Abstract
Agmatine is a polyamine and has been considered as a novel neurotransmitter or neuromodulator in the central nervous system. In the present study, the neuroprotective effect of agmatine against cell damage caused by N-methyl-d-aspartate (NMDA) and glutamate was investigated in cultured rat hippocampal neurons. Lactate dehydrogenase (LDH) activity assay, β-tubulin III immunocytochemical staining and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate (dUTP) nick end-labeling (TUNEL) assay were conducted to detect cell damage. Exposure of 12-day neuronal cultures of rat hippocampus to NMDA or glutamate for 1 h caused a concentration-dependent neurotoxicity, as indicated by the significant increase in released LDH activities. Addition of 100 µM agmatine into media ablated the neurotoxicity induced by NMDA or glutamate, an effect also produced by the specific NMDA receptor antagonist dizocilpine hydrogen maleate (MK801). Arcaine, an analog of agmatine with similar structure as agmatine, fully prevented the NMDA- or glutamate-induced neuronal damage. Spermine and putrescine, the endogenous polyamine and metabolic products of agmatine without the guanidine moiety of agmatine, failed to show this effect, indicating a structural relevance for this neuroprotection. Immunocytochemical staining and TUNEL assay confirmed the findings in the LDH measurement. That is, agmatine and MK801 markedly attenuated NMDA-induced neuronal death and significantly reduced TUNEL-positive cell numbers induced by exposure of cultured hippocampal neurons to NMDA. Taken together, these results demonstrate that agmatine can protect cultured hippocampal neurons from NMDA- or glutamate-induced excitotoxicity, through a possible blockade of the NMDA receptor channels or a potential anti-apoptotic property.
Keywords: Agmatine, Hippocampus, Glutamate, NMDA, Apoptosis, Lactate dehydrogenase
1. Introduction
Agmatine is an endogenous polyamine derived from enzymatic decarboxylation of l-arginine (Tabor and Tabor, 1984). In the past decade, accumulating evidence indicated agmatine's several levels of pharmacological and physiological importance. Agmatine is present in the brain and other tissues of mammals (Lortie et al., 1996; Li et al., 1994). In neuronal tissues, agmatine is present in axon terminals associated with synaptic vesicles (Reis et al., 1998) and can be taken up into synaptosomes via a Na+-independent system (Sastre et al., 1997). Agmatine has been reported to act as a ligand of the imidazoline receptor (Li et al., 1994). It inhibits all isoforms of nitric oxide synthase (NOS; Galea et al., 1996) and blocks nicotinic receptor (Loring, 1990), voltage-gated Ca2+ channels and N-methyl-d-aspartate (NMDA) receptor channels (Yang and Reis, 1999). All these functional characteristics suggest that agmatine may play a role as a neurotransmitter or neuromodulator in the brain.
Agmatine has previously been shown to exert its neuroprotective action by reducing the size of ischemic infarctions or the loss of cerebellar neurons after focal or global ischemia in vivo (Gilad et al., 1996b; Kim et al., 2004). Its neuroprotective effects also include attenuating the extent of neuronal loss following excitotoxic spinal cord injury (Fairbanks et al., 2000) and preventing neurotoxicity produced by glutamate in cultured cerebellar granule cells (Olmos et al., 1999). Our previous study demonstrated that agmatine can protect the cultured rat cortex neurons and PC12 cells from cell death after exposing to NMDA and glutamate (Zhu et al., 2003). However, more compelling evidence is needed for elucidating its neuroprotective role on different brain neurons.
The hippocampus plays a vital role in learning and memory (Eichenbaum and Otto, 1992). It also influences autonomic and vegetative functions (Jacobson and Sapolsky, 1991). Many studies have demonstrated that the hippocampus is one of the most vulnerable brain regions as regards to various neurological insults such as hypoxia–ischemia, seizure and prolonged stress. These insults produce excessive synaptic-glutamate accumulation, which triggers a series of intracellular biochemical changes and finally leads to degenerative events in the neuron, including cytoskeletal degradation, the collapse of mitochondrial potentials and protein misfolding. These glutamate-induced cell damages have been shown to contribute to pathological consequences in different kinds of disorders such as major depression, seizure and Alzheimer's diseases (Portera-Cailliau et al., 1995). Also, glutamate is a major excitatory neurotransmitter in the brain and a potent excitotoxin. Exposure of neuronal cultures to micromolar concentrations of glutamate has been reported to lead to neurotoxicity (Choi, 1988) and induce neuronal apoptosis (Kure et al., 1991). Given the abundance of agmatine in the hippocampus (Feng et al., 1997; Reis et al., 1998), studying the effects of agmatine on hippocampal neurons will provide important information regarding the potential role of this proposed neurotransmitter in this area.
In the present investigation using primary cultured hippocampal neurons of rats, we examined the neuroprotective effect of agmatine against neuronal damage induced by NMDA and glutamate. We observed that agmatine prevents hippocampal neurons from excitatory cell damage, an effect dependent on its capacity to block NMDA receptor channels, as well as its potential anti-apoptotic effects. Our data suggest that as an important polyamine in the hippocampus, agmatine may play a role in the physiology of hippocampal neurons. Further elucidation of the effects of agmatine on hippocampal neurons may lead to novel therapeutic strategies for the diseases related to the hippocampus.
2. Results
2.1. Neuroprotective effects of agmatine on NMDA- or glutamate-induced cell damage in cultured hippocampal neurons
As previously reported (Brewer et al., 1993), neurobasal medium supplemented with B-27 represents an optimized medium for sustaining the long-term survival of hippocampal neurons with little glial growth. The double immunocytochemical staining performed in 12-day hippocampal cultures with βT-III (to display putative neurons) and GFAP (for astrocytes) in the present study showed a nearly pure neuronal population with very little GFAP staining (less than 0.5%, data not shown), which is consistent with the report by Brewer et al. (1993).
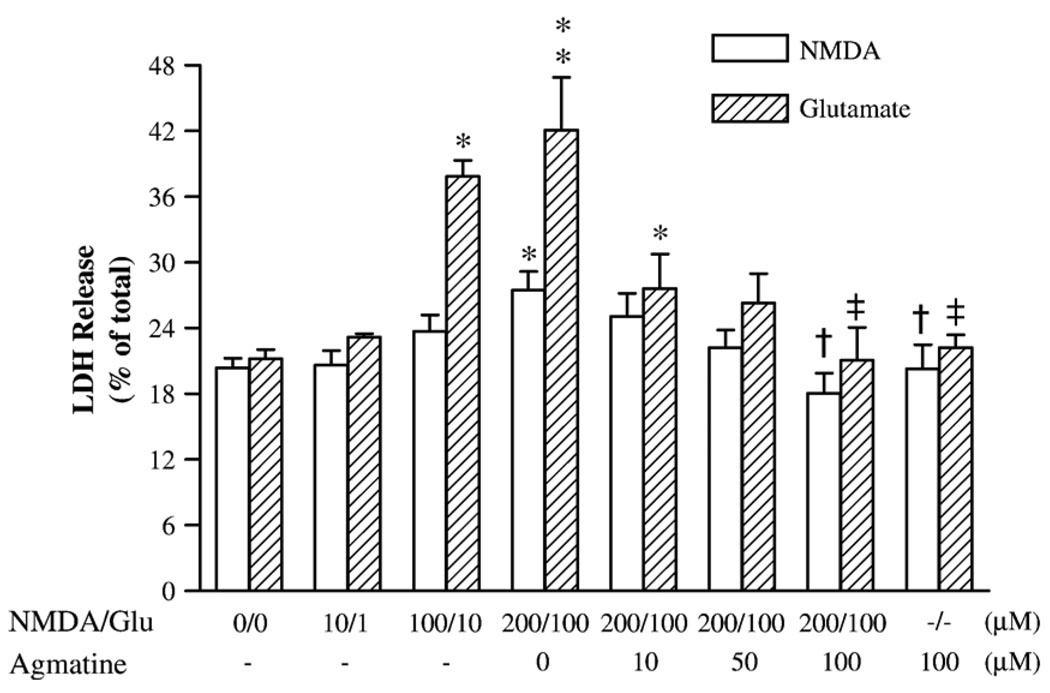
We examined the neuroprotective effects of agmatine on NMDA-induced neuronal damage. Twelve-day cultured hippocampal neurons were exposed to 10, 100, and 200 µM NMDA or 1, 10, and 100 µM glutamate in serum-free buffer for 1 h followed by continued incubation in normal growth medium for an additional 23 h. LDH assays showed that NMDA or glutamate caused a concentration-dependent neurotoxicity, as indexed by a significant increase in LDH activity after exposure to 200 µM NMDA (by 35%; F7,52 = 4.37, P < 0.05) or 10 and 100 µM glutamate (by 78 and 98%, respectively; F7,35 = 6.82, P < 0.01), respectively (Fig. 1). Concurrent exposure of these neurons to 200 µM NMDA or 100 µM glutamate with different concentrations of agmatine showed that 100 µM agmatine fully prevented increased LDH activities produced by NMDA or glutamate (Fig. 1). Agmatine (100 µM) alone did not cause a significant change in LDH activity.
Fig. 1.
The release of LDH activity in primary culture of rat hippocampal neurons illustrating the neurotoxic effects of NMDA or glutamate and neuroprotective effects of agmatine. Data are shown as mean ± SEM of 6–10 measurements. *P < 0.05, **P < 0.01, compared to controls (0). †P < 0.05, compared to the group treated with 200 µM NMDA. ‡P < 0.01, compared to the group treated with 100 µM glutamate (Glu).
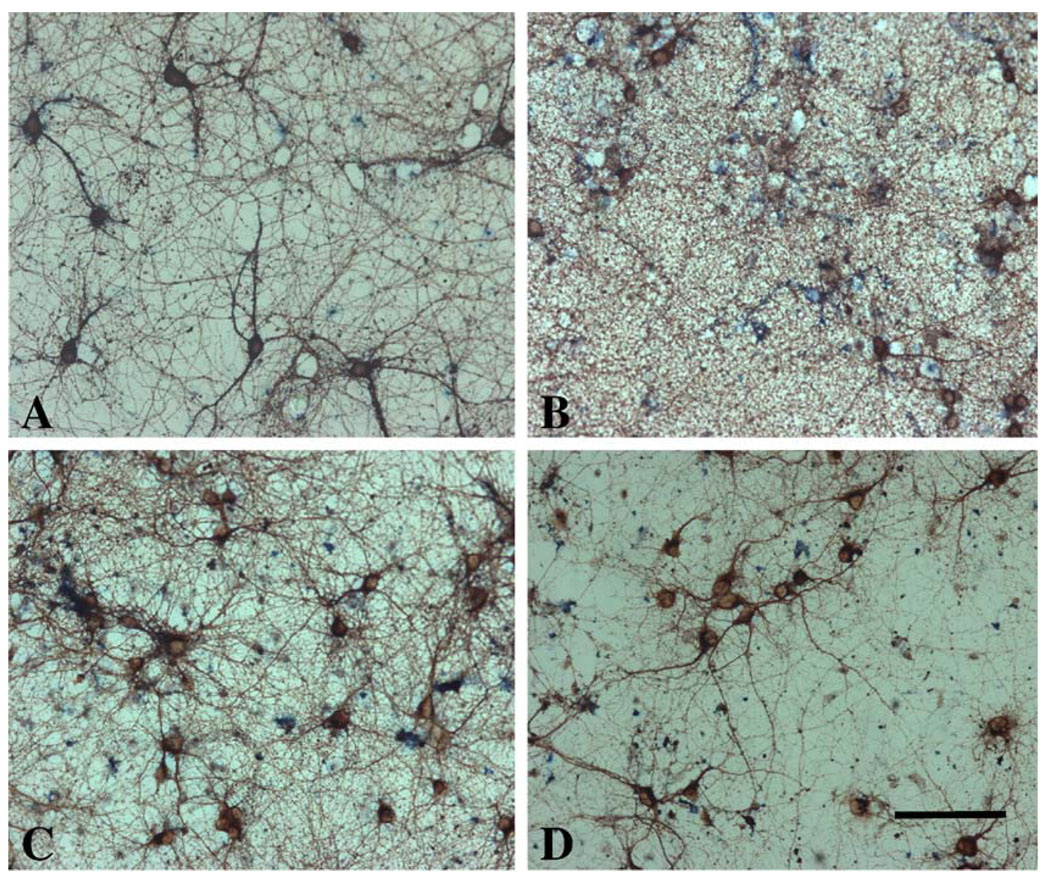
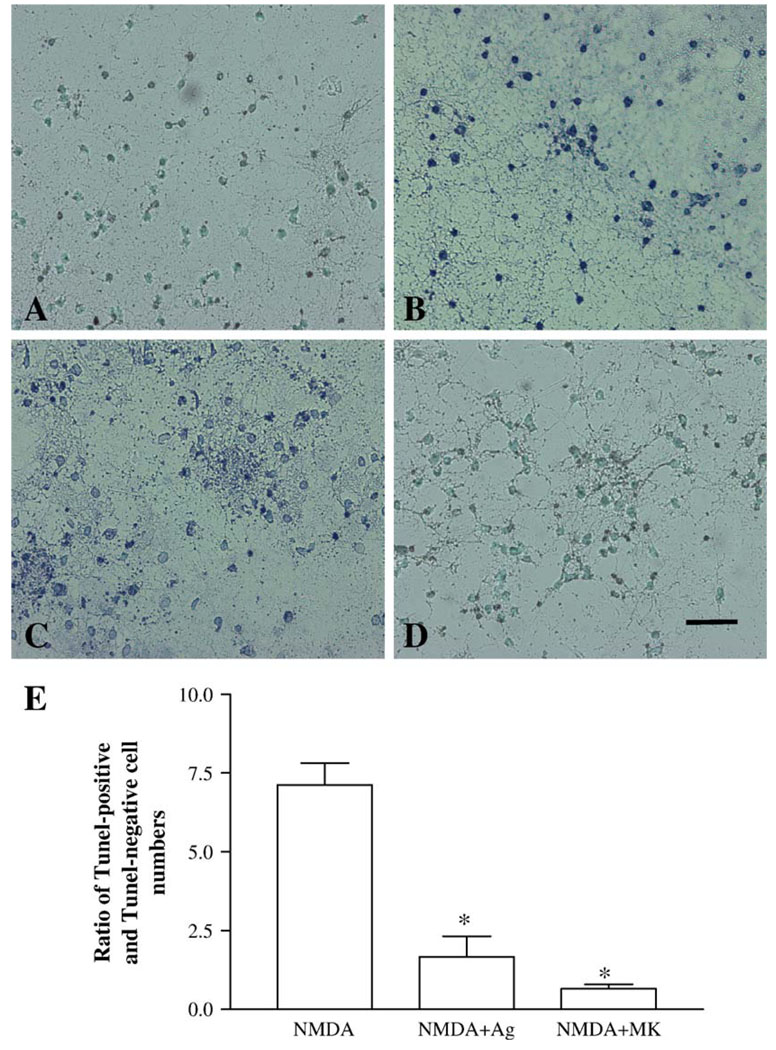
The neuroprotective effects of agmatine were further studied using immunocytochemical stainings with βT-III (Fig. 3) and TUNEL assay (Fig. 4) in cultured hippocampal neurons treated in the same way as those described in the experiment for LDH measurement. Control cultures exhibited βT-III-positive cells with homogeneous and compact morphology (Fig. 3A) or sparse TUNEL-labeled cells (Fig. 4A). Exposure of cultures to 200 µM NMDA resulted in a significant loss of βT-III-positive neurons (Fig. 3B), with the disappearance of neuritis, disrupted membranes, distorted somata, and condensed nuclei. The degree of cell death estimated by microscopic examination of immunostained cells on coverslips was consistent with the results obtained with LDH assay (data not shown). Also this exposure resulted in a large number of TUNEL-positive cells (Fig. 4B). The neuronal loss and increased TUNEL-positive cells were prevented by the addition of 100 µM agmatine (Figs. 3C and 4C) to the cultures. 100 µM agmatine by itself did not markedly affect the morphology of βT-III-positive neurons and the numbers of TUNEL-positive cells, compared to the control cultures (data not shown).
Fig. 3.
Agmatine reduces cell death in NMDA-treated rat hippocampal cultures. Hippocampal neurons (12 days) were exposed to vehicle (A), 200 µM NMDA either alone (B) or in combination with 100 µM agmatine (C), or 10 µM MK801 (D) for 1 h. The neuronal cultures were immunocytochemically stained with antibody to β-tubulin III. Calibration bar: 100 µm for all figures.
Fig. 4.
TUNEL assay in cultured rat hippocampal neurons (12 days) exposed to vehicle (A), 200 µM NMDA for 1 h either alone (B) or in combination with 100 µM agmatine (C), or 10 µM MK801 (D), followed by continue incubation in fresh growing media for additional 23 h. TUNEL assay shows the presence of DNA fragmentation in the nuclei of cultured hippocampal neurons as indicated by dark spots. Calibration bar: 100 µm for all figures. Above TUNEL assays were quantitatively analyzed (E). Data were expressed as ratio of TUNEL-positive to TUNEL-negative cells in 5–6 coverslips from 4 experiments. *P < 0.01 versus NMDA exposure.
2.2. Comparison of effects of agmatine, the analog of agmatine and MK801 on NMDA- or glutamate-induced neuronal damage
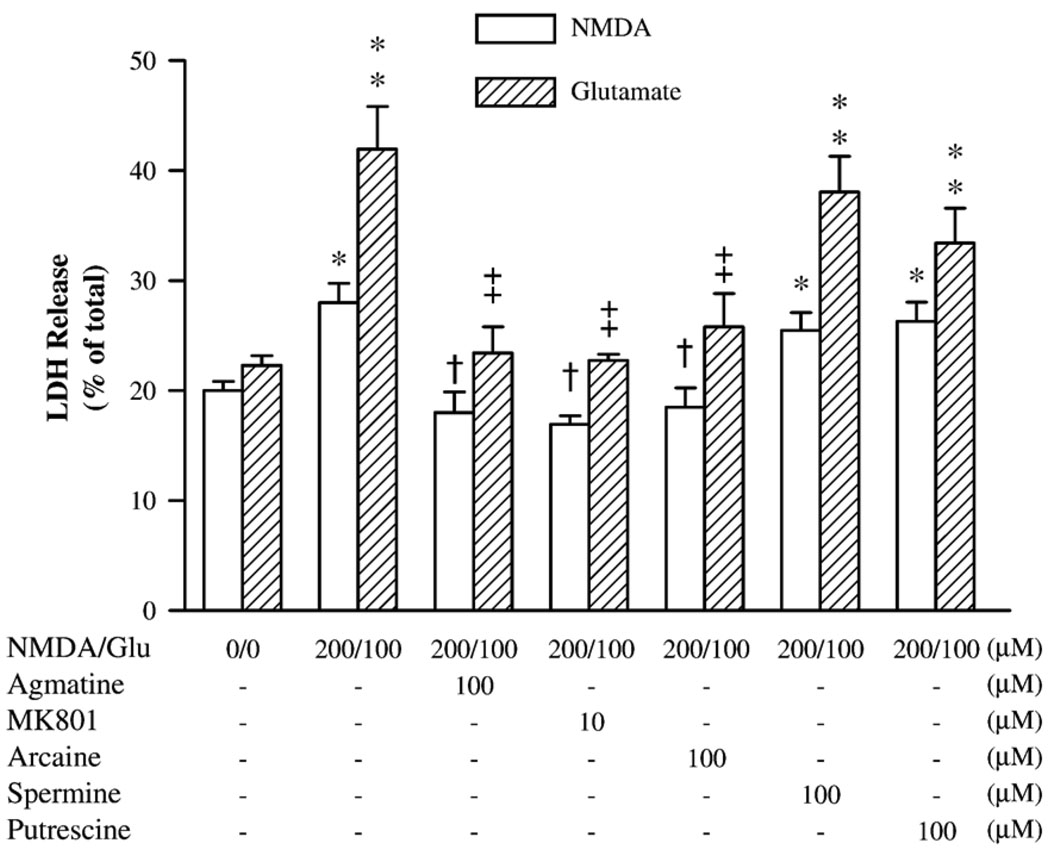
In these experiments, cultured hippocampal neurons were exposed to 200 µM NMDA or 100 µM glutamate in combination with either 100 µM agmatine, 10 µM MK801, 100 µM arcaine, spermine or putrescine by the same paradigm as described above. As illustrated in Fig. 2, 100 µM agmatine prevented the increase in LDH activity produced by 200 µM NMDA or 100 µM glutamate. In like manner, 10 µM MK801 fully abolished the NMDA- or glutamate-induced LDH increase (F6,41 = 7.09, P < 0.01), as did 100 µM arcaine, an agmatine analog with similar structures. However, spermine, an endogenous polyamine, and putrescine, a metabolic product of agmatine, both without the guanidino group, failed to prevent the increased LDH activity (F6,41 = 9.14, P < 0.01), after co-incubation with NMDA or glutamate.
Fig. 2.
The comparative neuroprotective effects of agmatine, MK801, arcaine, spermine, and putrescine against cell damage caused by NMDA or glutamate. Data are shown as mean ± SEM of 6 measurements. *P < 0.05, **P < 0.01, compared to the controls (0). †P < 0.05, compared to the group treated with 200 µM NMDA; ‡P < 0.01, compared to the group treated with 100 µM glutamate (Glu).
Similar effects between agmatine and MK801 were also evident in the studies of immunocytochemical staining and TUNEL assay. Co-incubation of NMDA with MK801 or agmatine prevented neuronal death (Figs. 3C and D) and significantly reduced TUNEL-labeled cells (Figs. 4C and D). This protective effect was substantial: the ratios of TUNEL-positive to TUNEL-negative cell numbers counted in the cultures that were co-incubated with NMDA and agmatine or MK801 cultures were 1.67 ± 0.65 and 0.65 ± 0.14, respectively, which were significantly lower than those of NMDA-treated cultures (7.10 ± 0.70) (F2,16 = 32.26, P < 0.01) (Fig. 4E).
3. Discussion
Since the last decade, a large body of in vitro and in vivo experimental evidence has demonstrated the neuroprotective effects of agmatine. This neuroprotective effect has been hypothesized to be related to its ability to block heteromeric NMDA receptor channels (Yang and Reis, 1999; Olmos et al., 1999). In the present study, 100 µM agmatine completely abolished the increased LDH levels and prevented neuronal death caused by NMDA or glutamate. This effect was shared by MK801, a non-competitive NMDA receptor antagonist, and arcaine, a synthetic analog of agmatine with two terminal guanidino groups required to block the NMDA receptor channels, but not by spermine and putrescine, two endogenous polyamines with no guanidino group. Our results extend previous observations and confirm that one mechanism of agmatine's neuroprotective effects is through its ability to block NMDA receptor channels.
While many studies support the neuroprotective role of agmatine, exposure of rat cerebellar granule neurons to 200–800 µM agmatine was found to result in cell death (Abe et al., 2003). The discrepancy between that study and ours may be in part due to different experimental conditions. It has been shown that agmatine exerted neurotoxicity in medium with high K+ (up to 27.5 mM) but not low K+ (under 10 mM) because high concentrations of K+ in the medium may trigger exocytosis and lead to release of glutamate from neurons. The K+ concentration in our exposure medium is less than 6 mM. Also, the highest concentration of agmatine used in the current study is 100 µM, a concentration that has been demonstrated to block NMDA receptor channels in rat hippocampal neurons (Yang and Reis, 1999). Nevertheless, our experiments and those of Abe et al. (2003) do suggest that whether agmatine exerts neuroprotection or neurotoxicity partly depends on the experimental conditions, including the concentrations of K+ and agmatine applied.
The ability of NMDA to induce apoptosis in cultured hippocampal neurons has been well documented (Diebaili et al., 2002; Vincent et al., 2002; Kajta et al., 2004; Prehn, 1996; Lee et al., 2003). Therefore, the NMDA induced neuronal death observed in the present study might also be due to apoptosis. Our TUNEL assay shows that exposure of cultured hippocampal neurons to NMDA significantly increased TUNEL-positive cell numbers, which were prevented by co-incubation with agmatine or MK801, suggesting that agmatine may exert its neuroprotective effect through its anti-apoptotic property. However, more study is needed to categorically define the anti-apoptotic characteristic of agmatine.
In conclusion, it is interesting to note that LDH measurements, immunocytochemical staining and TUNEL assay correlated well to demonstrate that agmatine fully protects hippocampal neurons against neurotoxicity induced by exposure to NMDA or glutamate. As glutamate-induced hippocampal dysfunction is implicated in seizure, Alzheimer's disease and major depression, the neuroprotection of agmatine against NMDA/glutamate induced excitotoxic insults has broad implications for potential new therapeutic strategies for these diseases.
4. Experimental procedures
4.1. Chemicals and reagents
Agmatine sulfate salt, N-methyl-d-aspartic acid (NMDA), l-glutamate acid monosodium salt, dizocilpine hydrogen maleate (MK801), arcaine sulfate salt, spermine dehydrochloride and putrescine dihydrochloride were purchased from Sigma Chemical Co. (St. Louis, MO).
4.2. Culture of hippocampal neurons
Hippocampi were dissected out from the brains of 18-day fetuses of Long Evans rats and placed in Hanks' balanced salt solution (HBSS) without Ca2+ and Mg2+ (Gibco BRL, Grand Island, NY) containing 1 mM sodium pyruvate and 10 mM HEPES. Then the hippocampal tissues were dissociated in HBSS solution containing 0.125% trypsin solution and 0.1 mg/ml deoxyribonuclease for 15 min at 37 °C. Subsequently, tissues were triturated by repeated passage through a constricted Pasteur pipette. The dispersed tissues were allowed to settle for 3 min. The supernatant was transferred to a fresh tube and centrifuged at 2000 rpm for 90 s. The pellet was resuspended in a neuron-defined culture medium, serum-free Neurobasal medium (Gibco BRL, Grand Island, NY), supplemented with B-27, 0.5 mM l-glutamine, 100 IU/ml penicillin, 100 mg/ml streptomycin and 25 µM glutamate. Trypan blue-excluding cells were counted, and cells were then plated onto 6-well plates coated with poly-d-lysine (100 µg/ml; BD Biosciences, Bedford, MA) at 2.5–3 × 105 per well. Cell cultures were kept in a humidified atmosphere of 95% air and 5% CO2 at 37 °C. Half of the medium was replaced with fresh medium without glutamate every 3–4 days. For immunocytochemical staining and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate (dUTP) nick end-labeling (TUNEL) assay, a 13-mm glass coverslip coated with poly-d-lysine (100 µg/ml) was placed in the middle of each well in 12-well plates which were not coated. 1 × 105 cells were plated onto each well. Cells adhered to and grew on the coverslip. The medium was changed as previously done for the 6-well plates.
4.3. Drugs exposures
The experiments to investigate the effect of agmatine on NMDA- or glutamate-induced cell damage were conducted using 12-day primary hippocampal cultures. The neuronal cultures were washed twice with Mg2+-free, HEPES-buffered saline (HBS, 146 mM NaCl, 10 mM HEPES, 2 mM CaCl2, 5 mM KCl, 10 mM d-glucose, pH 7.4) and incubated in the same solution in the absence (control) or presence of different concentrations of NMDA or glutamate, alone or in combination with agmatine (1–100 µM), MK801 (10 µM), arcaine (100 µM), spermine (100 µM) or putrescine (100 µM), for 1 h. The choice of 100 µM concentration for these polyamines is based on previously published data that 100 µM agmatine blocked NMDA receptor channels, but same concentration of putrescine or spermine failed to do so (Yang and Reis, 1999). However, the real concentrations of these polyamines in the hippocampus are different: agmatine 4.7 nmol/g wet wt (Feng et al., 1997), putrescine 7.1 nmol/g wet wt and spermine 213 nmol/g wet wt (Seiler and Schmidt-Glenewinkel, 1975). Therefore, the concentrations used in our in vitro experiment serve only for pharmacological comparisons. After two washes with fresh HBS solution, cells were subsequently re-incubated in culture media lacking any drugs for an additional period of 23 h. At the end of exposure, cultured neurons were harvested and LDH activity in the medium and cells measured. Cultured neurons used for immunocytochemical staining or TUNEL assay were treated the same way. The coverslips, where neurons were growing, were washed twice with Dulbecco's phosphate–saline (PBS) and fixed in 4% paraformaldehyde. Immunocytochemical staining or TUNEL assay was performed immediately on the fixed cultures.
4.4. Lactate dehydrogenase (LDH) assay
The release of LDH, a widely used index of cellular injury (Koh and Choi, 1987), was measured using a Cytotoxicity Detection kit (LDH) (Roche Diagnostics Corporation, Indianapolis, IN) according to manufacturer's instructions. Briefly, culture medium was collected and immediately centrifuged to remove any cells and debris. The cells were collected into microfuge tubes by adding 1 ml PBS, and cell homology was prepared by ultra-sonication for 25 s twice and centrifuging at 14,000 rpm for 4 min to remove cellular debris. LDH activities in the medium and cell homology were measured based on the protocol from the kit using a Microplate Reader (Bio-Rad, Hercules, CA). Results are expressed as percentage of LDH released into the medium compared to total LDH (medium + cells).
4.5. Immunocytochemical staining for β-tubulin III (βT-III)
Immunocytochemical staining was performed using a monoclonal antibody to βT-III (Chemicon, Temecula, CA). After fixing with 4% paraformaldehyde, coverslips bearing neuronal cultures were preincubated in 5% bovine serum in PBS supplemented with 0.2% Triton-X 100 for 1 h at room temperature, followed by incubation in primary antibody (1:500 dilution, in PBS containing 0.2% Triton-X 100) overnight at 4 °C. The following day, binding of βT-III antibody was detected with a biotinylated secondary antibody using ABC kit (Vector Laboratories, Burlingame, CA) according to the instructions of the manufacturer. 3,3-Diaminobenzidine tetra-hydrochloride (DAB) was used as the substrate. Staining for βT-III was then visualized and analyzed microscopically. To verify the concurrent presence of glial cells in the culture, double immunocytochemical staining for βT-III and glial fibrillary acidic protein (GFAP) was performed on some coverslips bearing neuronal cultures using a protocol of Multiple Antigen Labeling (Vector Laboratories, Burlingame, CA).
4.6. Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate (dUTP) nick end-labeling (TUNEL) assay
DNA damage in dying cells was identified using ApopTag Peroxidase In situ Apoptosis Detection kits (Chemicon, Temecula, CA). Briefly, coverslips bearing neuronal cultures were incubated in an equilibration buffer (30 min) followed by addition of terminal deoxynucleotidyl transferase (TdT) and digoxigenin–dUTP reaction buffer (60 min) at 37 °C. Coverslips were then washed in stop/wash buffer for 10 min at 37 °C, followed by incubation in anti-digoxigenin antibody conjugated to peroxidase for 30 min at room temperature. The digoxigenin–dUTP–peroxidase complex was visualized by reacting with DAB to generate a brown reaction product. Negative controls were performed by substituting distilled water for TdT in the working solution. TUNEL assay was then analyzed microscopically. Using a 10 × 10 reticule and 20× objective, the total numbers of TdT-positive cells were counted in five randomly chosen fields on each treated and control coverslip. The results are expressed as ratio of TdT-positive to TdT-negative cell numbers in 5–6 coverslips.
4.7. Statistics
Data are presented as means ± SEM values and analyzed by analysis of variance (ANOVA) using single-factor ANOVA (SuperANOVA program, Abacus concepts, Berkeley, CA). In the presence of significant F values, individual comparisons between means were made using the Student–Newman–Keuls test. For analyzing of the quantitative results of TUNEL labeling, original data which were expressed as ratio of TUNEL-positive to TUNEL-negative cells in coverslips were first converted to arcsin values using Excel program. Then these arcsin values were analyzed using one-way ANOVA as described above.
Acknowledgment
This work is supported by NIH grant RR17701.
Abbreviations
- ANOVA
analysis of variance
- DAB
3,3-diaminobenzidine tetrahydeochloride
- LDH
lactate dehydrogenase
- βT-III
β-tubulin III
- GFAP
glial fibrillary acidic protein
- MK801
dizocilpine hydrogen maleate
- NMDA
N-methyl-d-aspartate
- TUNEL
terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate (dUTP) nick end-labeling
REFERENCES
- Abe K, Abe Y, Saito H. Agmatine induces glutamate release and cell death in cultured rat cerebellar granule neurons. Brain Res. 2003;990:165–171. doi: 10.1016/s0006-8993(03)03454-1. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented neurobasal. A new serum-free medium combination. J. Neurosci. Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Diebaili M, De Bock F, Baille V, Bockaert J, Rondouin G. Implication of p53 and caspase-3 in kainic acid but not in N-methyl-d-aspartic acid-induced apoptosis in organotypic hippocampal mouse cultures. Neurosci. Lett. 2002;327:1–4. doi: 10.1016/s0304-3940(02)00137-4. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T. The hippocampus—What does it do? Behav. Neural Biol. 1992;57:2–36. doi: 10.1016/0163-1047(92)90724-i. [DOI] [PubMed] [Google Scholar]
- Fairbanks CA, Schreiber KM, Brewer KL, Yu CG, Stone LS, Kitto KF, Nguyen HO, Grocholski BM, Shoeman DW, Kehl LJ, Regunathan S, Reis DJ, Yezierski RP, Wilcox GL. Agmatine reverses pain induced by inflammation neuropathy, and spinal cord injury. Proc. Natl. Acad. Sci. U. S. A. 2000;97:10584–10589. doi: 10.1073/pnas.97.19.10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Halaris AE, Piletz JE. Determination of agmatine in brain and plasma using high performance liquid chromatography with fluorescence detection. J. Chromatogr. 1997;691:277–286. doi: 10.1016/s0378-4347(96)00458-6. [DOI] [PubMed] [Google Scholar]
- Galea E, Regunathan S, Eliopoulos V, Feinstein DL, Reis DJ. Inhibition of mammalian nitric oxide synthase by agmatine. An endogenous polyamine formed by decarboxylation of arginine. Biochem. J. 1996;316:247–249. doi: 10.1042/bj3160247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad GM, Salame K, Rabey JM, Gilad VH. Agmatine treatment is neuroprotective in rodent brain injury models. Life Sci. 1996;58:PL41–PL46. doi: 10.1016/0024-3205(95)02274-0. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic–pituitary–adrenocortical axis. Endocr. Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Kajta M, Lason W, Kupiec T. Effects of estrone on N-methyl-d-aspartic acid- and staurosporine-induced changes in caspase-3-like protease activity and lactate dehydrogenase-release: time- and tissue-dependent effects in neuronal primary cultures. Neuroscience. 2004;123:515–526. doi: 10.1016/j.neuroscience.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Kim JH, Yenari MA, Giffard RG, Cho SW, Park KA, Lee JE. Agmatine reduces infarct area in a mouse model of transient focal cerebral ischemia and protects cultured neurons from ischemia-like injury. Exp. Neurol. 2004;189:122–130. doi: 10.1016/j.expneurol.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Koh JY, Choi DW. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflus assay. J. Neurosci. Methods. 1987;20:83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- Kure S, Tominaga T, Yoshimoto T, Tada K, Narisawa K. Glutamate triggers internucleosomal DNA cleavage in neuronal cells. Biochem. Biophys. Res. Commun. 1991;179:39–45. doi: 10.1016/0006-291x(91)91330-f. [DOI] [PubMed] [Google Scholar]
- Lee J, Son D, Lee P, Kim S, Kim H, Kim C, Lim E. Alkaloid fraction of Uncaria rhynchophylla protects against N-methyl-d-aspartate-induced apoptosis in rat hippocampal slices. Neurosci. Lett. 2003;348:51–55. doi: 10.1016/s0304-3940(03)00613-x. [DOI] [PubMed] [Google Scholar]
- Li G, Regunathan S, Barrow C, Barrow CJ, Eshraghi J, Cooper R, Reis DJ. Agmatine: an endogenous clonidine-displasing substance in the brain. Science. 1994;263:966–969. doi: 10.1126/science.7906055. [DOI] [PubMed] [Google Scholar]
- Loring RH. Agmatine act as an antagonist of neuronal nicotinic receptors. Br. J. Pharmacol. 1990;99:207–211. doi: 10.1111/j.1476-5381.1990.tb14680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lortie MJ, Novontny WF, Peterson OW, Vallon V, Malvey K, Mendonca M, Satriano J, Insel P, Thomson SC, Blantz RC. Agmatine. a bioactiver metabolite of arginine. J. Clin. Invest. 1996;97:413–420. doi: 10.1172/JCI118430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos G, DeGregrio-Rocasolano N, Regalado MP, Gasull T, Boronat MA, Trullas R, Villarroel A, Lerma J, Garcia-Sevilla JA. Protection by imidazoline drugs and agmatine of aglutamate-induced neurotoxicity in cultured cerebellar granule cells through blockade of NMDA receptor. Br. J. Pharmacol. 1999;127:1317–1326. doi: 10.1038/sj.bjp.0702679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portera-Cailliau C, Hedreen JC, Price DL, Koliatsos W. Evidence for apoptotic cell death in Huntington disease and excitotoxic animal models. J. Neurosci. 1995;15:3775–3787. doi: 10.1523/JNEUROSCI.15-05-03775.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehn JHM. Marked diversity in the action of growth factors on N-methyl-d-aspartate-induced neuronal degeneration. Eur. J. Pharmacol. 1996;306:81–88. doi: 10.1016/0014-2999(96)00225-7. [DOI] [PubMed] [Google Scholar]
- Reis DJ, Yang XC, Milner TA. Agmatine containing axon terminals in rat hippocampus from synapses on pyramidal cells. Neurosci. Lett. 1998;250:185–188. doi: 10.1016/s0304-3940(98)00466-2. [DOI] [PubMed] [Google Scholar]
- Sastre M, Regunathan S, Reis DJ. Uptake of agmatine into rat brain synaptosomes: possible role of cation channels. J. Neurochem. 1997;69:2421–2426. doi: 10.1046/j.1471-4159.1997.69062421.x. [DOI] [PubMed] [Google Scholar]
- Seiler N, Schmidt-Glenewinkel T. Region of putrescine, spermidine and spermine in relation to the distribution of RNA and DNA in the rat nervous system. J. Neurochem. 1975;24:791–795. [PubMed] [Google Scholar]
- Tabor CW, Tabor H. Polyamines. Annu. Rev. Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Vincent VAM, Robinson CC, Simsek D, Murphy M., Jr Macrophage colony stimulating factor prevents NMDA-induced neuronal in hippocampal organotypic cultures. J. Neurochem. 2002;82:1388–1397. doi: 10.1046/j.1471-4159.2002.01087.x. [DOI] [PubMed] [Google Scholar]
- Yang XC, Reis DJ. Agmatine selectively blocks the NMDA subclass of glutamate receptor channels in cultured mouse hippocampal neurons. J. Pharmacol. Exp. Ther. 1999;288:544–549. [PubMed] [Google Scholar]
- Zhu MY, Piletz JE, Halaris A, Regunathan S. Effect of agmatine against cell death induced by NMDA and glutamate in neurons and PC12 cells. Cell. Mol. Neurobiol. 2003;23:865–872. doi: 10.1023/A:1025069407173. [DOI] [PMC free article] [PubMed] [Google Scholar]