Abstract
Objective
The goal was to investigate the association between maternal salivary cotinine levels (SCLs) and pregnancy outcome among African Americans smokers
Methods
In a randomized controlled trial conducted in 2001-2004 in Washington, D.C. 714 women (126 active smokers (18%)) were tested for SCLs at the time of recruitment and later in pregnancy. Sociodemographic health risks and pregnancy outcomes were recorded.
Results
Birth weights were significantly lower for infants born to mothers with baseline SCLs of ≥20 ng/ml compared to <20 ng/ml (p=0.024), ≥50 ng/ml compared to <50 ng/ml (p=0.002), ≥100 ng/ml compared to <100 ng/ml (p=0.002), in bivariate analyses. In linear regression analyses adjusting for sociodemographic and medical factors, SCLs of ≥20 ng/ml were associated with a reduction in birth weight of 88 grams when SCLs were measured at baseline (p=0.042) and 205 grams when SCLs were measured immediately before delivery (p<0.001). Corresponding results were 129 grams (p=0.006) and 202 grams (p<0.001) for ≥50 ng/ml and 139 grams (p=0.007) and 205 grams (p<0.001) for ≥100 ng/ml. Gestational age was not affected significantly at any SCL, regardless of when SCLs were measured.
Conclusions
Elevated SCLs early in pregnancy or before delivery were associated with reductions in birth weight. At any cutoff level, birth weight reduction was more significant for the same SCL measured late in pregnancy. Maintaining lower levels of smoking for women who are unable to quit may be beneficial.
Introduction
Despite decreases in rates of smoking during pregnancy,1,2 smoking remains the most preventable risk factor for poor pregnancy outcomes.3-7 Women who smoke before pregnancy are often unable to quit when pregnant, and early quitters may exhibit recidivism later.4,8-10 Behavioral interventions for smoking cessation delivered during prenatal care have shown limited success.11,12 Coexisting pyschobehavioral risk factors may modify the efficacy of such interventions.13,14 As a result, smoking reduction or quitting may be more attainable for some subgroups of pregnant smokers.
The literature is not consistent on the impact of cotinine levels on birth weight. African American (AA) smokers show higher cotinine levels than white smokers at each smoking dose.15 However, this does not translate into significant differences in birth weight reduction. Barnett reported that smoking was responsible for 30.7% of low birth weight births among non-Hispanic white births compared with 14.4% among AA births.16
The association between smoking and low birth weight has been reported in the literature for > 60 years.17 This relationship has not been found to be linear.4 Li et al18 showed the beneficial effect of cotinine-validated smoking reduction on birth weight. Studies using self-reported number of cigarettes smoked during pregnancy are limited by accuracy of reporting, especially over longer recall times. The importance of biomarker validation, especially in AA mothers, is related to genetically influenced metabolic variation. CYP1A1 expression in AA populations has been shown to influence the rates of nicotine metabolism.19 Mothers of different racial backgrounds, who smoke the same number of cigarettes, may not have similar nicotine/cotinine levels.
Few studies have examined the correlations between smoking-related biomarkers other than cotinine levels and pregnancy outcome. Gomez et al20 examined the relationship between exhaled carbon monoxide concentrations and decreased fetal growth, confirming the association between exhaled carbon monoxide of 6 to10 ppm and 450 gram reductions in birth weight. Although exhaled carbon monoxide is easy to collect, measurements may not be available in most prenatal care settings, and levels reflect only recent smoking activity. Another study correlated blood thiocyanate levels in pregnancy with birth weight reductions.21 Studies examining correlations of serum and urinary cotinine levels with fetal biometric findings and infant birth weight showed variable results.18,19,22-24 None reported cotinine cutoff levels associated with improvement in birth outcomes, and none focused exclusively on AA pregnant women.
This paper examines the effect of smoking, measured as salivary cotinine levels (SCLs), on birth weight. SCLs represent a reliable biomarker for smoking and are highly correlated with blood and urine levels.25-27 The correlation between SCLs and birth weight was examined both early and late in pregnancy, in an AA population. Our hypothesis was that reductions in maternal SCLs would be associated with improved birth outcomes.
Methods
The National Institutes of Health-DC Initiative to Reduce Infant Mortality in Minority Populations is a congressionally mandated, research collaboration between four major academic institutions in Washington, DC (Children's National Medical Center, Georgetown University, George Washington University, and Howard University), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Center for Minority Health and Health Disparities, and RTI International. As part of this collaboration, a randomized controlled trial was conducted to evaluate benefits of an integrated behavioral/psychosocial intervention delivered during routine prenatal care in reducing cigarette smoking, environmental tobacco smoke exposure, depression, and intimate partner violence during pregnancy. This study was reviewed and approved by the institutional review boards of the participating institutions, RTI International, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Data Collection
Between July 2001 and October 2003 women were screened for recruitment at six community based clinical sites in the District of Columbia that served mainly minority women. Women were recruited in their first and second trimester and were monitored to an average of ten weeks after delivery. Women were deemed demographically eligible if they self-identified as belonging to a minority group, were ≥18 years of age, ≤28 weeks pregnant, were residents of Washington, DC and spoke English. Demographically eligible women underwent a two-stage consent and enrollment process. After initial consent, participants were screened for the four risk factors (smoking, environmental tobacco smoke exposure, depression and/or intimate partner violence) using an audio-computer assisted self interview (A-CASI) which also confirmed their demographic eligibility. More detailed information on sociodemographic features, reproductive history and behavioral risks was collected during a baseline interview, which was conducted an average of nine days after A-CASI screening. After the baseline interview, women consented to participate in this randomized controlled trial. Site- and risk-specific block random assignment to the intervention or usual care groups was conducted. Follow-up data collection through telephone interviews occurred during the second and third trimesters of pregnancy (22-26 and 34-38 weeks of gestation, respectively). Details on recruitment were reported in El-Khorazaty et al.28
Interviewers were blinded to group assignment, whereas participants and the intervention team were not. Intervention and follow-up activities continued until July 2004. Details on the design and delivery of the intervention are reported in Katz et al. 29 and El-Mohandes et al.30 After delivery, maternal medical records were abstracted and data on infant and pregnancy outcomes were recorded.
Saliva Collection and Cotinine Level Measurement
Levels of cotinine, which is the major proximate metabolite of nicotine and has been widely used as a biomarker of tobacco exposure, were determined in saliva samples. Saliva samples were collected for SCL analysis at enrollment or at the next scheduled prenatal care visit. Pregnant women placed cotton salivettes in their mouth for 3-5 minutes. After removal, salivettes were placed in capped vials and transported in refrigerated coolers to the laboratory. The saliva samples were stored refrigerated until analyzed (maximum time until analysis was 5 days). SCLs were then determined by J2 Laboratories (Tucson, AZ) using gas chromatography–mass spectrometry with a lower detection limit of 10ng/ml.
Self-Reported Smoking
At each telephone interview, participants were asked about their current smoking habits. Women were considered to be self-reported smokers if they said they had smoked a cigarette in the week preceding the interview. Smokers also reported the average number of cigarettes smoked per day.
Study Population
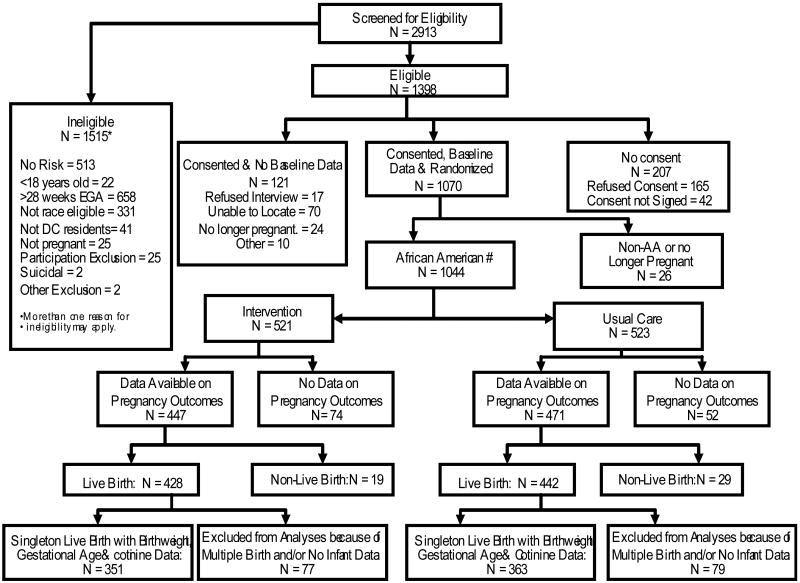
A total of 2,913 women were screened and 1,398 met eligibility criteria (See Figure 1). Of these 85% (n=1,191) consented to participate in a baseline telephone interview before random assignment; 1,070 (89.9%) were reached and participated. Eight women (6 intervention group and 2 usual care group) were identified (during intervention or data collection) as being suicidal. They were referred immediately to mental health care and were excluded from further participation. Of these women 1,044 were AA and still pregnant at the time of the interview. Data on pregnancy outcomes were available for 918 women and 870 had live births. Data are presented here for AA mothers with singleton live births for whom information available on infant's birth weight and gestational age as well as baseline SCLs was available (n=714 (82%)).
Figure 1. Profile of Project DC-HOPE Randomized Controlled Trial.
# Only African American (AA) women who were still pregnant at the time of the baseline interview are included in the analysis.
Statistical Methods
To preserve the randomization, participant data were analyzed according to their care group assignment, regardless of receipt of intervention, by using an intent-to-treat approach. SAS version 9.1.3 was used for all statistical analysis (SAS Institute Inc., Cary, NC).
Bivariate analyses compared women assigned to the two care groups with respect to sociodemographic characteristics, medical history and measures of smoking status (including SCLs with various cutoff points and number of cigarettes smoked) and birth outcomes. Characteristics with respect to which the participants were compared included maternal age, care group (intervention or usual care), gravidity, enrollment in Medicaid (yes/no), education (less than high school, high school graduate/general equivalency diploma (GED), or at least some college), alcohol and illicit drug use during pregnancy, gestational diabetes preconception diabetes, gestational hypertension and chronic hypertension. Low birth weight was defined as <2,500 grams whereas preterm birth as <37 weeks gestation. Small for gestational age (SGA) was defined as the lowest 10th percentile of birth weight for gestational age, on the basis of US data.31 For bivariate analyses, chi-square tests were used for categorical data and t-tests for continuous data. Average SCLs, infant weight, gestational age at birth, and SGA status were compared for women who were self-reported smokers or nonsmokers at baseline and at the follow-up interview closest to delivery. Similarly, the average self-reported number of cigarettes smoked per day, infant weight gestational age a birth, and SGA status were compared by using various SCL cutoff values at baseline and follow-up evaluations. Pearson correlations were used to explore the associations between self-reported numbers of cigarettes smoked and SCLs, and between SCLs and birth weight.
To evaluate the impact of cigarette smoking (e.g., measured SCLs, with various cutoff values, at baseline and at the follow-up interview immediately before delivery) on pregnancy outcomes, we used linear regression analyses to model pregnancy outcomes. Control variables included the characteristics identified previously. For birth weight analyses we also controlled for gestational age at delivery. These variables were selected for inclusion as covariates on the basis of previously published reports suggesting they might be related to adverse pregnancy outcomes.
Results
We report in this paper results on 714 AA mothers who had singleton live births and information available on baseline SCLs and infant weight and gestational age at birth. At baseline, the mean age of the mothers was 24.6 years and the mean gestational age was 19.2 weeks. A total of 75.8% of women were single, 72.1% had at least a high school diploma or GED, and 78.8% received Medicaid. The mean number of pregnancies (including current) was 3.7 and the mean number of previous live births was 1.4. In the study population the proportions of infants with low birth weight or preterm birth were 11.3% and 11.9%, respectively.
Table 1 compares the sociodemographic and reproductive characteristics at the baseline interview for smokers and nonsmokers. The estimated gestational age at the last follow-up before delivery was 34.0 weeks. The mean interval between the baseline interview and the last follow-up before delivery was 15.5 weeks. The rates of low birth weight and preterm birth among women who acknowledged smoking at baseline were 15.9% and 16.7%. These rates were higher, although not statistically significantly, than those for women who reported no smoking, that is, 10.4% (unadjusted p=0.077) and 10.9% (unadjusted p=0.069), respectively.
Table 1.
Characteristics of Mothers and Infants by Smoking Status at Baseline (BL) interview
| Characteristics | Category | BL Smoking (N=126) |
BL No smoking (N=588) |
Total (N=714) |
|---|---|---|---|---|
| Maternal age** | Mean ± SD | 27.0 ± 6.2 | 24.1 ± 5.0 | 24.6 ± 5.4 |
| No. of times pregnant (incl. current)** | Mean ± SD | 4.6 ± 2.8 | 3.5 ± 2.2 | 3.7 ± 2.3 |
| Number of live births** | Mean ± SD | 2.2 ± 1.9 | 1.3 ± 1.4 | 1.4 ± 1.5 |
| Gestational age at BL (weeks) | Mean ± SD | 19.3 ± 7.3 | 19.2 ± 6.7 | 19.2 ± 6.8 |
| Gender of the baby | Male | 60 (47.6%) | 281 (47.8%) | 341 (47.8%) |
| Female | 66 (52.4%) | 307 (52.2%) | 373 (52.2%) | |
| Relationship status (BL interview) | Single/separated/widowed/divorced | 97 (77.0%) | 444 (75.5%) | 541 (75.8%) |
| Married or living with partner | 29 (23.0%) | 144 (24.5%) | 173 (24.2%) | |
| Education level** | < High school (HS) | 58 (46.0%) | 141 (24.0%) | 199 (27.9%) |
| HS graduate/GED | 53 (42.1%) | 293 (49.8%) | 346 (48.5%) | |
| At least some college | 15 (11.9%) | 154 (26.2%) | 169 (23.7%) | |
| Currently receives Medicaid* | Yes | 112 (88.9%) | 448 (76.6%) | 560 (78.8%) |
| Alcohol use during pregnancy (BL interview)** | Yes | 46 (36.5%) | 101 (17.2%) | 147 (20.6%) |
| Illicit drug use during pregnancy** | Yes | 38 (30.2%) | 42 (7.1%) | 80 (11.2%) |
| Diabetes, gestational | Yes | 10 (8.1%) | 35 (6.0%) | 45 (6.4%) |
| Diabetes, preconception | Yes | 4 (3.2%) | 23 (3.9%) | 27 (3.8%) |
| Hypertension, gestational | Yes | 6 (4.8%) | 25 (4.3%) | 31 (4.4%) |
| Hypertension, chronic | Yes | 9 (7.1%) | 40 (6.8%) | 49 (6.9%) |
| Cotinine levels at BL** | <20 ng/ml | 16 (12.7%) | 529 (90.0%) | 545 (76.3%) |
| 20-49 | 11 (8.7%) | 21 (3.6%) | 32 (4.5%) | |
| 50-99 | 21 (16.7%) | 14 (2.4%) | 35 (4.9%) | |
| 100-199 | 30 (23.8%) | 14 (2.4%) | 44 (6.2%) | |
| 200+ | 48 (38.1%) | 10 (1.7%) | 58 (8.1%) | |
| Active smoking prior to delivery** | Yes | 85 (77.3%) | 15 (2.8%) | 100 (15.6%) |
| Cotinine levels (prior to delivery)** | <20 ng/ml | 21 (21.0%) | 440 (89.8%) | 461 (78.1%) |
| 20-49 | 9 (9.0%) | 16 (3.3%) | 25 (4.2%) | |
| 50-99 | 13 (13.0%) | 11 (2.2%) | 24 (4.1%) | |
| 100-199 | 30 (30.0%) | 19 (3.9%) | 49 (8.3%) | |
| 200+ | 27 (27.0%) | 4 (0.8%) | 31 (5.3%) | |
| Gestational age at delivery (weeks)# | Mean ± SD | 38.4 ± 2.2 | 38.7 ± 2.0 | 38.6 ± 2.1 |
| Infant birth weight (grams)* | Mean ± SD | 3018.9 ± 567.3 | 3184.6 ± 575.1 | 3155.3 ± 576.8 |
| Low birth weight (<2500 grams) | Yes | 20 (15.9%) | 61 (10.4%) | 81 (11.3%) |
| Very low birth weight (<1500 grams) | Yes | 1 (0.8%) | 7 (1.2%) | 8 (1.1%) |
| Preterm birth (<37 weeks) | Yes | 21 (16.7%) | 64 (10.9%) | 85 (11.9%) |
| Very preterm birth (<33 weeks) | Yes | 3 (2.4%) | 9 (1.5%) | 12 (1.7%) |
p<0.01;
p<0.001.
Mean interval between baseline interview and last follow-up prior to delivery was 15.5 weeks.
Table 2 compares the average SCL, infant weight and gestational age at birth, and SGA status for women who were self-reported smokers vs. nonsmokers at baseline and at the last follow-up interview before delivery. At both time points, women who reported smoking had significantly higher SCLs. Baseline SCLs of ≥20 ng/ml were reported for only 10.0% of women who denied smoking on the baseline interview. Birth weights were significantly lower for infants born to mothers who reported active smoking at baseline (unadjusted p=0.003) and those who reported smoking at the follow-up interview before delivery (unadjusted p=0.026). No significant differences in gestational age at birth or SGA status were observed for self-reported smokers at baseline or at the last follow-up interview before delivery.
Table 2.
Salivary Cotinine, Infant Birth weight, and Gestational Age by Smoking Status at Baseline and Follow-Up Interviews
| Characteristic | Smoking at Baseline | Smoking Prior to Delivery | ||
|---|---|---|---|---|
| Yes n=126 |
No n=588 |
Yes n=100 |
No n=543 |
|
| Cotinine Level at Baseline | 188.3 ± 163.9 | 20.1 ± 41.2 | 187.1 ± 154.1 | 21.7 ± 47.2 |
| p-value | <0.001 | <0.001 | ||
| Birth weight (grams) | 3018.9 ± 567.3 | 3184.6 ± 575.1 | 3078.1 ± 548.0 | 3211.4 ± 550.0 |
| p-value | 0.003 | 0.026 | ||
| Gestational Age (weeks) | 38.4 ± 2.2 | 38.7 ± 2.0 | 38.5 ± 2.0 | 38.8 ± 1.9 |
| p-value | 0.148 | 0.151 | ||
| Small-for-Gestational-Age | 19.8% | 14.6% | 17.0% | 14.5% |
| p-value | 0.143 | 0.527 | ||
Table 3 shows the self-reported number of cigarettes smoked per day, birth weight, gestational age and SCLs at both baseline and before delivery. Numbers of cigarettes smoked per day and SCLs were highly correlated at both time points. The Pearson correlation coefficients for the correlation between self report and biomarker findings was 0.62 at baseline (p<0.001) and 0.66 before delivery (p<0.001). These results validate the accuracy of mothers' self reports. There was an overall decreasing trend in birth weight with increased SCLs. No trend was observed for gestational age or SGA status. Table 4 shows infant birth weight and gestational age classified according to various SCL cutoff points (20 ng/ml, 50 ng/ml, or 100 ng/ml; at each cutoff levels, comparisons were made for less vs. greater than or equal to the specified level.) Birth weights were significantly lower for infants born to mothers with higher baseline SCL levels (p=0.024, p=0.002, and p=0.002, respectively), whereas gestational age at delivery was not significantly affected.
Table 3.
Number of Cigarettes Smoked/Day, Birth weight and Gestational Age by Salivary Cotinine Levels at Baseline (BL) and Prior to Delivery
| Cotinine Level at BL | <20 ng/ml (n=545) |
20-49 ng/ml (n=32) |
50-99 ng/ml (n=35) |
100-199 ng/ml (n=44) |
200+ ng/ml (n=58) |
|---|---|---|---|---|---|
| No. cigarettes smoked/day at BL: | |||||
| - Mean ± SD | 0.04 ± 0.40 | 1.10 ± 3.62 | 2.38 ± 2.61 | 4.07 ± 4.64 | 6.65 ± 6.97 |
| - Median | 0 | 0 | 1.0 | 4.0 | 5.0 |
| Birth weight: | |||||
| - Mean ± SD | 3183 ± 572 | 3275 ± 498 | 3105 ± 486 | 3042 ± 569 | 2950 ± 668 |
| - Median | 3221 | 3277 | 3124 | 3039 | 3034 |
| Gestational age: | |||||
| - Mean ± SD | 38.7 ± 2.0 | 38.9 ± 2.0 | 38.7 ± 1.7 | 38.4 ± 2.2 | 38.2 ± 2.7 |
| - Median | 39 | 39 | 39 | 39 | 39 |
| Cotinine Level Prior to Delivery | <20 ng/ml (n=561) |
20-49 ng/ml (n=25) |
50-99 ng/ml (n=24) |
100-199 ng/ml (n=49) |
200+ ng/ml (n=31) |
| No. cigarettes smoked/ day prior to delivery: | |||||
| - Mean ± SD | 0.05 ± 0.47 | 2.00 ± 4.41 | 2.25 ± 3.64 | 3.19 ± 3.33 | 6.67 ± 5.93 |
| - Median | 0 | 0 | 0 | 3.0 | 5.0 |
| Birth weight: | |||||
| - Mean ± SD | 3252 ± 525 | 3044 ± 449 | 3154 ± 459 | 2984 ± 591 | 3052 ± 579 |
| - Median | 3260 | 3025 | 3124 | 3044 | 3033 |
| Gestational age: | |||||
| - Mean ± SD | 38.9 ± 1.7 | 38.6 ± 1.3 | 39.3 ± 1.1 | 38.7 ± 2.9 | 38.7 ± 1.8 |
| - Median | 39 | 39 | 39 | 39 | 39 |
Table 4.
Infant Birth weight and Gestational Age Classified by Various Cutoff Points of Salivary Cotinine Levels
| Cotinine Level at Baseline | < 20 ng/ml (n=545) |
≥ 20 ng/ml (n=169) |
< 50 ng/ml (n=577) |
≥ 50 ng/ml (n=137) |
< 100 ng/ml (n=612) |
≥ 100 ng/ml (n=102) |
|---|---|---|---|---|---|---|
| No. cigarettes smoked/day: | ||||||
| - Mean ± SD | 0.04 ± 0.40 | 4.02 ± 5.49 | 0.10 ± 0.95 | 4.71 ± 5.64 | 0.23 ± 1.23 | 5.52 ± 6.17 |
| - Median | 0 | 3 | 0 | 4 | 0 | 4 |
| Birth weight (grams) | 3182.5 ± 572.4 | 3067.7 ± 583.9 | 3187.6 ± 568.6 | 3019.3 ± 593.5 | 3182.9 ± 564.1 | 2989.8 ± 625.6 |
| p-value | 0.024 | 0.002 | 0.002 | |||
| Gestational Age (weeks) | 38.7 ± 2.0 | 38.5 ± 2.3 | 38.7 ± 2.0 | 38.4 ± 2.3 | 38.7 ± 2.0 | 38.3 ± 2.5 |
| p-value | 0.371 | 0.203 | 0.138 | |||
| Small-for-Gestational-Age | 14.5% | 18.9% | 14.2% | 21.2% | 14.7% | 20.6% |
| p-value | 0.164 | 0.043 | 0.129 | |||
| Cotinine Level Prior to Delivery | < 20 ng/ml (n=461) |
≥ 20 ng/ml (n=129) |
< 50 ng/ml (n=486) |
≥ 50 ng/ml (n=104) |
< 100 ng/ml (n=510) |
≥ 100 ng/ml (n=80) |
| No. cigarettes smoked/day: | ||||||
| - Mean ± SD | 0.05 ± 0.47 | 3.61 ± 4.64 | 0.15 ± 1.15 | 3.99 ± 4.63 | 0.25 ± 1.44 | 4.53 ± 4.79 |
| - Median | 0 | 3 | 0 | 3 | 0 | 3.5 |
| Birth weight (grams) | 3251.8 ± 525.1 | 3043.5 ± 537.6 | 3241.1 ± 523.0 | 3043.4 ± 558.8 | 3237.0 ± 520.1 | 3010.2 ± 583.9 |
| p-value | <0.001 | <0.001 | <0.001 | |||
| Gestational Age (weeks) | 38.9 ± 1.7 | 38.8 ± 2.1 | 38.9 ± 1.7 | 38.8 ± 2.3 | 38.9 ± 1.7 | 38.7 ± 2.5 |
| p-value | 0.610 | 0.825 | 0.447 | |||
| Small-for-Gestational-Age | 13.0% | 18.6% | 13.4% | 18.3% | 13.5% | 18.8% |
| p-value | 0.108 | 0.195 | 0.214 | |||
Similar results were observed for SCLs before delivery. Birth weights were significantly lower for infants born to mothers with higher SCL levels at the follow-up interview closest to delivery (p<0.001 for the three cutoff points). In Pearson correlation analyses, both baseline (r=-0.125, p<0.001) and follow-up (r=-0.123, p=0.003) SCLs were significantly associated with a reduction in birth weight. No significant effect on gestational age was seen. No significant differences in SGA status were detected across various categories of SCLs except for the baseline cutoff level of 50 ng/ml of SCL (Table 4).
Linear regression models, as shown in Table 5, described the associations between pregnancy outcomes (birth weight and gestational age) and SCLs at baseline and before delivery at the three SCL cutoff levels. No regression models were created for SGA status because the bivariate results did not show significant differences. The models adjusted for care group, maternal age, education, gravidity, Medicaid status, gestational diabetes, gestational and chronic hypertension, illicit drug use and alcohol use during pregnancy, and in the case of birth weight, gestational age at delivery. We did not control for the gender of the baby because it was not significant in bivariate analysis. The regression model to predict birth weight using a baseline SCL cutoff of 20 ng/ml, indicated that SCL, gestational diabetes, pregnancy induced hypertension, and gestational age at delivery were all significant modifiers of infant weight. SCLs ≥20 ng/ml at baseline were associated with an average decrease in birth weight of 88 grams (p=0.042). Similar significant decreases in birth weight were observed for baseline SCLs of ≥50 ng/ml (decrease: 129 grams, p=0.006) and ≥100 ng/ml (decrease: 139 grams, p=0.007). Regression models predicting birth weight on the basis of SCLs before delivery showed similar results. In the model using a SCL cutoff value of 20 ng/ml, gestational diabetes and gestational age at delivery both were significant predictors of birth weight and SCLs seemed to play a greater role, compared with baseline levels. Follow-up SCLs of ≥20 ng/ml were associated with a 207-g decrease in birth weight (p<0.001). Regression models to predict birth weights with follow-up SCL cutoffs of 50 ng/ml and 100 ng/ml showed significant decreases in birth weights for women with higher SCLs (50 ng/ml: decrease: 202 grams, p<0.001 and 100 ng/ml: decrease: 205 grams, p<0.001). Whether baseline or follow-up SCLs were included as predictors, the models did not indicate that the intervention influenced birth weight.
Table 5.
Regression Models for Infant Birth weight and Gestational Age for Mothers with 20, 50 and 100 ng/ml Cutoff Cotinine Levels
| A. 20 ng/ml Cotinine | ||||
|---|---|---|---|---|
| Characteristic | Birth weight (grams) | Gestational Age (weeks) | ||
| Model 1 (n=700) |
Model 2 (n=579) |
Model 1 (n=700) |
Model 2 (n=579) |
|
| Care Group | -14.6 ± 33.9 | -21.9 ± 36.8 | 0.02 ± 0.16 | 0.03 ± 0.15 |
| Cotinine at BL ≥20 ng/ml | -87.8 ± 43.2* | --- | -0.08 ± 0.20 | --- |
| Cotinine Prior to Delivery ≥20 ng/ml | --- | -204.5 ± 47.8§§ | --- | -0.03 ± 0.19 |
| Maternal Age | -1.1 ± 3.9 | -1.1 ± 4.3 | -0.02 ± 0.02 | -0.02 ± 0.02 |
| Gravidity | 19.3 ± 8.7* | 17.2 ± 9.8 | -0.06 ± 0.04 | -0.09 ± 0.04* |
| Gestational Age at Delivery | 173.1 ± 8.2§§ | 159.9 ± 10.3§§ | --- | --- |
| Education: | ||||
| - HS/GED | 6.8 ± 41.6 | -37.1 ± 45.3 | -0.17 ± 0.19 | -0.13 ± 0.18 |
| - Some College | 57.7 ± 51.7 | -2.0 ± 55.2 | 0.15 ± 0.24 | 0.03 ± 0.22 |
| Medicaid | 43.6 ± 44.1 | 55.1 ± 45.9 | -0.18 ± 0.21 | -0.13 ± 0.19 |
| Alcohol Use During Pregnancy | 48.1 ± 43.6 | 36.8 ± 46.8 | -0.14 ± 0.20 | -0.17 ± 0.19 |
| Illicit Drug Use During Pregnancy | -66.9 ± 55.8 | -10.8 ± 62.6 | -0.07 ± 0.26 | -.0.04 ± 0.25 |
| Diabetes, Gestational | 283.5 ± 71.0§§ | 287.1 ± 76.0§§ | -0.47 ± 0.33 | -0.34 ± 0.31 |
| Hypertension, Gestational | -193.6 ± 83.9* | -161.1 ± 91.5 | -0.65 ± 0.39 | -0.78 ± 0.37* |
| Hypertension, Chronic | -25.8 ± 71.3 | -11.5 ± 80.3 | -0.30 ± 0.33 | 0.04 ± 0.33 |
| B. 50 ng/ml Cotinine | ||||
|---|---|---|---|---|
| Characteristic | Birth weight (grams) | Gestational Age (weeks) | ||
| Model 1 (n=700) |
Model 2 (n=579) |
Model 1 (n=700) |
Model 2 (n=579) |
|
| Care Group | -12.6 ± 33.8 | -24.1 ± 36.9 | 0.02 ±0.16 | 0.03 ± 0.15 |
| Cotinine at BL ≥50 ng/ml | -129.4 ± 46.4§ | --- | -0.17 ± 0.22 | --- |
| Cotinine Prior to Delivery ≥50 ng/ml | --- | -202.2 ± 51.4§§ | --- | 0.02 ± 0.21 |
| Maternal Age | -0.1 ± 3.9 | 1.2 ± 4.3 | -0.02 ± 0.02 | -0.02 ± 0.02 |
| Gravidity | 20.0 ± 8.7* | 16.2 ± 9.9 | -0.06 ± 0.04 | -0.10 ± 0.04* |
| Gestational Age at Delivery | 172.7 ± 8.2§§ | 160.4 ± 10.4§§ | --- | --- |
| Education: | ||||
| - HS/GED- Some College | -12.5 ± 41.5 | -37.1 ± 45.5 | -0.18 ± 0.19 | -0.13 ± 0.18 |
| - Some College | 50.1 ± 51.6 | 1.5 ± 55.3 | -0.13 ± 0.24 | 0.05 ± 0.22 |
| Medicaid | 44.7 ± 44.0 | 55.8 ± 46.0 | -0.14 ± 0.20 | -0.13 ± 0.19 |
| Alcohol Use During Pregnancy | 44.5 ± 43.4 | 41.4 ± 46.8 | -0.09 ± 0.20 | -0.16 ± 0.19 |
| Illicit Drug Use During Pregnancy | -63.4 ± 55.4 | -26.1 ± 62.2 | -0.06 ± 0.26 | -0.05 ± 0.25 |
| Diabetes, Gestational | 283.6 ± 70.8§§ | 291.1 ± 76.2§§ | -0.47 ± 0.33 | -0.34 ± 0.31 |
| Hypertension, Gestational | -190.8 ± 83.7* | -170.2 ± 91.7 | -0.65 ± 0.39 | -0.78 ± 0.37 |
| Hypertension, Chronic | -22.0 ± 71.1§§ | -7.7 ± 80.4 | -0.29 ± 0.33 | 0.05 ± 0.33 |
| C. 100 ng/ml Cotinine | ||||
|---|---|---|---|---|
| Characteristic | Birth weight (grams) | Gestational Age (weeks) | ||
| Model 1 (n=700) |
Model 2 (n=579) |
Model 1 (n=700) |
Model 2 (n=579) |
|
| Care Group | -14.1 ± 33.8 | -25.0 ± 36.9 | 0.02 ± 0.16 | 0.03 ± 0.15 |
| Cotinine at BL ≥100 ng/ml | -139.5 ± 51.1§ | --- | -0.27 ± 0.24 | --- |
| Cotinine Prior to Delivery ≥100 ng/ml | --- | -205.2 ± 57.3§§ | --- | -0.14 ± 0.23 |
| Maternal Age | -0.4 ± 3.9 | 1.8 ± 4.4 | -0.02 ± 0.02 | -0.02 ± 0.02 |
| Gravidity | 20.1 ± 8.7* | 15.5 ± 9.9 | -0.06 ± 0.04 | -0.09 ± 0.04* |
| Gestational Age at Delivery | 172.4 ± 8.2§§ | 159.2 ± 10.4§§ | --- | --- |
| Education: | ||||
| - HS/GED | -7.2 ± 41.2 | -35.2 ± 45.6 | -0.19 ± 0.19 | -0.15 ± 0.18 |
| - Some College - Some College | 56.3 ± 51.2 | 1.2 ± 55.6 | 0.12 ± 0.24 | 0.02 ± 0.22 |
| Medicaid | 42.2 ± 44.0 | 49.9 ± 46.1 | -0.14 ± 0.20 | -0.12 ± 0.19 |
| Alcohol Use During Pregnancy | 53.8 ± 43.1 | 46.6 ± 46.8 | -0.09 ± 0.20 | -0.17 ± 0.19 |
| Illicit Drug Use During Pregnancy | -67.4 ± 55.2 | -36.2 ± 62.1 | -0.05 ± 0.26 | -0.02 ± 0.25 |
| Diabetes, Gestational | 286.7 ± 70.9§§ | 292.7 ± 76.4§§ | -0.46 ± 0.33 | -0.34 ± 0.31 |
| Hypertension, Gestational | -187.2 ± 83.7* | -169.9 ± 91.9 | -0.64 ± 0.39 | -0.77 ± 0.37 |
| Hypertension. Chronic | -34.3 ± 71.2 | -14.7 ± 80.8 | -0.31 ± 0.33 | 0.03 ± 0.33 |
Note: Multivariable regression results for baseline data (Model 1) and for follow-up visit preceding delivery (Model 2).
p<0.001
p<0.01
p<0.05
In models to predict gestational age by using baseline or follow-up SCLs with a cutoff of 20 ng/ml, only gravidity and gestational hypertension were significantly associated with the outcome. For cutoff levels of 50 ng/ml and 100 ng/ml, only gravidity was significant. Table 6 shows the results of the multivariate regression modeling predicting birth weight and gestational age using the five SCL categories (<20, 20-49, 50-99, 100-199, and >200 ng/ml). When the four higher SCL categories were compared with <20 ng/ml, the results indicated that the higher SCLs, at baseline or before delivery, were associated with greater effects on birth weight reduction.
Table 6.
Regression Models for Infant Birth weight and Gestational Age for Mothers with Categorical Cotinine Levels
| Characteristic | Birth weight (grams) | Gestational Age (weeks) | ||
|---|---|---|---|---|
| Model 1* (n=700) |
Model 2* (n=579) |
Model 1* (n=700) |
Model 2* (n=579) |
|
| Care Group | -12.4 ± 33.8 | -22.7 ± 37.0 | +0.02 ± 0.16 | +0.02 ± 0.15 |
| Cotinine at BL: | ||||
| <20 ng/ml | Reference | --- | Reference | --- |
| 20-49 ng/ml | 52.4 ± 82.3 | --- | 0.18 ± 0.38 | --- |
| 50-99 ng/ml | -73.7 ± 80.9 | --- | 0.11 ± 0.37 | --- |
| 100-199 ng/ml | -115.5 ± 72.1 | --- | -0.04 ± 0.33 | --- |
| >200 ng/ml | -165.8 ± 66.3* | --- | -0.42 ± 0.31 | --- |
| Cotinine Prior to Delivery: | ||||
| <20 ng/ml | --- | Reference | --- | Reference |
| 20-49 ng/ml | --- | -161.1 ± 92.1 | --- | -0.19 ± 0.37 |
| 50-99 ng/ml | --- | -171.6 ± 93.9 | --- | 0.41 ± 0.38 |
| 100-199 ng/ml | --- | -234.5 ± 70.1§§ | --- | -0.22 ± 0.28 |
| >200 ng/ml | --- | -223.3 ± 88.1* | --- | 0.02 ± 0.36 |
| Maternal Age | -0.2 ± 3.9 | 1.4 ± 4.4 | -0.02 ± 0.02 | -0.02 ± 0.02 |
| Gravidity | 19.9 ± 8.7* | 17.1 ± 9.9 | -0.06 ± 0.04 | -0.09 ± 0.04 |
| Gestational Age at Delivery | 172.2 ± 8.3§§ | 159.7 ± 10.4§§ | --- | --- |
| Education: | ||||
| - HS/GED | -11.9 ± 41.7 | -40.3 ± 45.7 | -0.19 ± 0.19 | -0.15 ± 0.19 |
| - Some College | 51.4 ± 51.8 | -5.9 ± 55.5 | 0.13 ± 0.24 | 0.02 ± 0.23 |
| Medicaid | 43.3 ± 44.1 | 55.4 ± 46.1 | -0.14 ± 0.20 | -0.14 ± 0.19 |
| Alcohol Use During Pregnancy | 47.6 ± 43.8 | 36.9 ± 46.9 | -0.08 ± 0.20 | -0.16 ± 0.19 |
| Illicit Drug Use During Pregnancy | -66.7 ± 55.7 | -11.8 ± 62.8 | -0.07 ± 0.26 | -0.04 ± 0.25 |
| Diabetes, Gestational | 286.2 ± 71.1* | 286.7 ± 76.7§§ | -0.45 ± 0.33 | -0.36 ± 0.31 |
| Hypertension, Gestational | -188.5 ± 83.9* | -161.5 ± 92.0 | -0.64 ± 0.39 | -0.75 ± 0.37* |
| Hypertension, Chronic | -28.2 ±71.7 | -14.3 ± 80.7 | -0.33 ± 0.33 | 0.02 ± 0.33 |
Multivariable regression results for baseline data (Model 1) and for follow-up visit preceding delivery (Model 2).
p<0.001
p<0.01
p<0.05
The results were comparable when 85 preterm births were excluded. Baseline SCLs of <20 ng/ml was associated with a birth weight difference of 86 grams (p=0.067). With a baseline SCL cutoff value of 50 ng/ml, the birth weight difference was 144 grams (p=0.004). For a baseline SCL cutoff of 100 ng/ml, the birth weight difference of 139 grams was also significant (p=0.013). For SCLs later in pregnancy, similar models showed birth weight differences ranging from 173 to 203 grams (all significant at p<0.01). Small reductions in birth weight may be clinically significant, especially in populations that are already at increased risk for low birth weight.
Discussion
We confirmed the relationship between maternal smoking and reduced birth weight. The correlation found here between SCLs and birth weight among AA mothers with singleton live births is perhaps the first. Earlier studies with a similar approach have focused on Caucasians19,22-24,32 or Hispanic pregnant mothers.19 The SCLs reported for our study population seem to correlate with lower numbers of cigarettes smoked, compared with other studies. This is in agreement with previous results showing that AA women have higher cotinine levels than their white counterparts when exposed to the same self-reported number of cigarettes.15 In our study, women with SCLs ≥200 ng/ml before delivery reported smoking an average of 6.7 cigarettes per day compared with a reported level of 196 in women smoking 11.3 cigarettes.18 The significance of this finding for clinical practice may be more important for AA women because they seem to metabolize more cotinine when smoking fewer cigarettes. Such differences can be explained by the literature finding of higher prevalence rates of CYP1A1 and GSTT1 genotypes amongst AA individuals.33
Another finding in our study was that significant variations in birth weight were not associated with a similar effect on gestational age. This is in agreement with earlier findings by Secker-Walker and Vacek.22 for Caucasians smokers, with urinary cotinine and exhaled carbon monoxide as objective measures for tobacco smoking. In that study, no variation in gestational age could be correlated with high cotinine levels measured early or late in pregnancy. Variations in birth weight according to SCLs in our population were independent of gestational age. No significant reduction of gestational age was observed at any SCL cutoff level.
Of equal importance was the measurable influence of maternal cotinine on birth weight reduction at relatively low levels (<20 ng/ml vs. 20+ ng/ml). These lower levels seem to influence birth weight more strongly in later gestation. This finding has significant public health implications because 60% of the overall population effect of smoking on low birth weight is attributable to the category of light smokers (<11 cigarettes/day) 34. The clinical implication is that the management goal for smokers in pregnancy should remain complete cessation whenever possible. The reduction in birth weight associated with low cotinine levels close to the time of delivery was comparable to the effects of gestational diabetes and hypertension within this population of AA mothers. This finding is not surprising because cotinine levels later in gestation may have direct effects on placental blood flow and fetal growth patterns.23
Early in pregnancy, higher SCLs were associated with greater reductions in birth weight. A SCL cutoff of 50 ng/ml resulted in a birth weight reduction almost twice as large as the reduction for a SCL cutoff of 20 ng/ml. A cutoff level of ≥50 ng/ml in AA smokers seems to define an increased level of risk exposure for reduced birth weight.
The emphasis on quitting even in the late second and third trimesters of pregnancy may have measurable effects on birth weight. In our study, higher maternal SCLs were more significantly associated with birth weight reduction when measured in the later part of pregnancy. This was true across all SCLs except the highest category of ≥200 ng/ml. The reduction in birth weight did not vary significantly for the different cutoff levels when SCLs were measured close to delivery; therefore no amount of cigarette smoking could be considered safe (or safer).
A limitation of our study is that it was conducted with a population of women who demonstrated multiple risk factors (albeit relatively common and frequently co-occurring in this population). Determination of whether our results are generalizable would require confirmation by other studies conducted with similar populations. Another limitation was the use of a single biomarker as a guide to the potential benefits of a change in smoking behavior. Similarly low SCLs associated with nicotine replacement therapy rather than low-intensity smoking may be associated with higher birth weights than those reported in our study. We can only infer associations between certain SCLs and birth weight categories without having documented the potential additive effects of biotoxicants associated with cigarette smoking, such as carbon monoxide or thiocyanates. Studies examining similar cotinine levels in pregnant women using nicotine replacement rather than low-level smoking might help answer some of these questions.
Acknowledgments
Funding/Support: This study was supported by grants no. 3U18HD030445; 3U18HD030447; 5U18HD31206; 3U18HD03919; 5U18HD036104, Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center on Minority Health and Health Disparities
Footnotes
RTI International is a trade name of Research Triangle Institute.
References
- 1.US Dept of Health and Human Services. The Health Benefits of Smoking Cessation: A Report of the Surgeon General. Rockville, MD: Centers for Disease Control, Public Health Service; 1990. US Department of Health and Human Services publication CDC 90-8416. [Google Scholar]
- 2.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, Munson ML. Births: Final data for 2005, National vital statistics reports. no 6. Vol. 56. Hyattsville, MD: National Center for Health Statistics; 2007. [PubMed] [Google Scholar]
- 3.Bernstein IM, Mongeon JA, Badger GJ, Solomon L, Heil SH, Higgins ST. Maternal smoking ands its association with birth weight. Obstet Gynecol. 2005;106(5):986–991. doi: 10.1097/01.AOG.0000182580.78402.d2. [DOI] [PubMed] [Google Scholar]
- 4.England LJ, Kendrick JS, Wilson HG, Merritt RK, Gargiullo PM, Zahniser C. Effects of smoking reduction during pregnancy on the birth weight of term infants. Am J Epidemiol. 2001;154(8):694–701. doi: 10.1093/aje/154.8.694. [DOI] [PubMed] [Google Scholar]
- 5.Lightwood JM, Phibbs CS, Glantz SA. Short-term and Economic benefits of smoking cessation: Low birth weight. Pediatrics. 1999;104(6):1312–1320. doi: 10.1542/peds.104.6.1312. [DOI] [PubMed] [Google Scholar]
- 6.Zimmer-Gembeck MJ, Helfand M. Low birth weight in a public prenatal care program: Behavioral and Psychosocial risk factors and psychosocial intervention. Soc Sci Med. 1996;43(2):187–197. doi: 10.1016/0277-9536(95)00361-4. [DOI] [PubMed] [Google Scholar]
- 7.Windsor RA. Smoking cessation or reduction in pregnancy treatment methods: A meta-evaluation of impact of dissemination. Am J Med Sci. 2003;326(4):216–222. doi: 10.1097/00000441-200310000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Salihu HM, Wilson RE. Epidemiology of prenatal smoking and perinatal outcomes. Early Hum Dev. 2007;83:713–720. doi: 10.1016/j.earlhumdev.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Kirkland SA, Dodds LA, Brosky G. The natural history of smoking during pregnancy among women in Nova Scotia. CMAJ. 2000;163:281–282. [PMC free article] [PubMed] [Google Scholar]
- 10.Jaddoe VW, Troe EWM, Hofman A, Mackenbach LP, Moll HA, Steegers EA, Witteman JC. Active and passive maternal smoking during pregnancy and the risks of low birth weight and preterm: the generation R study. Paediatr Perinat Epidemiol. 2008;22:162–171. doi: 10.1111/j.1365-3016.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- 11.Windsor RA, Boyd NR, Orleans CT. A meta-evaluation of smoking cessation intervention research among pregnant women: improving the science and art. Health Educ Res. 1998;13:419–438. doi: 10.1093/her/13.3.419. [DOI] [PubMed] [Google Scholar]
- 12.De Bernabé JV, Soriano T, Albaladejo R, Juarranz M, Calle ME, Martinez D, Dominguez-Rojas V. Eur J Obstet Gynecol Reprod Biol. 2004;116:3–15. doi: 10.1016/j.ejogrb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Vander Weg MW, Ward KD, Scarinci IC, Read MC, Evans CB. Smoking-related correlates of depressive symptoms in low-income pregnant women. Am J Health Behav. 2004;28(6):510–521. doi: 10.5993/ajhb.28.6.4. [DOI] [PubMed] [Google Scholar]
- 14.Fox SH, Koepsell TD, Daling JR. Birth weight and smoking during pregnancy – effect modification by maternal age. Am J Epidemiol. 1994;139(10):1008–1015. doi: 10.1093/oxfordjournals.aje.a116940. [DOI] [PubMed] [Google Scholar]
- 15.English PB, Eskenazi B, Christianson RE. Black-white differences in serum cotinine levels among pregnant women and subsequent effects on infant birth weight. Am J Public Health. 1994;84:1439–1443. doi: 10.2105/ajph.84.9.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnett E. Race differences in the proportion of low birth weight attributable to maternal cigarette smoking in low-income population. Am J Health Promo. 1995;10(2):105–110. doi: 10.4278/0890-1171-10.2.105. [DOI] [PubMed] [Google Scholar]
- 17.Simpson WJ. A preliminary report on cigarette smoking and the incidence of prematurity. Am J Obstet Gynecol. 1957;73:808–815. [PubMed] [Google Scholar]
- 18.Li CQ, Windsor RA, Perkins L, Goldenberg RL, Lowe JB. The impact on infant birth weight and gestational age of cotinine-validated smoking reduction during pregnancy. JAMA. 1993;269(12):1519–1524. [PubMed] [Google Scholar]
- 19.Wang X, Tager IB, Van Vunakis H, Speizer FE, Harahan JP. Maternal smoking during pregnancy, urine cotinine concentrations, and birth outcomes. A prospective cohort study. Int J Epidemiol. 1997;26:978–988. doi: 10.1093/ije/26.5.978. [DOI] [PubMed] [Google Scholar]
- 20.Gomez C, Berlin I, Marquis P, Delcroix M. Expired air carbon monoxide concentration in mothers and their spouses above 5 ppm is associated with decreased fetal growth. Preventive Med. 2005;40:10–15. doi: 10.1016/j.ypmed.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 21.Jensen OH, Foss OP. Smoking in Pregnancy: Effects on the birth weight and on thiocyanate concentration in mother and baby. Acta Obstet Gynecol Scand. 1981;60:177–181. [PubMed] [Google Scholar]
- 22.Secker-Walker RH, Vacek PM. Relationships between cigarette smoking during pregnancy, gestational age, maternal weight gain, and infant birth weight. Addictive Behav. 2003;28:55–66. doi: 10.1016/s0306-4603(01)00216-7. [DOI] [PubMed] [Google Scholar]
- 23.Kalinka J, Hanke W, Sobala W. Impact of prenatal tobacco smoke exposure, as measured by midgestation serum cotinine levels, on fetal biometry and umbilical flow velocity waveforms. Am J Perinatol. 2005;22:41–47. doi: 10.1055/s-2004-837266. [DOI] [PubMed] [Google Scholar]
- 24.Peacock JL, Cook DG, Carey IM, et al. Maternal cotinine level during pregnancy and birth weight for gestational age. Int J Epidemiol. 1998;27:647–656. doi: 10.1093/ije/27.4.647. [DOI] [PubMed] [Google Scholar]
- 25.Benowitz N. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18:188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 26.Benowitz N, et al. SRNT Subcommittee on Biochemical Verification Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 27.Hukkanen J, Jacob P, III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharm Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 28.El-Khorazaty MN, Johnson AA, Kiely M, El-Mohandes AAE, Subramanian S, Laryea HA, Murray KB, Thornberry JS, Joseph JG. Recruitment and retention of low-income minority women in a behavioral intervention to reduce smoking, depression, and intimate partner violence during pregnancy. BMC Public Health. 2007;7:233. doi: 10.1186/1471-2458-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katz KS, Blake SM, Milligan RA, Sharps PW, White DB, Rodan MF, Rossi MA, Murray KB. The design, implementation and acceptability of an integrated intervention to address multiple behavioral and psychosocial risk factors among pregnant African American women. BMC Pregnancy and Childbirth. 2008;8:22. doi: 10.1186/1471-2393-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Mohandes AAE, Kiely M, Joseph JG, Subramanian S, Johnson AA, Blake SM, Gantz MG, El-Khorazaty MN. An Intervention to improve postpartum outcomes in African-American Mothers: A Randomized Controlled Trial. Obstet Gynecol. 2008;112:611–619. doi: 10.1097/AOG.0b013e3181834b10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 32.Secker-Walker RH, Vacek PM, Flynn BS, Mead PB. Smoking in pregnancy, exhaled carbon monoxide, and birth weight. Obstet Gynecol. 1997;89(5):648–653. doi: 10.1016/s0029-7844(97)00103-8. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, Niu T, Wise PH, Bauchner H, Xu X. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287(2):195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- 34.Magee BD, Hattis D, Kivel NM. Role of smoking in low birth weight. J Reprod Med. 2004;49:23–27. [PubMed] [Google Scholar]