Abstract
Objective
Endothelin-1 has been implicated in the pathogenesis of many cardiovascular-related diseases, including diabetes. The goal of this study was to examine the influence of endothelin-1 receptors (ETA) in impaired responses of cerebral (pial) arterioles in Type 1 diabetic rats.
Methods
We measured responses of cerebral arterioles in nondiabetic rats to eNOS-dependent (5′-adenosine diphosphate (ADP)), nNOS-dependent (N-methyl-D-aspartic acid (NMDA)) and NOS-independent (nitroglycerin) agonists before and during application of BQ-123, an ETA receptor antagonist. In addition, we harvested brain tissue from nondiabetic and diabetic rats to measure the production of superoxide anion under basal conditions and during inhibition of ETA receptors.
Results
We found that diabetes specifically impaired eNOS- and nNOS-dependent reactivity of cerebral arterioles, but did not alter NOS-independent vasodilation. In addition, while BQ-123 did not alter responses in nondiabetic rats, BQ-123 restored impaired eNOS- and nNOS-dependent vasodilation in diabetic rats. Further, superoxide production was higher in brain tissue from diabetic rats compared to nondiabetic rats under basal conditions and BQ-123 decreased basal production of superoxide in diabetic rats.
Conclusion
We suggest that activation of ETA receptors during T1D plays an important role in impaired eNOS- and nNOS-dependent dilation of cerebral arterioles.
Keywords: Brain, NMDA, ADP, Nitroglycerin, Oxidative Stress, Superoxide Anion
Introduction
Macro- and microvascular diseases are important risk factors associated with Type 1 diabetes mellitus (T1D). Endothelial dysfunction, defined as an imbalance between endothelium-dependent vasodilation and vasoconstriction, plays an important role in the pathogenesis of vascular dysfunction during many disease states, including T1D [13, 24, 31]. Many studies have shown that T1D affects endothelial cell function of large peripheral vessels by influencing the generation of nitric oxide via nitric oxide synthase (NOS) and/or via the formation of oxygen radicals [12, 25, 45, 47, 49]. In addition, we have shown that responses of cerebral resistance arterioles to endothelial nitric oxide synthase (eNOS)- and neuronal nitric oxide synthase (nNOS)-dependent agonists are decreased during T1D, presumably via the formation of oxygen radicals [37, 41, 54]. Thus, since alterations in vascular function will influence the regulation of tissue blood flow, especially blood flow to the brain, it is critical to determine factors that may influence vascular dysfunction during T1D.
Endothelins are proteins that are involved in the control of vascular tone in large and small blood vessels [51]. Three isoforms of endothelins (ET-1, -2, -3), expressing two types of receptors (ETA and ETB), have been identified. ETA receptors are found on vascular smooth muscle cells and when activated produce vasoconstriction [43]. ETB receptors are found on the interior lining of the endothelium of blood vessels and are capable of stimulating vasodilatation (via nitric oxide), as well as vasoconstriction [29, 32, 43]. Investigators have suggested that the production of ET-1 may play an important role in disease-related vascular complications, including those associated with type 1 and type 2 diabetes [26, 29, 52]. Substrate/enzymes that are responsible for the synthesis of ET-1, and ETA and ETB receptors are found in the blood vessels and cells of the brain [1, 6, 59, 60, 63-65]. However, to our knowledge no studies have examined the role of activation of ETA receptors in impaired responses of cerebral resistance arterioles during T1D nor have previous studies examined potential mechanisms by which inhibition of ETA receptors might influence reactivity of cerebral arterioles during T1D. Thus, the goal of our study was to determine whether inhibition of ETA receptors could influence impaired responses of cerebral arterioles in T1D. To accomplish this goal, we measured in vivo responses of cerebral (pial) resistance arterioles to eNOS- and nNOS-dependent agonists before and during inhibition of ETA receptors. In addition, we measured superoxide anion production in nondiabetic and diabetic rats before and during inhibition of ETA receptors.
Materials and Methods
Preparation of animals
All rats were housed in an animal care facility at the University of Nebraska Medical Center that is approved by the American Association for the Accreditation of Laboratory Animal Care, and all protocols were reviewed and approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (body weight 200–220 grams) were randomly assigned to a nondiabetic group that was injected with vehicle (sodium citrate buffer) or a diabetic group that was injected with streptozotocin (50 mg/kg IP). On the day of the experiment (2-3 months after injection of vehicle or streptozotocin), rats were anesthetized with thiobutabarbital sodium (Inactin; 100 mg/kg body weight, i.p.) and a tracheotomy was performed. The rats were mechanically ventilated with room air and supplemental oxygen. A catheter was placed in a femoral vein for infusion of supplemental anesthetic (10–20 mg/kg, as needed) and a femoral artery was cannulated to measure arterial blood pressure, to obtain a blood sample for the measurement of blood glucose concentration and for the measurement of arterial pH, pCO2 and pO2.
To visualize the microcirculation of the cerebrum, a craniotomy was prepared over the left parietal cortex [42]. The cranial window was suffused with artificial cerebral spinal fluid (flow rate = 2 ml/min) that was bubbled continuously (95% nitrogen and 5% carbon dioxide). The temperature of the suffusate was maintained at 37±1° C. The cranial window was connected via a three-way valve to an infusion pump that allowed infusion of agents into the suffusate, and thus onto the cerebral microcirculation. This method, which we have used previously [38, 40], maintained a constant temperature, pH, pCO2, and pO2 of the suffusate during infusion of agents.
Measurement of pial arteriolar reactivity
In vivo diameter of pial arterioles was measured using a video image-shearing device (model 908, Instrumentation for Physiology and Medicine, Inc.). We examined reactivity of the largest arteriole exposed by the craniotomy. Diameter of arterioles was measured immediately before application of agonists and every minute during a 5-minute application period. After application of the agonist was stopped, the diameter of pial arterioles returned to baseline within 3-5 minutes. Agonists were mixed in artificial cerebral spinal fluid, and then superfused over the cerebral microcirculation in a random manner. Application of each dose of agonist was separated by a 5-minute period and application of different agonists was separated by a 10-minute period.
Superoxide anion production
In groups of nondiabetic (n=8) and diabetic (n=13) rats we measured superoxide anion production using lucigenin-enhanced chemiluminescence [3]. Rats were anesthetized and exsanguinated, the brain was then removed and immersed in a modified Krebs-HEPES buffer containing (in mmol/L): 118 NaCl, 4.7 KCl, 1.3 CaCl2, 1.2 MgCl2, 1.2 KH2PO4, 25 NaHCO3, 10 HEPES, 5 glucose (20 glucose for brains from diabetic rats) (pH 7.4). Tissue samples from the parietal cortex were placed in polypropylene tubes containing 5 μmol/L lucigenin, then read in a Fentomaster FB12 (Zytox) luminometer, which reports relative light units (RLU) emitted integrated over 30 second intervals for 5 minutes. Data were corrected for background activity and normalized to tissue weight. In these studies, we measured superoxide anion production under basal conditions, during exposure to BQ-123 (1.0 μM), during exposure to ET-1 (0.01 and 0.1 μM), and during exposure to ET-1 in the presence of BQ-123.
Experimental protocol
The cranial window was suffused for 30–45 minutes before testing responses to the agonists. Then, we examined responses of pial arterioles in nondiabetic (n=7) and diabetic (n=7) rats to an eNOS-dependent agonist (5′-adenosine diphosphate; ADP (10 and 100 μM), an nNOS-dependent agonist (N-metyl-D-aspartic acid; NMDA (100 and 300 μM) and to a NOS-independent agonist (nitroglycerin (1.0 and 10 μM)). After this initial examination of reactivity, we started a continuous application of BQ-123 (1.0 μM), a specific inhibitor of ETA receptors [36], over the cerebral microcirculation. Sixty minutes after starting suffusion with BQ-123, we again examined responses to ADP, NMDA and nitroglycerin.
Statistical analysis
An unpaired t test was used to compare baseline parameters between nondiabetic and diabetic rats. Analysis of variance with Fischer’s test for significance was used to compare functional responses of cerebral arterioles between groups of rats before and during treatment with BQ-123, and superoxide anion production under basal conditions, during treatment with ET-1 and BQ-123. A p value of 0.05 or less was considered to be significant.
Results
Control conditions
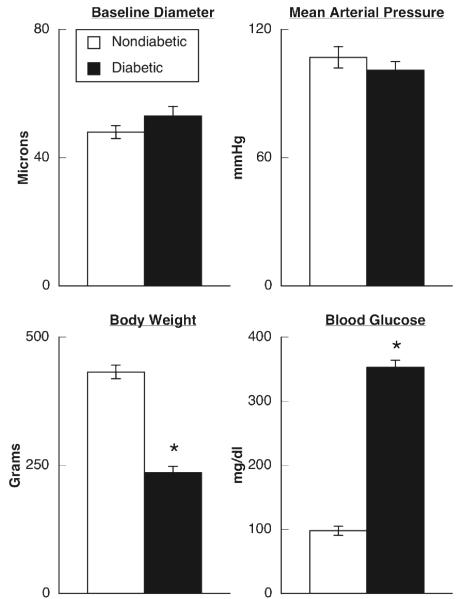
There were no significant differences in baseline diameter of pial arterioles or mean arterial blood pressure between nondiabetic and diabetic rats (Figure 1). However, blood glucose concentration was significantly higher in diabetic rats than in nondiabetic rats and body weight was significantly lower in diabetic compared to nondiabetic rats (Figure 1).
Figure 1.
Baseline diameter of pial arterioles, mean arterial pressure, body weight and blood glucose concentration in nondiabetic (open bars) and diabetic (closed bars) rats. Values are means±SE. * p < 0.05 versus nondiabetic rats.
Responses to the agonists
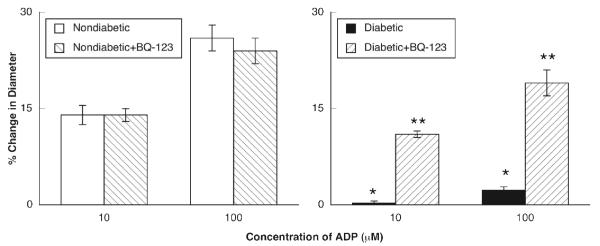
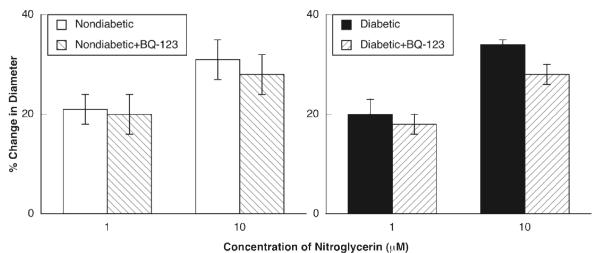
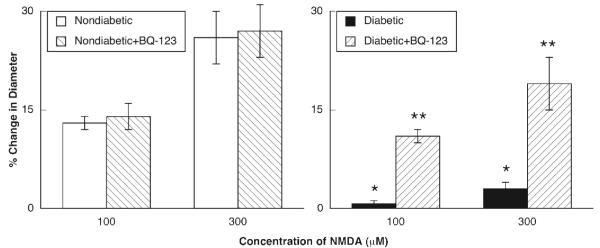
ADP, NMDA and nitroglycerin produced dilation of pial arterioles in nondiabetic and diabetic rats (Figures 2-4). However, the magnitude of vasodilation in response to ADP (Figure 2) and NMDA (Figure 3) was greater in nondiabetic than in diabetic rats. In contrast, dilation of pial arterioles in response to nitroglycerin (Figure 4) was similar in nondiabetic and diabetic rats.
Figure 2.
Responses of pial to ADP in nondiabetic and diabetic rats before and during superfusion with BQ-123 (1.0 μM for 60 minutes). Values are means±SE. * p < 0.05 versus response in nondiabetic rats. ** p < 0.05 versus response in diabetic rats before superfusion with BQ-123.
Figure 4.
Responses of pial to nitroglycerin in nondiabetic and diabetic rats before and during superfusion with BQ-123 (1.0 μM for 60 minutes). Values are means±SE.
Figure 3.
Responses of pial to NMDA in nondiabetic and diabetic rats before and during superfusion with BQ-123 (1.0 μM for 60 minutes). Values are means±SE. * p < 0.05 versus response in nondiabetic rats. ** p < 0.05 versus response in diabetic rats before superfusion with BQ-123.
Topical application of BQ-123 (1.0 μM for 1 hour) to the cranial window produced a small, but significant change in baseline diameter of pial arterioles in nondiabetic rats (47±2 μm before versus 51±2 μm after treatement with BQ-123; p < 0.05) and diabetic rats (53±2 μm before versus 56±3 μm after treatment with BQ-123; p < 0.05). BQ-123 did not affect dilation of pial arterioles in response to ADP (Figure 2), NMDA (Figure 3) or nitroglycerin (Figure 4) in nondiabetic rats. However, application of BQ-123 to the cranial window in diabetic rats restored impaired responses of cerebral arterioles to ADP (Figure 2) and NMDA (Figure 3) observed in diabetic rats towards that observed in nondiabetic rats. In contrast, application of BQ-123 did not alter vasodilation to nitroglycerin (Figure 4) in diabetic rats.
Superoxide production
Basal superoxide production was increased in parietal cortex samples from diabetic rats compared to nondiabetic rats (Figure 5). Treatment with BQ-123 (1.0 μM for 60 minutes) did not affect superoxide levels in nondiabetic rats, but significantly decreased basal superoxide production in diabetic rats (Figure 5). Incubation of cortex tissue with ET-1 (0.1 μM for 30 minutes) produced an increase in superoxide anion in nondiabetic and diabetic rats and this increase could be inhibited by pre-treatment with BQ-123 (1.0 μM) (Figure 5).
Figure 5.
Production of superoxide anion by parietal cortex tissue in nondiabetic rats (open bars) and diabetic rats (closed bars) under basal conditions, during application of ET-1 (0.01 and 0.1 μM), during application of BQ-123 (1.0 μM) and during application of ET-1 in the presence of BQ-123. Values are means±SE. “a” p < 0.05 versus nondiabetic rats, “b” p < 0.05 versus basal superoxide production, “c” p < 0.05 versus basal levels of superoxide production, and “d” p < 0.05 versus response to ET-1.
Discussion
There are two major findings from this study. First, impaired eNOS- and nNOS-dependent dilation of cerebral arterioles in diabetic rats can be restored towards that observed in nondiabetic rats by treatment with an ETA receptor antagonist (BQ-123). This finding appeared to be specific for NOS-dependent reactivity since responses to nitroglycerin were not altered by treatment with BQ-123. Second, the production of superoxide anion was increased from parietal cortex tissue in diabetic rats and BQ-123 inhibited this basal superoxide production. We suggest that activation of ETA receptors during T1D plays an important role in cerebrovascular dysfunction by a mechanism related to an increase in superoxide anion production.
Consideration of Methods
We used ADP and NMDA to examine eNOS- and nNOS-dependent responses of cerebral arterioles. We and others have suggested that ADP dilates cerebral arterioles via activation of NOS [5, 17, 39]. Other investigators [34, 66], however, have suggested that relaxation of the rat middle cerebral artery to purines is related to the synthesis/release of nitric oxide and EDHF. We did not examine a role for EDHF in response to ADP in the present study, and thus we cannot exclude the possibility that EDHF contributes to dilatation of cerebral arterioles in response to ADP. However, others [7, 9, 18, 21, 56] also have suggested that activation of potassium channels, presumably by EDHF, does not play a significant role in dilatation of cerebral vessels to the agonists used in the present study. In addition, we and others have shown that application of NMDA dilates cerebral arterioles via the synthesis/release of nitric oxide by activation of nNOS [18-20, 55]. Thus, we suggest that the use of ADP and NMDA are appropriate to evaluate NOS-dependent dilatation of cerebral arterioles.
We used BQ-123 to inhibit ETA receptors in order to determine whether activation of these receptors could influence cerebrovascular function during T1D. Many previous studies have shown that BQ-123 is specific for ETA receptors [8, 10, 35, 36] and in the present study we found that BQ-123 could inhibit superoxide production by parietal cortex tissue during application of ET-1. Thus, similar to that reported by other investigators for other vascular beds, it appears that BQ-123 is specific for ETA receptors. In addition, we measured responses of cerebral arterioles before and following application of BQ-123. One might suggest that there may be a time-dependent component of reactivity of cerebral arterioles to the agonists. However, we have shown previously that application of the agonists elicit reproducible vasodilation [2, 16]. Thus, we suggest that restoration of responses of cerebral arterioles to the agonists by BQ-123 is not due to a time-dependent effect of the agonists on vasoreactivity.
We have reported previously that impaired responses of cerebral arterioles during T1D can be improved/restored to that observed in nondiabetic rats by inhibition of various pathways including the formation of superoxide using superoxide dismutase [41], NADPH oxidase [37], poly(ADP-ribose) polymerase [3] or angiotensin type 1 receptors [4]. In the present study, we now show a role for ETA receptors in impaired responses of cerebral arterioles in T1D. Thus, one might ask how these findings fit with the results from these previous studies? It remains uncertain how inhibition of several specific, but presumably distinct pathways, can restore responses of cerebral arterioles during T1D. However, a study by Nishikawa et al [46] may provide some insight. These investigators found that inhibition of mitochondrial sources of superoxide was a causal link between hyperglycemia-induced damage and activation of three very distinct pathways (protein kinase C, advanced glycation end-products and the aldose reductase pathway). Thus, it is conceivable that mitochondrial sources of superoxide may contribute to activation of various pathways that have been implicated in diabetes-induced vascular dysfunction.
We measured production of superoxide anion by brain tissue in an attempt to determine the role of superoxide formation, by activation of ETA receptors, in cerebrovascular dysfunction during T1D. We have used this method for measuring production of superoxide in many previous studies [3, 4, 37]. It is not possible for us to determine the precise cellular source of superoxide using this methodology. However, based upon our findings using BQ-123, which is a specific inhibitor of ETA receptors that reside on endothelium, we suggest that the endothelium is a likely source of superoxide.
Consideration of previous studies
Smooth muscle cells/endothelial cells express ETA and ETB receptors [32]. In addition, these receptors proliferate in response to an increase in circulating/local levels of ET-1. Increased plasma levels of ET-1 have been reported in both type 1 and type 2 diabetic animals and patients [11, 22, 23, 33, 53, 57, 58]. Mechanisms by which T1D can lead to an increase in the synthesis/release of ET-1 are not entirely clear. Investigators have reported that hyperglycemia can induce the expression of ET-1 in cell cultures of endothelium via an increase in protein kinase C [48] and/or an increase in poly (ADP-ribose) polymerase, which in turn activates protein kinase C [44]. In addition, various cytokines and growth factors are increased during diabetes mellitus [30] and it has been reported that the production of ET-1 can be induced in human vascular smooth muscle cells by inflammatory cytokines [61, 62]. Further, it has been reported that ET-1 mRNA and protein can be increased in vascular cell walls by reactive oxygen species [27]. Thus, it appears that hyperglycemia during T1D can induced the synthesis/release of ET-1 through a variety of pathways.
Given that ET-1 is a very potent vasoconstrictor agent, it has been suggested that elevated levels of ET-1 during diabetes may contribute to vascular dysfunction [26, 29, 50]. Several studies have investigated this concept by examining the influence of inhibition of ET-1 receptors on vascular function. Dhein, et al. [14] reported that long-term (6 month) inhibition of ETA receptors using LU 135252 could inhibit T1D-induced impairment in relaxation of mesenteric arterioles. However, the mechanism by which LU 135252 restored endothelial function was not investigated. Kanie and Kamata [28] examined the influence of chronic treatment with a mixed ETA and ETB receptor antagonist (J-104132) on endothelial dysfunction of the aorta in diabetic rats. These investigators found that inhibition of ETA and ETB receptors could restore acetylcholine-induced relaxation of the aorta in diabetic rats [28]. The mechanism by which chronic treatment with J-104132 could restore endothelial function during T1D appeared to be related to inhibition of oxidative stress since J-104132 significantly decreased levels of superoxide anion and expression of p22phox mRNA (an important subunit for NAD(P)H oxidase). Finally, Dumont et al. [15] examined the influence of bosentan (a mixed ETA and ETB receptor antagonist) on myogenic tone and NOS-dependent relaxation of the middle cerebral artery in T1D. These investigators found that the enhanced myogenic tone observed in diabetic rats could be prevented by treatment with bosentan. In addition, impaired relaxation of the middle cerebral artery to bradykinin in diabetic rats could be reversed by treatment with bosentan. Unfortunately, these investigators [15] did not examine mechanisms responsible for the effects of bosentan on restoring impaired relaxation of the middle cerebral artery in diabetic rats. In the present study, we found that treatment of diabetic rats with BQ-123, a specific inhibitor of ETA receptors, could restore impaired eNOS- and nNOS-dependent dilation of cerebral resistance arterioles. Further, we found that treatment of cerebral cortex tissue with BQ-123 decreased elevated basal production of superoxide anion and prevented ET-1-induced increases in superoxide anion observed in diabetic rats.
In summary, we found that treatment of cerebral arterioles with BQ-123 could prevent T1D-induced impairment in eNOS- and nNOS-dependent responses of cerebral arterioles. In addition, we found that the increase in basal production of superoxide anion production observed during T1D could be inhibited by BQ-123. We suggest that increased circulating/local levels of endothelin-1 during T1D can activate ETA receptors and contribute to impaired responses of cerebral arterioles by an increase in oxidative stress.
Acknowledgments
This study was supported by National Institutes of Health Grants HL-090657, AA-11288 and funds from the University of Nebraska Medical Center.
References
- 1.Adner M, You J, Edvinsson L. Characterization of endothelin-A receptors in the cerebral circulation. Neuroreport. 1993;4:441–443. doi: 10.1097/00001756-199304000-00026. [DOI] [PubMed] [Google Scholar]
- 2.Arrick DM, Mayhan WG. Acute infusion of nicotine impairs nNOS-dependent reactivity of cerebral arterioles via an increase in oxidative stress. J Appl Physiol. 2007;103:2062–2067. doi: 10.1152/japplphysiol.00411.2007. [DOI] [PubMed] [Google Scholar]
- 3.Arrick DM, Sharpe GM, Sun H, Mayhan WG. Diabetes-induced cerebrovascular dysfunction: Role of poly(ADP-ribose) polymerase. Microvasc Res. 2007;73:1–6. doi: 10.1016/j.mvr.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Arrick DM, Sharpe GM, Sun H, Mayhan WG. Losartan improves impaired nitric oxide synthase-dependent dilatation of cerebral arterioles in type 1 diabetic rats. Brain Res. 2008;1209:128–135. doi: 10.1016/j.brainres.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayajiki K, Okamura T, Toda N. Involvement of nitric oxide in endothelium-dependent, phasic relaxation caused by histamine in monkey cerebral arteries. Japanese Journal of Pharmacology. 1992;60:357–362. doi: 10.1254/jjp.60.357. [DOI] [PubMed] [Google Scholar]
- 6.Bacic F, Uematsu S, Mccarron RM, Spatz M. Secretion of immunoreactive endothelin-1 by capillary and microvascular endothelium of human brain. Neurochemical Research. 1992;17:699–702. doi: 10.1007/BF00968008. [DOI] [PubMed] [Google Scholar]
- 7.Brayden JE. Hyperpolarization and relaxation of resistance arteries in response to adenosine diphosphate. Circulation Research. 1991;69:1415–1420. doi: 10.1161/01.res.69.5.1415. [DOI] [PubMed] [Google Scholar]
- 8.Cardillo C, Campia U, Bryant MB, Panza JA. Increased activity of endogenous endothelin in patients with type II diabetes mellitus. Circulation. 2002;106:1783–1787. doi: 10.1161/01.cir.0000032260.01569.64. [DOI] [PubMed] [Google Scholar]
- 9.Chrissobolis S, Ziogas J, Chu Y, Faraci FM, Sobey CG. Role of inwardly rectifying K+ channels in K+-induced cerebral vasodilatation in vivo. American Journal of Physiology. 2000;279:H2704–H2712. doi: 10.1152/ajpheart.2000.279.6.H2704. [DOI] [PubMed] [Google Scholar]
- 10.Clozel M, Watanabe H. BQ-123, a peptidic endothelin ETA receptor antagonist, prevents the early cerebral vasospasm following subarachnoid hemorrhage arfter intracisternal but not intravenous injection. Life Sciences. 1993;825:834–1629. doi: 10.1016/0024-3205(93)90081-d. [DOI] [PubMed] [Google Scholar]
- 11.Collier A, Leach JP, Mclellan A, Jardine A, Morton JJ, Small M. Plasma endothelinlike immunoreactivity levels in IDDM patients with microalbuminuria. Diabetes Care. 1992;15:1038–1040. doi: 10.2337/diacare.15.8.1038. [DOI] [PubMed] [Google Scholar]
- 12.Coppey LJ, Gellett JS, Davidson EP, Yorek MA. Preventing superoxide formation in epineurial arterioles of the sciatic nerve from diabetic rats restores endothelium-dependent vasodilation. Free Radic Res. 2003;37:33–40. doi: 10.1080/1071576021000028442. [DOI] [PubMed] [Google Scholar]
- 13.Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108:1527–1532. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 14.Dhein S, Hochreuther S, Spring CAD, Bollig K, Hufnagel C, Raschack M. Long-term effects of the endothelinA receptor antagonis LU 135252 and the angiotensin-converting enzyme inhibitor trandolapril on diabetic angiopathy and nephropathy in a chronic Type I diabetes mellitus rat model. Journal of Pharmacology and Experimental Therapuetics. 2000;293:351–359. [PubMed] [Google Scholar]
- 15.Dumont AS, Dumont RJ, Mcneill JH, Kassell NF, Sutherland GR, Verma S. Chronic endothelin antagonism restores cerebrovascular function in diabetes. Neurosurgery. 2003;52:653–60. doi: 10.1227/01.neu.0000048187.74897.7e. discussion 659-60. [DOI] [PubMed] [Google Scholar]
- 16.Fang Q, Sun H, Mayhan WG. Impairment of nitric oxide synthase-dependent dilatation of cerebral arterioles during infusion of nicotine. Am J Physiol Heart Circ Physiol. 2003;284:H528–34. doi: 10.1152/ajpheart.00752.2002. [DOI] [PubMed] [Google Scholar]
- 17.Faraci FM. Role of endothelium-derived relaxing factor in cerebral circulation: Large arteries vs. microcirculation. American Journal of Physiology. 1991;261:H1038–H1042. doi: 10.1152/ajpheart.1991.261.4.H1038. [DOI] [PubMed] [Google Scholar]
- 18.Faraci FM, Breese KR. Nitric oxide mediates vasodilatation in response to activation of N-methyl-D-aspartate receptors in brain. Circulation Research. 1993;72:476–480. doi: 10.1161/01.res.72.2.476. [DOI] [PubMed] [Google Scholar]
- 19.Faraci FM, Breese KR, Heistad DD. Responses of cerebral arterioles to kainate. Stroke. 1994;25:2080–2084. doi: 10.1161/01.str.25.10.2080. [DOI] [PubMed] [Google Scholar]
- 20.Faraci FM, Brian JE. 7-nitroindazole inhibits brain nitric oxide synthase and cerebral vasodilatation in response to N-methyl-D-aspartate. Stroke. 1995;26:2172–2176. doi: 10.1161/01.str.26.11.2172. [DOI] [PubMed] [Google Scholar]
- 21.Faraci FM, Heistad DD. Role of ATP-sensitive potassium channels in the basilar artery. American Journal of Physiology. 1993;264:H8–H13. doi: 10.1152/ajpheart.1993.264.1.H8. [DOI] [PubMed] [Google Scholar]
- 22.Francavilla S, Properzi G, Bellini C, Marino G, Ferri C, Santucci A. Endothelin-1 in diabetic and nondiabetic men with erectile dysfunction. Journal of Urology. 1997;158:1770–1774. doi: 10.1016/s0022-5347(01)64125-9. [DOI] [PubMed] [Google Scholar]
- 23.Guvener N, Aytemir K, Aksoyek S, Gedik O. Plasma endothelin-1 levels in non-insulin dependent diabetes mellitus patients with macrovascular disease. Coronary Artery Disease. 1997;8:253–258. doi: 10.1097/00019501-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Hadi H, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag. 2007;3:853–876. [PMC free article] [PubMed] [Google Scholar]
- 25.Hattori Y, Kawasaki H, Abe K, Kanno M. Superoxide dismutase recovers altered endothelium-dependent relaxation in diabetic rat aorta. American Journal of Physiology. 1991;261:H1086–H1094. doi: 10.1152/ajpheart.1991.261.4.H1086. [DOI] [PubMed] [Google Scholar]
- 26.Hopfner RL, Gopalakrishnan V. Endothelin: emerging role in diabetic vascular complications. Diabetologia. 1999;42:1383–1394. doi: 10.1007/s001250051308. [DOI] [PubMed] [Google Scholar]
- 27.Kaehler J, Sill B, Koester R, Mittmann C, Orzechowski HD, Muenzel T, Meinertz T. Endothelin-1 mRNA and protein in vascular wall cells is increased by reactive oxygen species. Clin Sci (Lond) 2002;103(Suppl 48):176S–178S. doi: 10.1042/CS103S176S. [DOI] [PubMed] [Google Scholar]
- 28.Kanie N, Kamata K. Effects of chronic administration of the novel endothelin antagonist J-104132 on endothelial dysfunction in streptozotocin-induced diabetic rat. Br J Pharmacol. 2002;135:1935–1942. doi: 10.1038/sj.bjp.0704659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan ZA, Chakrabarti S. Endothelins in chronic diabetic complications. Can J Physiol Pharmacol. 2003;81:622–634. doi: 10.1139/y03-053. [DOI] [PubMed] [Google Scholar]
- 30.Kunz M, Ibrahim S. Cytokines and cytokine profiles in human autoimmune diseases and animal models of autoimmunity. Mediators Inflamm. 2009;2009:1–20. doi: 10.1155/2009/979258. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part II. Circulation. 2003;108:1655–1661. doi: 10.1161/01.CIR.0000089189.70578.E2. [DOI] [PubMed] [Google Scholar]
- 32.Luscher TF. Endothelin. Journal of Cardiovascular Pharmacology. 1991;18(Suppl. 10):S15–S22. [PubMed] [Google Scholar]
- 33.Makino A, Kamata K. Elevated plasma endothelin-1 level in streptozotocin-induced diabetic rats and responsiveness of the mesenteric arterial bed to endothelin-1. British Journal of Pharmacology. 1998;123:1065–1072. doi: 10.1038/sj.bjp.0701704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marrelli SP, Khorovets A, Johnson TD, Childres WF, Bryan RM., Jr. P2 purinoceptor-mediated dilations in the rat middle cerebral artery after ischemia-reperfusion. Am J Physiol. 1999;276:H33–41. doi: 10.1152/ajpheart.1999.276.1.H33. [DOI] [PubMed] [Google Scholar]
- 35.Mather KJ, Lteif A, Steinberg HO, Baron AD. Interactions between endothelin and nitric oxide in the regulation of vascular tone in obesity and diabetes. Diabetes. 2004;53:2060–2066. doi: 10.2337/diabetes.53.8.2060. [DOI] [PubMed] [Google Scholar]
- 36.Mather KJ, Mirzamohammadi B, Lteif A, Steinberg HO, Baron AD. Endothelin contributes to basal vascular tone and endothelial dysfunction in human obesity and type 2 diabetes. Diabetes. 2002;51:3517–3523. doi: 10.2337/diabetes.51.12.3517. [DOI] [PubMed] [Google Scholar]
- 37.Mayhan WG, Arrick DM, Sharpe GM, Patel KP, Sun H. Inhibition of NAD(P)H oxidase alleviates impaired NOS-dependent responses of pial arterioles in Type 1 diabetes mellitus. Microcirculation. 2006;13:567–575. doi: 10.1080/10739680600885194. [DOI] [PubMed] [Google Scholar]
- 38.Mayhan WG. Impairment of endothelium-dependent dilatation of cerebral arterioles during diabetes mellitus. American Journal of Physiology. 1989;256:H621–H625. doi: 10.1152/ajpheart.1989.256.3.H621. [DOI] [PubMed] [Google Scholar]
- 39.Mayhan WG. Endothelium-dependent responses of cerebral arterioles to adenosine 5′-diphosphate. Journal of Vascular Research. 1992;29:353–358. doi: 10.1159/000158951. [DOI] [PubMed] [Google Scholar]
- 40.Mayhan WG. Impairment of endothelium-dependent dilatation of the basilar artery during diabetes mellitus. Brain Research. 1992;580:297–302. doi: 10.1016/0006-8993(92)90957-b. [DOI] [PubMed] [Google Scholar]
- 41.Mayhan WG. Superoxide dismutase partially restores impaired dilatation of the basilar artery during diabetes mellitus. Brain Research. 1997;760:204–209. doi: 10.1016/s0006-8993(97)00282-5. [DOI] [PubMed] [Google Scholar]
- 42.Mayhan WG, Heistad DD. Permeability of blood-brain barrier to various sized molecules. American Journal of Physiology. 1985;248:H712–H718. doi: 10.1152/ajpheart.1985.248.5.H712. [DOI] [PubMed] [Google Scholar]
- 43.Miller RC, Pelton JT, Huggins JP. Endothelins--from receptors to medicine. TIPS. 1993;14:54–60. doi: 10.1016/0165-6147(93)90031-e. [DOI] [PubMed] [Google Scholar]
- 44.Minchenko AG, Stevens MJ, White L, Abatan OI, Komjati K, Pacher P, Szabo C, Obrosova IG. Diabetes-induced overexpression of endothelin-1 and endothelin receptors in the rat renal cortex is mediated via poly(ADP-ribose) polymerase activation. Faseb J. 2003;17:1514–1516. doi: 10.1096/fj.03-0013fje. [DOI] [PubMed] [Google Scholar]
- 45.Nassar T, Kadery B, Lotan C, Da’As N, Kleinman Y, Haj-Yehia A. Effects of the superoxide dismutase-mimetic compound tempol on endothelial dysfunction in streptozotocin-induced diabetic rats. European Journal of Pharmacology. 2002;436:111–118. doi: 10.1016/s0014-2999(01)01566-7. [DOI] [PubMed] [Google Scholar]
- 46.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 47.Ohishi K, Carmines PK. Superoxide dismutase restores the influence of nitric oxide on renal arterioles in diabetes mellitus. Journal of the American Society of Nephrology. 1995;5:1559–1566. doi: 10.1681/ASN.V581559. [DOI] [PubMed] [Google Scholar]
- 48.Park J, Takahara N, Gabriele A, Chou E, Naruse K, Suzuma K, Yamauchi T, Ha S, Meier M, Rhodes C, King G. Induction of endothelin-1 expression by glucose: an effect of protein kinase C activation. Diabetes. 2000;49:1239–1248. doi: 10.2337/diabetes.49.7.1239. [DOI] [PubMed] [Google Scholar]
- 49.Pieper GM, Mei DA, Langenstroer P, O’Rourke ST. Bioassay of endothelium-derived relaxing factor in diabetic rat aorta. American Journal of Physiology Heart Circulatory Physiology. 1992;263:H676–H680. doi: 10.1152/ajpheart.1992.263.3.H676. [DOI] [PubMed] [Google Scholar]
- 50.Potenza M, Gagliardi S, Nacci C, Carratu’ M, Montagnani M. Endothelial dysfunction in diabetes: from mechanisms to therapeutic targets. Curr Med Chem. 2009;16:94–112. doi: 10.2174/092986709787002853. [DOI] [PubMed] [Google Scholar]
- 51.Rubanyi GM, Polokoff MA. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacological Reviews. 1994;46:325–415. [PubMed] [Google Scholar]
- 52.Schiffrin EL. Role of endothelin-1 in hypertension and vascular disease. American Journal of Hypertension. 2001;14:83S–89S. doi: 10.1016/s0895-7061(01)02074-x. [DOI] [PubMed] [Google Scholar]
- 53.Schneider JG, Tilly N, Hierl T, Sommer U, Hamann A, Dugi K, Leidig-Bruckner G, Kasperk C. Elevated plasma endothelin-1 levels in diabetes mellitus. Am J Hypertens. 2002;15:967–972. doi: 10.1016/s0895-7061(02)03060-1. [DOI] [PubMed] [Google Scholar]
- 54.Sun H, Molacek E, Zheng H, Fang Q, Patel KP, Mayhan WG. Alcohol-induced impairment of neuronal nitric oxide synthase (nNOS)-dependent dilation of cerebral arterioles: role of NAD(P)H oxidase. Journal of Molecular and Cellular Cardiology. 2006;40:321–328. doi: 10.1016/j.yjmcc.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Sun H, Patel KP, Mayhan WG. Impairment of neuronal nitric oxide synthase-dependent dilatation of cerebral arterioles during chronic alcohol consumption. Alcoholism: Clinical and Experimental Research. 2002;26:663–670. [PubMed] [Google Scholar]
- 56.Taguchi H, Heistad DD, Kitazono T, Faraci FM. Dilatation of cerebral arterioles in response to activation of adenylate cyclase is dependent on activation of Ca2+-dependent K+ channels. Circulation Research. 1995;76:1057–1062. doi: 10.1161/01.res.76.6.1057. [DOI] [PubMed] [Google Scholar]
- 57.Tarquini B, Perfetto F, Tarquini R, Cornelissen G, Halberg F. Endothelin-1’s chronome indicates diabetic and vascular disease chronorisk. Peptides. 1997;18:119–132. doi: 10.1016/s0196-9781(96)00258-6. [DOI] [PubMed] [Google Scholar]
- 58.Vural P, Cevik A, Curgunlu A, Canbaz M. Effects of diabetes mellitus and acute hypertension on plasma nitric oxide and endothelin concentrations in rats. Clin Chim Acta. 2002;320:43–47. doi: 10.1016/s0009-8981(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 59.Warner TD, Budzik GP, Mitchell JA, Huang ZJ, Murad F. Detection by bioassay and specific enzyme-linked immunoscorbent assay of phosphoramidon-inhibitable endothelin-converting activity in brain and endothelium. Journal of Cardiovascular Pharmacology. 1992;20:S19–S21. doi: 10.1097/00005344-199204002-00007. [DOI] [PubMed] [Google Scholar]
- 60.Warner TD, Schmidt HHHW, Kuk J, Mitchell JA, Murad F. Human brain contains a metalloprotease that converts big endothelin-1 to endothelin-1 and is inhibited by phosphoramidon and EDTA. British Journal of Pharmacology. 1992;106:505–506. doi: 10.1111/j.1476-5381.1992.tb14364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woods M, Mitchell J, Wood E, Barker S, Walcot N, Rees G, Warner T. Endothelin-1 is induced by cytokines in human vascular smooth muscle cells: evidence for intracellular endothelin-converting enzyme. Mol Pharmacol. 1999;55:902–929. [PubMed] [Google Scholar]
- 62.Woods M, Wood E, Mitchell J, Warner T. Signal transduction pathways involved in cytokine stimulation of endothelin-1 release from human vascular smooth muscle cells. J Cardiovasc Pharmacol. 2000;36:S407–9. doi: 10.1097/00005344-200036051-00119. [DOI] [PubMed] [Google Scholar]
- 63.Yamada G, Hama H, Kasuya Y, Masaki T, Goto K. Possible sources of endothelin-1 in damaged rat brain. Journal of Cardiovascular Pharmacology. 1995;26:S486–S490. [PubMed] [Google Scholar]
- 64.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 65.Yoshimoto S, Ishizaki Y, Kurihara H, Sasaki T, Yoshizumi M, Yanagisawa M, Yazaki Y, Masaki T, Takakura K, Murota S. Cerebral microvessel endothelium is producing endothelin. Brain Research. 1990;508:283–285. doi: 10.1016/0006-8993(90)90407-3. [DOI] [PubMed] [Google Scholar]
- 66.You J, Johnson TD, Marrelli SP, Mombouli JV, Bryan RM. P2u receptor-mediated release of endothelium-derived relaxing factor/nitric oxide and endothelium-derived hyperpolarizing factor from cerebrovascular endothelium in rats. Stroke. 1999;30:1125–1133. doi: 10.1161/01.str.30.5.1125. [DOI] [PubMed] [Google Scholar]