Abstract
Functional magnetic resonance imaging (fMRI) studies have shown that repetition priming of visual objects is typically accompanied by a reduction in activity for repeated compared to new stimuli (repetition suppression). However, the spatial distribution and direction (suppression vs. enhancement) of neural repetition effects can depend on the pre-experimental familiarity of stimuli. The first goal of this study was to further probe the link between repetition priming and repetition suppression/enhancement for visual objects and how this link is affected by stimulus familiarity. A second goal was to examine whether priming of familiar and unfamiliar objects following a single stimulus repetition is supported by the same processes as priming following multiple repetitions within the same task. In this endeavor, we examined both between and within subjects correlations between priming and fMRI repetition effects for familiar and globally unfamiliar visual objects during the first and third repetition of the stimuli. We included reaction time of individual trials as a linear regressor to identify brain regions whose repetition effects varied with response facilitation on a trial-by-trial basis. The results showed that repetition suppression in bilateral fusiform gyrus, was selectively correlated with priming of familiar objects that had been repeated once, likely reflecting facilitated perceptual processing or the sharpening of perceptual representations. Priming during the third repetition was correlated with repetition suppression in prefrontal and parietal areas for both familiar and unfamiliar stimuli, possibly reflecting a shift from top-down controlled to more automatic processing that occurs for both item types.
Keywords: priming, neural repetition suppression, neural repetition enhancement, fMRI, stimulus familiarity
1. Introduction
Repetition priming refers to the improvement in the speed and/or accuracy with which subjects respond to the repeated compared to the first presentation of stimuli. It is typically accompanied by repetition suppression (RS), a reduction in the neural response to repeated relative to new items (for reviews, see Grill-Spector, Henson, & Martin, 2006; Henson, 2003). As both repetition priming and RS are repetition-related phenomena and neither depends on explicit memory retrieval, it has been conjectured that RS reflects the neural plasticity that causes repetition priming (Henson, 2003; Wiggs & Martin, 1998).
In support of the causal relationship between RS and repetition priming, a transcranial magnetic stimulation study showed that RS in left inferior frontal cortex is necessary for semantic priming (Wig, Grafton, Demos, & Kelley, 2005). Furthermore, several neuroimaging studies have demonstrated correlations between the magnitude of repetition priming and RS in prefrontal cortex (e.g., Bunzeck, Schutze, & Duzel, 2006; Dobbins, Schnyer, Verfaellie, & Schacter, 2004; Lustig & Buckner, 2004; Maccotta & Buckner, 2004; Orfanidou, Marslen-Wilson, & Davis, 2006; Turk-Browne, Yi, & Chun, 2006), further supporting the link between these two phenomena.
Despite this evidence, the precise relationship between repetition priming and RS remains unclear because RS in at least some brain regions does not appear to be correlated with repetition priming across subjects or conditions (e.g., Habeck, Hilton, Zarahn, Brown, & Stern, 2006; Henson, Rylands, Ross, Vuilleumeir, & Rugg, 2004; Maccotta & Buckner, 2004; Race, Shanker, & Wagner, 2009; Reber, Gitelman, Parrish, & Mesulam, 2005; Sayres & Grill-Spector, 2006). A few studies have reported dissociations between priming and RS, suggesting that RS in some brain regions may not be sufficient for behavioral priming (Sayres & Grill-Spector, 2006; Xu, Turk-Browne, & Chun, 2007). Furthermore, some studies found that repetition priming of unfamiliar stimuli can be accompanied by increases in neural activity for the repeated material, also known as repetition enhancement (RE). Such RE effects have been reported in some of the same brain regions that show RS for familiar stimuli (Fiebach, Gruber, & Supp, 2005; Henson, Shallice, & Dolan, 2000; Schacter et al., 1995; Soldan, Zarahn, Hilton, & Stern, 2008; Thiel, Henson, & Dolan, 2002). Two of these studies only reported RE for the unfamiliar stimuli (Fiebach et al., 2005; Schacter et al., 1995), suggesting that not only RS, but also RE may be related to repetition priming. Others have reported RS, but not RE for unfamiliar objects, but only in a subset of the areas that showed RS for familiar objects (Vuilleumier, Henson, Driver, & Dolan, 2002). Taken together, these studies suggest that qualitatively different spatial networks and/or processes (RS and RE) may underlie priming for familiar and unfamiliar visual objects. Notably, however, brain regions demonstrating differential neural repetition effects as a function of stimulus familiarity have not been linked to behavioral priming. This leaves open the question of whether neural activity in these brain regions is indeed related to the behavioral expression of priming or simply co-occurs with it. Therefore, the first goal of this study was to further probe the link between repetition priming and RS/RE for visual objects and how this link is affected by the pre-experimental familiarity of the stimuli.
The second theoretical question we tried to address was whether single-exposure priming effects in object-decision tasks are indeed based on different underlying processes (i.e., mainly perceptual) than priming following multiple repetitions (mainly stimulus-response associations). This idea was proposed in a recent review article but has not yet been directly examined in a single experiment (Stevens, Wig, & Schacter, 2008). Thus, based on the finding that correlations between RS and priming are most robustly found in prefrontal but not occipital-temporal brain regions for familiar stimuli, Schacter, Wig, and Stevens (2007) suggested that priming effects are driven in a top-down manner by repetition effects in prefrontal regions. These prefrontal RS effects are thought to reflect a reduction in cognitive control mechanisms and increased reliance on automatic processing that occurs with stimulus and task repetition. Because in the current experiment, subjects performed the same task on repeated stimuli, such a reduction in top-down control mechanisms would be expected to occur for both the familiar and unfamiliar items and they would be expected to correlate with priming for both item types. Furthermore, these effects would be expected to be more prominent after multiple repetitions of an object than after a single repetition because automatization increases with additional repetitions.
At the same time, to the extent that perceptual, lexical, or semantic learning contributes to the observed behavioral priming effects, additional brain regions would be expected to show correlations between neural repetition effects and priming and these brain regions might differ for the familiar and unfamiliar items. For example, RS in occipital-temporal areas for familiar stimuli might reflect a sharpening of existing perceptual representations (Wiggs & Martin, 1998). RE in the same or other areas might reflect the formation of new perceptual, semantic, or lexical representations (Henson et al., 2000), which may also contribute to repetition priming effects. These perceptual and possibly lexical or semantic priming effects might be more evident following a single stimulus repetition, when learning is maximal and contribute progressively less to priming as the number of repetitions increases.
In order to investigate these questions, we re-analyzed the data from a previously published study that reported evidence for qualitatively different neural repetition effects as a function of stimulus familiarity (Soldan et al., 2008). Specifically, this study identified one spatial network of regions where both familiar and globally unfamiliar visual objects contributed similar repetition effects and a second spatial network where the familiar objects contributed an RS effect and the unfamiliar objects contributed an RE effect. Because the behavioral priming effects for the familiar and unfamiliar stimuli in this study were similar, but the neural repetition effects appeared to be qualitatively different, this data set is ideal for testing the hypothesis that distinct brain regions and neural processes (RS vs. RE) correlate with repetition priming for familiar and unfamiliar visual objects. Another advantage for using this data set is that all stimuli were presented four times throughout the experiment, allowing us to examine whether single repetition priming is associated with the same processes as multiple repetition priming.
In an effort to more directly assess the relation between behavioral and neural repetition effects, the current study not only tested for between-subjects correlations between priming and RS/RE but also for within-subjects correlations. Specifically, we examined correlations at the first level by modeling intra-individual variance in fMRI signal that varied linearly with trial-to-trial variance in correct RTs (see method). This provides another test of the hypothesized relationship between behavioral and neural processes. Moreover, if a particular brain region shows both a within and a between subjects correlation with repetition priming, it strengthens the conclusion that activity in this region is indeed related to behavioral priming.
In the current experiment, subjects saw an intermixed series of familiar and globally unfamiliar visual objects (see Figure 1). On each trial, the subjects' task was to judge whether each stimulus depicted a real or a non-real object. Each stimulus was presented four times. The stimulus repetitions occurred after a relatively short lag (2, 4, or 6 intervening items) to increase the magnitude of both priming and neural repetition effects. Behavioral and neural repetition effects as well as their relationship were assessed separately for the first and third repetition of stimuli and separately for the familiar and globally unfamiliar items. Specifically, priming was measured as the difference in reaction time to make the real/non-real decision for the first compared to the second presentation of stimuli and for the first compared to the fourth presentation of stimuli. Reliable priming at the within-subject level was detected in the majority of subjects for both familiar and unfamiliar stimuli, allowing us to validly examine within and between subjects correlations between priming and neural repetition effects.
Figure 1.

Examples of the types of stimuli used in this study.
2. Results
2.1 Behavioral performance
Overall accuracy in the task was very high [Mean familiar objects = 98.8, range = 96.8 − 100; Mean unfamiliar objects = 98.5, range = 94.9 − 100]. A repeated-measures analysis of variance (ANOVA) with object type (familiar vs. unfamiliar) and presentation (4 levels) as within-subject factors showed no significant effects of presentation and object type on classification accuracy [all p > 0.1]. Therefore, accuracy was not considered further.
Subjects' mean RT for familiar and unfamiliar objects across all four presentations is shown in Figure 2. An ANOVA on the RT values revealed main effects of presentation [F(3, 39) = 181.59, p < 0.0001], object type [F(1, 13) = 42.40, p < 0.0001], and an interaction between object type and presentation [F(3, 39) = 5.41, p = 0.014, Greenhouse-Geisser corrected]. The interaction reflected the fact that there was slightly more priming for the unfamiliar than the familiar items. However, when baseline differences in RT between stimulus types were taken into account by computing proportional priming scores, there was no difference in priming for the familiar and unfamiliar objects (and the interaction was not significant, p = 0.7). Importantly, all subjects except for two demonstrated reliable priming (as measured as the difference in RT from presentation 1 to 2 and 1 to 4) for both item types [all p < 0.05 two-tailed]. Specifically, one subject did not show reliable priming for the familiar objects at presentation 2 and another subject did not show priming for the unfamiliar items at presentations 2 and 4.
Figure 2.
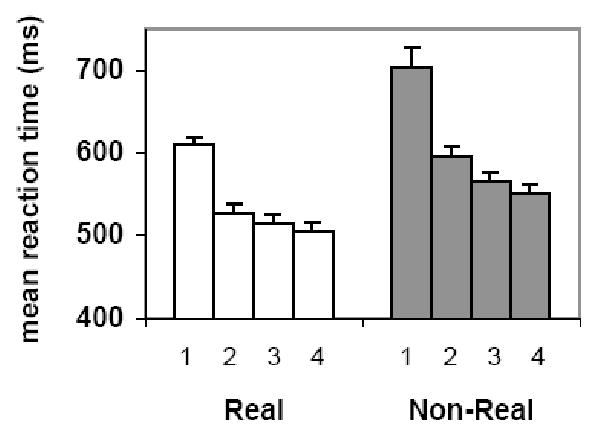
Behavioral Results. Mean reaction time (on y-axis) for classifying familiar and unfamiliar objects as a function of presentation number (on x-axis). Error bars represent the standard error of the mean.
2.2. fMRI Results: Neural repetition effects
Because the focus of this investigation was on correlations between priming and RS/RE, main effects of repetition are discussed only briefly (for more information see Soldan et al., 2008). Regions showing a significant neural repetition effect from presentation 1 to 2 and 1 to 4 for the familiar and unfamiliar stimuli are listed in Table 1. Consistent with many prior studies, the repetition of familiar items produced RS throughout occipital-temporal cortex, as well as in prefrontal and parietal regions (Henson, 2003). Repetition-related increases in neural activity were not observed for the familiar stimuli at this threshold.
Table 1. Neural repetition effects from the first to the second and from the first to the fourth presentation of a stimulus.
| Structure | BA | Z-score | k | X | Y | Z | 2nd | 4th |
|---|---|---|---|---|---|---|---|---|
| Repetition suppression for familiar objects | ||||||||
| R inferior frontal gyrus | 46, 47 | 4.61 | 40 | 36 | 13 | ✓ | ✓ | |
| 9, 45 | 4.40 | 53 | 13 | 20 | ✓ | ✓ | ||
| L inferior frontal gyrus | 9, 46 | 3.72 | 358 | -42 | 7 | 31 | ✓ | |
| R precentral gyrus | 6, 9 | 4.73 | 788 | 38 | 2 | 31 | ✓ | ✓ |
| R medial frontal gyrus | 6, 8 | 4.69 | 1085 | 6 | 27 | 41 | ✓ | ✓ |
| R middle frontal gyrus | 6 | 3.72 | 120 | 26 | 3 | 62 | ✓ | |
| L middle frontal gyrus | 46 | 3.56 | 237 | -44 | 30 | 17 | ✓ | |
| R superior frontal gyrus | 8, 9 | 3.41 | 136 | 2 | 32 | 48 | ✓ | |
| R postcentral gyrus | 2, 40 | 4.51 | 537 | 52 | -23 | 49 | ✓ | ✓ |
| R inferior parietal lobule | 40 | 4.00 | 48 | -46 | 59 | ✓ | ✓ | |
| R superior parietal lobule | 7 | 4.32 | 1097 | 28 | -66 | 42 | ✓ | ✓ |
| L superior parietal lobule | 7 | 3.53 | -30 | -55 | 56 | ✓ | ||
| L precuneus | 7 | 3.90 | 140 | -22 | -68 | 40 | ✓ | ✓ |
| R precuneus | 7 | 4.71 | 328 | 22 | -60 | 51 | ✓ | |
| L fusiform gyrus | 20 | 4.17 | -34 | -42 | -15 | ✓ | ||
| 19, 37 | 4.10 | 971 | -38 | -57 | -11 | ✓ | ✓ | |
| R fusiform gyrus | 19, 37 | 3.94 | 807 | 26 | -66 | -8 | ✓ | ✓ |
| L inferior temporal gyrus | 37 | 4.35 | -42 | -64 | -5 | ✓ | ||
| L parahippocampal gyrus | 19 | 4.17 | -24 | -55 | -6 | ✓ | ||
| R lingual gyrus | 19 | 3.94 | 32 | -62 | -4 | ✓ | ✓ | |
| L middle occipital gyrus | 19 | 4.96 | 753 | -24 | -87 | 8 | ✓ | |
| R middle occipital gyrus | 18, 19 | 4.30 | 26 | -84 | -4 | ✓ | ✓ | |
| L superior occipital gyrus | 19 | 4.54 | -32 | -78 | 24 | ✓ | ||
| R cuneus | 18 | 3.59 | 6 | -89 | 10 | ✓ | ||
| R cingulate gyrus | 24, 32 | 4.37 | 6 | 0 | 37 | ✓ | ✓ | |
| L insula | 13 | 5.24 | 278 | -34 | 21 | 1 | ✓ | ✓ |
| R insula/ inf. frontal gyrus | 13 | 5.15 | 737 | 40 | 22 | 6 | ✓ | ✓ |
| R claustrum | 5.10 | 160 | 36 | -12 | -4 | ✓ | ✓ | |
| R cerebellum | 5.14 | 84 | 18 | -59 | -12 | ✓ | ||
| Repetition enhancement for familiar objects | 2nd | 4th | ||||||
| N/A | ||||||||
| Repetition suppression for unfamiliar objects | 2nd | 4th | ||||||
| R inferior frontal gyrus | 45, 46 | 4.69 | 141 | 51 | 31 | 4 | ✓ | ✓ |
| 9 | 4.12 | 129 | 44 | 3 | 26 | ✓ | ||
| L inferior frontal gyrus | 9, 45 | 4.21 | 206 | -40 | 5 | 24 | ✓ | |
| L insula | 13 | 4.50 | 64 | -36 | 18 | 1 | ✓ | |
| R superior parietal lobule | 7 | 3.98 | 58 | 28 | -52 | 40 | ✓ | ✓ |
| L superior parietal lobule | 7 | 3.56 | 84 | -24 | -68 | 46 | ✓ | |
| R precuneus | 19 | 3.81 | 120 | 30 | -68 | 31 | ✓ | |
| L fusiform gyrus | 37 | 4.11 | 61 | -41 | -43 | -10 | ✓ | ✓ |
| R fusiform/ inferior temporal gyrus | 19, 37 | 3.78 | 164 | 46 | -62 | -4 | ✓ | |
| L middle occipital gyrus | 18, 19 | 3.68 | 90 | -34 | -87 | 4 | ✓ | |
| Repetition enhancement for unfamiliar objects | 2nd | 4th | ||||||
| L superior frontal gyrus | 8 | 3.80 | 145 | -20 | 20 | 47 | ✓ | |
| R superior frontal gyrus | 10, 9, 8 | 3.83 | 52 | 12 | 65 | 17 | ✓ | |
| R medial frontal gyrus | 10 | 3.83 | 102 | 12 | 57 | 12 | ✓ | |
| L postcentral gyrus | 2 | 3.86 | 97 | -46 | -28 | 53 | ✓ | ✓ |
| 40 | 3.82 | 73 | -57 | -21 | 14 | ✓ | ||
| L precuneus | 31, 7 | 3.35 | 639 | -14 | -65 | 24 | ||
| R precuneus | 31 | 4.39 | 585 | 6 | -53 | 32 | ||
| L inferior parietal lobule | 39, 40 | 3.53 | 24 | -30 | -37 | 50 | ✓ | |
| R inferior parietal lobule | 40 | 4.55 | 248 | 51 | -51 | 36 | ||
| L superior temporal gyrus | 39 | 3.70 | 45 | -44 | -49 | 23 | ✓ | |
| L angular gyrus | 39 | 4.54 | 497 | -51 | -66 | 36 | ✓ | |
| L traverse temporal gyrus | 41 | 3.34 | 60 | -48 | -25 | 12 | ✓ | ✓ |
| L lingual gyrus | 18 | 3.70 | 26 | -20 | -78 | 0 | ✓ | |
| L insula | 13 | 3.43 | 24 | -36 | -24 | 18 | ✓ | |
| Object type by repetition interaction | 2nd | 4th | ||||||
| R superior frontal gyrus | 9 | 3.40 | 75 | 8 | 50 | 27 | ✓ | |
| R medial frontal gyrus | 10 | 4.05 | 130 | 10 | 58 | 14 | ✓ | |
| L postcentral gyrus | 2, 4 | 3.80 | 83 | -48 | -34 | 54 | ✓ | ✓ |
| L precuneus | 7 | 4.03 | 150 | -8 | -48 | 50 | ✓ | |
| R inferior parietal lobule | 40 | 3.85 | 74 | 55 | -52 | 45 | ✓ | |
| L inferior parietal lobule | 40 | 3.50 | 53 | -50 | -22 | 28 | ✓ | |
| R fusiform gyrus | 19 | 3.43 | 24 | 26 | -64 | -4 | ✓ | |
| R superior temporal gyrus | 22 | 4.15 | 23 | 44 | -48 | 12 | ✓ | ✓ |
| L superior temporal gyrus | 41 | 3.94 | 42 | -38 | -30 | 14 | ✓ | ✓ |
| L middle occipital gyrus | 18 | 3.77 | 50 | -26 | -93 | 12 | ✓ | |
| L insula | 13 | 4.08 | 188 | -34 | -5 | 17 | ✓ | ✓ |
Note. Clusters that exceeded an extent threshold of 50 voxels at p < 0.001, uncorrected, are reported, except for the repetition enhancement effect for unfamiliar objects at presentation 2 where a cluster threshold of k=20 was used. The reported coordinates are cluster maxima. Cluster sizes (in number of voxels) are reported in the column labeled “k”, except for regions that were part of larger clusters. A checkmark (✓) in one of the last two columns indicates that the listed region demonstrated a repetition effect at presentation 2 (i.e., column labeled ‘2nd’) or presentation 4 (i.e., column labeled ‘4th’). If an area showed a repetition effect at both presentations 2 and 4, the reported coordinates, cluster sizes, and z-score are those associated with presentation 2.
For the unfamiliar items, both RS and RE were observed (see Table 1). The only significant RS effects for unfamiliar objects from presentation 1 to 2 were found in the right inferior frontal gyrus (BA 45), right superior parietal lobule, left fusiform gyrus (BA 37), and a region near the left parahippocampal gyrus (BA 30). Significant RE for the unfamiliar stimuli from presentation 1 to 2 was detected in bilateral parietal regions, superior temporal cortex, the left insula, and the left lingual gyrus (BA 18). At a lower threshold (p<0.005, k = 10), additional RE effects were detected in parietal and superior temporal cortex but not in occipital-temporal cortex. There was only one region where RE from presentation 1 to 2 reversed to RS from presentation 2 to 4: the left postcentral gyrus in the parietal lobe (BA 2).
The observation that there were greater RS effects (in terms of magnitude and spatial extent) for the familiar than the unfamiliar stimuli from presentation 1 to 2 was confirmed by an interaction between repetition and object type in several brain areas, including the right inferior and middle frontal gyrus, right fusiform gyrus, left middle occipital gyrus, and bilateral insula (see Table 1 for a complete lists). Areas demonstrating an interaction between repetition and object type at presentation 2 mostly showed a decrease with repetition for the familiar objects and no detectable repetition effect, or an increase with repetition for the unfamiliar objects.
From presentation 1 to 4, significant RS was detected for the unfamiliar objects in many of the same regions as for the familiar stimuli, including bilateral fusiform gyrus, bilateral inferior frontal cortex, and parietal cortex. Nonetheless, a number of areas showed an interaction between object type and repetition at presentation 4 (Table 1). Some of these regions demonstrated a large RE effect for the unfamiliar stimuli and no reliable repetition effect for the familiar objects, including the right medial frontal gyrus, left precuneus, and bilateral inferior parietal lobule. Other areas showed a decrease with repetition for the familiar objects and no repetition effect or an increase with repetition for the unfamiliar objects.
2.3. fMRI Results: Brain-behavior correlations
In order to examine whether any of the observed neural repetition effects were related to behavioral priming, we first isolated regions that demonstrated a significant RS or RE effect for the familiar or unfamiliar items from presentation 1 to 2 and 1 to 4 at the group level (thresholded at p < 0.001, k = 50). Within these regions only, we tested for the existence of voxels that showed a reliable association with priming by inclusively masking them with the relevant SPMs representing the within and/or between-subjects correlation with priming (at p < 0.005, one-tailed, k = 20 voxels unless stated otherwise), separately for each object type. For the purpose of visualization, scatterplots were constructed that represent the correlation between subjects' priming scores and subjects' mean signal difference between the first and second (or first and fourth) presentation of a stimulus in a 5 mm sphere (∼81 voxels) around the voxel showing the maximum correlation as determined in the inclusive masking analysis (see Figures 3 - 5). Visual inspection of these scatterplots was used to exclude regions from report where the correlations were obviously driven by a single outlier.
Figure 3.
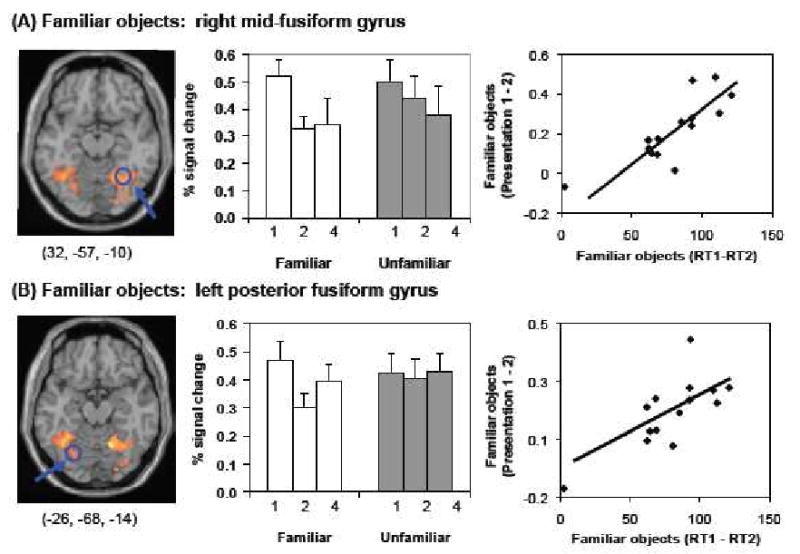
Brain regions showing between-subjects correlations between repetition suppression from presentation 1 to 2 and priming. For familiar objects, such correlations were detected in the right fusiform gyrus (A) and left fusiform gyrus (B). For unfamiliar objects, no such correlations were detected. The left panel shows areas demonstrating significant repetition suppression (contrast presentation 1 – 2, p < 0.001) for familiar objects. Areas showing the maximum correlation between repetition suppression and priming are circled in blue. Scatterplots of these correlations are displayed in the right panel. The middle panel shows mean fMRI activation for both types of objects at presentations 1, 2, and 4 at the coordinates displayed in the left panel. Note that both correlations were significant at the same threshold (right fusiform) or slightly lower threshold (left fusiform, p < 0.008) when the potential outlier was excluded from the GLM analysis.
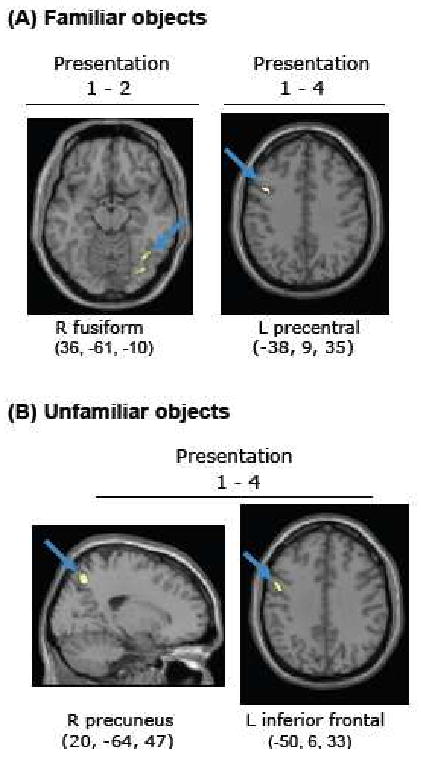
Figure 5.
Brain regions where the magnitude of repetition suppression (thresholded at p < 0.001) for familiar (A) and unfamiliar (B) objects was modulated by reaction time priming on a within-subjects basis are displayed. For familiar objects, we detected voxels in the right fusiform gyrus (A, left panel) where repetition suppression from presentation 1 to 2 correlated with priming at presentation 2 across subjects (at p < 0.05) and within subjects (at p < 0.05), combined probability p < 0.0000025. From presentation 1 to 4, repetition suppression in the left precentral gyrus (A, right panel) correlated with priming of familiar objects (at p < 0.005) and repetition suppression in the right precuneus (B, left panel) and left inferior frontal gyrus (B, right panel) correlated with priming of unfamiliar objects (at p < 0.005).
2.3.1. Familiar stimuli
For familiar objects, between-subjects correlations between RS from presentation 1 to 2 and priming were observed in right fusiform gyrus (BA 19, x = 32, y = -57, z = -10, k=92) and left fusiform gyrus (BA 19, x = -26, y = -68, z = -14, k = 49). Both of these regions were strongly activated relative to baseline during the first presentation of familiar objects, decreased in activation from presentation 1 to 2 but showed no further decrease from presentation 2 to 4. By comparison, there was no significant RS in these regions from presentation 1 to 2 for unfamiliar objects (Figure 3). Significant within-subject correlations between RS and priming at presentation 2 were not detected at this threshold. However, when we restricted the search volume to only those fusiform voxels showing both significant RS (at p < 0.001) and a between-subject correlation with priming (at p < 0.05, k=20), we detected an area in the right posterior fusiform gyrus where RS also weakly correlated with priming on a within-subjects basis (p < 0.05, combined probability p < 0.0000025; see Figure 6A).
From presentation 1 to 4, several frontal and parietal areas, but not occiptal-temporal regions, showed between-subjects correlations with priming from presentation 1 to 4. These were the right inferior frontal gyrus (BA 47, x = 38, y = 23, z = 0, k = 22), left precuneus (BA 7, x = -22, y = -68, z = 38), and right inferior parietal lobule (BA 40, x = 42, y = -40, z = 52). See Figure 4 for scatterplots. A significant within-subjects correlation between RS and priming was found in the left precentral gyrus (BA 9, x = -38, y = 9, z = 35, k = 23), see Figure 6A.
Figure 4.
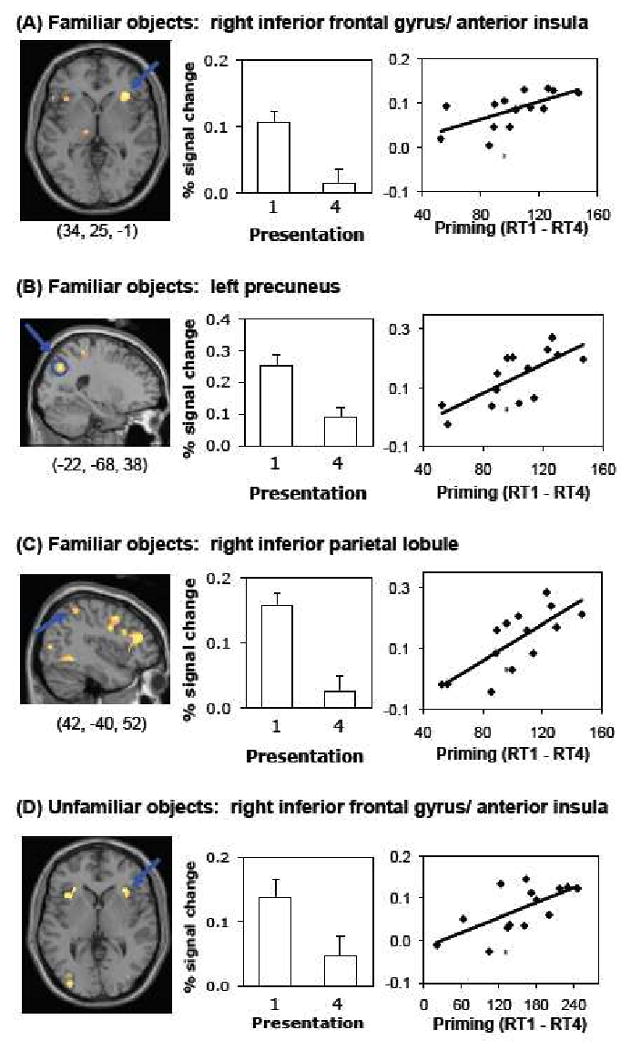
Brain regions showing between-subjects correlations between repetition suppression from presentation 1 to 4 and priming. Such correlations were detected in the right inferior frontal gyrus/anterior insula for both familiar (A) and unfamiliar objects (D) and in the left precuneus (B) and right inferior parietal lobule (C), for the familiar objects only. The left panel shows areas demonstrating significant repetition suppression (contrast presentation 1 – 4, p < 0.001) for the familiar items. Areas showing the maximum correlation between repetition suppression and priming are indicated by the blue arrow. Scatterplots of these correlations are displayed in the right panel. The middle panel shows mean fMRI activation for the familiar objects at presentations 1 and 4 at the corresponding coordinates displayed in the left panel.
A more detailed examination of the detected between-subject brain-behavior correlations revealed that the magnitude of RS for familiar objects from presentation 1 to 2 in the left and right fusiform gyrus was correlated with activation at presentation 1. Specifically, the more subjects activated these regions during the first presentation of familiar objects, the more RS they showed during the second presentation of familiar objects (right fusiform gyrus: r = 0.68, p < 0.01; left fusiform gyrus: r = 0.79, p < 0.001; all other regions p >0.2). For this reason, we tested whether the correlations between priming and RS were still significant after accounting for the magnitude of activation at presentation 1. Regressions of subjects' priming scores on their RS values and fMRI activation values at presentation 1 indicated that the magnitude of RS from presentation 1 to 2 of familiar objects in the right fusiform [t(13) = 5.78, p =0.0001] and the left fusiform [t(13) = 2.91, p = 0.014] gyri were still significant predictors of priming for familiar objects after accounting for activation at presentation 1 in these regions.
2.3.2. Unfamiliar stimuli
No regions were detected where RS from presentation 1 to 2 correlated with priming at presentation 2 across or within subjects for the unfamiliar objects. However, similarly to the familiar objects, frontal and parietal regions showed correlations between priming and RS for the unfamiliar objects from presentation 1 to 4. The magnitude of RS in the right inferior frontal gyrus, extending into the anterior insula correlated with priming across subjects (BA 47, x = 36, y = 19, z = -3), Figure 5A. The right precuneus (BA 7, x = 20, y = -66, z = 49) showed a within-subjects correlation between priming and RS (Figure 6B), as did the left inferior frontal gyrus at a lower extent threshold (x = -50, y = 6, z = 33, k = 15). There were no regions where RE correlated with priming between or within subjects.
2.3.3. Control Analysis: Relationship between RT-modulated repetition effects (i.e., within-subject correlations) and trial-by-trial fluctuations in attention
Notably, RT-differences for individual items (presentation 1 - 2 and 1 - 4) within a single subject may not only reflect priming, but also learning-unrelated processes that fluctuate across trials, particularly attention. Therefore, we wanted to confirm that the regions identified here as showing a within-subject correlation between RT-priming and RS in fact index activity that is related to stimulus repetition (and hence learning), rather than to repetition-unrelated factors that co-vary with RT and fMRI signal amplitude across trials.
We reasoned that repetition-unrelated factors should occur independently of the presentation number of a stimulus (i.e., changes in RT across trials should be associated with changes in fMRI amplitude across trials, independent of whether a given trial represents the first, second, or fourth presentation of a stimulus). Therefore, to isolate repetition-unrelated factors, we constructed two new design matrices for all subjects. For the first design matrix, we randomly switched approximately half (19 out of the 39) presentation 1 and presentation 2 onset times and RT values for each object type. This effectively breaks the link between presentations 1 and 2, while preserving all other aspects of the data. The reason is that for about half of the trials, the ordering between presentation 1 and presentation 2 will be correct, while for the other half of the trials, the ordering will be reversed, resulting in no net ordering. The onset and RT vectors for presentations 3 and 4 remained the same and the resulting data were analyzed in exactly the same manner as in the original analysis. For the second new design matrix, we randomly switched 19 out of the 39 presentation 1 and presentation 4 onset times and RT values for each subject, leaving the onset and RT vectors for presentations 2 and 3 unchanged. Time-series modeling and group-level analyses were performed in the same manner as with the original data. Finally, for each object type separately we inclusively masked regions showing a main effect of RS in the original analysis (at p < 0.001) with regions showing a within-subject correlation between priming and RS in the original analysis (at p < 0.005), and with regions showing repetition unrelated within-subject correlations between RT and fMRI activation in the control analysis (at p < 0.005). The results indicated that none of the regions identified in the original analysis as showing within subject correlations between priming and RS overlapped with regions identified in the control analysis. This suggests that activity in those regions demonstrating both RS and within-subjects correlations between RS and priming indeed indexes repetition-related learning effects that correlate with behavioral priming, not priming-unrelated processes that fluctuate across trials.
In a second control analysis, we used SPM to identify regions showing a within-subjects correlation between RT and fMRI signal amplitude at presentation 1, collapsing across object type to increase power. In these regions the magnitude of activation on each trial is directly proportional to subjects' RT across trials. Because variation in RT across trials within subjects presumably reflects, among other things, differences in attention across trials, activation in these regions should also represent fluctuations in attention that are unrelated to stimulus repetition. Next, we performed inclusion analyses to determine if those regions identified as showing both RS (at p < 0.001) and a within subjects correlations between RS and priming (at p < 0.005) overlapped with regions identified in the second control analysis (at p < 0.005). No such overlap was found for any region. This provides further support for the view that the within subjects correlations reported here do not occur in regions demonstrating trial-by-trial fluctuations in attention. Rather, these regions appear to demonstrate repetition-related learning effects that co-vary with priming.
3. Discussion
This study found that both the pre-experimental familiarity of visual objects and the number of stimulus repetitions affect brain-behavior correlations between neural repetition effects and behavioral priming. The main findings from this study can be summarized as follows. First, we found that the magnitude of RS in occipital-temporal brain regions specifically correlated with the magnitude of priming of familiar but not globally unfamiliar visual objects (Figure 3). These occipital-temporal correlations were present for familiar objects repeated once but not for familiar objects repeated thrice. Second, priming-correlated RS effects were detected in prefrontal and parietal cortex for both familiar and unfamiliar objects that had been repeated three times (Figures 4 and 5). Some of these areas showed correlations between priming and RS for both stimulus types, while other regions showed brain-behavior correlations for only one of the two types of objects. Third, reliable RE effects were detected for the unfamiliar but not the familiar stimuli in a distributed set of regions. The magnitude of these RE effects, however, was not reliably correlated with behavioral priming in any region. Each of these findings is discussed in more detail below.
3.1. Perceptual facilitation and neural sharpening
It has long been hypothesized that priming of visual objects can be mediated, at least partially, by a neural tuning or sharpening mechanism, whereby repeated stimuli are represented with fewer neurons (Wiggs & Martin, 1998), a reduced neuronal firing rate (Grill-Spector et al., 1999; McMahon & Olson, 2007), or a shorter duration of neuronal activity (Henson & Rugg, 2003). In support of this view, evidence from single-cell recordings in monkeys has demonstrated stimulus-dependent decreases in the neuronal firing rate for repeated visual stimuli in inferior temporal cortex and prefrontal cortex (Desimone, 1996; Ringo, 1996; Sawamura, Orban, & Vogels, 2006). However, the precise neuronal mechanisms giving rise to macroscopic RS effects as measured by fMRI during repetition priming tasks remain unclear (Grill-Spector et al., 2006; Henson & Rugg, 2003).
Interestingly, there has been little direct support in the form of brain-behavior relationships for the view that repetition priming can reflect facilitated perceptual processing (but see Golby et al., 2005; Habeck et al., 2006). Thus, although repetition priming of visual stimuli is typically accompanied by RS in visual brain regions, correlations between priming and RS in these regions are rarely observed and this lack of correlations has been discussed in the literature (e.g., Bunzeck et al., 2006; Horner & Henson, 2008; Maccotta & Buckner, 2004; Sayres & Grill-Spector, 2006; Schacter, Wig, & Stevens, 2007; Xu et al., 2007). Similarly, a recently developed monkey version of a repetition-priming task failed to find a correlation between reductions in neural spiking rate in inferotemporal cortex and priming across trials (McMahon & Olson, 2007). To our knowledge, the current study provides the first evidence for a familiarity-dependent link between RS in ventral visual areas and repetition priming and provides strong support for the view that some kind of neuronal sharpening mechanism can contribute to behavioral priming of familiar visual objects.
In particular, we found that the magnitude of RS in bilateral fusiform gyrus during the first repetition of familiar objects correlated with behavioral priming of these objects across subjects. The magnitude of RS in the right fusiform gyrus also correlated with priming on a trial-by-trial basis within subjects, further supporting the link between these two phenomena. Because the priming-correlated RS effects in the fusiform gyrus occurred for the familiar but not the globally unfamiliar stimuli, they might involve preexisting global perceptual representations of the objects, which are not available for the globally unfamiliar items.
It seems that there are at least three possible reasons why prior studies often failed to find reliable correlations between RS and priming in occipital-temporal cortex. First, in some studies the behavioral priming effects were relatively small or unreliable even at the group level (e.g., Reber et al., 2005) and in many studies priming was probably not reliable at the individual subject level. This greatly reduces the power to detect brain-behavior correlations. Second, some prior studies have examined brain-behavior correlations only for items that were repeated several times (Golby et al., 2005; Maccotta & Buckner, 2004; Race et al., 2009). As the present study showed, however, RS in a least some occipital-temporal regions may only be correlated with priming during the first repetition, but not following multiple repetitions. Third, and perhaps most importantly, many studies using visual stimuli used tasks that required subjects to make semantic or conceptual decisions on the items but did not have a strong perceptual component (e.g., Daselaar, Veltman, Rombouts, Raaijmakers, & Jonker, 2005; Dobbins et al., 2004; Horner & Henson, 2008; Maccotta & Buckner, 2004; Race et al., 2009). In the present study, by comparison, the visual inspection of the perceptual features of the objects and the integration of these features into a Gestalt percept were necessary for making the real/non-real object decision. It is quite possible that correlations between occipital-temporal RS for familiar objects and priming will only emerge when the priming task has a stronger perceptual component. When priming is based primarily on conceptual or semantic processes, neural activity reductions in ventral visual regions might reflect post-decision processes, such as the reduction in attention once a decision has been made (Horner & Henson, 2008), or the bypassing of perceptual stages of stimulus analysis (Schacter et al., 2007).
3.2 Transition from perceptual priming to stimulus-decision priming
It is interesting that RS in the ventral visual stream was associated with RT priming only for familiar objects that had been repeated once, but not three times. This might indicate that initially there was rapid learning of perceptual information (or sharpening of perceptual representations) that contributed to behavioral priming. By comparison, primarily non-perceptual processes may have mediated priming effects beyond the first repetition. One possibility is that priming during the third repetition was primarily based on more automatic stimulus-decision and/or stimulus-response associations, which are thought to contribute to behavioral priming when stimuli are repeated multiple times within the same task. In this view, activity reductions in prefrontal cortex are thought to reflect the bypassing of attention-demanding controlled processes and detailed stimulus analyses, as subjects are able to rely on more automatic processing (Dobbins et al., 2004; Horner & Henson, 2008; Schacter et al., 2007). Because such stimulus-decision/response associations become stronger as the number of stimulus presentations within a task increases, priming effects following multiple stimulus repetitions are more likely to be supported by such associations than priming effects following a single repetition (Dobbins et al., 2004).
Although the present study did not directly examine to what extent stimulus-response associations contributed to behavioral priming during the third stimulus repetition, the finding that RS in prefrontal cortical areas correlated with priming of both familiar and unfamiliar objects during the third repetition is consistent with this interpretation. In particular, a significant RS effect was detected in the right inferior frontal cortex (BA 45/47), extending into the anterior insula for both types of objects and the magnitude of this effect was correlated with priming across subjects for both stimulus types (Figures 4A, 4D). These results reinforce the findings from prior studies that have reported correlations between prefrontal RS and priming for stimuli repeated within the same task (Bergerbest, Ghahremani, & Gabrieli, 2004; Bunzeck et al., 2006; Dobbins et al., 2004; Horner & Henson, 2008; Maccotta & Buckner, 2004; Orfanidou et al., 2006; Thiel et al., 2005; Turk-Browne et al., 2006; Wig et al., 2005). Because the correlation between priming and RS in right inferior frontal/insular cortex was present for the familiar and unfamiliar objects, this region is likely engaged in stimulus and response-independent processes, such as general task set configuration and central response selection processes. This interpretation is consistent with studies implicating this region in the coordination and evaluation of task performance (Eckert et al., 2009).
Other regions in prefrontal cortex demonstrated RS effects that correlated with priming for one of the two types of objects during the third repetition. For familiar objects, the left precentral gyrus showed an RS effect at presentation 4 that was modulated by RT priming on a within-subjects basis. For the unfamiliar objects, RS in the left inferior frontal gyrus correlated with RT priming on a within-subjects basis. These data might provide evidence that stimulus-response mappings have a stimulus-specific organization, as has previously been shown for other stimuli (e.g., faces and scenes, Bunzeck et al., 2006).
In addition to the prefrontal areas, some parietal structures also showed reductions in activity during the third stimulus repetition that correlated with response time facilitation. Thus, RS in the left precuneus and right inferior parietal lobule correlated with priming of familiar objects, and RS in the right precuneus correlated with priming of unfamiliar objects. Although RS effects in parietal cortex are not uncommon (e.g., Fiebach et al., 2005; Golby et al., 2005; Koutstaal et al., 2001; Reber et al., 2005; Thiel, Henson, Morris, Friston, & Dolan, 2001), few studies have reported correlations between parietal RS and repetition priming (Golby et al., 2005; also see Habeck et al., 2006). These parietal correlations, however, are consistent with the view that repetition-related reductions in neural activity during repetition priming might reflect a decrease in attention-demanding control processes. Considerable evidence suggests that not only prefrontal, but also parietal areas have an important role in cognitive control (for a recent review, see Collette, Hogge, Salmon, & Van der Linden, 2006).
3.3. Repetition enhancement and priming
Although the repetition of unfamiliar objects was associated with repetition enhancement effects in many regions both at the first and third repetition, there were no regions where RE reliably correlated with priming. Some of the regions showing RE were initially deactivated relative to baseline, while other regions showed an initially positive response, including the left lingual gyrus and the left posterior insula. While these latter regions may index the formation of novel perceptual, lexical, or semantic representations (Fiebach et al., 2005; Henson, 2003), priming was apparently not related to the formation of such representations in this study. This result, however, represents a null finding and should be interpreted with caution. Furthermore, when priming is expressed as an increase in identification or classification accuracy for briefly presented or degraded stimuli, RE in perceptual regions may be more directly coupled to behavioral performance (Schacter et al., 1995; Turk-Browne, Yi, Leber, & Chun, 2007).
The current study failed to find reliable correlations between priming and neural repetition effects at presentation 2 for the unfamiliar stimuli. One possible explanation for this finding is that the observed behavioral priming effects resulted from the contribution of neural changes occurring in many different regions and at different levels of the stimulus processing hierarchy (early perceptual to late decision or semantic processes). Each of these distributed neural changes may have been too subtle to be detected with the current design but their combined effect nonetheless gave rise to strong behavioral priming at the output level of the system.
3.4. Conclusion
To our knowledge the current study is the first to provide evidence from within the same experiment for the presence of two distinct processes that may contribute to behavioral repetition priming (Schacter et al., 2007). First, neural activity reductions in ventral visual regions during the first repetition of familiar stimuli may reflect facilitated perceptual processing. Second, as stimuli continue to be repeated, there is a shift from facilitation at the perceptual level to facilitation at the decision, response, or task-level, as indexed by activity reductions in prefrontal and parietal cortex for both familiar and unfamiliar objects. The data also suggest that repetition enhancement effects that are evident for unfamiliar visual objects may largely co-occur with priming but not be directly related to it, at least in the type of task employed here. Taken together, our results provide new insight into how brain plasticity may mediate observable changes in behavior.
4. Experimental Procedure
4.1. Participants
Fourteen Columbia University students between the ages of 19 and 29 years participated in this study in return for payment. All subjects reported being right-handed, having normal or corrected-to-normal vision, and being free of psychiatric and neurological disorders. Three additional subjects were excluded from the analysis because of data acquisition problems (N=3). All subjects gave written informed consent, as approved by the Internal Review Board of the College of Physicians and Surgeons of Columbia University.
4.2. Stimuli
Examples of the familiar and globally unfamiliar stimuli are shown in Figure 1. Subjects saw 39 line drawings of familiar real-world objects (Snodgrass & Vanderwart, 1980) and 39 line drawings of globally unfamiliar non-real stimuli. The unfamiliar stimuli consisted of smoothly connected features of real Snodgrass and Vanderwart (1980) objects. Although the unfamiliar objects were composed of real parts/local features, the global structure and meaning of the objects was unfamiliar to subjects, as indicated by subjects' fast and highly accurate ability to discriminate the familiar from the unfamiliar items (see Results section). None of the unfamiliar objects shared any features with the familiar objects (i.e., there were two non-overlapping sets of objects).
4.3. Procedure
An event-related fMRI design was used that consisted of three scanning sessions, with the order of sessions counterbalanced between subjects. In each session, subjects viewed a distinct set of 26 objects (i.e., 13 familiar and 13 unfamiliar objects) that were each presented four times. Each session was exactly 8 min in duration and consisted of 4 buffer trials, followed by an intermixed series of 104 stimulus presentations: 13 familiar and 13 unfamiliar objects * 4 presentations/per object. The number of stimuli intervening between repetitions was kept relatively low (2, 4, or 6 items) so as to maximize the likelihood of generating sufficiently robust repetition effects (Henson et al., 2004). Three different repetition sequences were used to lower the predictability of stimuli to participants: [2, 4, 6], [4, 6, 2], and [6, 2, 4]. The three repetition sequences, which were counterbalanced over stimuli across subjects in a Latin square, were distributed equally throughout the task. However, given that 13 objects of each type were presented in a session, each session had 5 repetition sequences of one type and 4 of each of the two others. An additional 52 blank trials were interspersed throughout each session to provide a comparison to baseline and to maintain the required object spacing. During blank trials, the fixation cross was presented, but no stimulus followed and no response was required. Note that the number of initial stimulus presentations and repeated stimulus presentations within each of the three blocks was almost the same during the first half (14 vs. 12) and the second half (38 vs. 40) of each block. This means that changes in general arousal or attention across a block will not influence the results.
Each trial, including blank trials, lasted 3 sec. It began with a 500 ms inter-trial interval (ITI), followed by a 500 ms fixation cue, a 50 ms blank screen, and the stimulus presentation for 1000 ms. A 1950 ms response period started coterminously with stimulus onset. Subjects were instructed to indicate whether each stimulus represented a “real” or “non-real” object using their right and left index fingers, respectively. Subjects made their responses via differential button press (LUMItouch button boxes; Photon Control Company). Both speed and accuracy were emphasized. Task administration and data collection was controlled with PsyScope 1.2.5. All timing was facilitated by the use of the external PsyScope Button Box, which interfaced directly with the PsyScope software, the LUMItouch button boxes, and the MRI acquisition computer. Stimulus presentations were automatically synched to the video retrace signal. Prior to scanning, subjects viewed examples of both types of objects and completed five practice trials. They were not informed that the procedure was a memory test.
4.4. fMRI acquisition and preprocessing
A 1.5 T magnetic resonance scanner (Phillips) and a standard quadrature head coil were used to acquire functional T2*-weighted BOLD images using a gradient echo EPI pulse sequence (TE = 50 ms; TR = 3 sec; flip angle = 90°; 64 × 64 matrix, 400 cm 2 field of view, in-plane voxel size = 3.124 mm × 3.124 mm; slice thickness = 8mm, no gap; 17 transaxial slices per volume). At the end of the experiment, high resolution (in plane) T2 images were also acquired from each subject at the same slice locations as in the fMRI run using a fast echo spin sequence (TE = 100 ms; TR = 3 sec; 256 × 256 matrix; 400 cm2 field of view; in-plane resolution = 0.781 mm × 0.781 mm; slice-thickness = 8mm, no gap). Task stimuli were back-projected onto a screen located at the foot of the MRI bed using an LCD projector. Subjects viewed the stimuli via a mirror system located in the head coil.
The data were processed using SPM99 (Wellcome Department of Cognitive Neurology, London) so that the analyses would be consistent with those reported by Soldan, Zarahn, et al. (2008). For each subject, images were first corrected for timing of slice acquisition (slice acquisition was ascending, interleaved). All functional volumes were then realigned to the first volume of the first session. The T2 structural image was then co-registered to the first functional volume using the mutual information co-registration algorithm implemented in SPM99. This co-registered high-resolution image was then used to determine parameters (7 × 8 × 7 nonlinear basis functions) for transformation into a Talairach standard space defined by the Montreal Neurological Institute template brain supplied with SPM99. These normalization parameters were then applied to the functional data using sinc interpolation to re-slice the images to 2 mm × 2mm × 2mm.
4.5. fMRI time-series (i.e., first-level) modeling
Accuracy was ignored in the imaging analysis, as it was at ceiling for all participants. The regressors for the first-level general linear model were constructed by convolving the default SPM99 hemodynamic response function with the basis functions for each trial type (a rectangular pulse) aligned with stimulus onset. High-pass filtering eliminated information below (1/117) Hz.
As one aim of this investigation was to identify brain regions where intra-individual variability in RT-priming correlated with intra-individual variability in RS/RE, we included RT as a parametric modulator. Whereas the height of the unmodulated regressors remained constant across trials, the height of the modulated regressors (modeled linearly) varied on a trial-by-trial basis, depending on RT. For each subject, the RT values were mean centered for each level of presentation, object type, and session. The resulting GLM for each subject had one modulated and one unmodulated regressor for each crossing of object type (2), presentation (4), and session (3). In addition, for presentations 2, 3, and 4, RT-modulated regressors were included to control for RT at presentation 1, one for each crossing of presentation (3), object type (2), and session (3). These regressors always stayed silent, i.e., they were not used for the computation of any contrast images. They were included because preliminary analyses indicated that the magnitude of the RT-priming effect (i.e., difference between RT at presentation 1 and RT at presentations N > 1) for individual objects was positively correlated with RT at presentation 1, such that objects with higher RTs at presentation 1 showed a greater RT-priming effect at presentations N > 1. Thus, for each session and object type the following regressors were used (where pres = presentation; unmod = unmodulated, mod = modulated): pres1_unmod, pres1_mod_RT1, pres2_unmod, pres2_mod_RT1, pres2_mod_RT2, pres3_unmod, pres3_mod_RT1, pres3_mod_RT3, pres4_unmod, pres4_mod_RT1, pres4_mod_RT4.
Note that because this design controls for subject and stimulus-specific differences in RT at presentation 1 (via inclusion of the RT-modulated predictor for presentation 1), the RT-modulated predictors for presentations N > 1 can be used as a measure of the correlation between RT priming and RS/RE from presentations 1 to presentations N > 1, not just as a measure of the correlation between RT-priming and signal amplitude at presentations N > 1. See appendix for the mathematical explanation.
Next, linear contrast images were computed for each subject (implicitly with respect to baseline), averaging across sessions. There were 8 contrast images for the unmodulated predictors, one for each crossing of object type and presentation, and 6 contrast images for the modulated predictors, one for each level of object type and presentation (2, 3, and 4). All contrast images were intensity normalized by dividing each voxel by its time series average, masked with an image that represented the intersection of useable data from all subjects and had a gray matter prior probability of > 0.25 (to eliminate ventricles from the search volume), and spatially smoothed using a Gaussian kernel of 8 mm full width-half maximum. These images were then used for subsequent second-level univariate analysis.
4.6. SPM Group Analysis
Contrasts of the parameter estimates from single-subject models were entered into random-effects analyses (one-sample t tests) comparing the mean parameter estimate over subjects to zero. Unless reported otherwise, main effects of repetition and repetition by object type interactions were thresholded at p < 0.001 uncorrected, and a cluster threshold of k = 50 voxels. Contrast images representing the within-subjects correlation between priming and RS/RE from single-subject models were combined at the group level in the same manner as the other contrasts (by entering them into random-effects analyses and comparing the mean parameter estimate over subjects to zero) and thresholded at p < 0.005, k = 20. Between-subjects correlations between priming and RS/RE were computed by entering subjects' mean priming scores for familiar or unfamiliar objects as a covariate in the SPM analysis. Anatomic labels for cluster maxima were provided by Talairach Daemon (http://ric.uthscsa.edu/projects/talairachdaemon.html).
When interpreting the correlations, it is important to keep in mind that regions demonstrating between-subjects correlations (which are computed at the second, or group level) do not necessarily also show within-subjects correlations and vice versa. For example, it is possible that in some subjects, each 1-unit change in behavior (i.e., priming) is associated with a 1-unit change in neural signal (i.e., RS/RE), whereas in other subjects, a 1-unit change in behavior is associated with a 0.7-unit change in neural signal in the same brain region (implying differences in “neural efficiency” across subjects). When such differences in the slope between neural and behavioral repetition effects across subjects are present, then group-level correlations may not accurately represent brain-behavior correlations that are present at the individual-subject level.
Acknowledgments
Anja Soldan, Department of Psychology, University of Massachusetts - Dartmouth; Christian Habeck, Yunglin Gazes, and Yaakov Stern, Cognitive Neuroscience Division of the Taub Institute, Columbia University Medical Center.
This work was supported by NIA grant RO1-AG16714 to Yaakov Stern.
We would like to thank Eric Zarahn for assistance with fMRI data analysis. Correspondence concerning this article should be addressed to Anja Soldan, 285 Old Westport Road, North Dartmouth, MA 02747. E-mail: asoldan@umassd.edu.
Appendix A
In this study, the following general linear model was applied to the data, where
yi = fMRI response amplitude for presentation i
μ1 = mean fMRI amplitude at presentation 1
δir1 = modulation of fMRI signal at current presentation i by RT at presentation 1
βiri = modulation of fMRI signal at current presentation i by RT at current presentation i
εi= error term for presentation i.
We have for presentation i: yi = μi + βiri + δir1 + εI with the special case of i = 1 where β1=0.
Thus, the difference in fMRI amplitude from presentation 1 to presentation 2 is as follows:
| (1) |
| (2) |
| (3) |
Note that β2, which is the regression coefficient on the modulated predictor for presentation i = 2, represents the relationship between RS/RE and RT priming between presentations 1 and 2. The mathematical reason why the formula works out in such a manner is that this model also takes into account the relationship with the reaction time at baseline, to control for innate speed differences in reaction time unrelated to priming. In equation (2), we inserted zero as the sum of italicized terms - β2r1 + β2r1, enabling us to rewrite equation (2) as equation (3), with the terms relating to reaction time priming and to the baseline reaction time clearly separated. [It can easily be checked that these considerations hold for presentations 3 and 4 also. Index 2 can be substituted by a general index a > 1.]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergerbest D, Ghahremani DG, Gabrieli JD. Neural correlates of auditory repetition priming: reduced fMRI activation in the auditory cortex. J Cogn Neurosci. 2004;16(6):966–977. doi: 10.1162/0898929041502760. [DOI] [PubMed] [Google Scholar]
- Bunzeck N, Schutze H, Duzel E. Category-specific organization of prefrontal response-facilitation during priming. Neuropsychologia. 2006;44(10):1765–1776. doi: 10.1016/j.neuropsychologia.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Collette F, Hogge M, Salmon E, Van der Linden M. Exploration of the neural substrates of executive functioning by functional neuroimaging. Neuroscience. 2006;139(1):209–221. doi: 10.1016/j.neuroscience.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Aging affects both perceptual and lexical/semantic components of word stem priming: An event-related fMRI study. Neurobiol Learn Mem. 2005;83(3):251–262. doi: 10.1016/j.nlm.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Desimone R. Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci U S A. 1996;93(24):13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Schnyer DM, Verfaellie M, Schacter DL. Cortical activity reductions during repetition priming can result from rapid response learning. Nature. 2004;428(6980):316–319. doi: 10.1038/nature02400. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Menon V, Walczak A, Ahlstrom J, Denslow S, Horwitz A, Dubno JR. At the heart of the ventral attention system: the right anterior insula. Hum Brain Mapp. 2009;30(8):2530–2541. doi: 10.1002/hbm.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach CJ, Gruber T, Supp GG. Neuronal mechanisms of repetition priming in occipitotemporal cortex: spatiotemporal evidence from functional magnetic resonance imaging and electroencephalography. J Neurosci. 2005;25(13):3414–3422. doi: 10.1523/JNEUROSCI.4107-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Jahfari S, Scholte HS, Wolfensteller U, van den Wildenberg WP, Ridderinkhof KR. Function and structure of the right inferior frontal cortex predict individual differences in response inhibition: a model-based approach. J Neurosci. 2008;28(39):9790–9796. doi: 10.1523/JNEUROSCI.1465-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golby A, Silverberg G, Race E, Gabrieli S, O'Shea J, Knierim K, Stebbins G, Gabrieli J. Memory encoding in Alzheimer's disease: an fMRI study of explicit and implicit memory. Brain. 2005;128(Pt 4):773–787. doi: 10.1093/brain/awh400. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10(1):14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24(1):187–203. doi: 10.1016/s0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- Habeck C, Hilton HJ, Zarahn E, Brown T, Stern Y. An event-related fMRI study of the neural networks underlying repetition suppression and reaction time priming in implicit visual memory. Brain Res. 2006;1075(1):133–141. doi: 10.1016/j.brainres.2005.11.102. [DOI] [PubMed] [Google Scholar]
- Henson R, Shallice T, Dolan R. Neuroimaging evidence for dissociable forms of repetition priming. Science. 2000;287(5456):1269–1272. doi: 10.1126/science.287.5456.1269. [DOI] [PubMed] [Google Scholar]
- Henson RN. Neuroimaging studies of priming. Prog Neurobiol. 2003;70(1):53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD. Neural response suppression, haemodynamic repetition effects, and behavioural priming. Neuropsychologia. 2003;41(3):263–270. doi: 10.1016/s0028-3932(02)00159-8. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rylands A, Ross E, Vuilleumeir P, Rugg MD. The effect of repetition lag on electrophysiological and haemodynamic correlates of visual object priming. Neuroimage. 2004;21(4):1674–1689. doi: 10.1016/j.neuroimage.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Horner AJ, Henson RN. Priming, response learning and repetition suppression. Neuropsychologia. 2008;46(7):1979–1991. doi: 10.1016/j.neuropsychologia.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutstaal W, Wagner AD, Rotte M, Maril A, Buckner RL, Schacter DL. Perceptual specificity in visual object priming: functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia. 2001;39(2):184–199. doi: 10.1016/s0028-3932(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Lustig C, Buckner RL. Preserved neural correlates of priming in old age and dementia. Neuron. 2004;42(5):865–875. doi: 10.1016/j.neuron.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Maccotta L, Buckner RL. Evidence for neural effects of repetition that directly correlate with behavioral priming. J Cogn Neurosci. 2004;16(9):1625–1632. doi: 10.1162/0898929042568451. [DOI] [PubMed] [Google Scholar]
- McMahon DB, Olson CR. Repetition suppression in monkey inferotemporal cortex: relation to behavioral priming. J Neurophysiol. 2007;97(5):3532–3543. doi: 10.1152/jn.01042.2006. [DOI] [PubMed] [Google Scholar]
- Orfanidou E, Marslen-Wilson WD, Davis MH. Neural response suppression predicts repetition priming of spoken words and pseudowords. J Cogn Neurosci. 2006;18(8):1237–1252. doi: 10.1162/jocn.2006.18.8.1237. [DOI] [PubMed] [Google Scholar]
- Race EA, Shanker S, Wagner AD. Neural priming in human frontal cortex: Multiple forms of learning reduce demands on the prefrontal executive system. Journal of Cognitive Neuroscience. 2009 doi: 10.1162/jocn.2009.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097-1089. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Gitelman DR, Parrish TB, Mesulam MM. Priming effects in the fusiform gyrus: changes in neural activity beyond the second presentation. Cereb Cortex. 2005;15(6):787–795. doi: 10.1093/cercor/bhh179. [DOI] [PubMed] [Google Scholar]
- Ringo JL. Stimulus specific adaptation in inferior temporal and medial temporal cortex of the monkey. Behavioral Brain Research. 1996;76(1-2):191–197. doi: 10.1016/0166-4328(95)00197-2. [DOI] [PubMed] [Google Scholar]
- Sawamura H, Orban GA, Vogels R. Selectivity of neuronal adaptation does not match response selectivity: a single-cell study of the FMRI adaptation paradigm. Neuron. 2006;49(2):307–318. doi: 10.1016/j.neuron.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Sayres R, Grill-Spector K. Object-selective cortex exhibits performance-independent repetition suppression. J Neurophysiol. 2006;95(2):995–1007. doi: 10.1152/jn.00500.2005. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Reiman E, Uecker A, Polster MR, Yun LS, Cooper LA. Brain regions associated with retrieval of structurally coherent visual information. Nature. 1995;376(6541):587–590. doi: 10.1038/376587a0. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wig GS, Stevens WD. Reductions in cortical activity during priming. Curr Opin Neurobiol. 2007;17(2):171–176. doi: 10.1016/j.conb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol [Hum Learn] 1980;6(2):174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Soldan A, Zarahn E, Hilton HJ, Stern Y. Global familiarity of visual stimuli affects repetition-related neural plasticity but not repetition priming. Neuroimage. 2008;39(1):515–526. doi: 10.1016/j.neuroimage.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WD, Wig GS, Schacter DL. Implicit memory and priming. In: Roediger IHL, editor. Cognitive Psychology of Memory Vol [2] of Learning and Memory: A comprehensive reference. Oxford: Elsevier; 2008. pp. 623–644. [Google Scholar]
- Thiel A, Haupt WF, Habedank B, Winhuisen L, Herholz K, Kessler J, Markowitsch HJ, Heiss WD. Neuroimaging-guided rTMS of the left inferior frontal gyrus interferes with repetition priming. Neuroimage. 2005;25(3):815–823. doi: 10.1016/j.neuroimage.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Henson RN, Dolan RJ. Scopolamine but not lorazepam modulates face repetition priming: a psychopharmacological fMRI study. Neuropsychopharmacology. 2002;27(2):282–292. doi: 10.1016/S0893-133X(02)00316-0. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Henson RN, Morris JS, Friston KJ, Dolan RJ. Pharmacological modulation of behavioral and neuronal correlates of repetition priming. J Neurosci. 2001;21(17):6846–6852. doi: 10.1523/JNEUROSCI.21-17-06846.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk-Browne NB, Yi DJ, Chun MM. Linking implicit and explicit memory: common encoding factors and shared representations. Neuron. 2006;49(6):917–927. doi: 10.1016/j.neuron.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Turk-Browne NB, Yi DJ, Leber AB, Chun MM. Visual quality determines the direction of neural repetition effects. Cereb Cortex. 2007;17(2):425–433. doi: 10.1093/cercor/bhj159. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Henson RN, Driver J, Dolan RJ. Multiple levels of visual object constancy revealed by event-related fMRI of repetition priming. Nat Neurosci. 2002;5(5):491–499. doi: 10.1038/nn839. [DOI] [PubMed] [Google Scholar]
- Wig GS, Grafton ST, Demos KE, Kelley WM. Reductions in neural activity underlie behavioral components of repetition priming. Nat Neurosci. 2005;8(9):1228–1233. doi: 10.1038/nn1515. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Curr Opin Neurobiol. 1998;8(2):227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- Xu Y, Turk-Browne NB, Chun MM. Dissociating task performance from fMRI repetition attenuation in ventral visual cortex. J Neurosci. 2007;27(22):5981–5985. doi: 10.1523/JNEUROSCI.5527-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


