer 1a (L1a) receive a higher convergence of M/T cell input than pyramidal cells and mediate short-latency, disynaptic inhibition. However, target-specific differences in the short-term dynamics of M/T cell synapses lead to the early but transient recruitment of L1a interneurons during bursts of input. In contrast, perisomatic-targeting layer 3 fast-spiking (FS) cells receive excitation exclusively from active principal cells and mediate late-onset “feedback” inhibition during bursts. Individual layer 3 FS cells inhibit many pyramidal cells but preferentially target those that excite them. Using an optogenetic approach, we show that recurrent excitation to layer 3 FS cells is much stronger than excitation onto pyramidal cells themselves, resulting in somatic feedback inhibition that dominates excitation in local pyramidal cells.
Results
Bursts of LOT input elicit a shift in inhibition from dendrite to soma
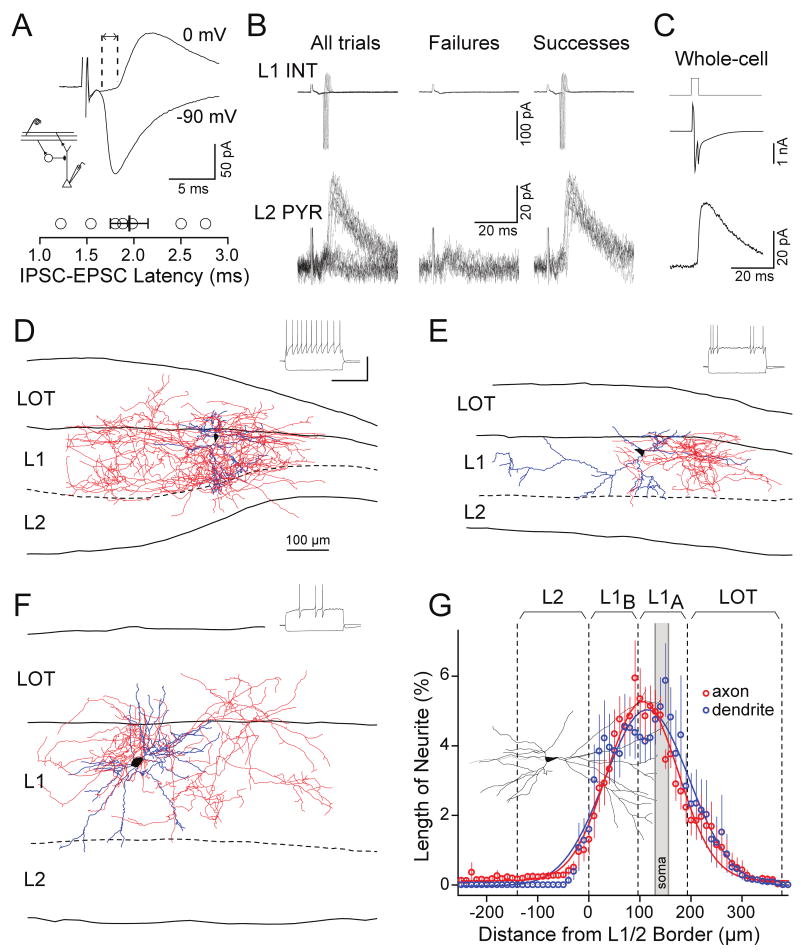
We studied synaptic responses using parasagittal slices of rat anterior piriform cortex (Franks and Isaacson, 2005, 2006; Poo and Isaacson, 2007). We made whole-cell recordings from deep layer 2 pyramidal cells and evoked synaptic responses via a stimulating electrode placed in the LOT (Fig. 1A, Methods). Pyramidal cells were voltage-clamped at the reversal potential for excitatory postsynaptic currents (EPSCs, Vm= 0 mV) to isolate inhibitory responses. We first examined the nature of inhibitory postsynaptic currents (IPSCs) evoked by bursts of LOT stimuli that approximate the respiratory-coupled activity of M/T cells in vivo (5 pulses at 20 Hz delivered every second). We could distinguish two distinct components of inhibition, one at the onset and one later during the burst, that showed different sensitivity to the strength of the stimulus. Low-intensity stimulation evoked short-latency IPSCs that were largest in response to the first stimulus and rapidly decreased in amplitude during the stimulus train (Fig. 1B). This “early-transient” inhibition grew in a graded fashion when stimulus strength was increased to recruit more LOT fibers (Supplemental Figure 1). As stimulus strength was further increased, IPSCs began to appear in response to late pulses in the stimulus train. These “late-onset” IPSCs could be readily distinguished from the transient component of inhibition, even when they overlapped, since they occurred with a longer latency after each individual stimulus pulse (Fig. 1C). Both types of IPSCs were abolished in the presence of the glutamate receptor antagonists NBQX (10 μM) and D-APV (50 μM) (Fig. 1D), confirming that these LOT-evoked responses are not due to direct activation of local interneurons; rather they require excitatory transmission from M/T cell axons.
Figure 1.
Bursts of LOT input evoke early-transient dendritic inhibition and late-onset somatic inhibition in piriform cortex. (A) Schematic of piriform cortex circuitry; individual M/T cell axons in the LOT project directly from OB to cortex and synapse on the apical dendrites of pyramidal cells (PYR) in layer 1a. Putative interneuron (INT) populations provide somatic and dendritic inhibition. (B) LOT-evoked IPSCs recorded in a L2 pyramidal cell (Vm=0mV). Low-intensity LOT stimulation elicits early, transient IPSCs while higher intensity stimulation elicits both early and late IPSCs. (C) Individual traces of IPSCs generated in another cell in response to the first (red traces) or fifth (black traces) stimulus pulse. (D) Responses from the cell in (C) before (Control) and after application of NBQX (20 μM) and APV (50 μM). (E-G) Focal dendritic application of gabazine selectively blocks the early-transient component of inhibition while somatic application of the antagonist blocks late-onset inhibition. (E) IPSCs recorded in a L2 pyramidal cell under control conditions and during focal dendritic (blue) or somatic (green) puffer application of gabazine (40 μM). (F) Time course of the experiment in (E). Peak IPSC amplitude was measured following the first (open circles) and fifth (closed circles) pulse. (G) Summary data of IPSC charge in response to the first pulse (open circles) or in response to the third through fifth pulses (closed circles). (n=4 for dendritic gabazine, n=5 for somatic gabazine).
We also observed that early-onset IPSCs had significantly slower rise times (10-90%) than the late-onset, long latency events (cf. Fig 1C, 2.2 ± 0.4 ms for IPSCs evoked by the first pulse vs. 0.6 ± 0.1 ms for IPSCs evoked by the fifth pulse, n=7, p=0.005). Given the somatic location of our recordings and the filtering properties of dendrites, the slower rise times of early-transient IPSCs suggest they may occur at more distal locations along the somato-dendritic axis of pyramidal cells than late-onset IPSCs. To determine the location of the two components of LOT-evoked inhibition onto pyramidal cells, we used focal application of the GABAA receptor antagonist gabazine (40 μM) via puffer pipette. Stimulus intensity was set above the threshold for recruiting both early-transient and late-onset IPSCs (Fig. 1E). When gabazine was focally applied to the distal apical dendritic region of the recorded pyramidal cell (245 ± 18 μm from soma, n=4), early-onset IPSCs were reversibly abolished (inhibitory charge evoked by the first pulse 8 ± 4% of control, p<0.01) while IPSCs late in the train remained essentially unchanged (IPSC charge during the last three pulses 104 ± 13% of control, Fig. 1 E-G). In contrast, somatic gabazine application (34 ± 5 μm from soma, n=5) reduced late-onset inhibition to 10 ± 4% of control levels while early inhibition was not significantly affected (86 ± 15% of control, p=0.4, Figure 1 E-G). These results indicate that during bursts of sensory input, inhibition shifts from the distal apical dendrite to the perisomatic compartment.
L1a interneurons mediate dendritic feedforward inhibition
What mechanisms underlie these spatio-temporally distinct sources of LOT-evoked inhibition? We first addressed the early dendritic component of inhibition by studying responses to single weak LOT stimuli. Under these conditions, onset of LOT-evoked IPSCs (Vm = 0 mV) followed monosynaptic EPSC onset (recorded at -90 mV, close to the reversal potential for inhibition) with a very brief delay (1.95 ± 0.2 ms, n = 7; Fig. 2A). This short latency indicates that the dendritic IPSC is recruited through a disynaptic, feedforward mechanism by LOT input rather than a feedback mechanism requiring the activation of pyramidal cells (Cruikshank et al., 2007; Gabernet et al., 2005).
Figure 2.
L1a interneurons underlie LOT-evoked feedforward inhibition onto the apical dendrites of pyramidal cells. (A) Early-onset IPSCs follow EPSCs with a brief latency, consistent with disynaptic recruitment of interneurons. Top, EPSC (-90 mV) and IPSC (0 mV) recorded from the same cell displaying the interval between the 10% rise times of the synaptic currents. Bottom, summary of latencies for 7 cells. (B) Simultaneous recording from a L1a interneuron in the cell-attached configuration (top) and a pyramidal cell in the whole-cell voltage-clamp configuration (0 mV). Stimulation of the LOT generated successes and failures of IPSCs in the pyramidal cell and APs in the L1a interneuron (All trials). Sorting the traces based on successes and failures of the interneuron APs revealed that the pyramidal cell IPSC only occurred when the interneuron fired. (C) Subsequent whole-cell recording of the interneuron (Vm=-80 mV) shows that an action current (elicited by a voltage step to 0 mV) evoked an IPSC in the pyramidal cell with an amplitude identical to the feedforward IPSCs evoked by LOT stimulation. (D-G) Dendritic (blue) and axonal (red) arbors of three biocytin-filled L1a interneurons (black cell bodies). Traces to the upper right are voltage responses to current steps (1 s) for each reconstructed cell (scale bar 500 ms, 50 mV). Dashed line, layer 1/2 border. (D) Cell with non-adapting firing pattern. (E) Cell with irregular-spiking firing pattern. (F) Cell with late-spiking firing pattern. (G) Summary of the laminar depths of neurites (10 μm bins) demonstrates localization of axon primarily within layer 1 (n=11 cells). Sample pyramidal cell included for comparison (black). Laminar borders (dashed lines) are drawn as mean measured distance from layer 1/2 border. Grey bar indicates the mean location (± SEM) of the cell bodies.
We hypothesized that interneurons located in L1a, close to the site of LOT inputs, would be poised to govern feedforward inhibition. To test this idea, we monitored spiking in L1a interneurons when LOT stimulus strength was set at the threshold for recruiting feedforward inhibition. We could resolve clear successes and failures of IPSCs on 50% of trials in a voltage-clamped pyramidal cell, and in a simultaneous cell-attached recording from a L1a interneuron, we observed successes and failures of individual APs that co-varied with the pyramidal cell IPSCs (Fig. 2B). Subsequent whole-cell recording of the interneuron confirmed that it made a direct unitary connection onto the pyramidal cell with an IPSC amplitude identical to that of successful IPSCs evoked by LOT stimulation (Fig. 2C). This indicates that L1a interneurons are a source of LOT-evoked feedforward inhibition in piriform cortex.
To determine whether the axons of L1a interneurons target the distal apical dendrites of pyramidal cells as suggested by our focal application of gabazine, we filled cells with biocytin and reconstructed their morphology. Despite variability in their spike-firing patterns, all L1a interneurons had dendritic and axonal arbors restricted to layer 1 (Fig. 2D-F, Supplemental Figure 2). On average, 92 ± 16% of the total axon length of L1a interneurons (n = 11) was distributed superficial to layer 2 (Fig. 2G). Thus, L1a interneurons have axons that spread laterally for hundreds of microns and exclusively target the apical dendrites of principal cells.
L1a interneurons receive strong LOT input
Do L1a interneurons sample from the same sensory afferents as neighboring pyramidal cells? To address this, we used simultaneous recordings from L1a interneurons and pyramidal cells (within 300 μm) to compare the properties of M/T cell inputs onto these two cell types. LOT stimulation evoked short-latency, monosynaptic EPSCs in all L1a interneurons (Fig. 3), confirming that they can be activated in a feedforward manner. We first measured the amplitude of LOT-evoked EPSCs in response to LOT stimulation at progressively stronger intensities (Fig. 3B,C). In virtually all paired recordings (n=12/13), LOT-evoked EPSCs were larger in interneurons than pyramidal cells over a range of stimulus intensities (Fig. 3D). On average, the compound EPSC was 6.1 + 1.2 times larger (Fig. 3E; at stimulus strengths 4-8 times threshold for recruiting single fibers). This difference in LOT-evoked compound EPSC amplitude could be due either to stronger unitary connections between M/T cells and L1a interneurons or a higher convergence of M/T cell axons onto L1a interneurons.
Figure 3.
L1a interneurons and pyramidal cells are contacted by the same M/T cells, but interneurons receive a higher convergence of synaptic input with different short-term dynamics. (A) Recording schematic. (B-G) L1a interneurons are contacted by more M/T cell axons than pyramidal cells. (B) EPSCs recorded simultaneously in a L1a interneuron and pyramidal cell as LOT stimulus strength is gradually increased. (C) Plot of EPSC amplitudes indicates that increasing LOT stimulus intensity (8.6V to 50 V) always recruits larger responses in the interneuron compared to the pyramidal cell. (D) Summary showing that compound EPSC amplitudes are typically larger in interneurons compared to simultaneously recorded pyramidal cells (n = 12 pairs). (E) Ratio of EPSC amplitudes in the same cell pairs. (F) Minimal stimulation evoked failures and successes of EPSCs that co-varied in a paired recording. Top, trials sorted based on successes or failures in the pyramidal cell (black) show matching successes and failures in the interneuron (blue). Bottom, plot of EPSC amplitudes for each trial in the interneuron and pyramidal cell. (G) Summary of single-fiber LOT inputs onto simultaneously recorded pairs of L1a interneurons and pyramidal cells (n=12 pairs). Filled circles, mean amplitude. (H) During a burst of LOT input (5 pulses, 20 Hz), EPSCs depress in an interneuron (blue) and facilitate in a pyramidal cell (black). (I) Summary plot (n=12 pairs) of short-term dynamics of EPSCs evoked at 20 Hz onto L1a interneurons (blue circles) and pyramidal cells (black circles). Blue lines represent responses of individual interneurons.
To differentiate between these two possibilities, we determined the strength of single M/T axon connections in simultaneously recorded L1a interneurons and pyramidal cells using minimal LOT stimulation (Franks and Isaacson, 2006). In most cases this required adjusting the stimulus strength first to isolate a single fiber in one cell, then readjusting it to isolate a single fiber in the other cell. In one example, minimal LOT stimulation evoked successes and failures of transmission that were perfectly correlated in the two cells (Fig. 3F), indicating that the same M/T cell axon could contact both a pyramidal cell and interneuron. The strength of single fiber inputs varied over a large range in L1a interneurons (Fig. 3G), similar to previous observations for pyramidal cells (Franks and Isaacson, 2006). The distributions of single-fiber EPSC amplitude measured in cell pairs were virtually identical (mean 43.1 ± 12 pA and 38.6 ± 9.6 pA for interneurons and pyramidal cells, respectively; Kolmogorov-Smirnov (KS) test, p = 0.2, n = 18; Fig. 3G). Together, these data suggest that feedforward L1a interneurons and pyramidal cells sample sensory input from the same complement of M/T cells. Since the amplitudes of single-fiber EPSCs were equivalent in both cell types, our results suggest that there is a 6-fold greater convergence of LOT fibers onto L1a interneurons.
Synaptic properties underlying early-transient activation of L1a interneurons
We next used bursts of stimuli to compare the temporal dynamics of LOT-evoked EPSCs in simultaneously recorded pairs of L1a interneurons and pyramidal cells (5 pulses, 20 Hz; Fig. 3H). In striking contrast to the short-term facilitation of EPSC amplitude in pyramidal cells, we found across the population of L1a interneurons that LOT-evoked EPSCs depressed during stimulus trains (Fig. 3H,I). Although a minority of interneurons showed weak facilitation on the 2nd pulse, all cells had strongly depressed EPSCs by the last pulse in the train. On average, the EPSC amplitude of the 2nd pulse relative to the first (P2/P1) was 2.36 ± 0.43 for pyramidal cells and 0.97 ± 0.10 for interneurons (n=12 pairs), whereas the ratio P5/P1 was 1.53 ± 0.31 vs. 0.45 ± 0.06 for pyramidal cells and interneurons, respectively. This relationship held true for a range of stimulus frequencies (10 Hz: P5/P1 pyramidal=1.64 ± 0.22, interneuron=0.51 ± 0.04, n=7 pairs; 50 Hz: P5/P1 pyramidal=1.06 ± 0.27, interneuron=0.21 ± 0.03, n=9 pairs). These results indicate that there are target cell-specific differences in the short-term dynamics of synaptic inputs to pyramidal cells and L1a interneurons and suggest that L1a interneurons may preferentially fire early in response to bursts of LOT activity.
We therefore investigated synaptic integration and threshold firing of L1a interneurons in response to trains of LOT stimuli (5 pulses, 20 Hz). We determined AP threshold by making cell-attached recordings and adjusting the strength of LOT stimulation such that APs were evoked on 50% of individual trials (Franks and Isaacson, 2006). We then ruptured the membrane patch and recorded the underlying EPSCs (Vm=-90 mV) at the same stimulus setting. L1a interneurons were most likely to fire APs only in response to the first or second pulse during the stimulus train (Fig. 4A, n=16). APs were precisely time-locked: they occurred within a very narrow time window (standard deviation of latencies for APs on the first pulse = 0.28 ± 0.03 ms, n=10) and their latency from the onset of the underlying EPSC was brief (1.89 ± 0.31 ms). Furthermore, the amplitude of the EPSC producing threshold firing averaged 482 ± 53 pA (n=11), only moderately larger than the EPSC amplitude (∼300 pA) found previously to bring pyramidal cells to spike threshold (Franks and Isaacson, 2006). Given the average single-fiber EPSC amplitude of ∼40 pA, these experiments suggest that the coincident activation of only ∼12 M/T cell inputs is sufficient to bring L1a interneurons to AP threshold. Together, these results indicate that while L1a interneurons can be precisely activated by relatively few M/T cells, depressing synaptic input favors their early-transient recruitment during bursts of activity.
Figure 4.
Early-transient recruitment of L1a interneurons during bursts of LOT input and the properties of their unitary connections. (A) Top, cell-attached responses to LOT stimulation adjusted to elicit threshold firing in a L1a interneuron. Middle, summary histogram of AP latencies (n= 16 interneurons). Bottom, average EPSC from the same cells. Grey shading is SEM. (B) Top, L1a interneuron action potential (5 ms, 1 nA step) evokes a short-latency, bicuculline-sensitive IPSC in a connected pyramidal cell (-40 mV). Bottom, summary of connectivity between pairs of L1a interneurons and pyramidal cells. (C) Unitary IPSCs between L1 interneurons and pyramidal cells display short-term depression.
Connectivity of L1a interneurons
The influence of L1a interneurons within the cortical circuit depends on their connectivity with cortical pyramidal cells. For connected pairs of L1a interneurons and pyramidal cells, single APs in the interneuron evoked short-latency (0.6 ± 0.1 ms, n=10) unitary IPSCs (uIPSCs) in pyramidal cells (Vm=-40 mV, Fig. 4B). The mean unitary conductance was 0.6 ± 0.1 nS (n = 23) and uIPSCs were blocked by the GABAA antagonist bicuculline (50 μM, Fig. 4B). The rise times of uIPSCs (2.0 ± 0.3 ms, n = 10) were identical to those of early-transient feedforward IPSCs evoked by LOT stimulation (2.2 ms), consistent with the idea that L1a interneurons are a source of feedforward inhibition. L1a interneurons inhibited 29% of local pyramidal cells (n = 46 pairs, Fig. 4B, Methods). Feedback projections (unitary excitatory connections from pyramidal cells) were only rarely observed (2%, n = 46). These results suggest that individual L1a interneurons provide feedforward inhibition onto a large number of pyramidal cells but are unlikely to be a major source of feedback inhibition. Additionally, when interneurons were driven to fire bursts of spikes (5 APs, 20 Hz) uIPSC amplitude depressed (P2/P1= 0.58 ± 0.02; P5/P1=0.39 ± 0.02, n = 23; Fig. 4C). Therefore, the short-term synaptic depression of both their excitatory input and inhibitory output ensures L1a interneurons produce only early-transient dendritic inhibition during bursts of M/T cell activity.
Dendritic inhibition enforces late temporal integration of bursting input
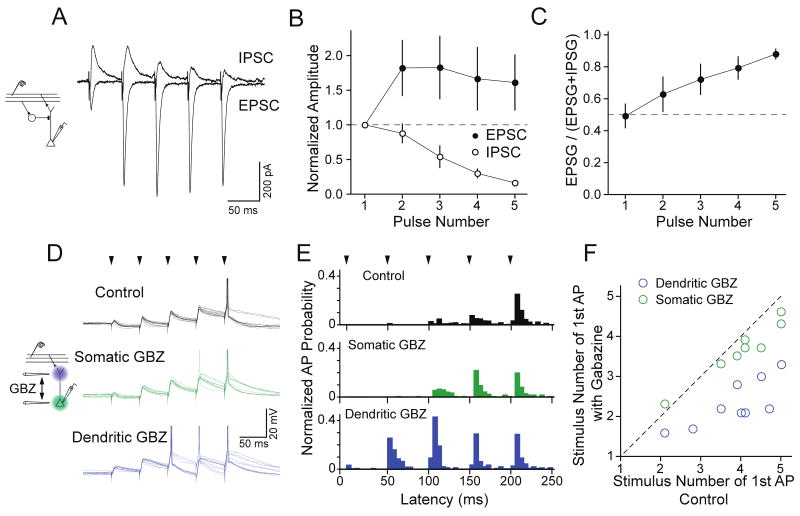
What role does early-transient dendritic feedforward inhibition play in regulating the integration and firing of pyramidal cells in response to M/T cell input? Given that the transformation of synaptic input to spike output relies on the relative balance of excitation and inhibition, we compared the amplitude of excitation and dendritic inhibition in pyramidal cells during trains of weak LOT stimuli. The ratio of the EPSC on the fifth pulse relative to the first (P5/P1) averaged 1.61 ± 0.4 (n=5) while for the IPSC this value fell to 0.16 ± 0.02 (Fig. 5A, B). This relationship was also observed at higher stimulus frequencies (50 Hz: P5/P1 EPSC=3.3±1, IPSC=0.25±0.04, n=5). To determine the relative magnitudes of excitation and inhibition, we measured the fraction of the total conductance contributed by the excitatory postsynaptic conductance (EPSG). While excitation and inhibition were perfectly balanced on the first pulse (fractional EPSG=0.49 ± 0.08, n=5), by the last pulse in the train, the total synaptic conductance was dominated by excitation (fractional EPSG=0.88 ± 0.03; Fig. 5C). Thus, bursts of sensory input cause a dramatic shift in the balance of feedforward inhibition and excitation received by pyramidal cell dendrites.
Figure 5.
Transient dendritic feedforward inhibition gates early integration of LOT input. (A) A train of LOT stimulation (5 pulses, 20 Hz) causes strong facilitation of monosynaptic EPSCs and marked depression of feedforward IPSCs in the same pyramidal cell. (B) Summary (n=5) of the normalized amplitude of the monosynaptic EPSCs and feedforward IPSCs during a 20 Hz burst of LOT input. (C) The initial ratio of excitation to inhibition is balanced but bursting input causes excitation to overwhelm inhibition during stimulus trains. Data from cells in B. (D) Overlay of individual traces showing the voltage response of a pyramidal cell to a burst of LOT stimuli (arrowheads, 20 Hz). Under control conditions (black traces), integration of successive EPSPs and depression of IPSPs allowed the pyramidal cell to reach AP threshold late in the burst on most trials. Focal puffer application of gabazine (40 μM) at the soma (green traces) had little effect on spiking while focal gabazine application in L1a (blue traces) caused the cell to reach threshold earlier during the train and fire APs on more trials. (E) Average normalized AP probability distributions under control conditions (black) and during somatic (green) or dendritic (blue) application of gabazine (n = 9). (F) Summary plot of the same cells showing that dendritic but not somatic gabazine application reduces the number of successive LOT stimuli required to bring pyramidal cells to AP threshold.
This relationship suggests that dendritic inhibition may act synergistically with excitation to enforce temporal summation and limit early spiking by pyramidal cells in response to trains of weak stimuli. We explored this possibility by recording pyramidal cells in current clamp to monitor spike output, under conditions in which they were just above threshold to fire a single AP in response to bursts of LOT stimuli (fraction of trials eliciting an AP=0.63 ± 0.07, n=9). Weak LOT stimulation ensured that only early-transient inhibition was recruited during bursts. In control conditions, AP latency was skewed toward EPSPs later in the train (Fig. 5D, E). Focal application of gabazine at the soma had little effect on the likelihood that a cell would reach AP threshold (0.61 ± 0.10 of trials) or on the timing of APs (Fig. 5E, F) during stimulus trains, indicating that somatic inhibition was not limiting integration of weak LOT input under these conditions (Methods). In contrast, focal dendritic application of the antagonist increased the probability that APs were evoked (0.94 ± 0.04 of trials) and often led to multiple APs in response to the stimulus (average number of spikes per trial = 1.96 ± 0.33 versus 0.66 ± 0.07 for control). Blocking dendritic inhibition also shifted AP latencies to earlier EPSPs during the stimulus train (Fig. 5 E, F). These results indicate that dendritic feedforward inhibition limits the temporal integration and spike output of pyramidal cells in response to early phases of bursting input.
L3 fast-spiking interneurons mediate late-onset somatic inhibition
We next sought to identify the source of the late-onset somatic inhibition recruited by bursts of LOT input at higher stimulus intensity. In contrast to the short latencies between individual LOT stimulus pulses and the onset of dendritic IPSCs, the longer latencies we observe for somatic IPSCs (cf. Fig. 1C) suggest they are not recruited in a simple disynaptic, feedforward fashion. Given the preferential late firing of pyramidal cells in response to LOT stimulus trains, we hypothesized that late-onset IPSCs might arise from a circuit involving perisomatic-targeting feedback interneurons that receive recurrent excitation from pyramidal cells. Layer 3 (L3) of piriform cortex contains large numbers of multipolar GABAergic interneurons, the majority of which are “basket cells” whose axons preferentially contact cell bodies (Ekstrand et al., 2001). We thus explored L3 interneurons as a possible source of the late-onset somatic inhibition recruited by strong bursts of LOT input.
We recorded IPSCs evoked by LOT bursts in pyramidal cells (Vm= 0 mV) while simultaneously monitoring APs in nearby (<150 μm) L3 multipolar cells in cell-attached mode. When LOT stimulus strength was set to evoke late-onset IPSCs in pyramidal cells, we found cells in L3 that fired at or near threshold in the late phases of stimulus trains (Fig. 6A). Subsequent whole-cell current clamp recordings from these targeted L3 cells revealed that the majority were fast-spiking (FS) cells (Markram et al., 2004) with low input resistance (85.5 ±11.3 MΩ, n=16), narrow AP half-width (0.43 ± 0.02 ms), and little adaptation during spike trains (adaptation ratio 0.79 ± 0.07; firing rate in last 100 ms/first 100 ms). Threshold firing of L3 FS cells recorded in cell-attached mode in response to trains of LOT stimuli was remarkably consistent with the timing of late-onset inhibition (Fig. 6A,B). The population response of L3 FS cells revealed that the majority of APs were elicited in response to later pulses of stimulus trains with most spikes occurring in response to the fourth (32 ± 0.6% of all APs) or fifth (28 ± 0.6%) stimulus (Fig. 6B; n=8). Thus, the firing of L3 FS cells indicates that they are poised to contribute to late-onset inhibition.
Figure 6.
Layer 3 fast-spiking cells preferentially fire late during trains of LOT stimuli and receive only recurrent excitation. (A) Traces from a voltage-clamped pyramidal cell (black; 0mV) and cell-attached L3 FS cell (green) demonstrating that threshold L3 FS cell firing late during trains of LOT stimuli overlaps with late-onset inhibition. (B) Summary histogram of AP latencies during threshold firing of L3 FS cells (black, n=8). Threshold firing distribution of L1a interneurons from Figure 4 (grey bars) is plotted for comparison. Histograms represent average probability distribution in 2-ms time bins for the two populations. (C) Simultaneous whole-cell voltage-clamp recording (same cell pair in A; -90 mV) demonstrating monosynaptic LOT-evoked EPSCs in the pyramidal cell (black) and delayed, polysynaptic EPSCs in the L3 FS cell (green). Inset, FS cell spiking (scale bar, 500 ms, 50 mV). (D) Top, expanded and normalized traces of individual EPSCs during the first stimulus pulse for the L3 FS cell (green) and the L2 pyramidal (black) cell in C. Bottom, summary of latency differences (n=6 pairs). (E) Facilitation of LOT-evoked polysynaptic EPSCs in L3 FS cells (green) and direct LOT-evoked EPSCs in pyramidal cells (black).
Additionally, FS cells fired at a longer latency after each stimulus pulse than L1a interneurons (Fig. 6B), suggesting that L3 FS cells are not recruited by monosynaptic LOT excitation. To test this directly, we made voltage-clamp recordings to study the underlying EPSCs evoked by trains of LOT input (Fig. 6C). The onset of LOT-evoked EPSCs onto L3 FS cells occurred 4.4 ± 0.9 ms after monosynaptic LOT-evoked EPSCs onto pyramidal cells (first pulse, Fig. 6D; fifth pulse latency, 3.1+0.5 ms, n=6). This delay indicates that LOT-evoked responses in L3 FS cells are not mediated by direct excitation from M/T cell axons, rather they arise through recurrent excitatory connections from local pyramidal cells. Indeed, during trains of LOT stimulation, the amplitude of recurrent excitation in L3 FS cells facilitated in parallel with the monosynaptic excitation received by pyramidal cells (Fig. 6E). Similar to L1a interneurons, the amplitude of the EPSC producing threshold firing in L3 FS cells averaged 454 ± 129 pA (n=11). These results show that late-onset firing of L3 FS cells is due to the progressive increase in recurrent excitation from pyramidal cells, which are themselves recruited to fire by facilitating M/T cell inputs.
In a subset of simultaneously recorded cell pairs (n=2), we directly confirmed that L3 FS cells underlie polysynaptic inhibition evoked by LOT stimulation (Fig. 7A,B). For these pairs, when stimulus strength was set at the threshold for eliciting late-onset inhibition, successes and failures of long-latency IPSCs in the pyramidal cell co-varied with APs from the cell-attached FS cell (Fig. 7A). Subsequent whole-cell recording from the FS cell revealed that it made a direct inhibitory connection onto the pyramidal cell with an IPSC amplitude identical to that of successful long-latency IPSCs evoked by LOT stimulation (Fig. 7B).
Figure 7.
Fast-spiking cells in layer 3 mediate feedback inhibition and target the perisomatic compartment of layer 2 pyramidal cells. (A) Simultaneous recording from a cell-attached L3 FS cell (top) and voltage-clamped pyramidal cell (0 mV, bottom). The third pulse in a burst of LOT stimuli generated a reliable feedforward IPSC followed by successes and failures of long-latency feedback IPSCs. APs in the L3 FS cell co-sorted with successes of large, long-latency IPSCs. (B) Subsequent current-clamp recording confirmed that the L3 interneuron was a FS cell (scale bar 500 ms, 50 mV) and that single APs evoked uIPSCs in the pyramidal cell with an amplitude equivalent to the large feedback IPSCs elicited by LOT stimulation. (C) Reconstruction of biocytin-filled L3 FS cell axon (red) and dendrites (blue). Inset shows threshold firing response (scale bar, 500 ms, 50 mV). Dashed line, layer 1/2 border. (D) Laminar depth of FS cell neurites (n=3 cells, 10 μm bins) demonstrates segregation of dendrites within L3 and high density of axons within the pyramidal cell layer. Sample pyramidal cell included for comparison. Dashed lines indicating laminar borders are drawn at mean distance from layer 1/2 border; grey bar indicates the mean location of the soma (± SEM).
Somatic-targeting L3 FS cells are highly interconnected with local pyramidal cells
To determine if L3 FS cells provide polysynaptic inhibition that targets the somatic compartment (Fig. 1), we reconstructed the dendritic and axonal arbors of biocytin-filled L3 FS interneurons. The dendrites of L3 FS cells were most concentrated within L3; more importantly, their dendrites did not extend into layer 1 (Fig. 7C,D). These anatomical data are consistent with our findings that L3 FS cells do not receive direct excitatory input from the LOT but rather are situated to receive recurrent collateral input from L2/3 pyramidal cell axons. The axons of L3 FS cells ramified extensively in L2/3 (on average, 95 ± 3% of total axon length, n=3), avoiding L1 and the distal apical dendrites of pyramidal cells (Fig. 7D). Furthermore, axon segments often gave rise to boutons surrounding the somata of L2/3 cells (not shown), indicating they formed perisomatic “baskets”. Thus, L3 FS interneurons have anatomical properties ideal for governing somatic feedback inhibition.
We further examined the connectivity of L3 FS interneurons and nearby pyramidal cells using paired whole-cell recording. In a connected cell pair, single APs in the FS cell caused short latency (0.65 ± 0.09 ms, n=10) IPSCs in the pyramidal cell. In some pairs, single APs in the pyramidal cell could also generate a unitary excitatory postsynaptic current (uEPSC) in the interneuron, indicating a reciprocal connection (Fig. 8A). Across all paired recordings, the mean uIPSC conductance was 1.1 ± 0.3 nS (n=22). Consistent with the idea that FS cells target the perisomatic compartment, unitary IPSCs from FS cells onto pyramidal cells had rapid rise times (0.68 ± 0.15 ms, n=10). These rise times were identical to those of the late-onset IPSCs evoked by trains of LOT stimulation (0.6 ms) and significantly faster than those of the dendritic uIPSCs generated by L1a interneurons (2.0 ms; p = 0.001). The amplitudes of uEPSCs onto FS cells averaged 43 ± 17 pA (range 9 to 209 pA, Vm=-90 mV, n=11) and their kinetics were fast (decay tau=2.63 ± 0.4 ms). Given that FS cells require ∼450 pA of excitatory input to reach AP threshold, these results suggest that the coincident activity of relatively few pyramidal cells can elicit somatic feedback inhibition.
Figure 8.
L3 FS cells are highly interconnected with pyramidal cells in piriform cortex. (A) Paired recording of a L3 FS cell (green) and connected pyramidal cell (black). A single AP in the L3 FS cell (current clamp) produces a short-latency IPSC in the voltage-clamped (-40 mV) pyramidal cell. A single AP in the pyramidal cell (current clamp) produces a short-latency EPSC in the FS cell. Left inset, responses of the cells to current steps (scale bar, 500 ms, 50 mV). (B) Summary of connectivity between L3 FS and pyramidal cells. (C) Unitary EPSCs from L2 pyramidal cells onto L3 FS cells display short-term depression. (D) Unitary IPSCs from L3 FS cells onto L2 pyramidal cells also display short-term depression.
Individual L3 FS cells made highly divergent connections, inhibiting 35% of local pyramidal cells and receiving direct excitatory connections from 18% of pyramidal cells tested (n=64 pairs; Fig. 8B). Intriguingly, the rate of reciprocal connections was higher than predicted by random connectivity: in a cell pair without an excitatory connection, the likelihood that the interneuron would connect to the pyramidal cell was 26% (14/53 pairs) while the probability of an inhibitory connection was significantly higher in cell pairs with an excitatory connection (73%, 8/11 pairs, p < 0.01, Fisher's Exact Test). The strength of unitary inhibitory connections tended to be stronger between reciprocally-connected pairs compared to one-way connections (1.73 ± 0.65, n=14 and 0.66 ± 0.21 nS, n=8, respectively; p = 0.1, KS test). In response to trains of APs from pyramidal cells, the amplitudes of uEPSCs in L3 FS cells strongly depressed (P5/P1 = 0.32 ± 0.05, n=11, Fig. 8C). Unitary inhibitory connections from L3 FS cells onto pyramids also depressed during AP trains (P5/P1 = 0.53 ± 0.03, n=22, Fig. 8D). Together, these results show that L3 FS cells mediate widespread recurrent inhibition across the cortical population and are biased to inhibit those pyramidal cells that directly excite them.
Strong recurrent excitation of L3 FS cells drives widespread feedback inhibition
To determine the relative strength of recurrent inhibition and excitation evoked by local activity, we used an optogenetic approach to selectively activate small populations of pyramidal cells in olfactory cortex. Ntsr1-cre mice (line Ntsr1-creGN209 from the GENSAT project) express Cre recombinase in a fraction of L2/3 pyramidal cells of piriform cortex, but not in pyramidal cells of other cortical regions or in inhibitory cells (Fig. 9A, Methods). We used an adeno-associated virus (rAAV-FLEX-rev-ChR2-tdTomato) to drive Cre-dependent co-expression of the light-activated channel channelhodopsin-2 (ChR2) (Atasoy et al., 2008; Petreanu et al., 2009; Zhang et al., 2006) and the fluorescent protein tdTomato. We took advantage of both the restricted expression of Cre recombinase and focal delivery of virus to ensure that ChR2 was only expressed in anterior piriform cortex (Supplemental Fig. 3). Slices from these animals revealed tdTomato expression restricted to sparse populations of piriform cortex L2/3 cells (Fig. 9A); all fluorescent cells had pyramidal morphology and targeted recordings demonstrated firing properties consistent with pyramidal cells (Fig. 9B, n=7).
Figure 9.

Recurrent inhibition dominates excitation between pyramidal cells while L3 FS cells receive the strongest recurrent excitation. (A-B) Selective expression of ChR2 in L2/3 pyramidal cells in Ntsr1-cre mice. (A) Left, fixed thin section of anterior piriform cortex from a mouse generated by a cross between an Ntsr1-cre and a Rosa-YFP mouse. Green, YFP; red, GAD-67 immunofluorescence. Cells expressing YFP do not overlap with those immunolabeled for GAD-67. Right, acute slice of piriform cortex from an Ntsr1-cre mouse injected focally with AAV-FLEX-ChR2-tdTomato. (B) Current-clamp recording shows regular-spiking firing pattern of a ChR2-tdTomato expressing pyramidal cell. A brief (1 ms) pulse of blue light (470 nm) reliably generates a single action potential in the same cell (7 traces overlaid). (C) A brief (1 ms) flash of 470 nm light elicits a small EPSC and large IPSC in a neighboring uninfected pyramidal cell. Both excitatory and inhibitory currents were abolished in the presence of glutamate receptor antagonists (+APV/NBQX). (D) Summary plot of the inhibitory and excitatory conductance elicited in pyramidal cells by activation of ChR2. Inset: ratio of inhibition to excitation. (E) ChR2-evoked EPCSs in a simultaneously recorded L3 FS (green) and pyramidal cell (black). (F) Summary data showing that recurrent excitation is larger in L3 FS cells. Filled circles, average EPSC amplitude (-90 mV) in L3 FS (green) and pyramidal (black) cells. (G) A brief light pulse evokes a large EPSC in a L3 FS cell (green) but no response in a simultaneously recorded L1a interneuron (blue) (H) Summary data showing that recurrent excitation is larger in L3 FS cells than in L1a interneurons. Filled circles, average EPSC amplitude (-90 mV) in L3 FS cells (green) and L1a interneurons (blue). Scale bars, 500 ms, 50 mV.
In cells expressing tdTomato-ChR2, brief (1 ms) pulses of 470-nm light activated large ChR2-mediated currents (>1 nA, not shown) that were sufficient to cause reliable, short-latency AP firing (Fig. 9B). We examined the relative balance of recurrent excitation and inhibition within the cortical circuit by making voltage-clamp recordings from neighboring pyramidal cells that did not express ChR2. While brief light pulses evoked both EPSCs and IPSCs, synaptic responses were dominated by inhibition (Fig. 9C,D). Application of NBQX (10 μM) and APV (50 μM) abolished ChR2-evoked IPSCs (n=4), confirming they were elicited by recurrent excitation. On average, the inhibitory conductance was 8.4 ± 2.4 times larger than the excitatory conductance (Fig. 9D, n=7). Thus, pyramidal cell activity favors the recruitment of local recurrent inhibition rather than excitation across the pyramidal cell population.
We measured the amplitude of light-evoked EPSCs in pyramidal cell-L3 FS cell pairs (Fig. 9E) to compare the relative amount of recurrent excitation these two cell types receive. The amplitude of light-evoked EPSCs was always larger in L3 FS cells than in pyramidal cells (117 ± 28 pA and 11 ± 7 pA, respectively; n=13 pairs, Fig. 9F). Given the low rate of excitatory connections between pyramidal cells and L1a interneurons, we hypothesized that L1a interneurons would receive little recurrent excitation in response to ChR2-evoked activation of the local circuit. Indeed, while brief light pulses always elicited excitation in L3 FS cells (9/9 cells) we rarely observed EPSCs in simultaneously recorded L1a interneurons (3/9 cells, Fig. 9G). In these paired recordings, EPSC amplitude averaged 247 ± 152 pA in L3 FS cells but only 12 ± 11 pA in L1a interneurons (n=9, Fig. 9H). These results reveal that compared to pyramidal cells and layer 1a interneurons, L3 FS cells receive the most recurrent excitation, further highlighting their importance as a major circuit for feedback inhibition in piriform cortex
Discussion
Routing of inhibition from dendrite to soma
Weak LOT input triggers short-latency disynaptic inhibition exclusively onto the apical dendritic compartment of pyramidal cells in piriform cortex. However, dendritic feedforward inhibition rapidly wanes during bursts of LOT stimulation that mimic the firing pattern of M/T cells in response to odors in vivo. We find that increasing stimulus intensity leads to the subsequent appearance of somatic inhibition that is preferentially recruited late during bursts of input. Intriguingly, this progressive shift of inhibition along the somato-dendritic axis in piriform cortex occurs in the opposite direction to that generally found in other brain regions. For example, in hippocampal and neocortical circuits, excitatory stimuli elicit transient somatic inhibition and delayed dendritic inhibition (Gabernet et al., 2005; Higley and Contreras, 2006; Kapfer et al., 2007; Pouille and Scanziani, 2001, 2004; Silberberg and Markram, 2007; Tan et al., 2008). In piriform cortex, afferent sensory input is uniquely localized to the distal apical dendritic layer, thus the shift in inhibition in piriform cortex represents a logical transition from a region of synaptic integration to a region of spike output.
M/T cell input drives transient dendritic feedforward inhibition from L1a interneurons
We identified dendritic-targeting L1a interneurons as a major source of disynaptic feedforward inhibition in olfactory cortex. In neocortex, feedforward interneurons are driven reliably by powerful thalamic input and single thalamic fibers make stronger connections onto interneurons compared to principal cells (Cruikshank et al., 2007; Gabernet et al., 2005; Hull et al., 2009). In piriform cortex, while the strengths of single M/T cell axon inputs onto feedforward interneurons and pyramidal cells are similar, L1a interneurons are contacted by more inputs. If axons from M/T cells of different glomeruli make random connections, this would mean that L1a interneurons receive excitation from a broader pool of glomeruli than pyramidal cells. Our results also suggest that M/T cell inputs have target cell specific differences in transmitter release probability: the short-term depression of LOT inputs onto interneurons compared to the facilitation onto pyramidal cells suggests a higher release probability at synapses onto interneurons (Koester and Johnston, 2005; Scanziani et al., 1998; Zucker and Regehr, 2002). Thus multiple mechanisms ensure that L1a interneurons are reliably activated by M/T cell spiking. A recent study of neurogliaform and horizontal cells in L1a of GAD67-GFP mice is generally consistent with our description of L1a interneurons, and our results likely include both cell types (Suzuki and Bekkers, 2010).
We find that the strength of dendritic feedforward inhibition (IPSC conductance) and direct excitation (EPSC conductance) onto pyramidal cells is balanced during low-frequency activation of LOT inputs. This is similar to observations in thalamocortical circuits where the relative strength of short-latency inhibition is equal to or even exceeds that of monosynaptic excitation (Cruikshank et al., 2007; Gabernet et al., 2005; Higley and Contreras, 2006; Wehr and Zador, 2003). In thalamocortical circuits both excitation and inhibition depress during trains (Gabernet et al., 2005; Higley and Contreras, 2006), however bursts of M/T cell input lead to a strong facilitation of direct excitation and depression of feedforward inhibition in piriform cortex. We predicted that the interplay between these two opposing dendritic signals would act synergistically to enforce temporal summation. Indeed, we found that dendritic inhibition curtails early spike output during bursts of M/T cell input.
Late-onset feedback somatic inhibition is mediated by layer 3 interneurons
Given that piriform cortex pyramidal cells preferentially fire late during bursts of input, recurrent inhibition should track this activity. Indeed, we show that LOT stimulation recruits late-onset inhibition from L3 FS interneurons, which are driven exclusively through a feedback mechanism. L3 FS cells have anatomical features and spike firing patterns consistent with parvalbumin (PV)-positive, somatic-targeting interneurons found in many brain regions (Markram et al., 2004; Somogyi et al., 1998). In piriform cortex, L3 FS cells have axons that exclusively target the somatic compartment and dendrites that receive no direct M/T cell inputs. Our findings are in agreement with a recent description of PV+ L3 multipolar cells in piriform cortex of GAD67-GFP mice (Suzuki and Bekkers, 2010). In addition, a somatic inhibitory current (Itrunc) recruited by LOT stimulation has been proposed to limit synaptic integration via a disynaptic feedforward mechanism (Luna and Schoppa, 2008). However, the long latency (∼10 ms) between excitation and Itrunc as well as its somatic location are more consistent with the recurrent inhibition mediated by L3 FS cells we describe here.
L3 FS cells make unitary inhibitory connections onto a large fraction (35%) of pyramidal cells, similar to the high connectivity rates reported for somatic targeting FS cells in other circuits (Glickfeld et al., 2008; Holmgren et al., 2003; Yoshimura and Callaway, 2005). The majority of unitary excitatory connections from pyramidal cells onto FS cells were reciprocated by inhibitory connections, as previously found in visual cortex (Yoshimura and Callaway, 2005). While the high reciprocal connectivity suggests a degree of specificity in their wiring, unidirectional inhibitory connections were most common. Since individual FS cells always inhibit more pyramidal cells than those that directly excite them, this divergence of FS cell output provides a mechanism for “lateral” inhibition.
We sought to define the relative influence of recurrent excitation vs. recurrent inhibition in the cortical population, however the piriform cortex receives extrinsic input from a variety of cortical areas (Haberly, 2001) making it impossible to selectively activate recurrent connections with a conventional stimulating electrode. To circumvent this problem, we used viral delivery of Cre-dependent ChR2 to selectively activate pyramidal cells of anterior piriform cortex. We found that recurrent inhibition greatly outweighed recurrent excitation. L3 FS cells received much stronger recurrent excitation than pyramidal cells and likely underlie the strong recurrent inhibition in response to activation of local pyramidal cells. While the properties of L3 FS cells are most consistent with late-onset somatic inhibition, our results do not rule out the possibility that other subtypes of interneurons participate in recurrent inhibition.
Implications for olfactory information coding
Inhibition is an important feature of odor-evoked responses in both the piriform cortex of rodents (Poo and Isaacson, 2009) and the mushroom body, an analogous region of the insect brain (Laurent, 2002). In both systems, odors evoke sparse population responses that arise from specific excitation and broadly tuned inhibition. In locusts, feedforward interneurons located in the lateral horn govern broadly tuned inhibition and short integration time windows for precise spike timing during odor-evoked oscillations in synaptic activity (Laurent, 2002; Perez-Orive et al., 2002). In piriform cortex, global (widespread and nonselective) inhibition has been proposed to result from local interneurons that receive ubiquitous and broadly tuned excitation (Poo and Isaacson, 2009), and odor-evoked oscillations (15-30 Hz) in excitation and inhibition are thought to constrain spike timing (Poo and Isaacson, 2009). Both local feedforward and feedback circuits are likely to contribute to global inhibition and the regulation of spike output in piriform cortex.
L1a interneurons have many features optimized for producing broadly tuned feedforward inhibition in response to odors: they receive a high convergence of M/T cell inputs (likely sampled from different glomeruli), require coincident activity from few inputs to fire, and make inhibitory contacts onto the dendrites of many pyramidal cells. We show that dendritic feedforward inhibition is most effective at limiting the integration of excitatory sensory input under conditions when M/T cells fire sparsely (i.e. one or few APs). However, bursts of APs from strongly activated M/T cells reduce the effectiveness of feedforward inhibition, permitting pyramidal cells to reach spike threshold via the temporal summation of EPSPs. Together, these features suggest that dendritic feedforward inhibition limits cortical responses to temporally sparse M/T cell activity while promoting the representation of odors causing bursts of activity. Our results do not exclude the possibility that dendritic feedforward inhibition could contribute to coincidence detection (Pouille and Scanziani, 2001) in piriform cortex: pyramidal cells receiving inputs only from very weakly activated M/T cells (each firing a single AP) could reach spike threshold only if M/T cells were active within a very narrow (<2 ms) time window before the onset of feedforward inhibition. However, whether weakly activated M/T cells fire with such synchrony under physiological conditions is unclear. Rather, we propose that temporally dynamic feedforward inhibition acts largely as a salience filter to selectively enhance the cortical representation of strongly active (bursting) M/T cells.
L3 FS interneurons read out pyramidal cell activity and produce somatic inhibition across the local cortical population. Individual L3 FS cells contact many pyramidal cells and thus can mediate lateral inhibition across the cortical population. Pyramidal cells in piriform cortex that respond to a given odorant are broadly distributed without spatial preference (Stettler and Axel, 2009). Given that L3 FS cell dendrites branch extensively, they likely receive excitatory input from pyramidal cells that are preferentially tuned to respond to different odorants. This makes it likely that L3 FS cells participate in odor-evoked inhibition that is broadly tuned; however it is also possible that pyramidal cells co-tuned to respond to the same odorant form specific subcircuits with particular interneurons. The reciprocal connectivity between L3 FS cells and pyramidal cells also suggests a mechanism for oscillations in recurrent excitation and inhibition in response to odor-evoked input. Indeed, in other systems FS cells have been proposed to contribute to the phasing of pyramidal cell firing during oscillations (Cardin et al., 2009; Cobb et al., 1995). In the simplest case, this feedback circuit contributes to sparse odor representations by regulating the amplification and distribution of excitation by associative connections.
Materials and Methods
Experiments followed approved national and institutional guidelines for animal use. Rats (Sprague-Dawley, P14-P25) were anesthetized with ketamine and xylazine (100 mg and 10 mg per kg, respectively) and decapitated. The cortices were quickly removed and placed into ice cold artificial CSF (aCSF) containing (in mM): 83 NaCl, 2.5 KCl2, 0.5 CaCl2, 3.3 MgSO4, 1 NaH2PO4, 26.2 NaHCO3, 22 glucose, and 72 sucrose, equilibrated with 95% O2 and 5% CO2. Parasagittal slices (350 μm) of anterior piriform cortex were cut using a vibrating slicer (Vibratome) and incubated at 34°C for 30 min. For some experiments, we confirmed that coronal slices gave identical results. Slices were transferred to a recording chamber on an upright microscope equipped with infrared, differential interference contrast optics (BX50WI; Olympus) and superfused with aCSF containing (in mM): 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgSO4, 1 NaH2PO4, 26.2 NaHCO3 and 22 glucose, equilibrated with 95% O2 and 5% CO2. All experiments were conducted at 28-30°C.
Recordings were collected using a Multiclamp 700B amplifier (Molecular Devices), digitized at 10 kHz and analyzed with AxographX software. For voltage clamp recordings, pipettes (3-5 MΩ) typically contained a cesium-based internal solution (in mM: 130 D-Gluconic acid, 130 CsOH, 5 NaCl, 10 HEPES, 10 EGTA, 12 phosphocreatine, 3 Mg-ATP and 0.2 Na-GTP (pH 7.3). For all current clamp (and some voltage clamp recordings), pipettes contained a K+-based internal (in mM: 150 potassium gluconate, 1.5 MgCl2, 5 HEPES buffer, 0.1 EGTA 10 phosphocreatine and 2.0 Mg-ATP; pH 7.4; 0.2% biocytin added for interneuron recordings). For most pyramidal cell recordings, a fluorescent dye (Alexa 488, 20 μM, Invitrogen) was added to the internal solution to confirm the presence of intact apical dendrites. Voltages were corrected for a junction potential of 15 mV. Series resistance was <20 MΩ and compensated by >80% and recordings were terminated if series resistance increased by >20%. Unless noted otherwise, synaptic responses were evoked at 0.1-0.2 Hz with a bipolar stimulating electrode placed in the LOT. The stimulating electrode was placed >300 μm lateral of the recorded cells to reduce monosynaptic activation of interneurons. Unless stated otherwise, individual traces show the average of 10-20 trials and values are mean ± SEM.
L1a and L3 interneurons could be easily identified by position of their soma, morphology, and their intrinsic properties. Deep L2 pyramidal cells were identified by their position in the lower half of the dense cell body layer, a triangular soma with prominent apical dendrite, and intrinsic properties (Suzuki and Bekkers, 2006). For studying connections between L1a interneurons and pyramidal cells, we made recordings from 317 interneuron-pyramidal cell pairs, of which 27 (8.8%) had unitary connections. However, the laminar distance between the cell bodies in these paired recordings could be >200 μm. To control for slices in which the interneuron axonal arbor may have been partially severed, we first identified an interneuron that evoked an IPSC in a pyramidal cell. Then, while keeping the interneuron, we recorded sequentially from additional pyramidal cells. Thus, the probability of connections from interneurons to pyramidal cells was computed exclusively using the additional pairs—that is, it did not include the initial pair used to identify the intact interneuron. For L3 FS cells, all cell pairs (n=64) were used to determine the probability of connections, since connections were tested in cells with nearby somata (<150 μm). Biocytin-filled cells were revealed by a DAB reaction with nickel intensification. Cells were manually reconstructed and neurite length measured using Neurolucida (MBF).
Gabazine was applied focally via a pipette (10 μm tip diameter) using pressure (10-20 p.s.i, 20 ms, Picospritzer). Pulses (2-4) were applied at the start of stimulus trials first close to the soma of the recorded cell and then after the pipette was translated parallel to the apical dendrite and positioned in L1a. In half the experiments, gabazine was first applied to the dendrites and following recovery of the response the antagonist was applied to the cell body. For current clamp experiments examining the role of dendritic inhibition, pyramidal cells were held depolarized by 10 mV. This allowed us to study integration of bursts of weak LOT excitation in individual pyramidal cells without recruiting widespread recurrent inhibition from the cortical population.
Ntsr1-cre animals (Tg(Ntsr1-cre)209Gsat) were obtained from the Gensat Project and the full expression pattern of Cre-recombinase in this line can be viewed at http://www.gensat.org/creGeneView.jsp?founder_id=44880&gene_id=511. Ntsr1-cre mice were crossed with the Rosa-YFP reporter line (Gt(ROSA)26Sortm1(Smo/ (EYFP)Cos/J, Jackson Laboratory) to produce mice expressing YFP in pyramidal cells of piriform cortex. For immunohistochemistry, whole brains were fixed in 4% PFA and frozen in 20% sucrose/PBS before being cut into thin (50 μm) sections using a sliding microtome. GABAergic interneurons were revealed using anti-GAD-67 (clone 5406, Millipore) and goat-anti mouse conjugated with Alexa 594 (A-11032, Invitrogen). Slices were mounted in Vectashield with DAPI (Vector Labs). All YFP-expressing cells targeted in acute slices had regular spike-firing properties typical of pyramidal cells (7/7, not shown).
High-titer (1.2*1012) stock of AAV-FLEX-rev-ChR2-tdTomato was produced from Addgene plasmid #18917 by the University of Pennsylvania Vector Core. Mouse pups from crosses of Ntsr1-cre heterozygotes and ICR WT mice were injected at p0-2 since the skull at this age is soft enough to be penetrated by an injection pipette. Pups were isoflurane-anesthetized and positioned in a custom-made mold. Injections were targeted to the anterior piriform cortex based on empirically determined landmarks including the posterior border of the eye and the superficial temporal vein. Injections (13 nl) were made using a Nanoject II injector (Drummond Scientific) fitted with a pulled glass beveled micropipette. Location and depth were controlled using a 3-axis micromanipulator. Anterior piriform cortex was injected at a depth of (0.25-0.5 mm) and the pipette was kept at each site for 30 seconds to allow virus to spread locally. Experiments were performed from animals (p30-40) in which only one hemisphere expressed ChR2-tdTomato and ipsilateral slices were used for recording. We confirmed that the major anatomical and functional properties of L3 FS and L1a interneurons were equivalent to those characterized in rats (not shown). For photostimulation, light from a 470-nm LED (Thorlabs) was collimated and delivered via the 40× objective positioned over L2/3.
Supplementary Material
Supplemental Figure 1. The temporal structure of inhibition depends on LOT stimulation intensity. (A) Total inhibitory charge transfer (pC) evoked in L2/3 pyramidal cells (n=6) during bursts at three LOT stimulus intensities. Low LOT stimulation intensity (blue) only evokes early-transient (feedforward) inhibition. Increasing stimulation intensity enhances early-transient inhibition and begins to recruit late-onset (feedback) inhibition (green). Higher stimulus intensity that is suprathreshold for recruiting both feedforward and feedback inhibition causes late-onset IPSCs to dominate total charge transfer. (B) The distribution of total inhibitory charge with respect to pulse number at the three stimulus intensities depicted in (A).
Supplemental Figure 2. Intrinsic electrical properties and axonal morphologies of L1a interneurons grouped by spike firing patterns. (A) Spiking profiles generated in response to depolarizing current steps could be characterized as one of three patterns: non-adapting, irregular spiking, and late-spiking. (B) Intrinsic electrical properties were indistinguishable between L1a interneurons grouped by these firing patterns. Black bars are average ± SEM for all cells. (C) L1a interneurons with different spike firing patterns have similar laminar distributions of their axons. The amount of total axon length concentrated in layer 1 was not different between categories (non-adapting, 81 ± 5%; late-spiking, 79 ± 7%; irregular-spiking, 83 ± 6%). We also found no consistent differences in the synaptic properties or connectivity of L1a interneurons when grouped by spike-firing patterns.
Supplemental Figure 3. Whole mount of the brain from an Ntsr1-cre mouse showing focal expression of ChR2-tdTomato in the anterior piriform cortex. (A) Ventral view of the brain highlighting the olfactory bulbs (OB), lateral olfactory tract (LOT), and anterior piriform cortex (APC). (B) Fluorescence image showing focal tdTomato expression (red spot). (C) Overlay of the two images indicates localized expression of tdTomato in anterior piriform cortex.
Acknowledgments
We thank Dan Padgett for his contribution to the early phase of this project. We are indebted to M. Scanziani for helpful discussions and to C. Poo, H. Adesnik, and R. Malinow for advice and encouragement. We are thankful to Paul Abelkop for technical assistance. Supported by NIDCD (R01DC04682, J.S.I.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson JS, Carandini M, Ferster D. Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. J Neurophysiol. 2000;84:909–926. doi: 10.1152/jn.2000.84.2.909. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathellier B, Buhl DL, Accolla R, Carleton A. Dynamic ensemble odor coding in the mammalian olfactory bulb: sensory information at different timescales. Neuron. 2008;57:586–598. doi: 10.1016/j.neuron.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Cang J, Isaacson JS. In vivo whole-cell recording of odor-evoked synaptic transmission in the rat olfactory bulb. J Neurosci. 2003;23:4108–4116. doi: 10.1523/JNEUROSCI.23-10-04108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci. 2007;10:462–468. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- Ekstrand JJ, Domroese ME, Feig SL, Illig KR, Haberly LB. Immunocytochemical analysis of basket cells in rat piriform cortex. J Comp Neurol. 2001;434:308–328. [PubMed] [Google Scholar]
- Ferster D, Jagadeesh B. EPSP-IPSP interactions in cat visual cortex studied with in vivo whole-cell patch recording. J Neurosci. 1992;12:1262–1274. doi: 10.1523/JNEUROSCI.12-04-01262.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks KM, Isaacson JS. Synapse-specific downregulation of NMDA receptors by early experience: a critical period for plasticity of sensory input to olfactory cortex. Neuron. 2005;47:101–114. doi: 10.1016/j.neuron.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Franks KM, Isaacson JS. Strong single-fiber sensory inputs to olfactory cortex: implications for olfactory coding. Neuron. 2006;49:357–363. doi: 10.1016/j.neuron.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48:315–327. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Glickfeld LL, Atallah BV, Scanziani M. Complementary modulation of somatic inhibition by opioids and cannabinoids. J Neurosci. 2008;28:1824–1832. doi: 10.1523/JNEUROSCI.4700-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Higley MJ, Contreras D. Balanced excitation and inhibition determine spike timing during frequency adaptation. J Neurosci. 2006;26:448–457. doi: 10.1523/JNEUROSCI.3506-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren C, Harkany T, Svennenfors B, Zilberter Y. Pyramidal cell communication within local networks in layer 2/3 of rat neocortex. J Physiol. 2003;551:139–153. doi: 10.1113/jphysiol.2003.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, Isaacson JS, Scanziani M. Postsynaptic mechanisms govern the differential excitation of cortical neurons by thalamic inputs. J Neurosci. 2009;29:9127–9136. doi: 10.1523/JNEUROSCI.5971-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfer C, Glickfeld LL, Atallah BV, Scanziani M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat Neurosci. 2007;10:743–753. doi: 10.1038/nn1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester HJ, Johnston D. Target cell-dependent normalization of transmitter release at neocortical synapses. Science. 2005;308:863–866. doi: 10.1126/science.1100815. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature. 1999;398:338–341. doi: 10.1038/18686. [DOI] [PubMed] [Google Scholar]
- Laurent G. Olfactory network dynamics and the coding of multidimensional signals. Nat Rev Neurosci. 2002;3:884–895. doi: 10.1038/nrn964. [DOI] [PubMed] [Google Scholar]
- Luna VM, Schoppa NE. GABAergic circuits control input-spike coupling in the piriform cortex. J Neurosci. 2008;28:8851–8859. doi: 10.1523/JNEUROSCI.2385-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margrie TW, Schaefer AT. Theta oscillation coupled spike latencies yield computational vigour in a mammalian sensory system. J Physiol. 2003;546:363–374. doi: 10.1113/jphysiol.2002.031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Neville KR, Haberly LB. Olfactory Cortex. 5th. New York: Oxford University Press; 2004. [Google Scholar]
- Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. An early critical period for long-term plasticity and structural modification of sensory synapses in olfactory cortex. J Neurosci. 2007;27:7553–7558. doi: 10.1523/JNEUROSCI.1786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. Odor representations in olfactory cortex: “sparse” coding, global inhibition, and oscillations. Neuron. 2009;62:850–861. doi: 10.1016/j.neuron.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Routing of spike series by dynamic circuits in the hippocampus. Nature. 2004;429:717–723. doi: 10.1038/nature02615. [DOI] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Sparse odor coding in awake behaving mice. J Neurosci. 2006;26:8857–8865. doi: 10.1523/JNEUROSCI.0884-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BD, Katz LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron. 1999;23:499–511. doi: 10.1016/s0896-6273(00)80803-x. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Gahwiler BH, Charpak S. Target cell-specific modulation of transmitter release at terminals from a single axon. Proc Natl Acad Sci U S A. 1998;95:12004–12009. doi: 10.1073/pnas.95.20.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg G. Polysynaptic subcircuits in the neocortex: spatial and temporal diversity. Curr Opin Neurobiol. 2008;18:332–337. doi: 10.1016/j.conb.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron. 2007;53:735–746. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Soucy ER, Albeanu DF, Fantana AL, Murthy VN, Meister M. Precision and diversity in an odor map on the olfactory bulb. Nat Neurosci. 2009;12:210–220. doi: 10.1038/nn.2262. [DOI] [PubMed] [Google Scholar]
- Spors H, Grinvald A. Spatio-temporal dynamics of odor representations in the mammalian olfactory bulb. Neuron. 2002;34:301–315. doi: 10.1016/s0896-6273(02)00644-x. [DOI] [PubMed] [Google Scholar]
- Stettler DD, Axel R. Representations of odor in the piriform cortex. Neuron. 2009;63:854–864. doi: 10.1016/j.neuron.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Bekkers JM. Neural coding by two classes of principal cells in the mouse piriform cortex. J Neurosci. 2006;26:11938–11947. doi: 10.1523/JNEUROSCI.3473-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Bekkers JM. Distinctive Classes of GABAergic Interneurons Provide Layer-Specific Phasic Inhibition in the Anterior Piriform Cortex. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z, Hu H, Huang ZJ, Agmon A. Robust but delayed thalamocortical activation of dendritic-targeting inhibitory interneurons. Proc Natl Acad Sci U S A. 2008;105:2187–2192. doi: 10.1073/pnas.0710628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Takahashi YK, Tanifuji M, Mori K. Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nat Neurosci. 2000;3:1035–1043. doi: 10.1038/79857. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron. 2001;32:723–735. doi: 10.1016/s0896-6273(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- Wilent WB, Contreras D. Dynamics of excitation and inhibition underlying stimulus selectivity in rat somatosensory cortex. Nat Neurosci. 2005;8:1364–1370. doi: 10.1038/nn1545. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Kadohisa M, Fletcher ML. Cortical contributions to olfaction: plasticity and perception. Semin Cell Dev Biol. 2006;17:462–470. doi: 10.1016/j.semcdb.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Callaway EM. Fine-scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nat Neurosci. 2005;8:1552–1559. doi: 10.1038/nn1565. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods. 2006;3:785–792. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. The temporal structure of inhibition depends on LOT stimulation intensity. (A) Total inhibitory charge transfer (pC) evoked in L2/3 pyramidal cells (n=6) during bursts at three LOT stimulus intensities. Low LOT stimulation intensity (blue) only evokes early-transient (feedforward) inhibition. Increasing stimulation intensity enhances early-transient inhibition and begins to recruit late-onset (feedback) inhibition (green). Higher stimulus intensity that is suprathreshold for recruiting both feedforward and feedback inhibition causes late-onset IPSCs to dominate total charge transfer. (B) The distribution of total inhibitory charge with respect to pulse number at the three stimulus intensities depicted in (A).
Supplemental Figure 2. Intrinsic electrical properties and axonal morphologies of L1a interneurons grouped by spike firing patterns. (A) Spiking profiles generated in response to depolarizing current steps could be characterized as one of three patterns: non-adapting, irregular spiking, and late-spiking. (B) Intrinsic electrical properties were indistinguishable between L1a interneurons grouped by these firing patterns. Black bars are average ± SEM for all cells. (C) L1a interneurons with different spike firing patterns have similar laminar distributions of their axons. The amount of total axon length concentrated in layer 1 was not different between categories (non-adapting, 81 ± 5%; late-spiking, 79 ± 7%; irregular-spiking, 83 ± 6%). We also found no consistent differences in the synaptic properties or connectivity of L1a interneurons when grouped by spike-firing patterns.
Supplemental Figure 3. Whole mount of the brain from an Ntsr1-cre mouse showing focal expression of ChR2-tdTomato in the anterior piriform cortex. (A) Ventral view of the brain highlighting the olfactory bulbs (OB), lateral olfactory tract (LOT), and anterior piriform cortex (APC). (B) Fluorescence image showing focal tdTomato expression (red spot). (C) Overlay of the two images indicates localized expression of tdTomato in anterior piriform cortex.