Abstract
Abstract:
Asthma poses a significant burden on patients, families, health care providers, and the medical system. While efforts to standardize care through guidelines have expanded, difficulty in managing severe asthma has encouraged research about its pathobiology and treatment options. Novel biologic therapeutics are being developed for the treatment of asthma and are of potential use for severe refractory asthma, especially where the increased cost of such agents is more likely justified. This review will summarize currently approved (omalizumab) and investigational biologic agents for asthma, such as antibodies, soluble receptors, and other protein-based antagonists, and highlight recent published data on efficacy and safety of these therapies in humans. As these newer agents with highly targeted pharmacology are tested in asthma, we are also poised to learn more about the role of cytokines and other molecules in the pathophysiology of asthma.
Key Words: asthma, biologic therapies, cytokines, monoclonal antibodies
INTRODUCTION
There is an increasing sense of urgency in addressing the problems of morbidity and mortality caused by asthma. Despite the well-known and fairly consistent efficacy of drugs such as inhaled corticosteroids, leukotriene modifiers and β2 agonists for the majority of asthmatics, as many as 10% suffer from severe disease inadequately controlled by conventional therapy. Severe and sustained symptoms lead to poor quality of life, disproportionate use of health care resources, and significant adverse effects from hospitalizations and frequent need for systemic steroids. Current asthma guidelines offer inadequate assistance in the management of severe refractory asthmatic patients, in part because of a paucity of other treatment options. Novel biologic therapeutics are being developed for the treatment of asthma and are of potential use for severe refractory asthma, especially where the increased cost of such agents is more likely justified. This review will briefly summarize what is meant by “biologic therapies” and then highlight recent published data on efficacy and safety of these therapies for asthma.
WHAT ARE BIOLOGIC THERAPIES?
Biologic therapies have revolutionized the treatment of many diseases including asthma. By definition, the term “biologics” or “biologicals” include a variety of protein-based therapeutics, such as antibodies, soluble receptors (eg, etanercept), recombinant protein-based receptor antagonists (eg, pitrakinra) and other related structures. Their main advantages include the duration of action and highly specific and strong binding to the target of interest; their main disadvantages are the cost and need for parenteral administration.
Most biologicals in clinical use are antibodies, and their generic names contain standard nomenclature as a suffix to indicate their origins (Fig. 1). Initially, pure murine antibodies were created with hybridoma technology, generating therapies that were 100% mouse with generic names given the suffix “momab” (eg, ibritumomab); however, immunogenicity of mouse antibodies in human subjects caused reduced efficacy and increased risk of infusion reactions including anaphylaxis and death. To reduce immunogenicity, chimeric antibodies (“ximabs” like rituximab) were engineered. These antibodies are a marriage of murine variable regions combined with human constant regions, creating antibodies that are ≈80% human. These were a step forward but still had the potential for being immunogenic. Humanized monoclonal antibodies (“zumabs” such as omalizumab) go one step further, where now only the hypervariable regions of the mouse antibody are retained, while the remaining 95% of the antibody is molecularly replaced by human sequences. In the latest approach, fully human antibodies (“umabs” such as adalimumab) can be created by using phage display technology and molecular biology or more directly by immunizing mice that have had their immunoglobulin genes replaced with human versions.1 Newer artificial antibody structures (Fig. 2) such as bispecific antibodies, mix 2 separate arms with 2 different binding specificities to target 2 different types of antigens [eg, a single antibody where one arm binds interleukin (IL)-4 and the other arm binds IL-13]. None of these newer antibody structures have been tried in asthma, so the remainder of this review will focus on available data with standard biologicals (see Table 1).
FIGURE 1.
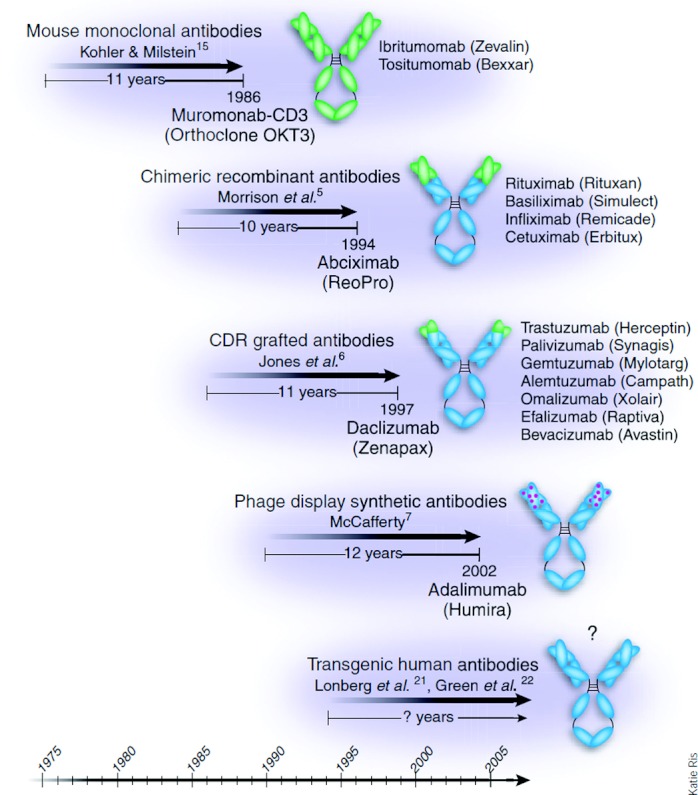
In general, FDA-approved mAbs have emerged between 10 and 12 years after the date that the new technologies on which they were based were reported in the scientific literature. Reprinted by permission from reference 1.
FIGURE 2.
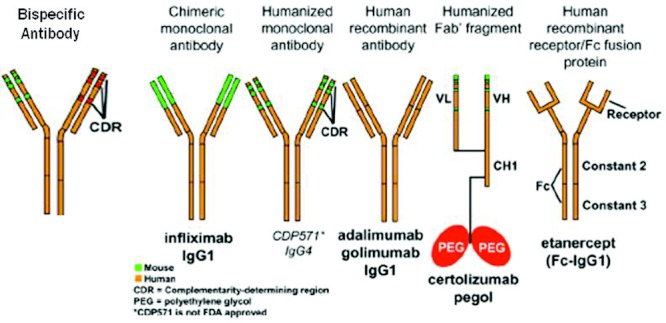
Standard nomenclature for mAbs identifies their source with the last 4 or 5 letters: -omab, murine: -ximab, chimeric: -zumab, humanized: and –umab, human. The middle part of the name reflects the disease indication for which the mAb was initially intended: -lim for immune and inflammatory diseases, -cir for cardiovascular disorders, and -tu for tumors or neoplastic conditions. The first 3 or 4 letters may be chosen by the sponsor. Modified (by adding the structure of a bispecific antibody) from reference 28, with permission.
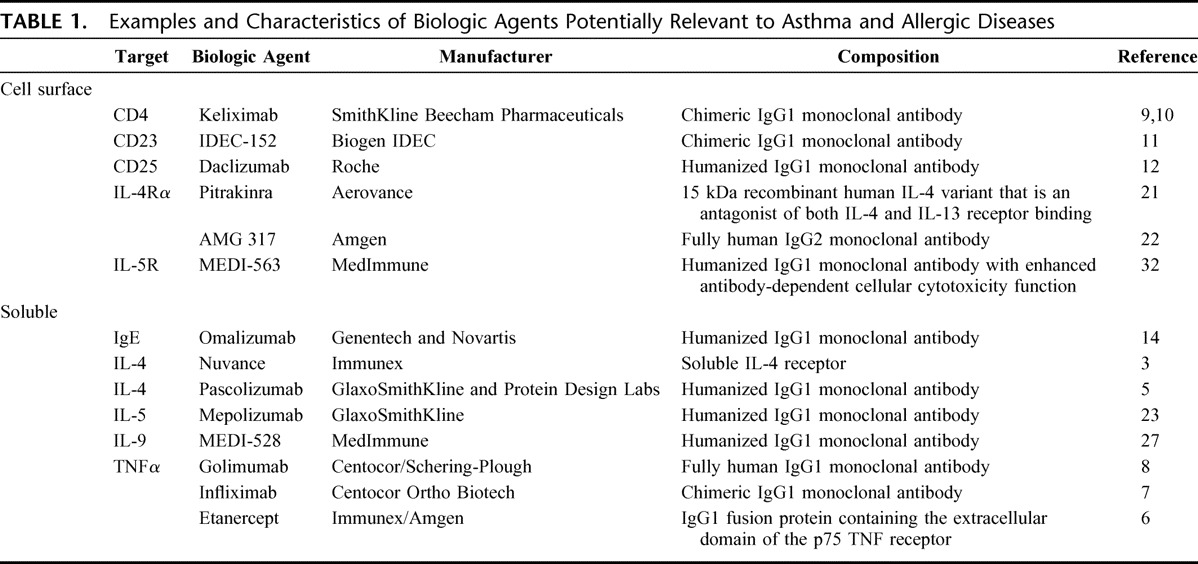
TABLE 1.
Examples and Characteristics of Biologic Agents Potentially Relevant to Asthma and Allergic Diseases
IL-4
IL-4 is important to the development of allergic inflammation. It induces the IgE isotype switch and up-regulates expression of vascular cell adhesion molecule-1 on endothelium and a variety of TH2 chemokines, thus promoting recruitment of T lymphocytes, monocytes, basophils, and eosinophils to sites of allergic inflammation. Furthermore, IL-4 induces mucin production and promotes the differentiation of naive TH0 lymphocytes into TH2 lymphocytes that secrete IL-4, IL-5, IL-9, and IL-13.2,3
A clinical trial studied the soluble recombinant human IL-4 receptor (IL-4R), Nuvance in asthma. Nuvance inhibited a decline in FEV1 during inhaled corticosteroid withdrawal and was overall well tolerated.2,3 However, in subsequent clinical trials in patients taking only beta agonist, soluble IL-4R failed to demonstrate significant clinical efficacy. In addition, Steinke et al found no clinical benefit with soluble IL-4R as inhaled corticosteroids were tapered.4
A phase I randomized double blind placebo controlled study evaluated the effects of pascolizumab, a humanized anti-IL-4 antibody, in 24 patients with mild to moderate asthma. Pascolizumab was well tolerated and no serious adverse events occurred.5 However, a phase IIa clinical trial in steroid-naive, mild to moderate asthmatics, did not demonstrate clinical efficacy. Because the IL-4 targeting studies have failed to demonstrate clinical efficacy, one can justify concluding that either IL-4 is not a disease-perpetuating cytokine in asthma, or perhaps that IL-4 was not adequately neutralized for a sufficient amount of time, or in the right patient population, or at the relevant site of IL-4 action. While the former seems most likely, without convincing evidence documenting the latter issues, the exact reason for the failure of these studies remains speculative.
TNFα
Tumor necrosis factor (TNF)α is a multifunctional proinflammatory cytokine produced by inflammatory cells including monocytes, macrophages, mast cells, smooth muscle cells, and epithelial cells. TNFα may initiate airway inflammation by up-regulating adhesion molecules, mucin hypersecretion, and airway remodeling, and by synergizing with TH2 cytokines. Berry et al demonstrated that severe refractory asthmatics have evidence of up-regulation of TNFα as compared with healthy controls and mild asthmatics.6
Entanercept was evaluated in a small, randomized, double-blind placebo-controlled crossover study in 10 patients with severe refractory asthma and elevated TNFα levels, 10 patients with mild to moderate asthma, and 10 control patients. Entanercept treatment was associated with improved FEV1, asthma related quality of life, and the concentration of methacholine needed to provoke a 20% decrease in FEV1. No serious adverse reactions were noted.6
In another double-blind, placebo-controlled, parallel-group study, 38 patients with moderate asthma on inhaled corticosteroids were treated with infliximab. Although infliximab treatment did not improve the primary end point of morning peak expiratory flow, it decreased diurnal variation of the peak expiratory flow rate and asthma exacerbations. No serious adverse events were noted.7
Golimumab was recently evaluated in the largest randomized, double-blind, placebo-controlled study in 309 patients with severe, uncontrolled asthma. No significant differences were observed for the change in FEV1 or exacerbations. Several serious adverse events occurred in patients receiving active drug including cellulitis, pneumonia, active tuberculosis, and sepsis (resulting in death); various malignancies in the active treatment group terminated the study prematurely.8 Despite some initially encouraging results, there remains no clear indications for the use of these agents in asthma, and by inference, there is no clear role for TNFα in perpetuating asthma or asthma exacerbations.
CD4
CD4+ T cells are likely to be involved as a source of proinflammatory cytokines in asthma. Keliximab is a monoclonal antibody that causes a transient reduction in the number of CD4+ T cells.9 A double blind, randomized, placebo-controlled study with 22 severe oral corticosteroid dependent asthmatics patients was completed. A subset of patients received the highest dose of keliximab (3.0 mg/kg). There was significant improvement of peak expiratory flow rates in the high dose treatment arm. However, CD4+ T cells remained transiently reduced 14 days postinfusion, raising safety concerns.10
CD23
CD23 is a low-affinity immunoglobulin E receptor (FcεRII) and is important in regulating IgE production. IDEC-152 is a chimeric monoclonal antibody directed against CD23. CD23 is expressed on T and B cells, neutrophils, monocytes, and macrophages. CD23 is overexpressed in allergic disease and may be involved in IgE overproduction, which can lead to mast cell degranulation. A phase I dose-escalating placebo-controlled study in 30 asthmatics demonstrated that IDEC-152 caused a dose-dependent reduction in serum IgE concentrations. No significant adverse events were reported.11
CD25
Airway inflammation is associated with activated CD25+ T cells, IL-2, and soluble IL-2 receptors. Daclizumab is a humanized monoclonal antibody directed against the alpha subunit of the high affinity IL-2 receptor (CD25). This inhibits IL-2 binding and release of inflammatory cytokines. A randomized, double-blind, placebo-controlled, parallel-group study was performed to evaluate the efficacy of daclizumab in patients with moderate to severe asthma poorly controlled on inhaled corticosteroids. There were 115 patients enrolled in the study, 88 patients were randomized to the treatment arm and 27 patients were randomized to the placebo arm. Treatment with daclizumab led to improvements in FEV1, daytime asthma symptoms, and rescue β2 agonist use, but the effects were modest. A similar frequency of adverse advents was reported in the treatment and placebo groups. However, 3 patients reported serious adverse events (anaphylaxis, varicella zoster virus meningitis and myelitis, and breast cancer) in the treatment arm, likely related to the study drug.12,13 One final consideration is that T-regulatory cells also express CD25 and are believed to play a role in down-regulating TH2 responses. Whether these cells are targeted in a detrimental way in these studies is not known.
IgE
Immunoglobulin (Ig)E is an important mediator of allergic reactions thought to be responsible for the induction and maintenance of airway inflammation and related symptoms in many asthmatics. Omalizumab is a humanized monoclonal anti-IgE antibody that binds free circulating IgE and prevents the interaction between IgE and high affinity (FcεRI) and low affinity (FcεRII) IgE receptors on inflammatory cells.14 Omalizumab also down-regulates the surface expression of FcεRI on basophils, mast cells, and dendritic cells (Fig. 3). Omalizumab was the first and only biologic agent approved by the Food and Drug Administration (FDA) for the treatment of asthma and has been in use since June 2003.
FIGURE 3.
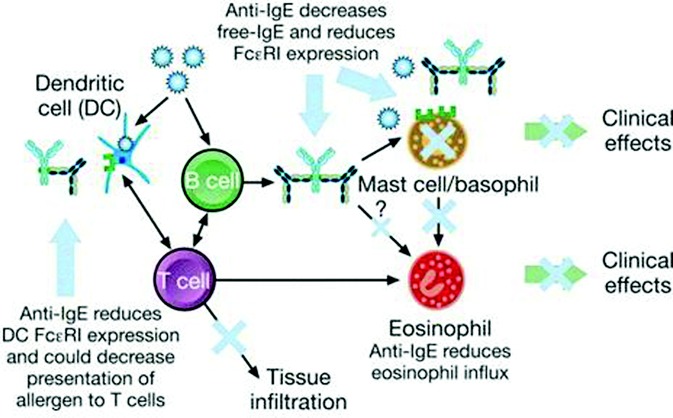
Proposed mechanisms of action of omalizumab. Omalizumab decreases free IgE levels and reduces FcεRI receptor expression on mast cells and basophils. This results in decreased mast cell activation and sensitivity, leading to a reduction in eosinophil influx and activation. Anti-IgE treatment with omalizumab might result in decreased mast cell survival. Omalizumab also reduces dendritic cell FcεRI receptor expression. Reprinted from reference 29, with permission.
The efficacy of omalizumab in the treatment of inhaled corticosteroid–dependent asthma has been demonstrated in numerous studies. Busse et al completed a phase III, double-blind, placebo-controlled trial involving 525 subjects with severe allergic asthma requiring daily inhaled corticosteroids. The primary end point was the number of exacerbation episodes during the steroid reduction period and the stable steroid period. During the stable steroid phase, fewer omalizumab subjects than placebo subjects experienced one or more exacerbations (14.6 vs. 23.3%; P = 0.009). During the steroid reduction phase, the omalizumab group had fewer subjects with exacerbations (21.3 vs. 32.3%; P = 0.04). The median reduction in inhaled corticosteroid dose was significantly greater in the omalizumab group than in the placebo group (75 vs. 50%; P < 0.001).15,16
The efficacy of omalizumab was demonstrated in other clinical trials including INNOVATE. INNOVATE was a double-blind, parallel-group study in which 419 subjects were randomized to receive omalizumab or placebo for 28 weeks. The omalizumab group had a 26% reduction in the rate of clinically significant exacerbations compared with placebo (.68 vs. .91, P = 0.042).17
A recent omalizumab observational study of 280 subjects demonstrates similar findings. After 6 months, they found a reduction in daily symptoms by 80%, nocturnal symptoms by 86%, asthma exacerbations by 82%, hospitalizations by 76%, unscheduled health care visits by 81%, and improvement in quality of life (Mini Asthma Quality of Life Questionnaire increased from 2.9 to 4.5 after 6 months of treatment).14
Brown et al determined the incremental cost effectiveness ratio of adding omalizumab to standard therapy (inhaled corticosteroids and long-acting beta agonist). The base case lifetime analysis of standard therapy versus standard therapy plus omalizumab for the first 5 years, gave an incremental cost effectiveness ratio of 31,209 Euros. This study suggests that add-on omalizumab therapy is cost-effective in patients with severe persistent allergic asthma.18
Regarding safety, the FDA recently issued an early communication notice after receiving interim data from an ongoing postmarketing surveillance study that showed a disproportionate increase in heart problems potentially caused by omalizumab side effects. EXCELS (Evaluating the Clinical Effectiveness and Long-Term Safety in Patients with Moderate to Severe Asthma) is an observational study to try to determine long-term side effects of omalizumab. The FDA reports that interim data from EXCELS show a disproportionate increase in ischemic heart disease, arrhythmias, cardiomyopathy, and cardiac failure, pulmonary hypertension, cerebrovascular disorders, and embolic, thrombotic, and thrombophlebitic events in patients treated with omalizumab compared with the control group. No causal relationship has been identified and the FDA will provide additional information as analyses become available.19 In addition, Limb et al evaluated omalizumab and anaphylaxis events through postmarketing surveillance from June 2003 through December 2006. An estimated 57,300 patients received omalizumab during this period and 124 cases of anaphylaxis associated with omalizumab administration were described. Many cases had a delayed onset of anaphylaxis and a protracted progression of symptoms resulting in a black box warning.20
IL-4/IL-13
TH2 cytokines such as IL-4 and IL-13 may contribute to airway inflammation. Pitrakinra is a recombinant human IL-4 variant that engages the IL-4Rα receptor complex to interfere with binding of both IL-4 and IL-13. Wenzel et al evaluated the effects of pitrakinra in atopic asthmatics in 2 randomized, double blind placebo controlled phase II a studies. In the first study, patients received either pitrakinra 25 mg subcutaneous daily or placebo. There were fewer asthma related adverse events (P = 0.069) and lower beta agonist rescue use (P = 0.031). In the second study, patients received either pitrakinra 60 mg via nebulization twice daily or placebo followed by inhaled allergen challenge. Patients in the treatment arm had a remarkable reduction in the late phase response to allergen. No serious adverse events were reported.21
AMG 317 is a monoclonal antibody that inhibits both IL-4 and IL-13 by blocking the shared IL-4Rα chain. A 12-week randomized, double-blind, placebo-controlled phase II study evaluated AMG 317 in moderate to severe asthmatics. Patients were randomized to 12 weeks of weekly subcutaneous injections of AMG 317 or placebo. AMG 317 did not demonstrate clinical efficacy across the overall group of patients. Clinically significant improvements were observed in several outcome measures in patients with higher baseline Asthma Control Questionnaire scores. AMG 317 was safe and well tolerated in this study.22
IL-5
One experimental monoclonal antibody against IL-5, mepolizumab, has been studied in asthma and results have been published. Initial studies in mild and moderate asthmatics demonstrated significant reductions in blood and sputum eosinophils; however, there were no significant changes in any of the clinical endpoints measured including exacerbation rates, FEV1, morning peak expiratory flow, rescue beta agonist use, and quality of life.23
In a subsequent double-blind, placebo-controlled, parallel-group study, 61 subjects with refractory eosinophilic asthma were randomized to receive mepolizumab or placebo at monthly intervals, 29 subjects received mepolizumab and 32 received a placebo for 1 year. The mepolizumab group experienced fewer severe exacerbations than placebo (2.0 vs. 3.4 exacerbations per subject; P = 0.02) and greater improvement on the Asthma Quality of Life Questionnaire (mean increase from baseline .55 vs. .19, P = 0.02). There were also significant decreases in sputum and blood eosinophils in the active treatment group. Improvements in eosinophil counts after infusion of mepolizumab as compared with placebo were reduced by a factor of 2.1 in bronchial biopsy specimens (P = 0.68), by a factor of 8.2 in bronchoalveolar lavage specimens (P = 0.06), and by a factor of 16.0 in bronchial wash specimens (P = 0.02). In addition, airway wall thickness and total wall area measured by computed tomography (CT) were reduced in the treatment arm group as compared with placebo. There were no significant improvements in symptoms, airway hyperresponsiveness, or FEV1 after bronchodilator use.24
A second smaller double-blind, placebo-controlled, parallel-group study enrolled 20 asthmatic patients with persistent sputum eosinophilia and symptoms despite prednisone treatment. Nine patients were randomized to receive mepolizumab (5 monthly infusions) and 11 patients to receive placebo. During this period, 12 asthma exacerbations occurred in 10 patients who received placebo (9 of the subjects had sputum eosinophilia at the time of exacerbation). However, in the mepolizumab treatment group, only 1 patient had an exacerbation. Patients in the treatment group were able to use less prednisone than patients on placebo (84 vs. 48% of maximum possible dose, P = 0.04). There were also significant decreases in sputum and blood eosinophils in the active treatment group. Improvements in asthma control and eosinophil counts were maintained for 8 weeks after the last infusion.25
The 2 most recent studies highlight the importance of selecting the appropriate patient for this treatment. Mepolizumab therapy may be especially useful in a subgroup of asthmatics that have eosinophilic driven exacerbations that do not respond to corticosteroid treatment. In retrospect, it now seems likely that the initial IL-5 targeting studies may have failed because of enrolling patients in whom their asthma was not eosinophil driven. It remains to be determined whether IL-5 therapies can affect lung function and bronchial hyperreactivity especially if used for longer periods of time.
Reslizumab is a humanized monoclonal antibody against IL-5. Several clinical trials evaluating its safety and efficacy are in progress. A phase II, multicenter randomized, double-blind placebo-controlled trial in severe asthmatic adult patients with persistent symptoms despite inhaled corticosteroids and elevated eosinophils in induced sputum is underway. The primary end point of the study is improvement in the Asthma Quality of Life Questionnaire. Secondary endpoints include FEV1, asthma, exacerbations, and eosinophil values. No data are available at the time of this publication.26
Several studies examining the effect of humanized anti-IL-5Rα antibody (MEDI-563) are in progress. In vitro, MEDI-563 mediated the lysis of IL-5Rα transfected cells with a 200-fold higher potency in comparison to the parental mAb. Reed et al studied the safety and biologic activity in 6 mild asthmatics after a single intravenous dose of MEDI-563. Circulating eosinophils decreased below detection limits within 24–48 hours of dosing. Because basophils also express IL-5Rα, this treatment may affect basophil numbers as well. No serious adverse events were reported.27 Results of additional completed clinical studies should be forthcoming in the near future.30
IL-9
IL-9 increases airway inflammation, mucus production, and mast cell differentiation, in large part by inducing other TH2 cytokines. MEDI-528 is a novel human monoclonal antibody against IL-9. Preclinical murine models demonstrated inhibition of IL-9 caused reduced airway hyperresponsiveness and mast cell activation. A recent human phase I study was completed and no serious adverse events were reported.31 A phase IIa randomized, placebo-controlled, double-blinded study is currently in progress.32
CONCLUSION
Examining the effects of biologic agents provides unique and valuable insight into the pathobiology of asthma. Furthermore, it is an ideal opportunity to identify mechanisms inherent to severe refractory asthma. The development of biologic agents has been a slow and arduous process; however, a substantial amount of progress has been achieved. Although omalizumab is an expensive medical treatment, therapy may be cost effective in patients with uncontrolled severe persistent allergic asthma because the majority of the economic burden is in this population. Hopefully ongoing efforts with biologicals will lead to improved management options for our most severe asthma patients.
REFERENCES
- 1.Lonberg N. Human antibodies from transgenic animals. Nat Biotechnol. 2005;23:1117–1125 [DOI] [PubMed] [Google Scholar]
- 2.Borish LC, Nelson HS, Corren J, Bensch G, Busse WW, et al. Efficacy of soluble IL-4 receptor for the treatment of adults with asthma. J Allergy Clin Immunol. 2001;107:963–970 [DOI] [PubMed] [Google Scholar]
- 3.Borish LC, Nelson HS, Lanz MJ, Claussen L, Whitmore JB, et al. Interleukin-4 receptor in moderate atopic asthma. A phase I/II randomized, placebo-controlled trial. Am J Respir Crit Care Med. 1999;160:1816–1823 [DOI] [PubMed] [Google Scholar]
- 4.Steinke JW. Anti-interleukin-4 therapy. Immunol Allergy Clin North Am. 2004;24:599–614, vi. [DOI] [PubMed] [Google Scholar]
- 5.Hart TK, Blcakburn MN, Brighman-Burke M, Dede K, Al-Mahdi N, Zia-Amirhosseini P, Cook RM. Preclinical efficacy and safety of pascolizumab (SB 240683): a humanized anti-interleukin-4 antibody with therapeutic potential in asthma. Clin Exp Immunol. 2002;130:93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med. 2006;354:697–708 [DOI] [PubMed] [Google Scholar]
- 7.Erin EM, Leaker BR, Nicholson GC, Tan, Green LM, et al. The effects of a monoclonal antibody directed against tumor necrosis factor-alpha in asthma. Am J Respir Crit Care Med. 2006;174:753–762 [DOI] [PubMed] [Google Scholar]
- 8.Wenzel SE, Barnes PJ, Bleecker ER, Bousquet J, Busse W, et al. A randomized, double-blind, placebo-controlled study of tumor necrosis factor-alpha blockade in severe persistent asthma. Am J Respir Crit Care Med. 2009;179:549–558 [DOI] [PubMed] [Google Scholar]
- 9.Kon OM, Sihra BS, Compton CH, Leonard TB, Kay AB, Barnes NC. Randomised, dose-ranging, placebo-controlled study of chimeric antibody to CD4 (keliximab) in chronic severe asthma. Lancet. 1998;352:1109–1113 [DOI] [PubMed] [Google Scholar]
- 10.Kon OM, Sihra BS, Loh LC, Barkans J, Compton CH, et al. The effects of an anti-CD4 monoclonal antibody, keliximab, on peripheral blood CD4+ T-cells in asthma. Eur Respir J. 2001;18:45–52 [DOI] [PubMed] [Google Scholar]
- 11.Rosenwasser LJ, Busse WW, Lizambri RG, Olejnik TA, Totoritis MC. Allergic asthma and an anti-CD23 mAb (IDEC-152): results of a phase I, single-dose, dose-escalating clinical trial. J Allergy Clin Immunol. 2003;112:563–570 [DOI] [PubMed] [Google Scholar]
- 12.Busse WW, Israel E, Nelson HS, Baker JW, Charous BL, et al. Daclizumab improves asthma control in patients with moderate to severe persistent asthma: a randomized, controlled trial. Am J Respir Crit Care Med. 2008;178:1002–1008 [DOI] [PubMed] [Google Scholar]
- 13.Antoniu SA. Daclizumab a novel corticosteroid-sparing therapy for asthma? Expert Opin Investig Drugs. 2009;18:369–371 [DOI] [PubMed] [Google Scholar]
- 14.Korn S, Thielen A, Seyfried S, Taube C, Kornmann O, Buhl R. Omalizumab in patients with severe persistent allergic asthma in a real-life setting in Germany. Respir Med. 2009;103:1725–1731 [DOI] [PubMed] [Google Scholar]
- 15.Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–190 [DOI] [PubMed] [Google Scholar]
- 16.Nowak D. Management of asthma with anti-immunoglobulin E: a review of clinical trials of omalizumab. Respir Med. 2006;100:1907–1917 [DOI] [PubMed] [Google Scholar]
- 17.Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60:309–316 [DOI] [PubMed] [Google Scholar]
- 18.Brown R, Turk F, Dale P, Bousquet J. Cost-effectiveness of omalizumab in patients with severe persistent allergic asthma. Allergy. 2007;62:149–153 [DOI] [PubMed] [Google Scholar]
- 19.Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm172406.htm Accessed May 18, 2010.
- 20.Limb SL, Starke PR, Lee CE, Chowdhury BA. Delayed onset and protracted progression of anaphylaxis after omalizumab administration in patients with asthma. J Allergy Clin Immunol. 2007;120:1378–1381 [DOI] [PubMed] [Google Scholar]
- 21.Wenzel S, et al. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007;370:1422–1431 [DOI] [PubMed] [Google Scholar]
- 22.Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, et al. A Randomized, controlled, Phase 2 study of AMG 317, an IL-4Rα antagonist, in patients with asthma. Am J Respir Crit Care Med. 2010;181:788–796 [DOI] [PubMed] [Google Scholar]
- 23.Flood-Page P, Swenson C, Faiferman I, Matthews J, Williams M, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. 2007;176:1062–1071 [DOI] [PubMed] [Google Scholar]
- 24.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993 [DOI] [PubMed] [Google Scholar]
- 26.Available at: http://clinicaltrials.gov/ct2/show/NCT00587288 Accessed May 18, 2010.
- 27.Reed J, Kolbeck R, Molfino N, Kozhich A, Humbles A, et al. MEDI-563, a humanized anti-IL-5Rα antibody with enhanced effector function, induces reversible blood eosinopenia in mild asthmatics. J Allergy Clin Immunol. 2008;121:S47(abstract). [Google Scholar]
- 28.Lee SJ, Chinen J, Kavanaugh A. Immunomodulator therapy: monoclonal antibodies, fusion proteins, cytokines, and immunoglobulins. J Allergy Clin Immunol. 2010;125(Suppl 2):S314–S323 [DOI] [PubMed] [Google Scholar]
- 29.Holgate S, Casale T, Wenzel S, Bousquet J, Deniz Y, Reisner C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J Allergy Clin Immunol. 2005;115:459–465 [DOI] [PubMed] [Google Scholar]
- 30.Kolbeck R, Kozhich A, Koike M, Peng L, Andersson C, et al. MEDI-563, a humanized anti-interleukin-5 receptor-alpha monoclonal antibody with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010. In Press. [DOI] [PubMed] [Google Scholar]
- 31.White B, Leon F, White W, Robbie G. Two first-in-human, open-label, phase I dose-escalation safety trials of MEDI-528, a monoclonal antibody against interleukin-9, in healthy adult volunteers. Clin Ther. 2009;31:728–740 [DOI] [PubMed] [Google Scholar]
- 32.Available at: www.medscape.com/viewarticle/5844047 Accessed May 18, 2010.


