Abstract
Therapeutic immunization leading to cancer regression remains a significant challenge. Successful immunization requires activation of adaptive immunity, including tumor specific CD4 + T cells and CD8+ T cells. Generally speaking, the activation of T cells is compromised in patients with cancer due to immune suppression, loss of tumor antigen expression, and dysfunction of antigen presenting cells (APC). APC such as dendritic cells (DC) are key for the induction of adaptive anti-tumor immune responses. Recently, attention has focused on novel adjuvants that enhance DC function and their ability to prime T cells. Agonists that target toll-like receptors (TLR) are being used clinically either alone or in combination with tumor antigens and showing initial success both in terms of enhancing immune responses and eliciting anti-tumor activity. This review summarizes the application of these adjuvants to treat cancer and the potential for boosting responses in vivo.
Keywords: Toll-like receptors, Cancer vaccines, Dendritic cells, Vaccine adjuvants
Background
Considerable evidence exists showing that the immune system protects the host against progressive growth of primary non-viral cancers and influences the immunogenicity of tumors, a concept known as immune surveillance (1). This has propelled studies to identify effective immune therapeutic approaches to eradicate or reduce outgrowth of human cancers. Immunotherapies fall into two broad categories: those that target antigen presenting cells (APC) by enhancing their ability to stimulate the immune system, and those that target the adaptive immune response i.e. T cells and B cells. In this review we discuss approaches that enhance the activity of APC such dendritic cells (DC). DC are the most potent APC and function to activate innate (e.g. natural killer cells (NK)), and adaptive immune responses which are mediated by B cells and T cells (Figure 1). Both innate and adaptive arms of the immune system are key for recognizing and eliminating tumor cells. Since vaccines generally elicit humoral and T cell responses, efforts are being directed towards approaches that elicit tumor-specific, integrated B cell, CD4+ and CD8+ T cell responses in vivo. A challenge in the field of cancer vaccines has been how to elicit these responses and ensure they are not only antigen-specific, but effective, durable and safely administered. In this review we discuss the use of Toll-like receptor agonists, novel compounds that activate DC and other members of the immune system in vivo, and are showing promise in the clinic as vaccine adjuvants in cancer patients.
Figure 1. Expression of Toll-like receptors on innate immune cells.
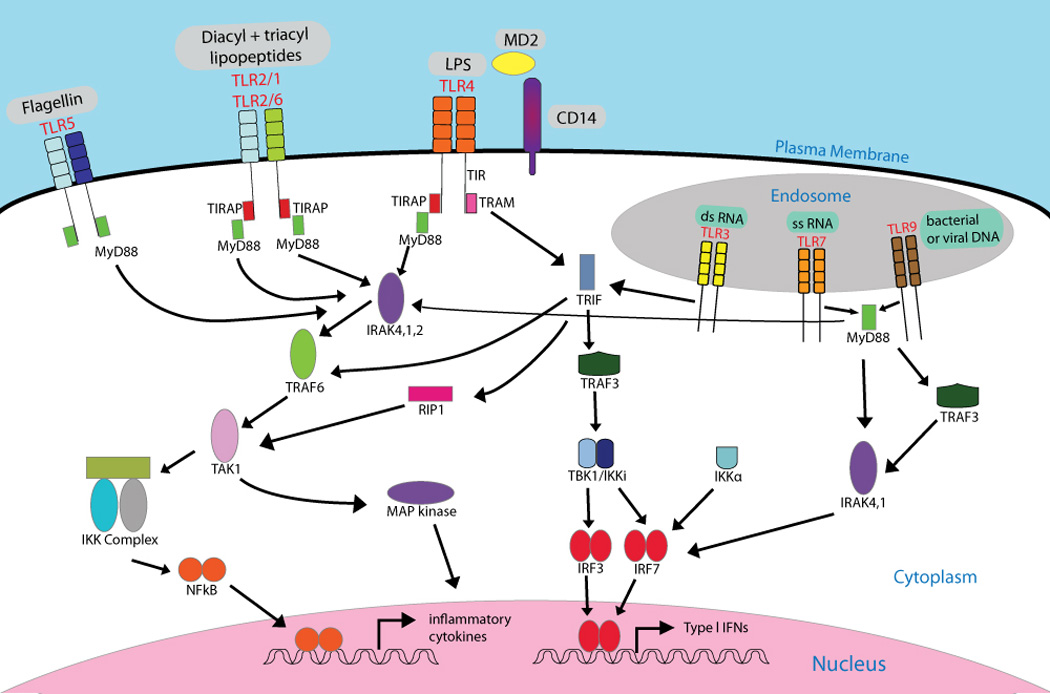
TLR 1,-2,-4, -5 and -6 are expressed in the plasma membrane where TLR2 associates with either TLR1 or TLR6. TLR3, -7, -8 and -9 traffic from the endoplasmic reticulum to the endosome where they encounter their ligands. MYD88 (myeloid differentiation primary response protein 88) and TRIF (TIR domain-containing adaptor protein inducing IFN) are signalling adaptors that link Toll-like receptors (TLRs) are to downstream kinases that define a given signalling pathway. All TLRs use MyD88, except for TLR3 which uses TRIF. The sorting adaptor TIRAP (TIR domain-containing adaptor protein) is used by TLR1, TLR2, TLR4 and TLR6 and links the TIR domain to MyD88, whereas TRIF is recruited by both TLR4 and TLR3. An additional adaptor TRAM, links the TIR domain of TLR4 with TRIF. TLRs which use the MyD88 dependent pathway recruit the IRAK family of proteins and TRAF6 resulting in the activation of TAK1. This in turn leads to the activation of NFKB and the MAPK pathway and results in the induction of pro-inflammatory cyokines and upregulation of phenotypic markers of activation (CD80, CD86). TLR4 (which relies on additional accessory molecules MD2 and CD14) and TLR3 both trigger the TRIF-dependent pathway, which also leads to activation of inflammatory cytokines via NFKB and MAP Kinase. In addition, TRIF recruits TRAF, leading to the activation of TBK1/IKKi, IRF3 and IRF7 and transcription of type I IFN. MyD88 also associates with the IRAK family of proteins. A complex of proteins (TRAF3, IRAK1 and Ikkα) subsequently activates IRF7. Examples of ligands binding the TLRs are shown. (Adapted from Kumar et al.,(125)).
Abbreviations: LPS, lipopolysaccharide; PtdIns(4,5)P2, phosphatidylinositol-4,5-bisphospate; TRAF3, TNFR-associated factor 3.
Toll-like receptors (TLR)
Toll like receptors (TLR) are a family of pattern recognition receptors (PRR) that function as primary sensors of the innate immune system to recognize microbial pathogens. They were initially discovered as factors involved in the embryonic development and resistance of the fly Drosophila to bacterial and fungal infection (2, 3). TLR recognize distinct structures in microbes, often referred to as “PAMPs” (pathogen associated molecular patterns). Ligand binding to TLR invokes a cascade of intra-cellular signaling pathways that induce the production of factors involved in inflammation and immunity (4, 5). PRR also include intracellular proteins e.g. Nod-like receptors, RIG-1 like helicases (RLHs) as well as extracellular receptors, e.g. scavenger receptors and C-type lectin receptors (4, 5). TLR, the topic of this review, are typically activated by microbial signals but may also be activated by endogenous ligands (e.g. heat shock proteins, fibronectin and fibrinogen) or synthetic compounds (Table 1). TLR can be expressed on members of the innate and adaptive immune system (DCs, macrophages, granulocytes, T cells, B cells, NK cells and mast cells), as well as by endothelial and epithelial cells (5). More recently, TLR have been found on tumor cells, including melanoma (6). In humans, ten TLR have been identified (Figure 1). These receptors comprise a family of conserved membrane spanning molecules containing an ectodomain of leucine-rich repeats, a transmembrane domain and an intracellular TIR (Toll/IL-1R) domain (7). TLR that are expressed on the surface of cells detect pathogens within the local environment (TLR1,-2,-4,-5,-6). TLR4 recognizes bacterial cell wall component lipopolysaccharide (LPS) through its ectodomain (8), in addition to MPL A (monophosphoryl lipid A). Lipoprotein and lipoteichoic acid are recognized by TLR2 in combination with TLR1 and TLR6, respectively (9). TLR5 recognizes bacterial flagellin (10). In contrast, certain TLR (TLR-3, -7/8, -9) are located within the endoplasmic reticulum (ER) and rapidly recruited to endosomal-lysosomal compartments, where they can detect microbial nucleic acids (dsRNA, ssRNA and ss DNA containing unmethylated CpG motifs, respectively (4, 5)).
Table 1.
Natural and synthetic ligands of Toll-like receptors
| Receptor | Pathogen Associated ligands (PAMPs) | Endogenous ligands | Synthetic ligands |
|---|---|---|---|
| TLR 1/2 | Triacylated lipopeptides (Bacteria and Mycobacteria)* | Not known | Pam3Cys * |
| TLR 2 | Peptidoglycan (gram positive bacteria); Bacterial lipoprotein; Lipoteichoic acid; LPS (Porphyromonas gingivalis, Leptospira interrogans); GPI-anchor proteins (Trypanosoma cruzi); Neisserial porins, Hemagglutinin (MV); phospholipomannan (Candida); LAM (Mycobacteria) |
Not known | CFA MALP2** Pam2Cys** FSL-1** Hib-OMPC |
| TLR 3 | ssRNA virus (WNV), dsRNA virus (RSV, MCMV) |
Not known | Poly I:C; poly A:U |
| TLR 4 | LPS (Gram-negative bacteria); F-protein (RSV); Mannan (Candida); Glycoinositolphospholipids (Trypanosoma); Envelope proteins (RSV and MMTV) |
Hsp60, Hsp70, fibronectin domain A surfactant protein A, hyaluronan; HMGB-1 |
AGP MPL A RC-529 MDF2β CFA |
| TLR 5 | Flagellin (Flagellated bacteria) | Not known | Flagellin |
| TLR 2/6 | Phenol-soluble modulin (Staphylococcus epidermidis) Diacylated lipopeptides (Mycoplasma); LTA (Streptococcus); Zymosan (Saccharomyces) |
Not known | MALP-2** Pam2Cys** FSL-1** |
| TLR 7 | Viral ssRNA (Influenza, VSV, HIV, HCV) | Human RNA | Guanosine analogs; imidazoquinolines (e.g. Imiquimod, Aldara ® R848, Resiquimod®); Loxoribine |
| TLR 8 | ssRNA from RNA virus | Human RNA | Imidazoquinolines; Loxoribine; ssPolyU 3M-012 |
| TLR 9 | dsDNA viruses (HSV, MCMV); Hemozoin (Plasmodium); Unmethylated CpG DNA (bacteria and viruses) |
Human DNA/chromatin, LL37-DNA |
CpG-oligonucleotides |
| TLR10 | Not known | Not known | Not known |
Ligand binding to TLR induces the recruitment of intracellular adaptors which form signal transduction complexes within the cytoplasm (7). This leads to the activation of signaling pathways including NF-KB and the MAP kinases p38 and JNK, which regulate the expression of genes involved in inflammation (cytokines) and immunity (MHC molecules, adhesion molecules). As different TLR signal through different combinations of adaptors, there is recruitment of dissimilar transcription factors and diverse gene induction. The endosomal receptors TLR7, -8, -9 are activated after ligand engagement and interact with the adaptor MyD88 (myeloid differentiation primary response gene), following which there is association with several signaling complexes ultimately leading to the activation of IRF7, NF- KB and MAP kinases (Figure 2, (7)). The expression of IRF7 facilitates the induction of high levels of type I interferons (IFN). TLR3, also located within endosomes, recognizes dsRNA or its synthetic mimic poly I:C. Unlike other TLR, TLR3 binding induces signal transduction via a MyD88 independent pathway, associating with the TRIF adaptor, signaling through IRF3, and inducing IFNβ production (7). LPS, after engaging TLR4, recruits several adaptors (TIRAP, MyD88, TRAM and TRIF) to the TIR intracellular domain. These adaptors subsequently engage both the MyD88 and TRIF- dependent signaling pathways (7).
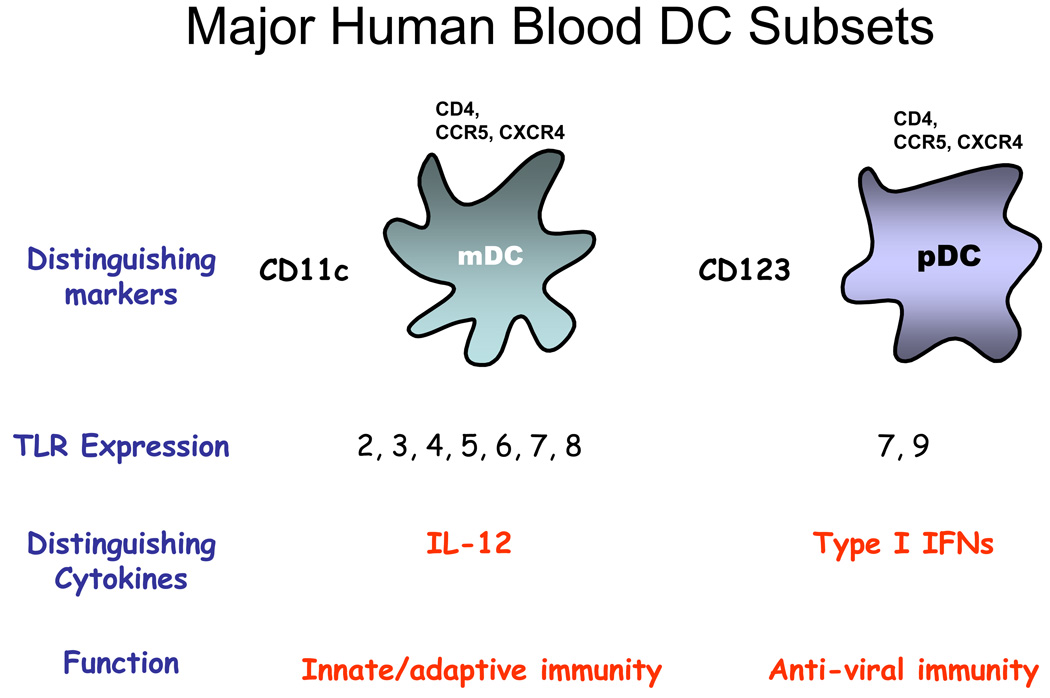
Figure 2. Major DC subsets in blood.
There are two subsets in blood, the myeloid DC (mDC) or the plasmacytoid DC (pDC). They are distinguished by surface marker expression, TLR expression, cytokine production and primary functional roles. It is now appreciated that pDC can also participate in the induction of adaptive immune responses although their precise roles need to be determined. While pDC do not synthesize IL-12, mDC can produce type I IFN via TLR3 ligation.
Basis for using TLR agonists to treat cancer
William Coley, a NY surgeon, was a pioneer in using bacterial components “Coley’s toxins” to treat cancer. He documented an association between infection and cancer (11), and subsequently tested extracts of Streptococcus pyogenes and Serratia marcescens in his patients, thus laying the foundation for using synthetic PAMPs in cancer therapy. PAMPs function by activating many types of APCs through their effects on epithelial and tumor cells. Human DC subsets express distinct TLR, and their response to stimulation is correspondingly differential. When stimulated, the myeloid or “conventional” subset of DC (mDC) which expresses TLR 1–8, upregulates activation markers (e.g. CD80, CD86, MHC class I and II, CCR7), produces pro-inflammatory cytokines and chemokines (e.g. TNF, IL-1, IL-6, MIP-1α, MIP-1β, MIP-3α) and primes antigen-specific CD4+ and CD8+ T cells (Figure 2). Moreover, these DCs acquire an enhanced capacity to take up antigens and present them in an appropriate form to T cells shown in Figure 3 (12). The plasmacytoid subset of DC (pDC) expresses only TLR7 and TLR9. Following activation via TLR7 or -9, pDC produce high levels of type I IFNs and chemokines, prime and boost T cells, and activate NK cells (13, 14). Therefore, TLR ligation of these APCs are likely to have consequential effects on stimulating immunity in the host. Given that dying tumor cells may adversely affect DC function (15, 16), activating DC with TLR agonists may be critical for priming anti-tumor immunity. TLR are also expressed on other immune related cells (macrophages, NK cells), epithelial cells and even some epithelial cell derived cancers, including breast cancer, squamous cell cancers, and melanoma (17). While the biological function of TLR expression remains to be determined, it has been suggested that TLR ligation may promote tumor progression, through induction of immune suppressive factors, by conferring resistance to apoptosis stimulating, regulatory T cell function or even promoting angiogenesis. Other studies involving TLR3 and TLR9 agonists, however, have shown enhanced production of pro-inflammatory cytokines and even induction of apoptosis (6, 18, 19). There is increasing evidence that TLR polymorphisms also influence the risk to cancer. Certain TLR4 polymorphisms for example have been associated with an increased risk in prostate cancer (TLR4), gastric cancer (TLR4), and colorectal cancer in certain populations. Follicular lymphoma has been associated with TLR2 polymorphisms, while variants of TLR3 and TLR10 may influence susceptibility to nasopharyngeal carcinoma in Chinese (17). There is evidence to suggest that the efficacy of radiation and chemotherapy in breast cancer requires TLR4 activation via endogenous agonists (high mobility group box-1, HMGB-1) released by dying tumor cells (Table 1). The TLR4 polymorphism (TLR4 Asp299Gly) is associated with worse outcomes in breast cancer patients receiving chemotherapy (20). Altogether, these findings provide a strong rationale for using TLR agonists in the clinic to promote anti-tumor immune responses.
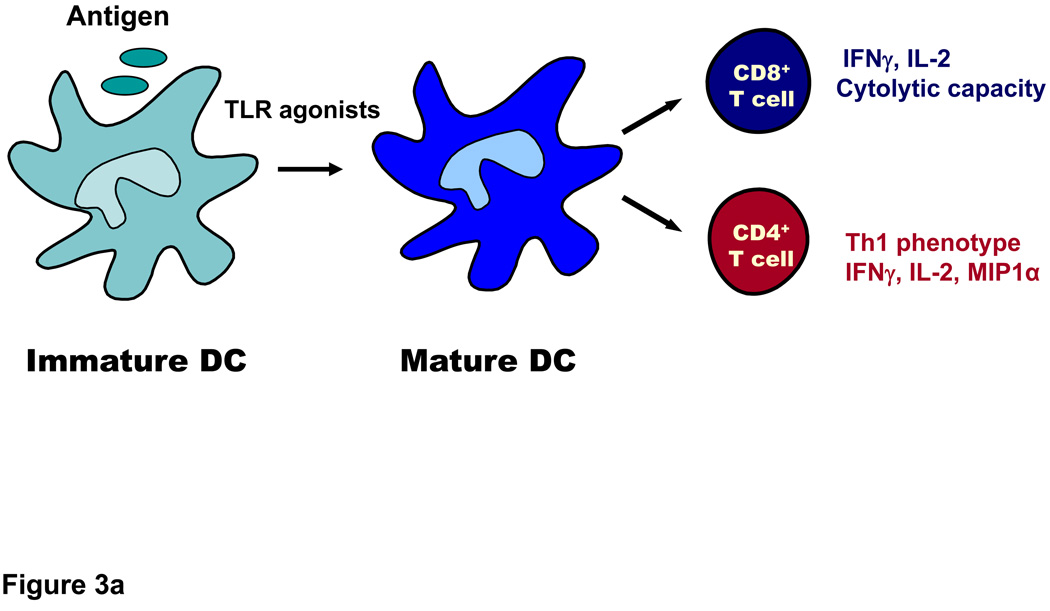
Figure 3. DC undergo activation following ligation of TLR and prime CD4+ and CD8+ T cells.
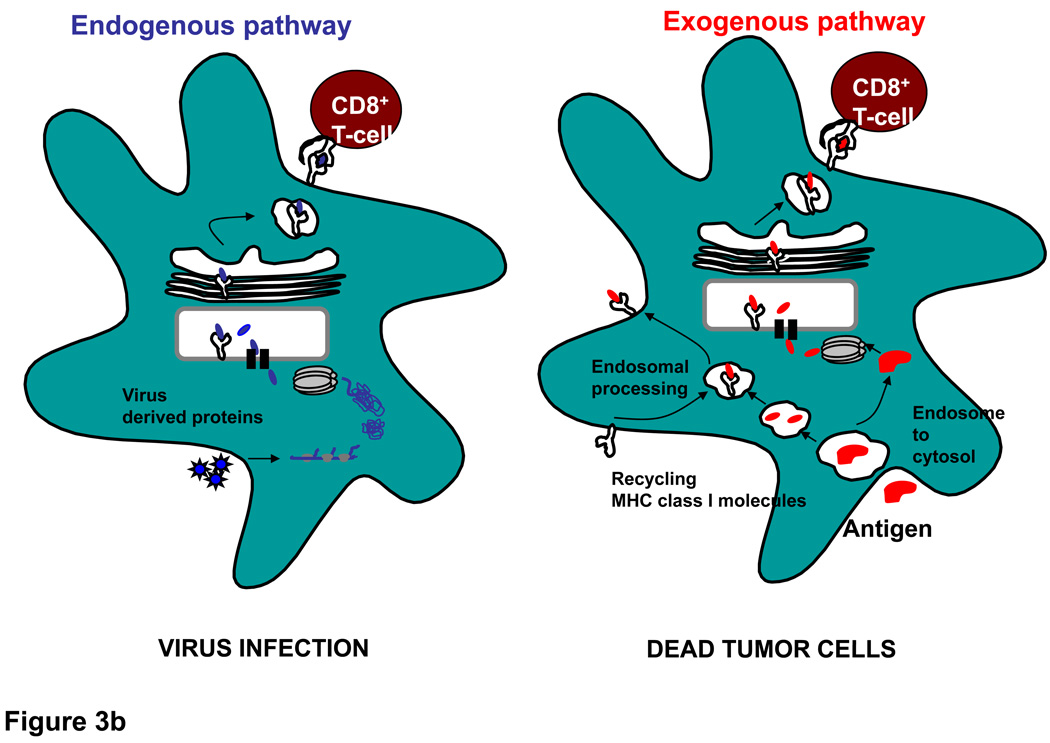
A. DC are most efficient at acquiring antigen when they are in their immature state through mechanisms that include phangocytosis, endocytosis and receptor mediated uptake. After encountering TLR ligands, they undergo maturation and upregulate HLA molecules (which present peptide antigens to T cell receptors on T cells) as well as co-stimulatory molecules such as CD80 and CD86, which interact with CD28 on T cells. DC also produce cytokines (IL-12, type I IFN) that aid in priming of CD4+ helper cells and cytolytic T cells. B. DC utilize endogenous and exogenous pathways to process and present antigens to CD8+ T cells. In the endogenous pathway, exemplified by virus infection or transduction of cells with RNA or DNA encoding antigens, antigen is processed in the cytoplasm by the proteosome and then transported into the ER where further processing can take place and peptides access newly synthesized HLA class I molecules. The peptide-HLA complex is then transported to the cell surface where it can interact with the T cell receptor. In the exogenous pathway, dying virus- infected cells or tumor cells (e.g. following chemotherapy or irradiation) are phagocytosed by DC and crosspresented to T cells. Dying tumor cells also release factors that activate DC via TLRs or components of the inflammasome. Antigens from these cells may access the cytoplasm and intersect with the conventional endogenous pathway of antigen processing. Alternatively, they may be processed within the endosomes themselves and acquired by recycling class I molecules which return to the cell surface. The exogenous pathway explains how antigens from dead cells can be acquired and presented to T cells.
Clinical application and efficacy of TLR agonists
Although TLR agonists have shown great promise in early stage cancers, their anti-tumor activity remains to be established in the adjuvant or metastatic setting. Furthermore, the mechanism(s) of anti-tumor activity has not been fully elucidated and will require further investigation. Agonists either in synthetic form, or as components of bacterial or viral vectors have been evaluated in the clinic. They have been studied either as single agents or in combination with tumor antigens (21). Evidence obtained thus far is consistent with findings in animal models, namely that they can induce potent immunity in humans in addition to clinical responses. Below, we summarize pre-clinical and clinical experience, including our own, with various TLR agonists.
Synthetic TLR agonists
TLR3 agonists
TLR3 is expressed on tissue and blood dendritic cells, monocytes, mast cells, NK cells and epithelial cells. Polyribosinic:polyribocytidic acid (Poly I:C), is a synthetic dsRNA complex, which directly activates DC and also triggers NK cells to kill tumor cells (22). In addition to being recognized by endosomal TLR3 (23), it induces high levels of type I interferons and activates several nuclear and cytoplasmic enzyme systems (oligoadenylate synthetase [OAS], the dsRNA dependent protein kinase [PKR], RIG-I Helicase, and MDA5 [melanoma differentiation associated gene]. that are involved in antiviral and antitumor host defenses (5). It has been shown to have broad gene regulatory actions as well. Poly I:C induces prolonged survival of tumor bearing rodents following IP or IV administration, and enhances antigen-specific responses to viral antigens, especially with anti-CD40 (24–27). It appears to exert its therapeutic effects through eliciting antibody responses (28, 29), enhancing cross-priming (23), stimulating anti-tumor CD8+ T cells (30–32), and antigen-specific CD4+ T cells (33). Relative to several other toll-like receptor (TLR) agonists, poly IC is the most effective inducer of type I interferon (IFN), which seems to be required for DC maturation and development of CD4+ T cell mediated immunity. Besides TLR3, poly I:C also relies on the intracellular signaling molecule MDA5, to optimize the production of type I IFN (33). It is the TLR3 agonist formulation most extensively tested as a single agent in humans with infectious diseases and cancers including glioblastoma, renal cell cancer, melanoma, leukemia, ovarian cancer, breast cancer (34–41) (A. Salazar personal communication).
Other molecular TLR3 mimics include polyadenosine-polyuridylic acid (poly AU) Ampligen (polyI:polyC (12)U; Hemispherx Biopharma) and Polyinosinic-Polycytidylic acid stabilized with poly-L-lysine and carboxymethylcellulose (Poly-ICLC, Hiltonol®). The latter is a more stable version of the TLR3 agonist and has been given IV or IM, 2 to 3 times weekly at doses of 10 to 50 µg/kg in patients with gliomas (42). A phase II study in adult patients with recurrent supratentorial anaplastic glioma treated with single agent poly-ICLC, showed no improvement in 6-month progression free survival compared to a historical database. Furthermore no objective radiographic response rates were observed. The authors suggested that poly-ICLC may confer a better outcome in combination with agents such as temozolomide. Administration of poly IC-LC in subjects with melanoma (35) and advanced renal cell cancer (43), was also well-tolerated and while no clinical benefit was observed, it was possible to consistently detect type I IFN in the serum 8 h after a single injection (median titer 199 U/ml), indicating a systemic effect. (35). Ongoing clinical trials are evaluating this agent more extensively in melanoma, prostate, cervical, ovarian, breast, colon and pancreatic cancers, including by intra-tumoral administration. Poly-ICLC has also been used nasally in a recent phase I randomized dose escalation trial in humans (N=50), and was well tolerated at all doses (A. Salazar personal communication). The most common side effects of low-dose Poly-ICLC are temporary discomfort at the injection site and occasional transient malaise, with flu-like symptoms in some patients.
Surprisingly, the agonist is only now being evaluated in humans directly in conjunction with tumor antigens. A phase I/II trial of patients with recurrent malignant glioma receiving intra-lymph-node injections of DCs loaded with HLA-A2 restricted peptides (derived from various glioma-associated antigens such as gp100, and 13Rα2) is currently under review. In addition participants received twice weekly IM injections of 20 ug/kg poly-ICLC. The frequency of CD8+ cells reactive to EphA2- or IL-13Rα2-tetramers was increased post-vaccination in the blood mononuclear cells in 9 of 11 participants evaluated. An interim analysis showed an association between positive tetramer response and 6-month progression-free survival, suggesting a possible correlation between antigen-specific responses and clinical response (44). In our own laboratory, we have found that poly I:C of most TLR agonists tested is the most potent activator of human mDC in vitro, and elicits strong anti-tumor immune responses to melanoma antigens (45). These data provide strong support for testing poly I:C together with tumor antigens in humans with cancer.
TLR4 agonists
Bacterial (LPS) and its derivatives are a commonly used vaccine adjuvants. Monophosphoryl lipid A (MPL) a detoxified component of LPS is derived from Salmonella minnesota, and contains the lipid A moiety that ligates TLR4 (46). Because it is substantially less toxic than LPS, has a history of safety and potently activates human DC, it has been incorporated into several vaccines. It also produces a different pro-inflammatory profile compared to LPS, including the production of type I IFNs, possibly because it may preferentially activate the TRIF vs. MyD88 signaling pathways (47). MPL has been approved for inclusion in a vaccine for Hepatitis B, Fendrix™ (48) and cervical cancer, Cervarix™ (49, 50). Cervarix, a GSK product, is indicated for the prevention of diseases caused by oncogenic human papillomavirus (HPV) types 16 and 18, including cervical cancer, cervical intraepithelial neoplasia (CIN) grade 1 and 2 or worse and adenocarcinoma in situ. Besides MPL, the vaccine contains L1 capsid proteins from HPV and the adjuvant aluminum hydroxide. 100% protection of the vaccine was demonstrated in phase II trials against types 16 and 18 HPV, (51). Over 30,000 women have received this vaccine and it is now approved in both Europe and America. Vaccination offers protection for >6 years, making this vaccine a milestone in the prevention of cancer. A clinical trial to determine whether Cervarix is more effective than Merck's HPV vaccine Gardasil™, which contains L1 capsid proteins and aluminum hydroxyphosphate sulfate, in addition to a yeast protein is under evaluation. MPL is also a component of Glaxo Smith Kline’s vaccine formulations for NSCLC and melanoma in combination with TLR9 agonists (see below). Phase IIB studies showed improved median survival time in patients with stage IIIB NSLC devoid of metastases (52).
MPL is a constituent of the DETOX adjuvant, an oil-droplet complex which also contains purified mycobacterial cell-wall skeleton (CWS) and has been used in combination with melanoma cell lysates (MelacineTM) or irradiated tumor cells (53). Melacine was granted approval in Canada based upon Phase III results demonstrating superior quality of life during active therapy for Stage IV melanoma as compared to a four-drug chemotherapy control, although both therapies achieved similar efficacy results. Furthermore, a meta-analysis of therapies for Stage IV melanoma showed that amongst Melacine recipients, the median survival of 11 months was better than that achieved by other therapies. Moreover, patients who were clinical responders to Melacine had a longer median survival. Melacine was also tested in resected stage II melanoma in a study conducted by the Southwest Oncology Group (SWOG). The primary endpoint was disease-free survival (DFS) in patients who received Melacine or no adjuvant therapy after surgical resection. While Melacine vaccination had no significant benefit in terms of prolongation of disease free survival in the total patient population, 38 percent of patients who expressed two or three of five different HLA genes (HLA-A2, HLA-A28, HLA-B44, HLA-B45, and HLA-C3) showed some benefit from vaccination (54).
Sialyl-Tn (STn) is a carbohydrate associated with the MUC1 mucin on a number of human cancer cells, is associated with more aggressive disease and has been incorporated into cancer vaccines for breast cancer and other epithelial tumors (THERATOPE, Biomera Inc.,). When linked to the neoantigen keyhole limpet haemocyanin (KLH), and given with DETOX, it is safe and may lead to occasional tumor regression in subjects with breast cancer (55). Immune responses in the form of both antibodies or cellular responses have been observed in patients with breast, colon and pancreatic cancers using sialyl-Tn (STn) or ras epitopes as antigens and DETOX (55–58) verifying the ability of MPL to stimulate an immune response to tumor associated antigens. MUC1 has also been incorporated into vaccines (BLP25, Stimuvax, Biomira/Merck) targeting non small cell lung cancer (NSCLC). These vaccines are composed of MUC1 peptide incorporated into liposomes containing MPL (52). In a randomized phase IIB trial, sc immunization with Stimuvax improved medium survival time by 17.3 months for patients with stage IIIB NSLC. Phase III studies are in progress.
Finally, results of a placebo controlled randomized phase II study testing GSK’s AS-02b vaccine platform, (comprising MPL and the additional adjuvant QS21, a saponin), and the cancer testis antigen, MAGE A3, showed prolonged disease free survival in patients with resected stage Ib-IIIa NSLC (59, 60). Ongoing randomized phase III studies are now evaluating MPL in GSK’s advanced adjuvant platform AS-15 which also includes TLR9 agonists (see below).
TLR7/8 agonists
Imiquimod (3M)and Resiquimod (R848; 3M) are imidazoquinolines, synthetic immune modulators which target TLR7 and TLR8, TLR that typically recognize viral ssRNA (13, 14, 61, 62). They have the advantage of activating both mDC and pDC stimulate innate and adaptive immune responses, while also activating NK cells (63, 64). Type I IFN production by pDC facilitates direct priming of CD8+ T cells, as well as cross-priming through promotion of MHC and transporter of antigen peptides (TAP) molecules on DC (65, 66). Interestingly, both compounds can activate caspase-1 through intracellular PRRs Nod-like receptors (NLR): cryopyrin/Nalp 3 to induce the production of IL-1β and IL-18 (67) which also facilitate adaptive immune responses. Imiquimod (formulated as 5% cream, Aldara™) is the only approved TLR7 agonist for treatment of genital warts (C. acuminata), actinic keratoses, basal cell carcinoma, and lentigo maligna (reviewed in (68)), where it has proven efficacy. It has also been used off-label to treat other HPV-associated lesions, as well as cutaneous melanoma (68). Imiquimod has been useful as adjunctive therapy to treat HIV-infected patients with intra-anal cancer by reducing recurrences of lesions (69). Resiquimod, related to Imiquimod binds to both TLR7 and -8, is a considerably more potent analog, and is in testing stages for treatment of genital HSV. In animal models, when administered together with peptides, proteins, or bacterial vectors and DNA constructs encoding tumor antigens (e.g. melanoma associated antigens, MAA), these agents augment anti-tumor activity (70). DC activity against tumor antigens, including MAA, can also be substantially enhanced in vivo if the APC are first activated with TLR7/8 agonists (71–73).
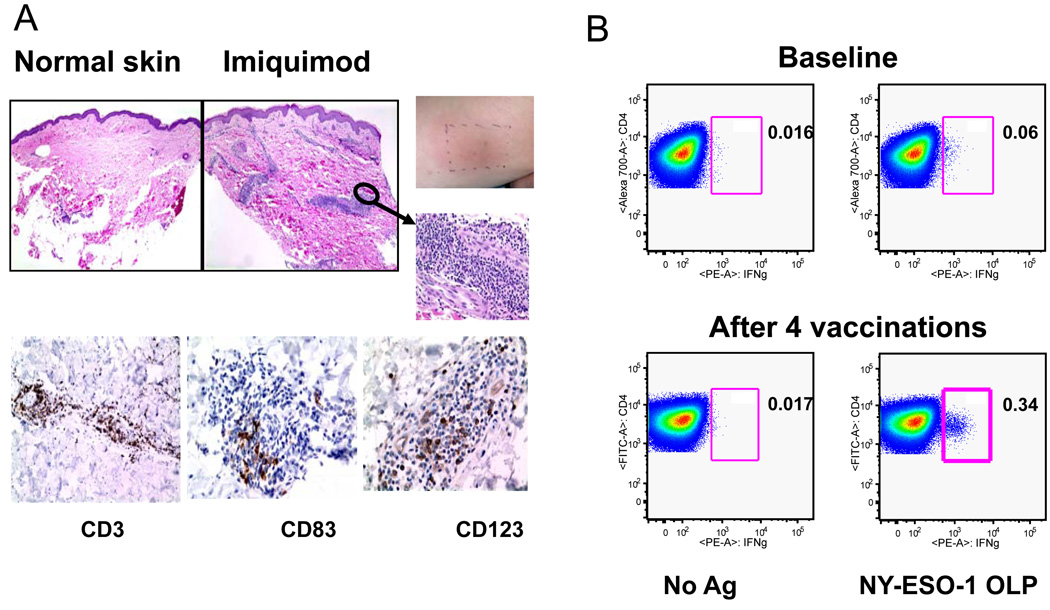
In humans, topical Imiquimod treatment enhanced the immunogenicity of a melanoma peptide vaccine when given with systemic Flt-3 ligand, which mobilizes DC systemically (74). Injection of immature human DCs into imiquimod-pretreated skin lead to DC activation in situ and enhanced migratory capacity to draining lymph nodes in cancer patients (75). Recently, we and other investigators demonstrated that imiquimod rapidly recruits significant numbers of human mDC and pDC into topically treated areas (Figure 4; (76)), enhances their survival, induces their trafficking to draining lymph nodes (73, 77), and confers human mDC and pDC with cytolytic acivity against tumors in a perforin/granzyme B and TRAIL dependent fashion, respectively (78). Topical application of Imiquimod also caused reversal of T regulatory cell infiltration and suppressive activity in squamous cell cancers of the skin and restored the expression of E-selectin in skin blood vessels (79). In a vaccine trial, our group showed that intradermal injection of the CT antigen NY-ESO-1, as whole protein, into Imiquimod-treated skin of resected melanoma patients, primed new humoral and helper T cell responses and induced local infiltration of T, B, NK and activated mDC and pDC subsets (Figure 4 (21, 76)). This study demonstrated, for the first time, the safety and adjuvant activity of Imiquimod when administered simultaneously with protein antigen. It also confirmed the agonist’s mDC and pDC activating potential in vivo. Of note, no indoleamine 2’3’-dioxygenase (IDO) was detected in vivo. We have shown that IDO is triggered in vitro by ligation of TLR7/TLR9 on pDC. IDO metabolizes tryptophan to kynenurenine, which is responsible for the induction of T regulatory cells (14).
Figure 4. Imiquimod induces local inflammation and NY-ESO-1 specific CD4+ T cell responses.
A. Representative H and E stained sections of control skin and Imiquimod treated skin (left panels). Right upper panels show inflammation at the Imiquimod treated of one patient. Representative immunohistochemistry sections for three tested markers are shown (CD3: T cells; CD83: mature DC; CD123: plasmacytoid DC). B. Quantification of IFNγ-secreting NY-ESO-1-specfic CD4+ T cells. Representative before and after vaccine samples for one patient are shown. Following a one week in vitro stimulation with pooled NY-ESO-1 overlapping peptides, cells were re-stimulated and stained for intracellular IFNγ. CD4 staining is shown on the y axis and IFNγ staining is shown on the x-axis.(76). Copyright 2008. The American Association of Immunologists, Inc.
We are currently undertaking a randomized controlled study evaluating the immunogenicity of topical Resiquimod in combination with the cancer testis antigen NY-ESO-1 protein delivered SC in Montanide ISA 51. Given the proven efficacy of Imiquimod in treating cutaneous pre-malignant and malignant lesions, it is likely that these agents, in the absence of potent systemically administered formulations (80, 81) will gain greater use in the treatment of additional cutaneous pre-malignant and malignant conditions e.g. cervical intraepithelial neoplasia.
TLR9 agonists
We and others have shown that synthetic oligonucleotides containing unmethylated CpG dinucleotide (CpG-ODN) bind TLR9 on pDC, leading to their activation and type I IFN production (13, 14). Ligation of TLR9 on B cells induces their activation and proliferation (82). CpG-ODNs have been classified into three families: D-, K or C-type ODNs. These differ based on their backbones (phosphodiester or phosphorothioate), location and number of CpG dinucleotides, and palindromic sequences. In animal models, these constructs have anti-tumor effects when given either as monotherapy, or together with vaccines or other treatments (82–85). In humans, the responses to monotherapy, whether used to treat HCV (86) or cancers (non small cell lung cancer, cutaneous T-cell lymphoma, renal cell cancer, non-Hodgkin’s lymphoma, chronic lymphocytic leukemia) regardless of delivery route (i.v., s.c, or intratumorally) or if given with adjunct chemotherapy have been generally low (68, 82, 87, 88). Early phase 1 trials have shown that CPG ODN are well-tolerated at levels that can stimulate immune activation: NK cell activation, inflammatory cytokine production and reduction of regulatory T cell number in draining lymph nodes (89). These findings suggest that CpG should be used with additional agents to achieve maximal effects. Indeed, Romero et al., (90, 91) showed that the addition of CpG to a Melan A/MART-1 HLA A2-restricted peptide and the “water in oil” adjuvant Montanide, dramatically increased the number of antigen-reactive cells elicited (upto 1.15% of circulating CD8+ T cells). Moreover, enhanced tumor-reactive CD8+ T-cell responses were also observed after vaccination with NY-ESO-1 peptide, CpG 7909 and Montanide ISA-51 in patients with NY-ESO-1 expressing tumors and responses were associated with survival (92).
In a similar manner, we tested the immunogenicity of CpG plus NY-ESO-1 antigen emulsified in Montanide in patients with resected melanoma. Specific and strong integrated CD4+ T cell and antibody responses were elicited in most vaccinated patients, along with CD8+ T cell responses in approximately half the patients (93). These findings clearly established that protein antigen, presented on the right platform can elicit class I restricted responses. Given that peptide/Montanide combinations are significantly immunogenic (90, 91), it will be important to dissect the precise contribution of Montanide vs. CpG agonist in additional trials.
Recently, GSK reported on immune responses in subjects with metastatic melanoma in a phase II randomized trial receiving their vaccine AS02B comprising MPL, MAGE A3 antigen, QS21 in oil/water emulsion vs. AS15, comprising CpG in addition to MPL, MAGEA3 antigen, QS21 and liposome formulation. The addition of CpG to the vaccine formulation significantly enhanced the induction of antigen-specific CD4+ T cell and antibody responses. A difference between arms in time to treatment failure was also documented (94). Phase III trials of MAGE A3/AS15 are in progress in patients with resected stage 1b-IIIa NSCLC and resected, high risk melanoma.
TLR9 agonists have also been used in combination with chemotherapy, radiotherapy or monoclonal antibodies targeting CD20 molecules on B cells (Rituximab) with evidence of clinical activity. However, Phase III studies exploring the combination of PF-3512676 (a Pfizer product formerly known as CpG7909) with paclitaxel/carboplatin or gemcitabine/cisplatin vs. chemotherapy alone as first-line treatment of patients with advanced NSLC indicated no improvement in progression free survival or overall survival with the addition of the CpG-ODN (86, 89)
Overall TLR9 agonists appear to be generally well tolerated with side effects including flu-like symptoms local injection site reactions and in some cases hematological side effects. Evidence is accumulating that they have potential as components of immunotherapies that are administered in the adjuvant setting, and are likely to work best when combined with other immunomodulators or treatments.
Pathogens expressing TLR agonists
Several inactivated or attenuated pathogens are components of vaccines against infectious agents (Table 1). Many are now also being tested as vectors for cancer vaccines. Mycobacterium bovis, (Bacillus-Calmette Guérin, BCG), is the only vaccine currently available for tuberculosis and is also approved to treat superficial bladder cancer and bladder cancer in situ. BCG cell wall skeleton and peptidoglycan activate TLR 2 and TLR4 signaling (95), and induce local inflammation in addition to tumor-specific immunity (96, 97). Addition of BCG to vaccination with NY-ESO-1 protein has also led to induction of antibody and CD4+ T cells in humans (98). Yellow fever vaccine stimulates innate and adaptive immune responses through its ability to activate DC via TLR2, -7, -8 and -9 (99), and is being actively investigated as a vaccine adjuvant. Pox vectors, such as vaccinia or canarypox are being evaluated as vaccine vectors in melanoma and ovarian cancer amongst other tumors. Pox vectors expressing the cancer testis antigen NY-ESO-1 have proven immunogenicity (100) and current modifications involving the inclusion of co-stimulatory molecules may enhance their function further. Fowlpox vectors (ALVAC) have recently been used as a component of a preventive vaccine for HIV infection and may have a modest beneficial effect, (101). Pox vectors (e.g. Modified Vaccinia Ankara, MVA) can signal APC via TLR2 (102–104). Bacterial vectors recombinant for cancer-testis antigens such as Salmonella typhimurium may provide efficient stimulation by engaging multiple TLRs (105). Finally, adenovirus vectors expressing various tumor associated antigens including telomerase and cancer-testis antigens are in evaluation in the clinic. Adenovirus is reported to activate DC via TLR9 (106, 107). Besides BCG, it remains to be seen whether other pathogens expressing TLR agonists will prove to be efficacious in treating either early or advanced stages of cancer.
Prospects for TLR agonists
We predict that synthetic TLR agonists will be most efficacious when used in optimal combinations together with antigen(s) and combined with other modalities including other vaccines, adjuvants and immune modulators. Activation of TLR9 with CpG ODNs for example, increases the immunogenicity of peptide-, DNA-, tumor cell- or DC-based vaccines (108). Fusion of antigen to the TLR agonist presents yet another attractive approach to enhance the immune response to poorly immunogenic antigens (109–111)
Route of injection is also likely to influence outcome. Intra-tumoral administration of TLR agonists may directly activate locally infiltrating DC, directly promote tumor cell apoptosis or sensitize tumor cells to cytotoxic agents. Intratumoral injection is safe when delivered in combination with rituximab (anti-CD20), an antibody which targets B cells (112, 113), and the effects of local injection of poly I:C into cutaneous melanoma is currently under evaluation. Although clinical efficacy has not yet been shown, with the advent of more potent analogs it seems likely that intratumoral injection approaches in combination with other interventions will yield clinical responses of targeted lesions. Imiquimod, for example, is used off label to treat small in transit melanomas
Therapeutic interventions such as chemotherapy and radiation induce cell death leading to the release of tumor antigens and endogenous cellular factors (e.g. heat shock proteins, HMGB-1) that activate TLRs on DC, in addition to triggering various intracellular signaling pathways through the release of ATP (e.g. NLR members of the inflammasome, (114). Tumor antigens released as a consequence of cell death can be acquired by DC and crosspresented to T cells (20, 115). In animal models, lymphodepletion (through radiation or chemotherapy) activates TLR4, an essential step in promoting the effectiveness of adoptively transferred T cells in preclinical models (116). Preclinical models also indicate that combining chemotherapy or radiation with systemic administration of synthetic TLR agonists, or vaccines which incorporate synthetic TLR agonists, are synergistic and enhance stimulation of anti-tumor immunity as well as tumor regression.
Certain adjuvants selectively activate other cellular non-TLR sensors a prominent example being aluminum hydroxide, an adjuvant that is the component of many FDA approved vaccines, (117). Alum formulations induce the secretion of IL-1β and IL-18 in vitro, and in vivo (IL-1β) and recruit and activate monocytes and granulocytes (118). Their role as inducers of the inflammasome has come under question but it is clear that they exert pro-inflammatory effects (117). Sharp et al. found that poly(lactic-co-glycolic acid) (PLGA) and polystyrene microparticles activated the NLRP3 pathway of the inflammasome (119), and TLR agonists (e.g. LPS) in conjunction with these experimental adjuvants or approved adjuvants such as aluminum hydroxide may be more effective when given in combination, as they mimic viruses and other pathogens which can target multiple pathways (118, 120). Moreover, as TLR agonists may sensitize tumor cells to cytotoxic agents, their future lies in combination with other therapies including cancer vaccines and monoclonal antibodies. Interestingly, GSK’s Cervarix vaccine contains MPL and aluminum hydroxide while its AS15 vaccine platform contains MPL, CpG ODN and QS21 (QuilA, a saponin extracted from the bark of the Quillaria saponaria tree) which triggers IL-1 release in an inflammasome-dependent way (121). New immune modulators such as anti-CTLA-4 and anti-PD-1 which block regulatory molecules on T cells and improve their anti-tumor function, may also improve the immunogenicity of TLR agonists.
Preclinical models indicate that combinations of TLR agonists are superior to individual use in vivo (122, 123). TLR4 agonists act synergistically with TLR7, TLR8 or TLR9 agonists in the induction of a selected set of genes, including the Th1 polarizing cytokines IL-12 and IL-23, thereby conferring potent Th1 polarizing activity to human DC. Ligands for 3 TLRs (TLR2/6, TLR3, and TLR9) increased protective efficacy in mice towards a viral protein by enhancing the avidity of antigen-specific T cells, when compared with using ligands for any 2 of these TLRs (122). On the other hand, ligation of TLR2 and the PRR dectin-1 with zymosan, induces DC to produce IL-10 but not IL-12 or IL-6, and in vivo administration of zymosan suppresses antigen-specific responses response in a IL-10, and TGFβ dependent manner (124). Studies are needed to carefully evaluate stimulatory vs. inhibitory combinations of TLR agonists. In humans trials are investigating a combinatorial approach which include GSK’s AS15 platform which includes agonists that activate both TLR4 and TLR9, thereby targeting both mDC and pDC subsets and allowing for optimal activation of both. The next decade is likely to bring several new synthetic agonists into the clinic such as acylated monosacharides that are structurally related to lipid-A, flagellin and novel TLR7 and TLR8 agonists. By taking advantage of the divergent signaling pathways used by various TLR to enhance DC activation, it should be possible to improve the overall immune response. An important goal will be to ascertain which combination of TLR agonists induces desirable anti-tumor immune responses (Th1, cytolytic T cells, NK cell activation) overcomes tolerance and reverses the immunosuppressive effects of T regulatory cells.
Challenges for the future
For TLR agonists to achieve recognition in the clinic it will be critical to undertake side-by-side comparisons against the same antigen using selected immune monitoring assays that measure the quantity and quality of responses (e.g. avidity, memory cell generation, durability). Through a program of the Cancer Vaccine Collaborative, a joint program of the Cancer Research Institute and the Ludwig Institute for Cancer Research, a coordinated global network of clinical trial sites has been conducting a series of parallel early-stage clinical trials to identify the optimal composition of successful therapeutic cancer vaccines. The antigens selected for these trials were primarily cancer/testis antigens, such as NY-ESO-1 and MAGE-A3, as well as melanoma differentiation antigens, such as Melan-A/MART-1. As discussed above, various forms of these antigens (peptides, protein, long peptides) have been mixed or co-administered with a series of Toll-like receptor ligands: CpG, Imiquimod, Resiquimod, PolyIC-LC, OK-432, Monophosphoryl lipid A (MPL), ISCOMatrix®, BCG. Additionally, these antigens have also been formulated as recombinant viruses (Vaccinia, Folwpox) or DNA endowed with natural CpG signals. Efforts such as these will yield important new information regarding successful vaccine platforms. Despite this progress, these studies highlight an endemic problem in the field of cancer vaccines: the lack of commercial availability of most of the TLR ligands discussed in this review. Efforts to systematically test these reagents, let alone to combine them, are thwarted by proprietary issues that should hopefully become less pronounced as these reagents prove their value in the clinic and become readily available.
Acknowledgments
Sources of support:
NIH: 3R37AI044628-11S1
NIH: 4R37AI044628
NIH: 1RC1AI087097
NIH: 5R01AI071078
NIH: 5P01AI057127
NIH: 5R01AI06684
Bill & Melinda Gates Foundation
Cancer Research Institute
Emerald Foundation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
All authors have no conflict of interest to declare.
Contributor Information
Nina Bhardwaj, New York University School of Medicine, 522 First Avenue, Smilow Research Building, Room 1303, New York, NY 10016, Office: (212) 263-5814, Fax: (212) 263-6729, nina.bhardwaj@nyumc.org.
Sacha Gnjatic, Ludwig Institute for Cancer Research.
Nikhil B. Sawhney, Newark Academy
REFERENCES
- 1.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Advances in immunology. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 2.Anderson KV, Bokla L, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell. 1985;42:791–798. doi: 10.1016/0092-8674(85)90275-2. [DOI] [PubMed] [Google Scholar]
- 3.Lemaitre B, Reichhart JM, Hoffmann JA. Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14614–14619. doi: 10.1073/pnas.94.26.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. Innate immune recognition of viral infection. Nature immunology. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 6.Salaun B, Lebecque S, Matikainen S, et al. Toll-like receptor 3 expressed by melanoma cells as a target for therapy? Clin Cancer Res. 2007;13:4565–4574. doi: 10.1158/1078-0432.CCR-07-0274. [DOI] [PubMed] [Google Scholar]
- 7.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nature reviews. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 8.Medzhitov R, Preston- Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi F, Smith KD, Ozinsky A, et al. The innate immune response to bacterial flagellin is mediated by Toll- like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 11.Coley WB., II Contribution to the Knowledge of Sarcoma. Ann Surg. 1891;14:199–220. doi: 10.1097/00000658-189112000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 13.Beignon AS, McKenna K, Skoberne M, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. The Journal of clinical investigation. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manches O, Munn D, Fallahi A, et al. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. The Journal of clinical investigation. 2008;118:3431–3439. doi: 10.1172/JCI34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skoberne M, Somersan S, Almodovar W, et al. The apoptotic-cell receptor CR3, but not alphavbeta5, is a regulator of human dendritic-cell immunostimulatory function. Blood. 2006;108:947–955. doi: 10.1182/blood-2005-12-4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skoberne M, Beignon AS, Bhardwaj N. Danger signals: a time and space continuum. Trends Mol Med. 2004;10:251–257. doi: 10.1016/j.molmed.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Yu L, Chen S. Toll-like receptors expressed in tumor cells: targets for therapy. Cancer Immunol Immunother. 2008;57:1271–1278. doi: 10.1007/s00262-008-0459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Droemann D, Albrecht D, Gerdes J, et al. Human lung cancer cells express functionally active Toll-like receptor 9. Respir Res. 2005;6:1. doi: 10.1186/1465-9921-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salaun B, Coste I, Rissoan MC, et al. TLR3 can directly trigger apoptosis in human cancer cells. J Immunol. 2006;176:4894–4901. doi: 10.4049/jimmunol.176.8.4894. [DOI] [PubMed] [Google Scholar]
- 20.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature medicine. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 21.Adams S. Toll-like receptor agonists in cancer therapy. Immunotherapy. 2010;1 doi: 10.2217/imt.09.70. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sivori S, Falco M, Della Chiesa M, et al. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10116–10121. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz O, Diebold SS, Chen M, et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 24.Boscardin SB, Hafalla JC, Masilamani RF, et al. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. The Journal of experimental medicine. 2006;203:599–606. doi: 10.1084/jem.20051639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trumpfheller C, Finke JS, Lopez CB, et al. Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. The Journal of experimental medicine. 2006;203:607–617. doi: 10.1084/jem.20052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soares H, Waechter H, Glaichenhaus N, et al. A subset of dendritic cells induces CD4+ T cells to produce IFN-gamma by an IL-12-independent but CD70-dependent mechanism in vivo. The Journal of experimental medicine. 2007;204:1095–1106. doi: 10.1084/jem.20070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trumpfheller C, Caskey M, Nchinda G, et al. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2574–2579. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sloat BR, Cui Z. Nasal immunization with anthrax protective antigen protein adjuvanted with polyriboinosinic-polyribocytidylic acid induced strong mucosal and systemic immunities. Pharmaceutical research. 2006;23:1217–1226. doi: 10.1007/s11095-006-0206-9. [DOI] [PubMed] [Google Scholar]
- 29.Ichinohe T, Kawaguchi A, Tamura S, et al. Intranasal immunization with H5N1 vaccine plus Poly I:Poly C12U, a Toll-like receptor agonist, protects mice against homologous and heterologous virus challenge. Microbes and infection / Institut Pasteur. 2007;9:1333–1340. doi: 10.1016/j.micinf.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Salem ML, Kadima AN, Cole DJ, et al. Defining the antigen-specific T-cell response to vaccination and poly(I:C)/TLR3 signaling: evidence of enhanced primary and memory CD8 T-cell responses and antitumor immunity. J Immunother. 2005;28:220–228. doi: 10.1097/01.cji.0000156828.75196.0d. [DOI] [PubMed] [Google Scholar]
- 31.Cui Z, Qiu F. Synthetic double-stranded RNA poly(I:C) as a potent peptide vaccine adjuvant: therapeutic activity against human cervical cancer in a rodent model. Cancer Immunol Immunother. 2006;55:1267–1279. doi: 10.1007/s00262-005-0114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu X, Nishimura F, Sasaki K, et al. Toll like receptor-3 ligand poly-ICLC promotes the efficacy of peripheral vaccinations with tumor antigen-derived peptide epitopes in murine CNS tumor models. Journal of translational medicine. 2007;5:10. doi: 10.1186/1479-5876-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longhi MP, Trumpfheller C, Idoyaga J, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. The Journal of experimental medicine. 2009;206:1589–1602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ewel CH, Urba WJ, Kopp WC, et al. Polyinosinic-polycytidylic acid complexed with poly-L-lysine and carboxymethylcellulose in combination with interleukin 2 in patients with cancer: clinical and immunological effects. Cancer research. 1992;52:3005–3010. [PubMed] [Google Scholar]
- 35.Hawkins MJ, Levin M, Borden EC. An Eastern Cooperative Oncology Group phase I-II pilot study of polyriboinosinic-polyribocytidylic acid poly-L-lysine complex in patients with metastatic malignant melanoma. Journal of biological response modifiers. 1985;4:664–668. [PubMed] [Google Scholar]
- 36.Krown SE, Kerr D, Stewart WE, 2nd, et al. Phase I trials of poly(I,C) complexes in advanced cancer. Journal of biological response modifiers. 1985;4:640–649. [PubMed] [Google Scholar]
- 37.Lampkin BC, Levine AS, Levy H, et al. Phase II trial of a complex polyriboinosinic-polyribocytidylic acid with poly-L-lysine and carboxymethyl cellulose in the treatment of children with acute leukemia and neuroblastoma: a report from the Children's Cancer Study Group. Cancer research. 1985;45:5904–5909. [PubMed] [Google Scholar]
- 38.Nakamura O, Shitara N, Matsutani M, et al. Phase I–II trials of poly(ICLC) in malignant brain tumor patients. Journal of interferon research. 1982;2:1–4. doi: 10.1089/jir.1982.2.1. [DOI] [PubMed] [Google Scholar]
- 39.Rettenmaier MA, Berman ML, DiSaia PJ. Treatment of advanced ovarian cancer with polyinosinic-polycytidylic lysine carboxymethylcellulose (poly(ICLC] Gynecologic oncology. 1986;24:359–361. doi: 10.1016/0090-8258(86)90313-6. [DOI] [PubMed] [Google Scholar]
- 40.Stevenson HC, Abrams PG, Schoenberger CS, et al. A phase I evaluation of poly(I,C)-LC in cancer patients. Journal of biological response modifiers. 1985;4:650–655. [PubMed] [Google Scholar]
- 41.Robinson RA, DeVita VT, Levy HB, et al. A phase I-II trial of multiple-dose polyriboinosic-polyribocytidylic acid in patieonts with leukemia or solid tumors. Journal of the National Cancer Institute. 1976;57:599–602. doi: 10.1093/jnci/57.3.599. [DOI] [PubMed] [Google Scholar]
- 42.Butowski N, Chang SM, Junck L, et al. A phase II clinical trial of poly-ICLC with radiation for adult patients with newly diagnosed supratentorial glioblastoma: a North American Brain Tumor Consortium (NABTC01-05) J Neurooncol. 2009;91:175–182. doi: 10.1007/s11060-008-9693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giantonio BJ, Hochster H, Blum R, et al. Toxicity and response evaluation of the interferon inducer poly ICLC administered at low dose in advanced renal carcinoma and relapsed or refractory lymphoma: a report of two clinical trials of the Eastern Cooperative Oncology Group. Invest New Drugs. 2001;19:89–92. doi: 10.1023/a:1006458232384. [DOI] [PubMed] [Google Scholar]
- 44.Okada H, Lieberman F, Ueda R, et al. Type-1 dendritic cell vaccines in combination with POLY-ICLC- association between positive tetramer response and 6-month progression-free survival. 2009 Joint Meeting of the Society for Neuro-Oncology (SNO) and the American Association of Neurological Surgeons/Congress of Neurological Surgeons (AANS/CNS) Section on Tumors.2009. [Google Scholar]
- 45.Bogunovic D, Manches O, Yewdall A, et al. Simultaneous TLR4 engagement inhibits TLR3-induced proinflammatory signaling in dendritic cells through IL-10 and p38-dependent pathways and limits CD8+ T cell priming. Journal ol Investigation. 2010 in revision. [Google Scholar]
- 46.Ribi E, Cantrell JL, Takayama K, et al. Lipid A and immunotherapy. Reviews of infectious diseases. 1984;6:567–572. doi: 10.1093/clinids/6.4.567. [DOI] [PubMed] [Google Scholar]
- 47.Okemoto K, Kawasaki K, Hanada K, et al. A potent adjuvant monophosphoryl lipid A triggers various immune responses, but not secretion of IL-1beta or activation of caspase-1. J Immunol. 2006;176:1203–1208. doi: 10.4049/jimmunol.176.2.1203. [DOI] [PubMed] [Google Scholar]
- 48.Thoelen S, De Clercq N, Tornieporth N. A prophylactic hepatitis B vaccine with a novel adjuvant system. Vaccine. 2001;19:2400–2403. doi: 10.1016/s0264-410x(00)00462-x. [DOI] [PubMed] [Google Scholar]
- 49.Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 50.Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 51.Kresge KJ. Cervical Cancer Vaccines: Introduction of vaccines that prevent cervical cancer and genital warts may foreshadow implementation and accetability issues for a future AIDS vaccine. The Newsletter on International AIDS Vaccine Research. 2005;9:1–5. [PubMed] [Google Scholar]
- 52.Butts C, Murray N, Maksymiuk A, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol. 2005;23:6674–6681. doi: 10.1200/JCO.2005.13.011. [DOI] [PubMed] [Google Scholar]
- 53.Mitchell MS, Kan-Mitchell J, Kempf RA, et al. Active specific immunotherapy for melanoma: phase I trial of allogeneic lysates and a novel adjuvant. Cancer research. 1988;48:5883–5893. [PubMed] [Google Scholar]
- 54.Sosman JA, Unger JM, Liu PY, et al. HLA-A2 and/or HLA-C3 expression defines a subset of T3N0 melanoma patients with improved overall survival from melacine vaccine: an updated analysis of SWOG 9035. Proc Am Soc Clin Oncol. 2002;21 [Google Scholar]
- 55.MacLean GD, Reddish M, Koganty RR, et al. Immunization of breast cancer patients using a synthetic sialyl-Tn glycoconjugate plus Detox adjuvant. Cancer Immunol Immunother. 1993;36:215–222. doi: 10.1007/BF01740902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khleif SN, Abrams SI, Hamilton JM, et al. A phase I vaccine trial with peptides reflecting ras oncogene mutations of solid tumors. J Immunother. 1999;22:155–165. doi: 10.1097/00002371-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Miles DW, Towlson KE, Graham R, et al. A randomised phase II study of sialyl-Tn and DETOX-B adjuvant with or without cyclophosphamide pretreatment for the active specific immunotherapy of breast cancer. Br J Cancer. 1996;74:1292–1296. doi: 10.1038/bjc.1996.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holmberg LA, Sandmaier BM. Theratope vaccine (STn-KLH) Expert Opin Biol Ther. 2001;1:881–891. doi: 10.1517/14712598.1.5.881. [DOI] [PubMed] [Google Scholar]
- 59.Vansteenkiste JF, Zielinski M, Dahabreh IJ, et al. Association of gene expression signature and clinical efficacy of MAGE-A3 antigen-specific cancer immunotherapeutic (ASCI) as adjuvant therapy in resected stage IB/II non-small cell lung cancer (NSCLC) J Clin Oncol. 2008;26 Abstract No. 7501. [Google Scholar]
- 60.Vansteenkiste J, Zielinski M, Linder A, et al. Final results of a multi-center, double-blind, randomized, placebo-controlled Phase II study to assess the efficacy of MAGE-A3 immunotherapeutics as adjuvant therapy in Stage Ib/II non-small cell lunch cancer (NSCLC) J Clin Oncol ASCO Annual Meeting Proceedings Part I. 2007;25 Abstract 7554. [Google Scholar]
- 61.Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 62.Jurk M, Heil F, Vollmer J, et al. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nature immunology. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 63.Liu C, Lou Y, Lizee G, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. The Journal of clinical investigation. 2008;118:1165–1175. doi: 10.1172/JCI33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fonteneau JF, Gilliet M, Larsson M, et al. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood. 2003;101:3520–3526. doi: 10.1182/blood-2002-10-3063. [DOI] [PubMed] [Google Scholar]
- 65.Cho HJ, Takabayashi K, Cheng PM, et al. Immunostimulatory DNA-based vaccines induce cytotoxic lymphocyte activity by a T-helper cell-independent mechanism. Nature biotechnology. 2000;18:509–514. doi: 10.1038/75365. [DOI] [PubMed] [Google Scholar]
- 66.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, Tough DF. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–1015. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 67.Kanneganti TD, Ozoren N, Body-Malapel M, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 68.Meyer T, Stockfleth E. Clinical investigations of Toll-like receptor agonists. Expert opinion on investigational drugs. 2008;17:1051–1065. doi: 10.1517/13543784.17.7.1051. [DOI] [PubMed] [Google Scholar]
- 69.Kaspari M, Gutzmer R, Kaspari T, et al. Application of imiquimod by suppositories (anal tampons) efficiently prevents recurrences after ablation of anal canal condyloma. The British journal of dermatology. 2002;147:757–759. doi: 10.1046/j.1365-2133.2002.04979.x. [DOI] [PubMed] [Google Scholar]
- 70.Smorlesi A, Papalini F, Orlando F, et al. Imiquimod and S-27609 as adjuvants of DNA vaccination in a transgenic murine model of HER2/neu-positive mammary carcinoma. Gene therapy. 2005;12:1324–1332. doi: 10.1038/sj.gt.3302559. [DOI] [PubMed] [Google Scholar]
- 71.Fong L, Engleman EG. Dendritic cells in cancer immunotherapy. Annu Rev Immunol. 2000;18:245–273. doi: 10.1146/annurev.immunol.18.1.245. [DOI] [PubMed] [Google Scholar]
- 72.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 73.Prins RM, Craft N, Bruhn KW, et al. The TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunity. J Immunol. 2006;176:157–164. doi: 10.4049/jimmunol.176.1.157. [DOI] [PubMed] [Google Scholar]
- 74.Shackleton M, Davis ID, Hopkins W, et al. The impact of imiquimod, a Toll-like receptor-7 ligand (TLR7L), on the immunogenicity of melanoma peptide vaccination with adjuvant Flt3 ligand. Cancer Immun. 2004;4:9. [PubMed] [Google Scholar]
- 75.Nair S, McLaughlin C, Weizer A, et al. Injection of immature dendritic cells into adjuvant-treated skin obviates the need for ex vivo maturation. J Immunol. 2003;171:6275–6282. doi: 10.4049/jimmunol.171.11.6275. [DOI] [PubMed] [Google Scholar]
- 76.Adams S, O'Neill DW, Nonaka D, et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J Immunol. 2008;181:776–784. doi: 10.4049/jimmunol.181.1.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Craft N, Bruhn KW, Nguyen BD, et al. The TLR7 agonist imiquimod enhances the anti-melanoma effects of a recombinant Listeria monocytogenes vaccine. J Immunol. 2005;175:1983–1990. doi: 10.4049/jimmunol.175.3.1983. [DOI] [PubMed] [Google Scholar]
- 78.Stary G, Bangert C, Tauber M, et al. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. The Journal of experimental medicine. 2007;204:1441–1451. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clark RA, Huang SJ, Murphy GF, et al. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. The Journal of experimental medicine. 2008;205:2221–2234. doi: 10.1084/jem.20071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dudek AZ, Yunis C, Harrison LI, et al. First in human phase I trial of 852A, a novel systemic toll-like receptor 7 agonist, to activate innate immune responses in patients with advanced cancer. Clin Cancer Res. 2007;13:7119–7125. doi: 10.1158/1078-0432.CCR-07-1443. [DOI] [PubMed] [Google Scholar]
- 81.Dummer R, Hauschild A, Becker JC, et al. An exploratory study of systemic administration of the toll-like receptor-7 agonist 852A in patients with refractory metastatic melanoma. Clin Cancer Res. 2008;14:856–864. doi: 10.1158/1078-0432.CCR-07-1938. [DOI] [PubMed] [Google Scholar]
- 82.Krieg AM. Development of TLR9 agonists for cancer therapy. The Journal of clinical investigation. 2007;117:1184–1194. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stern BV, Boehm BO, Tary-Lehmann M. Vaccination with tumor peptide in CpG adjuvant protects via IFN-gamma-dependent CD4 cell immunity. J Immunol. 2002;168:6099–6105. doi: 10.4049/jimmunol.168.12.6099. [DOI] [PubMed] [Google Scholar]
- 84.Miconnet I, Koenig S, Speiser D, et al. CpG are efficient adjuvants for specific CTL induction against tumor antigen-derived peptide. J Immunol. 2002;168:1212–1218. doi: 10.4049/jimmunol.168.3.1212. [DOI] [PubMed] [Google Scholar]
- 85.Baines J, Celis E. Immune-mediated tumor regression induced by CpG-containing oligodeoxynucleotides. Clin Cancer Res. 2003;9:2693–2700. [PubMed] [Google Scholar]
- 86.Schmidt C. Clinical setbacks for toll-like receptor 9 agonists in cancer. Nature biotechnology. 2007;25:825–826. doi: 10.1038/nbt0807-825. [DOI] [PubMed] [Google Scholar]
- 87.Hofmann MA, Kors C, Audring H, et al. Phase 1 evaluation of intralesionally injected TLR9-agonist PF-3512676 in patients with basal cell carcinoma or metastatic melanoma. J Immunother. 2008;31:520–527. doi: 10.1097/CJI.0b013e318174a4df. [DOI] [PubMed] [Google Scholar]
- 88.Pashenkov M, Goess G, Wagner C, et al. Phase II trial of a toll-like receptor 9-activating oligonucleotide in patients with metastatic melanoma. J Clin Oncol. 2006;24:5716–5724. doi: 10.1200/JCO.2006.07.9129. [DOI] [PubMed] [Google Scholar]
- 89.Krieg AM. Toll-like receptor 9 (TLR9) agonists in the treatment of cancer. Oncogene. 2008;27:161–167. doi: 10.1038/sj.onc.1210911. [DOI] [PubMed] [Google Scholar]
- 90.Romero P, Cerottini JC, Speiser DE. The human T cell response to melanoma antigens. Advances in immunology. 2006;92:187–224. doi: 10.1016/S0065-2776(06)92005-7. [DOI] [PubMed] [Google Scholar]
- 91.Speiser D, Romero P. Toward improved immunocompetence of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1467–1469. doi: 10.1172/JCI25427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Karbach J, Gnjatic S, Bender A, et al. Tumor-reactive CD8+ T-cell responses after vaccination with NY-ESO-1 peptide, CpG 7909 and Montanide ISA-51: association with survival. International journal of cancer. 2010;126:909–918. doi: 10.1002/ijc.24850. [DOI] [PubMed] [Google Scholar]
- 93.Valmori D, Souleimanian NE, Tosello V, et al. Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:8947–8952. doi: 10.1073/pnas.0703395104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lehman F, Louahed J, Gaulis S, et al. Clinical response to the MAGE-A3 immunotherapeutic in metastatic melanoma patients is associated with a specific gene profile present prior to treatment. Cancer Research Institute Symposium; 2008. Abstract. [Google Scholar]
- 95.Tsuji S, Matsumoto M, Takeuchi O, et al. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin: involvement of toll-like receptors. Infect Immun. 2000;68:6883–6890. doi: 10.1128/iai.68.12.6883-6890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jackson AM, Alexandroff AB, Kelly RW, et al. Changes in urinary cytokines and soluble intercellular adhesion molecule-1 (ICAM-1) in bladder cancer patients after bacillus Calmette-Guerin (BCG) immunotherapy. Clin Exp Immunol. 1995;99:369–375. doi: 10.1111/j.1365-2249.1995.tb05560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bruno S, Machi AM, Semino C, et al. Phenotypic, functional and molecular analysis of lymphocytes associated with bladder cancer. Cancer Immunol Immunother. 1996;42:47–54. doi: 10.1007/s002620050250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sharma P, Bajorin DF, Jungbluth AA, et al. Immune responses detected in urothelial carcinoma patients after vaccination with NY-ESO-1 protein plus BCG and GM-CSF. J Immunother. 2008;31:849–857. doi: 10.1097/CJI.0b013e3181891574. [DOI] [PubMed] [Google Scholar]
- 99.Querec T, Bennouna S, Alkan S, et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. The Journal of experimental medicine. 2006;203:413–424. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jager E, Karbach J, Gnjatic S, et al. Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14453–14458. doi: 10.1073/pnas.0606512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 102.Zhu J, Martinez J, Huang X, et al. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood. 2007;109:619–625. doi: 10.1182/blood-2006-06-027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Delaloye J, Roger T, Steiner-Tardivel QG, et al. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS pathogens. 2009;5:e1000480. doi: 10.1371/journal.ppat.1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104.Samuelsson C, Hausmann J, Lauterbach H, et al. Survival of lethal poxvirus infection in mice depends on TLR9, and therapeutic vaccination provides protection. The Journal of clinical investigation. 2008;118:1776–1784. doi: 10.1172/JCI33940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nishikawa H, Sato E, Briones G, et al. In vivo antigen delivery by a Salmonella typhimurium type III secretion system for therapeutic cancer vaccines. The Journal of clinical investigation. 2006;116:1946–1954. doi: 10.1172/JCI28045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhu J, Huang X, Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. Journal of virology. 2007;81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Perreau M, Pantaleo G, Kremer EJ. Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells. The Journal of experimental medicine. 2008;205:2717–2725. doi: 10.1084/jem.20081786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van Duin D, Medzhitov R, Shaw AC. Triggering TLR signaling in vaccination. Trends in immunology. 2006;27:49–55. doi: 10.1016/j.it.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 109.Kedl RM, Rees WA, Hildeman DA, et al. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000;192:1105–1114. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cuadros C, Lopez-Hernandez FJ, Dominguez AL, et al. Flagellin fusion proteins as adjuvants or vaccines induce specific immune responses. Infection and immunity. 2004;72:2810–2816. doi: 10.1128/IAI.72.5.2810-2816.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huleatt JW, Jacobs AR, Tang J, et al. Vaccination with recombinant fusion proteins incorporating Toll-like receptor ligands induces rapid cellular and humoral immunity. Vaccine. 2007;25:763–775. doi: 10.1016/j.vaccine.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 112.Warren TL, Weiner GJ. Synergism between cytosine-guanine oligodeoxynucleotides and monoclonal antibody in the treatment of lymphoma. Semin Oncol. 2002;29:93–97. doi: 10.1053/sonc.2002.30147. [DOI] [PubMed] [Google Scholar]
- 113.Friedberg JW, Kelly JL, Neuberg D, et al. Phase II study of a TLR-9 agonist (1018 ISS) with rituximab in patients with relapsed or refractory follicular lymphoma. Br J Haematol. 2009;146:282–291. doi: 10.1111/j.1365-2141.2009.07773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nature medicine. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 115.Ivanov S, Dragoi AM, Wang X, et al. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007;110:1970–1981. doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paulos CM, Wrzesinski C, Kaiser A, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. The Journal of clinical investigation. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McKee AS, Munks MW, MacLeod MK, et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009;183:4403–4414. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Spreafico R, Ricciardi-Castagnoli P, Mortellaro A. The controversial relationship between NLRP3, alum, danger signals and the next-generation adjuvants. European journal of immunology. 2010;40:638–642. doi: 10.1002/eji.200940039. [DOI] [PubMed] [Google Scholar]
- 119.Sharp FA, Ruane D, Claass B, et al. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:870–875. doi: 10.1073/pnas.0804897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Demento SL, Eisenbarth SC, Foellmer HG, et al. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine. 2009;27:3013–3021. doi: 10.1016/j.vaccine.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li H, Willingham SB, Ting JP, et al. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu Q, Egelston C, Gagnon S, et al. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. The Journal of clinical investigation. 120:607–616. doi: 10.1172/JCI39293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Napolitani G, Rinaldi A, Bertoni F, et al. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nature immunology. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Denning TL, Wang YC, Patel SR, et al. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nature immunology. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 125.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochemical and biophysical research communications. 2009;388:621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]