Abstract
Endogenous tri-potential neural stem cells (eNSCs) exist in the adult spinal cord and differentiate primarily into oligodendrocytes (OLs) and astrocytes. Previous in vivo and in vitro studies have shown that during development proliferation and differentiation of oligodendrocyte progenitor cells (OPCs) depend on activity in neighboring axons. However, this activity-dependent development of OPCs has not been examined in the adult CNS. In the present study, we stimulated unilateral corticospinal (CS) axons of the adult rat and investigated proliferation and differentiation of OPCs in dorsal corticospinal tract (dCST). eNSCs were labeled with the mitotic indicator 5-Bromo-2′-deoxyuridine (BrdU). Phenotypes of proliferating cells were identified by double-immunolabeling of BrdU with a panel of antibodies to cell markers: NG2, Nkx2.2, APC, GFAP, and Glut-1. Electrical stimulation of CS axons increased BrdU labeled eNSCs and promoted the proliferation and differentiation of OPCs, but not astrocytes and endothelial cells. Our findings demonstrate the importance of neural activity in regulating OPC proliferation/differentiation in the mature CNS. Selective pathway electrical stimulation could be used to promote remyelination and recovery of function in CNS injury and disease.
Keywords: electrical activity, cell birth, oligodendrocyte progenitor cell, myelination, corticospinal tract
eNSCs exist in the adult mammalian CNS. In the spinal cord, eNSCs distribute throughout the entire cord, but exist predominately in white matter tracts. The phenotypic fate is glial, largely OL, but not neuronal [13]. These dividing cells contribute to the turnover of OLs with a low rate in intact cord and have potential for remyelination in pathological demyelination events [12].
Neuronal spiking activity is important for OL development, as it is for pathway development [18]. Electrical stimulation, augmenting neuronal activity, promotes development of OPCs and subsequent myelination of adjacent dorsal root ganglion axons in vitro [25] [10]. This is consistent with studies in developing optic nerve showing that OPC proliferation and myelination depend on the activity of neighboring axons [1] [6].
In this study we determined if applied electrical activity promoted the birth and differentiation of myelinating cells in the mature CNS. We unilaterally stimulated the corticospinal tract (CST) in adult rats, at the level of the medullary pyramid, using an approach that promotes CST outgrowth and strengthens CST connections with spinal neurons [5]. We anterogradely labeled CST axons with biotinylated dextran amine (BDA) and used BrdU and a panel of antibodies to glial markers to identify proliferating glia. We found that electrical stimulation selectively increased the proliferation and differentiation of OPCs in dCST compared to the unstimulated side and sham animals. Our findings have important implications for explaining the mechanism of myelin formation, harnessing activity to promote remyelination and devising new approaches to the treatment of multiple sclerosis (MS) and spinal cord injury (SCI).
Eight female Sprague Dawley rats (250-275 gm) were randomly divided into stimulation and sham groups (n = 4/group). Surgeries were performed under general anesthesia (80 mg/kg ketamine; 10 mg/kg xylazine, i.p.). Experiments were conducted with the approval of Columbia University and New York State Psychiatric Institute Animal Care and Use Committees. First, 10% BDA (300 nL/injection; 15 injections) was pressure injected unilaterally into the forelimb and hindlimb areas of sensorimotor cortex to label the CST. Two weeks after BDA labeling, a craniotomy was made in the ventral occipital bone to expose the PT on the ventral brain surface. A tripolar electrode (two leads, one ground; 0.005″ PlasticsOne) was secured to the surface of pyramid ipsilateral to the BDA injections. Animals receiving stimulation were connected to a commutator (PlasticsOne) via a flexible cable. We stimulated 6 h/day for 10 days using the following parameters: 333 Hz, 45 ms burst, delivered every 2s, 35-120 μA. Stimulation current was the minimum needed to evoke forelimb muscle contraction, ensuring that the CST was activated [5]. Animals in the sham group underwent electrode implantation only but received no stimulation.
During the 10-day period of electrical stimulation, BrdU (50 mg/kg) was injected (i.p.) in all animals once every other day. Twenty-four hours after the last injection, animals were perfused with 200 ml saline followed by 400 ml 4% paraformaldehyde in 0.1M PBS. Spinal cords were removed, post-fixed for two hours, and transferred to 20% sucrose at 4°C. Frozen transverse sections (40 μm) through C2, C8, T6 and L3 segments were cut.
Randomly selected sections from the above segments were rinsed 3 × 10 min in PBS. They were pretreated with 2N HCl to denature DNA (37°C; 45 min) and 2 × 15 min borate buffer (pH 8.5) to neutralize HCl. Following 60 min in 10% normal goat serum (NGS) and 0.1% triton X-100 in PBS, sections were incubated in rat anti-BrdU antibody (1:600; Serotec) in the same solution as above at 4°C for 48 h. After rinses in PBS, sections were incubated in 1:400 Cy3-goat anti rat IgG and 1:400 Cy2-streptavidin at room temperature for 2 hours. In double immunostaining, the following primary antibodies were mixed with BrdU antibody: rabbit anti-NG2 (1: 200; Chemicon); mouse anti-APC-CC1 (1: 200; Oncogene); rabbit anti-Nkx2.2 (1:100; DSHB); rabbit anti-GFAP (1:4; ImmunoStar); rabbit anti-Glut-1 (1:100, Chemicon). Alexa 488-goat anti-rabbit or goat anti-mouse IgG (1:200) was mixed with Cy3-goat anti rat IgG. Cy5-streptavidin (1:400) was added to label CST axons. Sections were mounted onto slides, partially dried, and coverslipped.
Cells were counted in the dCST in stimulated animals contralateral to PT stimulation and cortex tracer injection (experimental group) and the following three control dCST regions: 1) stimulated animals, ipsilateral to stimulation; 2) sham animals, contralateral dCST; and 3) sham animals, ipsilateral dCST. Average numbers of BrdU+ cells were compared using a two-way analysis of variance (ANOVA). The factors were dCST groups (i.e., experimental dCST region and 3 control regions; total n = 4) and selected spinal regions (C2, C8, T6, and L3) with repeated measures on the second factor. For analysis of double-labeled cells, counts from four different spinal segments were summed. The comparison was performed in contralateral dCST between stimulated and sham animals with a t-test.
BrdU+ cells were identified by the presence of Cy3 (red) in the nuclei. A double-labeled cell was identified as an Alexa 488+ (green) cytoplasm with a Cy3 stained BrdU+ nucleus. BDA+ axons were seen using Cy2 (green) or Cy5 (orange under microscope and blue in figures). Color images were taken with Slidebook 4.1 and processed using Adobe Photoshop.
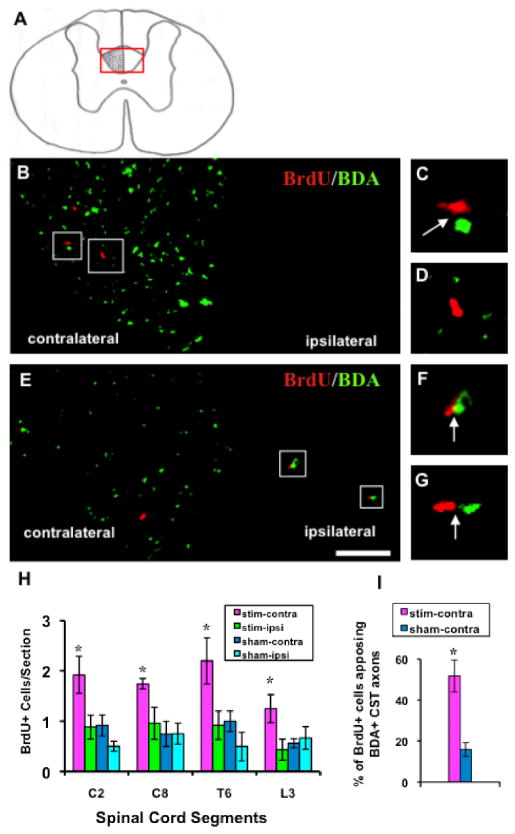
In all rats, greater than 95% of BDA+ axons were in the contralateral dCST (Fig. 2A). Uncrossed CST axons were occasionally observed in ipsilateral dCST and ventral CST. Only BDA+ axons and BrdU+ cells in the dCST were investigated (Fig. 2A-G). Rats that underwent electrical stimulation had more than double the number of BrdU+ nuclei in contralateral (i.e., stimulated) than ipsilateral (unstimulated) dCST (contralateral v/s ipsilateral; C2: 1.92±0.37 v/s 0.88±0.24 per section; C8: 1.74±0.11 v/s 0.96±0.32 per section; T6: 2.2±0.46 v/s 0.92±0.29 per section; L3: 1.25±0.28 v/s 0.43±0.21 per section). In sham rats, there was less than half the number of BrdU+ nuclei than in the stimulated group dCST and, in contrast to the stimulated rats, no systematic differences existed between the two sides (contralateral v/s ipsilateral; C2: 0.92±0.21 v/s 0.5±0.1 per section; C8: 0.75±0.25 v/s 0.75±0.21/section; T6: 1±0.2 v/s 0.5±0.29 per section; and L3: 0.56±0.09 v/s 0.67±0.2 per section). Repeated measures ANOVA indicated a significant effect of group across the four spinal levels (C2, C8, T6, and L3) where BrdU+ cell counts were made. Post-hoc test confirmed that the number of BrdU+ cells was significantly greater in the stimulated dCST than in the three control dCSTs (F=32.5, p< 0.0001; Fig. 2H). There were no differences among control dCSTs (p > 0.05). These results show that PT stimulation selectively promotes cell birth in the stimulated dCST.
Fig 2.
BDA+ CST axons (green) and BrdU+ nuclei (red) in electrically stimulated animals. A: Coronal spinal cord section. More than 95% BDA labeled CST axons project in the contralateral dCST. The red box indicates the micrographs in B and E. B: T6 section. C and D: higher magnification of boxed regions in B. BrdU+ nuclei distributed predominantly in contralateral dCST and most of them apposed BDA+ axons (C; arrow). D, BrdU+ cell not contacting a BDA+ axon. E: L3 section. Parts F and G, higher magnification of boxed regions in E. Sparse BDA+ axons in the ipsilateral dCST always closely apposed BrdU+ cells (arrows). Bar, 50 μm. H: BrdU+ nuclei in the dCST of the four groups. Number of labeled cells in experimental group (contralateral dCST of stimulated animals) was significantly greater than the three control groups (two-way ANOVA; *: p< 0.0001). I: There was a significantly higher percentage of BrdU+ cells contacting BDA+ axons in the contralateral dCST than in sham animals (t-test; *: p<0.01).
We commonly found BrdU+ cells in close proximity to labeled dCST axons in the stimulated (Fig. 2B, C), but seldom in sham, animals. We estimated the number of BrdU+ cells in apposition to labeled contralateral dCST axons by counting the Cy3+ nuclei located within 5 μm (i.e., half the width of OPC cytoplasm) of a BDA+ axon. There was more than a three-fold higher percentage of BrdU+ cells apposing labeled dCST axons in stimulated animals than in shams (51.74±7.92 %; v/s 15.75±3.45 %, p< 0.01; Fig. 2I). In stimulated animals, a small number of ipsilateral CST axons projecting from this pyramid received the same electrical stimulation as the contralateral axons. Although these uncrossed axons were too sparse to study quantitatively, most of them apposed a BrdU+ cell (Fig. 2E-G). In sham animals, uncrossed dCST axons never apposed BrdU+ cells. These results strongly suggest that active CST axons promote cell birth in the dCST.
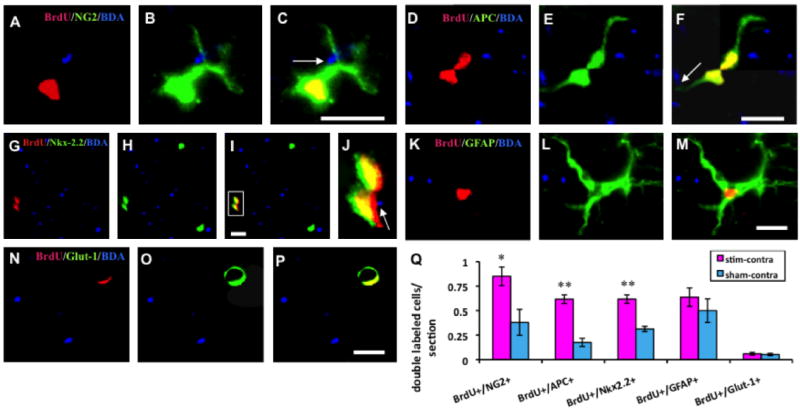
We next identified the cell class in the dCST labeled with BrdU. In stimulated animals, BrdU+ cells commonly expressed OL lineage markers. To investigate the phenotypic fate of newborn stem/progenitor cells, sections were double-immunostained for BrdU and different specific markers. The comparison was performed only in contralateral dCSTs, between stimulated and sham rats. Proliferating OPCs were identified by colocalization of BrdU in the nuclei and NG2 in cytoplasm. The processes always apposed BDA+ axons (Fig. 3A-C). The average number of BrdU+/NG2+ cells in stimulated rats (0.85±0.096/section) was double than in sham rats (0.38±0.132/section; p < 0.05; Fig. 3Q) indicating that PT stimulation promotes birth of OPCs.
Fig 3.
Double immunolabeled BrdU+ cells and different markers in contralateral dCST in stimulated animals. A, B, C: a NG2 stained BrdU+ OPC. D, E, F: a dividing BrdU+/APC+ mature oligodendrocyte. Arrows indicate processes of NG2+ and APC+ cells apposing BDA labeled axons. G, H, I, J: A pair of BrdU and Nkx-2.2 double-labeled nuclei contacting a stimulated axon. J. Higher magnification of box in I. K, L, M: a BrdU+/GFAP+ proliferating astrocyte. N, O, P: BrdU+/Glut-1+ cell. All bars in figures, 10 μm. Q: Bar graphs showing that numbers of BrdU+/NG2+, BrdU+/APC+, and BrdU+/Nkx-2.2+ cells in the experimental group are significantly greater then control and that there is no statistical difference between experimental and control groups for BrdU+/GFAP+ and BrdU+/Glut-1+ cells (t-test; *: p < 0.05; **: p<0.01).
Proliferating mature OLs were identified by colocalization of BrdU+ nuclear and APC+ cytoplasm (Fig. 3D-F). The number of OLs in contralateral dCST of stimulated rats (0.62±0.04/section) was three fold greater than in sham rats (0.18±0.04/section; p <0.01; Fig. 3Q).
The homeodomain transcription factor Nkx2.2, which promotes differentiation of OPCs into mature OLs [21], co-localized with BrdU in proliferating cells (Fig. 3G-J). In contralateral dCST of stimulated rats, BrdU+/Nkx2.2+ cells (0.62±0.04/section) were double the number observed in sham rats (0.31±0.03/section, p<0.01; Fig. 3Q). This suggests that PT stimulation promoted differentiation from OPCs into mature OLs.
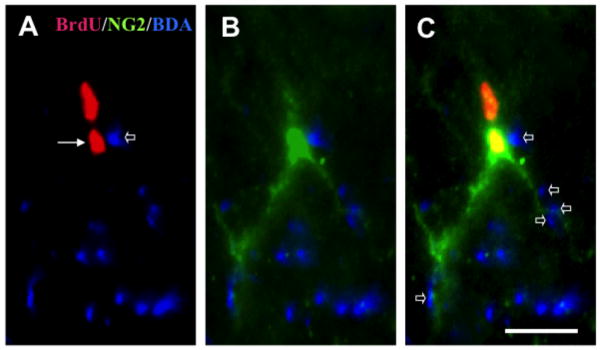
In the stimulated dCST, we observed cells asymmetrically dividing into two BrdU+ daughter cells. Figure 4 shows a pair of BrdU+ nuclei regarded to be derived from a single parent cell. Only the one contacting the BDA+/stimulated CST axons expressed the OPC marker, NG2. This suggests that contact with a stimulated axon determined the post-mitotic phenotypic fate of the progenitor cell.
Fig 4.
A BrdU+ cell in the stimulated group asymmetrically divided into two daughter cells. One cell (arrow in A) contacted labeled/stimulated dCST axons (open arrow in A) and differentiated into an NG2+ OPC (B). The sister cell, which did not contact dCST axons, did not develop this phenotype. Small open arrows in C indicate multiple appositions between stimulated axons and cell body or dendrites of the OPC. No asymmetrical divisions were found in control groups. Bar, 10 μm
Electrical stimulation of the CST did not augment the number of proliferating astrocytes or endothelial cells. In double immunostained sections, abundant numbers of BrdU+ cells expressed GFAP (Fig. 3K-M). These typical astrocytes had stellate cell bodies with numerous long processes. In contrast with the proliferating OPCs and OLs, BrdU+/GFAP+ cells and processes seldom contacted BDA labeled/stimulated axons. The average numbers of proliferating astrocytes did not differ significantly between stimulated (0.64±0.09/section) and sham (0.5±0.12/section) rats (p> 0.05; Fig.3Q). A small number of BrdU+ newborn cells were immunostained with Glut-1, an endothelial cell marker. Usually, the BrdU+ nuclei appeared crescent shaped and Glut-1 stained cytoplasm that formed a ring shaped capillary (Fig. 3N-P). There were no significant differences in the numbers of BrdU+/Glut-1+ cells in stimulated (0.06±0.01/section) and sham (0.05±0.01/section) rats (p>0.05; Fig.3Q). Our results show that electrical activity in adult dCST axons selectively promotes the development of myelinating glia.
Our findings provide the first in vivo demonstration in the adult CNS that electrical stimulation can selectively promote the proliferation and development of myelinating cells. Stimulating the adult PT promoted the proliferation and differentiation of OPCs contacting or in close proximity to neighboring CST axons in the spinal cord. These findings have important implications for promoting remyelination in the damaged CNS. There was no difference in the number of BrdU+ cells among three control groups indicating that electrode implantation and BDA injection did not increase glial cell birth.
Our data are in accord with earlier studies in developing animals indicating the importance of activity in OL development. Transection of the developing optic nerve or intravitreous injection of TTX produced a substantial decline in the number of proliferating OPCs and decreased myelination in the developing optic nerve [1] [7]. These observations suggest that proliferation of OPCs and myelination depends on spiking in neighboring axons.
PT stimulation increases the numbers of spikes conducted into the dCST and could increase glutamate release from the stimulated axons [14], thereby providing an activity signal to drive glial cell birth and differentiation. While there are many mechanisms that could explain our finding, we highlight three. First, by acting through AMPA/kainate receptors expressed on OPCs, glutamate can increase the phosphorylation of the cAMP response element binding protein (CREB) and promotes OPC mitosis [22]. Second, active axons release, or produce a signal that stimulates astrocytes to release, the mitogen of OPCs platelet-derived growth factor (PDGF) [1] [6]. The AA homodimeric form of PDGF induces a class of bi-potential glial progenitors (O-2A progenitor cells) to differentiate into OPCs by asymmetric division [8]. PDGF α receptor is mainly expressed in OPCs that generate myelinating cells in CNS [24]. Third, the findings of Fields and colleagues provide a plausible mechanism for how dCST activity promotes nearby myelinating glial cells [9] [10]. DRG neurons were co-cultured with OLs and Schwann cells in multi-compartment chambers. ATP was released from both DRG cell bodies and axonal compartments after electrical axon stimulation and this was blocked by TTX. Extracellular breakdown of ATP produced adenosine. By acting on purinergic and adenosine receptors that are selectively expressed in OPCs, ATP and adenosine releases calcium from intracellular stores and lead to differentiation from OPCs to mature OLs [25].
In the present study, we observed a significantly greater number of mature OLs that may derive from OPCs or directly from mitosis of APC+ OLs in stimulated dCST (Fig. 3D-F). The prevailing view is that mature OLs in the healthy CNS are post-mitotic and only the OPCs that give rise to them have the capacity to divide [23] [1]. Mitosis of mature OLs has been reported only in pathological conditions (e.g., CNS trauma [16]; experimental demyelination [27]; experimental autoimmune encephalomyelitis [20]). The capacity for increased neuronal activity to drive division of mature OLs is potentially important for remyelination [17].
Possible asymmetric division of BrdU+ newborn cells was observed in stimulated rats. Only the daughter cell contacting dCST axons developed into an OPC. Whereas the fate of its sister cell was not determined, we propose that it could be an undifferentiated endogenous stem cell or a pre-OPC glial progenitor. Plausibly, the cell not in apposition to the labeled dCST axon would be less activated than the one in contact. The less activated daughter cell should be less developed than the more active one [10].
We propose that in the intact mature CNS, there is a basal rate of formation of myelinating stem cells within spinal tracts that reflects a balance between local environmental cues (i.e., mitogens), which are normally limiting, and the activity of axons within the tract [6] [26]. After injury or a demyelinating event, mitogens become augmented in the damaged region and then cell proliferation can be regulated according to the activity of spared axons within the tract [11]. Consistent with this idea is our finding that decreasing neuronal activity after administration of the GABA-B agonist Baclofen decreased the number of regenerative OLs in an animal model of SCI [15]. Conversely, electrical stimulation of peroneal nerves enhances birth and differentiation of OLs selectively in spinal cord regions predicted to receive enhanced activation from circuits related to the peroneal nerve [2].
Demyelination contributes to functional impairments in MS and following SCI. Restoring myelination is a major goal of repair in patients with these conditions [4] [19]. Proliferation of endogenous OPCs and their subsequent differentiation into myelinating oligodendrocytes may be important for the observed remyelination after injury [12] [13]. Augmenting this intrinsic remyelination response is a potentially important therapeutic direction. In the present study, we show that electrical stimulation promotes OPC proliferation/differentiation in the intact spinal cord. We predict that these new born OPCs, after maturation, will contribute to the remyelination in demyelination events. Our findings have important implications for devising therapies for improving outcome after SCI. Activity of the CS system can be augmented non-invasively using repetitive transcranial magnetic stimulation (rTMS) [3] or transcranial direct current stimulation (TDCS). Our findings may also provide a novel strategy for promoting remyelination in MS, and other demyelination disorders.
Fig 1.
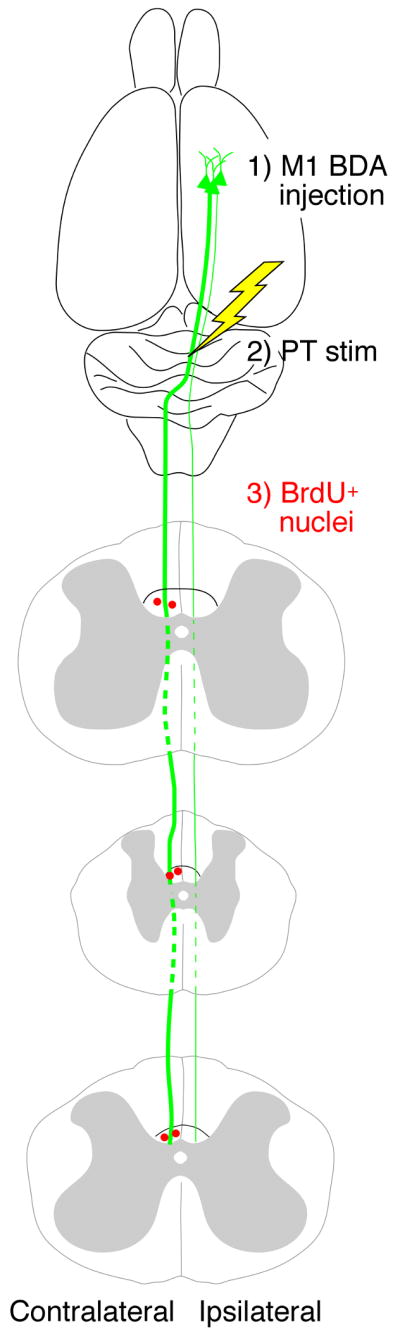
Experimental design. BDA was injected unilaterally into cortex to label the CST (green). Two weeks later, the PT electrode was implanted and stimulated daily (10 days; yellow). During the stimulation period, BrdU was administrated every other day. BrdU+ cells in dCST were counted (red).
Acknowledgments
This work was supported by Hugo W. Moser Research Institute at Kennedy Krieger (JWM), NIH NS064004 (JHM) and NYS Spinal Injury Research Program grant C022064 (JHM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells dependents on electrical activity in axons. Nature. 1993;361:258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- 2.Becker D, Gary DS, Rosenzweig ES, Grill WM, McDonald JW. Functional electrical stimulation helps replenish progenitor cells in the injured spinal cord of adult rats. Exp Neurol. 2010;222:211–218. doi: 10.1016/j.expneurol.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belci M, Catley M, Husain M, Frankel HL, Davey NJ. Magnetic brain stimulation can improve clinical outcome in incomplete spinal cord injured patients. Spinal Cord. 2004;42:417–419. doi: 10.1038/sj.sc.3101613. [DOI] [PubMed] [Google Scholar]
- 4.Bruce W, Kuhlmann T, Stadelmann C. Remyelination in multiple sclerosis. J Neurol Sci. 2003;206:181–185. doi: 10.1016/s0022-510x(02)00191-0. 2003. [DOI] [PubMed] [Google Scholar]
- 5.Brus-Ramer M, Carmel JB, Chakrabarty S, Martin JH. Electrical stimulation of spared corticospinal axons augments connections with ipsilateral spinal motor circuits after injury. J Neurosci. 2007;27:13793–13801. doi: 10.1523/JNEUROSCI.3489-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calver AR, Hall AC, Yu WP, Walsh FS, John K, Heath JK, Betsholtz C, William D, Richardson WD. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron. 1998;20:869–882. doi: 10.1016/s0896-6273(00)80469-9. [DOI] [PubMed] [Google Scholar]
- 7.Demerens D, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engel U, Wolswijk G. Oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells derived from adult rat spinal cord: In vitro characteristics and response to PDGF, bFGF and NT-3. Glia. 1996;16:16–26. doi: 10.1002/(SICI)1098-1136(199601)16:1<16::AID-GLIA3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 9.Fields RD, Stevens B. ATP: an extracellular signaling molecule between neurons and glia. Trends Neurosci. 2000;23:625–633. doi: 10.1016/s0166-2236(00)01674-x. [DOI] [PubMed] [Google Scholar]
- 10.Fields RD. Volume transmission in activity-dependent regulation of myelinating glia. Neurochem Internat. 2004;45:503–509. doi: 10.1016/j.neuint.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Frost EE, Nielsen JA, Le TQ, Armstrong RC. PDGF and FGF2 regulate oligodendrocyte progenitor responses to demylination. J Neurobiol. 2003;54:457–472. doi: 10.1002/neu.10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- 13.Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, Thal LJ, Gage FH. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- 15.Li Q, McDonald JW. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2003. Dose dependent effect of Baclofen on neural proliferation in damaged spinal coed: A quantitative analysis. Program No.245.21. CD-ROM. [Google Scholar]
- 16.Ludwin SK. Proliferation of mature oligodendrocytes after trauma to the central nervous system. Nature. 1984;308:274–275. doi: 10.1038/308274a0. [DOI] [PubMed] [Google Scholar]
- 17.Ludwin SK, Bakker DA. Can oligodendrocytes attached to myelin proliferate? J Neurosci. 1988;8:1239–1244. doi: 10.1523/JNEUROSCI.08-04-01239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin JH, Friel K, Salimi I, Chakrabarty S. Corticospinal Development. In: Squire L, editor. Encyclopedia of Neuroscience. Vol. 3. Oxford: Academic Press; 2009. pp. 302–214. [Google Scholar]
- 19.McDonald JW, Belegu V. Demyelination and remyelination after spinal cord injury. J Neurotrauma. 2006;23:345–359. doi: 10.1089/neu.2006.23.345. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen KB, Pender MP. Survival and mitosis of myelinating oligodendrocytes in experimental autoimmune encephalomyelitis: an immunocytochemical study with Rip antibody. Acta Neuropathol. 1999;98:39–47. doi: 10.1007/s004010051049. [DOI] [PubMed] [Google Scholar]
- 21.Qi Y, Cai J, Wu Y, Wu R, Lee J, Fu H, Rao M, Sussel L, Rubenstein J, Qiu M. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development. 2001;128:2723–2733. doi: 10.1242/dev.128.14.2723. [DOI] [PubMed] [Google Scholar]
- 22.Redondo C, Lopez-Toledano MA, Lobo MVT, Gonzalo-Gobernado R, Reimers D, Herranz AS, Paino CL, Bazan E. Kainic acid triggers oligodendrocyte precursor cell proliferation and neuronal differentiation from striatal neural stem cells. J Neurosci Res. 2007;85:1170–1182. doi: 10.1002/jnr.21245. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds R, Wilkin GP. Oligodendrocyte progenitor cells but not oligodendroglia divide during normal development of the rat cerebellum. J Neurocytol. 1991;20:216–224. doi: 10.1007/BF01186994. [DOI] [PubMed] [Google Scholar]
- 24.Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens B, Porta S, Haak LL, Gallo V, Fields RD. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron. 2002;36:855–868. doi: 10.1016/s0896-6273(02)01067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Heyningen P, Calver AR, Richardson WD. Control of progenitor cell number by mitogen supply and demand. Curr Biol. 2001;11:232–241. doi: 10.1016/s0960-9822(01)00075-6. [DOI] [PubMed] [Google Scholar]
- 27.Wood PM, Bunge RR. The origin of remyelinating cells in the adult central nervous system: the role of the mature oligodendrocyte. Glia. 1991;4:225–232. doi: 10.1002/glia.440040214. [DOI] [PubMed] [Google Scholar]