Abstract
Background
Difficulties in communication and reciprocal social behavior are core features of autism spectrum disorders (ASD) and are often present, to varying degrees, in other family members. This prospective longitudinal infant sibling study examines whether social-communicative features of family members may inform which infants are at increased risk for ASD and other developmental concerns.
Method
Two hundred and seventeen families participated in this study. Infant siblings were recruited from families with at least one older child diagnosed with an ASD (n = 135) or at least one typically developing older child (n = 82). Families completed the Social Responsiveness Scale to assess social and communication features of the broader autism phenotype (BAP), sometimes called quantitative autistic traits (QAT). Family affectedness was assessed in two ways: categorically, based on number of affected older siblings (i.e., typical, simplex, multiplex risk groups) and dimensionally, by assessing varying degrees of QAT in all family members. Infant siblings were assessed at 36 months of age and completed the Autism Diagnostic Observation Schedule and the Mullen Scales of Early Learning.
Results
In structural equation models, comparisons between multiplex, simplex and typical groups revealed the highest rates of QAT in the multiplex group followed by the simplex and typical groups. Infant sibling outcomes were predicted by gender, family risk group, proband QAT, and additional sibling QAT.
Conclusions
Replicating previous cross-sectional and family history findings, the present study found elevated social and communication features of the BAP in siblings and fathers of ASD families, but not in mothers. While social and communication features of the BAP in mothers, fathers, and undiagnosed siblings did not predict infant sibling outcomes, having more than one affected older sibling did. Infant siblings from multiplex families were at significantly higher risk for ASD than infant siblings from simplex families in this sample.
Keywords: Autistic disorder, Pervasive developmental disorder, family factors, siblings, structural equation modeling
In recent years, the emergence of autism spectrum disorder (ASD) symptoms has been studied within the context of infant siblings (Rogers, 2009). Within infant sibling studies, a child born to a family raising one or more children with an ASD is followed longitudinally to examine, among other things, what aspects of early development help predict outcomes. This prospective longitudinal design provides a unique opportunity to study ASD symptoms as they develop and to identify the earliest possible markers of later ASD diagnoses. Previous infant sibling studies have assessed whether differences in visual fixation and disengagement (Zwaigenbaum, 2005), face processing (Young et al., 2009), early communication and joint attention behaviors (Landa & Garrett-Mayer, 2006), object exploration (Ozonoff et al., 2008), and head circumference (Elder, Dawson, Toth, Fein & Munson, 2008) can predict which infant siblings later develop an ASD. These studies have reliably found differences in a variety of early social and communication behaviors in infants who have older siblings with ASD. The present study moves beyond aspects of the infant’s development to examine whether features of family members may help inform which infants are at increased risk for ASD or other developmental concerns.
Deficits in communication and reciprocal social behavior are not only core features of autism spectrum disorders but also are often present, to varying degrees, in other family members. Cross-sectional and family history studies have demonstrated that social and communication difficulties are more common in families with an ASD child than in families with only typically developing children (Bailey, Palferman, Heavey & Le Couteur, 1998; Ruser et al., 2007). These communication and relational differences are often studied as an element of the broader autism phenotype (BAP). The BAP is a constellation of subclinical behaviors associated with ASD that can be found in non-autistic family members. The BAP includes differences in social interactions, relationships, communication skills, and restricted patterns of behavior or intense interests. Previous reports detail higher rates of the BAP in fathers (Virkud et al., 2008) and siblings (Bailey et al., 1998; Constantino et al., 2006) of families with an ASD child, with the highest rates in multiplex families (Constantino et al., 2006; Virkud et al., 2008). The BAP is thought to be an index of family genetic risk for autism reflecting as yet undefined genetic factors (Piven, 2001). In infant sibling studies no specific genetic mechanisms have been identified; therefore, the present study does not examine genetic pathways but rather assesses the social and communication features of the BAP that are presumed to index genetic risk.
Previous studies posit that the social-communicative differences of the BAP are normally distributed across the general population and are genetically transmitted across generations (Constantino et al., 2003; Constantino & Todd, 2003; Virkud et al., 2008). The gradient of social-communication differences associated with the BAP have also been labeled quantitative autistic traits (QAT; Virkud et al., 2008). The Social Responsiveness Scale (SRS) is a widely used instrument that measures both typical features of autism and subclinical communication and relationship differences that may be seen among relatives. The SRS is particularly well-suited for the present study because it is well validated in family members of typically developing children and children with ASD (Constantino et al., 2006, 2007) and previous studies have documented the heritability of the reciprocal and social communication behaviors captured by the SRS (Constantino & Todd, 2005; Hoekstra, Bartels, Verweij & Boomsma, 2007).
Family affectedness may be conceptualized in several ways. When assessing the affectedness of siblings, diagnostic categories are often employed (i.e., ASD or non-ASD). Then affected families can be categorized on the basis of the number of children diagnosed with an ASD, resulting in simplex and multiplex groups. This approach has direct clinical relevance but does not capture the varying degrees of affectedness and subclinical difficulties that may be present in both siblings and parents. Therefore, the present study examined family affectedness in two ways: by assigning families to a risk group (i.e., simplex, multiplex or typical) on the basis of the older sibling(s)’ diagnostic status and by assessing varying degrees of QAT, as measured by the SRS, in parents and siblings.
The present prospective longitudinal study attempts to replicate differential levels of family affectedness (or QAT) in families with at least one child diagnosed with an ASD versus families with only typically developing children, something previously reported only in cross-sectional and family history studies (Bailey et al., 1998; Constantino et al., 2006, 2007). Additionally, this study aims to move the field forward by investigating QAT as a predictor of developmental outcomes in infant siblings.
Research Questions and Hypotheses
Research Question 1
Do quantitative autistic traits vary across families with only typically developing children, one child with an ASD (simplex), and more than one child with an ASD (multiplex)? Based on previous studies (Bailey et al., 1998; Constantino et al., 2006; Losh, Childress, Lam & Piven, 2008; Virkud et al., 2008), we expected elevated rates of QAT in families of children with an ASD, with the highest rates in multiplex families.
Research Question 2
Can a categorical measure of family risk status (i.e., simplex, multiplex and typical) inform infant sibling outcomes? We expected higher rates of ASD and other developmental disorders among infants in the multiplex group, followed by the simplex and typical groups.
Research Question 3
Can a dimensional measure of family affectedness (e.g., QAT) inform infant sibling outcomes? We expected children who developed ASD to have the highest levels of family QAT, followed by families of infants who developed other developmental concerns.
Methods
Sample
Two hundred and seventeen families participated in this collaborative study between the University of California, Davis (n = 115) and the University of California, Los Angeles (n = 102). Infant siblings were recruited from families with at least one older child diagnosed with an ASD (n = 135) or at least one typically developing older child and no children with ASD (n = 82). The older siblings (i.e., the proband) were diagnosed with autistic disorder, Asperger syndrome, or pervasive developmental disorder – not otherwise specified. Proband diagnostic status was confirmed with the Autism Diagnostic Observation Schedule (ADOS; Lord, Rutter, DiLavore, & Risi, 2002). In multiplex families, the oldest child with an ASD was considered the proband. Family demographic information stratified by risk group is provided in Table 1.
Table 1.
Sample demographic characteristics stratified by family risk group.
| Typical | Simplex | Multiplex | ||||
|---|---|---|---|---|---|---|
| N | 82 | 124 | 11 | |||
| Enrolled Males, n(% ) | 50 (61%) | 60 (48%) | 8 (73%) | |||
| Number of Children, M (SD)a | 1.72 (.85) | 2.23 (.65) | 3.07(1.12) | |||
| Married, n (%) | 80 98(98%) | 115 (93%) | 10 (91%) | |||
| Family Income, n (%) | ||||||
| Below $25,000 | 2 (3%) | 5 (4%) | 2 (18%) | |||
| $25,000-$49,999 | 8 (10%) | 25 (21%) | 2 (28%) | |||
| $50,000-74,999 | 15 (19%) | 16 (14%) | 3 (27%) | |||
| $75,000-$99,999 | 11 (14%) | 20 (17%) | 2 (18%) | |||
| $100,000-$124,999 | 11 (14%) | 21 (18%) | 0 (0%) | |||
| $125,000 and above | 32 (40%) | 31 (26%) | 2 (18%) | |||
| Education, n (%) | Mother | Father | Mother | Father | Mother | Father |
| High School/GED | 10 (13%) | 17 (21%) | 34 (28%) | 27 (23%) | 5 (50%) | 4 (36%) |
| College Degree | 34 (43%) | 33 (41%) | 56 (46%) | 53 (46%) | 4 (40%) | 5 (46%) |
| Graduate Degree | 35 (44%) | 31 (38%) | 31 (26%) | 36 (31%) | 1 (10%) | 2 (18%) |
At 36 months, infant siblings’ outcomes were categorized as typically developing (n = 151, 69%), with an ASD (n = 21, 10%), or with other developmental concerns (n = 45, 21%). Infant siblings were placed in the ASD outcome group if they scored over the ASD cutoff on the ADOS and met DSM-IV criteria for Autistic Disorder or Pervasive Developmental Disorder Not Otherwise Specified by an expert clinician. Children entered the developmental concerns (DC) outcome group if they scored more than 1.5 SD below the mean on one or more of the Mullen Scales of Early Learning (MSEL; Mullen, 1995) or had social communication difficulties as indexed by the ADOS (i.e., Module 1 scores above 4, Module 2 scores above 5) but did not meet criteria for an ASD. Children in the DC outcome group had clinical diagnoses of general developmental delay, speech/language delay, behavior disorders, social anxiety, and social difficulties that were sub-threshold for ASD. Infant siblings who did not show significant delays (i.e., did not score 1.5 SD below the mean) on the MSEL or have elevated ADOS scores were placed in the typical (TYP) outcome group.
Measures
Social Responsiveness Scale
The Social Responsiveness Scale (SRS) was used to index quantitative autistic traits (QAT) in family members. The SRS is a 65-item questionnaire which assesses social interactions, relationships, and communication skills. Items are rated on a 4-point scale of (1) not true, (2) sometimes true, (3) often true, and (4) almost always true. Studies using the SRS report high heritability and stability in SRS scores (Constantino & Todd, 2005; Constantino et al., 2009), and discriminant validity, differentiating well between typically developing, at-risk, and autism populations (Constantino et al., 2000; Reiersen, Constantino, Volk, & Todd, 2007). SRS raw scores range from 0 – 195, with an ASD raw score cut-off of 87 for males and 74 for females. Raw SRS scores were used for mother, father, and the proband QAT. To index QAT in all additional siblings (beyond the proband) mean SRS raw scores were used to avoid the confound of family size (i.e., equate all families regardless of number of additional siblings). SRS mean scores for additional siblings were calculated for 14 (17%) families in the typical group, 36 (29%) in the simplex group and 8 (73%) in the multiplex group. This disproportionate distribution was expected as additional siblings are a requirement for multiplex group membership. As suggested in the manual, mothers completed the SRS on the father and on each sibling. Fathers completed the SRS on the mother. All SRS forms were completed at time of enrollment at least two years before infant sibling outcomes were assessed. Descriptive SRS statistics stratified by risk group are provided in Table 2.
Table 2.
ANOVA results for father, mother, proband and additional sibling QAT in multiplex, simplex and typical groups.
| Mean Difference (SE) |
||||||
|---|---|---|---|---|---|---|
| F | Group | n | M (SD) | Simplex | Multiplex | |
| Father QAT | F (2, 214) = 4.69 ** | Typical | 82 | 20.72(14.85) | −6.52(2.66)* | −14.55(6.00)* |
| Simplex | 124 | 27.24(2047) | - | −8.03 (5.88) | ||
| Multiplex | 11 | 35.27(23.34) | - | |||
| Mother QAT | F (2, 203) = .25 | Typical | 81 | 23.48(14.43) | −.67(2.51) | −4.12(5.82) |
| Simplex | 115 | 24.14(19.11) | - | −3.44(5.23) | ||
| Multiplex | 10 | 27.60(18.32) | - | |||
| Proband QAT | F (2, 165) = 116.03** | Typical | 43 | 25.05(14.24) | −68.56(4.80)** | −96.55(9.43)** |
| Simplex | 115 | 93.61(29.57) | - | −27.99(8.86)** | ||
| Multiplex | 10 | 121.60(34.93) | - | |||
| Additional Siblings | F (2, 55) = 18.49** | Typical | 14 | 15.42(8.40) | −14.25(6.81)* | −57.29(9.59)** |
| Mean QAT | Simplex | 36 | 29.67(24.47) | - | −43.04(8.46)** | |
| Multiplex | 8 | 72.71(23.52) | - | |||
Note. QAT = quantitative autistic traits
p < .01
p < .05
Autism Diagnostic Observation Schedule
The ADOS (Lord et al., 2000) is a semi-structured standardized interaction and observation that measures symptoms of autism. It has two empirically derived cutoffs, one for ASD and one for autistic disorder. It was administered to the proband at the initial visit to confirm inclusion and to the infant siblings at 36 months of age to determine outcome. Each administrator completed an initial ADOS training and maintained 80% or greater agreement with a reliable trainer across all items.
Mullen Scales of Early Learning
The MSEL (Mullen, 1995) is a standardized developmental test for children ages birth to 68 months. The subscales administered include fine motor, visual reception, and expressive and receptive language. This measure was used in the outcome definition, as described above.
Procedure
This study was conducted under the approval of the Institutional Research Review Boards. The study was explained to parents orally and in writing, all their questions were answered, and consent was obtained before conducting assessments. The SRS was completed on each immediate family member at time of enrollment. Infant sibling assessments were completed at 36 months as part of a larger experimental battery administered at that age.
Data Analysis
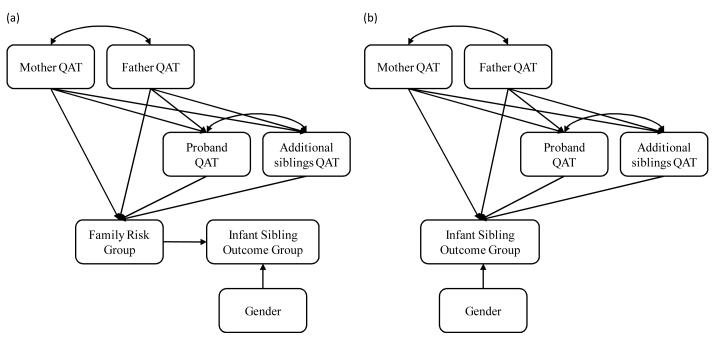
To address our research questions a series of structural equation models (SEM) were tested in Mplus 5 (Muthen & Muthen, 2007), followed by ANOVAs or two-way contingency tables to explicate group differences. SEM was chosen over other analytic approaches because it affords the simultaneous testing of each parameter of interest (assessing overall family contributions not just the affectedness of each family member independently) and can model the expected associations between variables. The first specified model tests our first two research questions (Figure 1a). Research question 1 aims to confirm different levels of family QAT in families with only typically developing children (typical group), one child with an ASD (simplex group), and more than one child with an ASD (multiplex group). Support for this hypothesis is a necessary element to address questions 2 and 3 (i.e., differential rates of family QAT are required if we expect they will inform later outcomes). Research question 2 asks whether family risk status can inform infant sibling outcomes. In the second model (Figure 1b), QAT was used to estimate parent and sibling contributions to infant sibling outcomes. The second model seems to determine whether infant siblings from families with more affected family members are at increased risk for negative developmental outcomes.
Figure 1.
Specified path models for (a) family risk group (multiplex, simplex or typical) and infant sibling outcomes group (autism spectrum disorders, other developmental concerns or typical) and (b) family quantitative autistic traits (QAT) and infant sibling outcomes.
The SEM models were specified, identified, and tested for assumption violations prior to model and path estimation and interpretation. Transformations were employed to reduce non-normality bias. Additionally, a full information maximum likelihood (FIML) procedure was used to address missing data. In the Mplus FIML procedure, individual missing data patterns are assessed, and means and covariances for each missing data pattern are calculated to inform the observed information matrix (Arbuckle, 1996; Kaplan, 2009). The observed information matrix is used to generate estimates (Kenward & Molenberghs, 1998). Addressing missing data via FIML assumes data missing at random (MAR; Little & Rubin, 1989) and is preferable to pairwise or list-wise deletion (Arbuckle, 1996).
Overall Model Fit
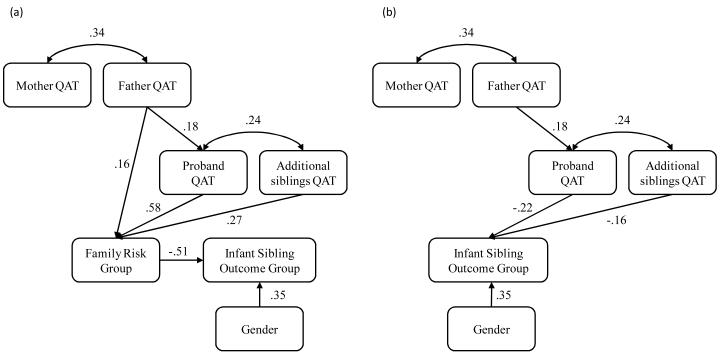
To examine the overall model fit, three indices were assessed: chi-square (Χ2), root mean square error of approximation (RMSEA), and the comparative fit index (CFI). The Χ2 index is a model of misspecification; therefore, a significant Χ2 means the model does not fit the sample data. Some statisticians suggest that the exact fit test in Χ2 is an unrealistic standard; therefore, indices of approximate fit like RMSEA were also assessed (Kaplan, 2009). RMSEA tests whether the model fits the population approximately. In RMSEA, .00 is the best possible fit, with higher values indicating poorer fit. Acceptable fit within the RMSEA index is generally .05 or lower (Browne & Cudeck, 1992). Within this study, the CFI compares the specified model to a null model. The null model posits that there are no associations among the variables. CFI ranges from 0 to 1, with higher values indicating better fit. CFI values above .90 are generally interpreted as acceptable model fit (Bagozzi & Yi, 1988). The specified models had acceptable fit (see Figure 2 note) indicating the specified paths adequately capture relations present in the sample.
Figure 2.
Significant standardized path coefficients (β) for models of family affectedness by (a) family risk group (multiplex, simplex or typical) and (b) quantitative autistic traits (QAT).
Note. Model fit indices for (a) Χ2(7) = 4.92; CFI = 1.0; RMSEA = .00 and (b) Χ2(4) =.75; CFI = 1.0; RMSEA = .00.
Path Estimation
After model fit was assessed, then individual path coefficients were interpreted. Estimates (Z) that reached the critical ratio of 1.96 were considered significant. Standardized path coefficients (β) are provided in Figure 2. The standardized path coefficients allow for direct comparisons of magnitude across paths.
Results
Model 1
This model was specified to capture the relations between (1) family risk status (i.e., simplex, multiplex, and typical) and family QAT and (2) family risk status and infant sibling outcomes (Figure 1a). This model also includes the expected relations between mother, father, and sibling QAT. For example, current theories of autism posit an assortative mating bias in families of children with ASD (Baron-Cohen, 2006); therefore, we specified an association between mother and father QAT. Additionally, infant sibling gender was included given the increased risk for ASD in male children.
Research Question 1
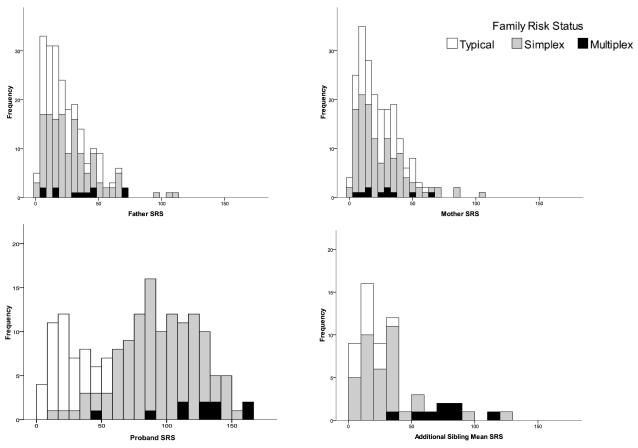
Families of children with an ASD reported elevated QAT (Figure 2a; Table 2). Father QAT was significantly higher in families of children with an ASD than families of typically developing children but there were no significant differences in father QAT between simplex and multiplex groups. Proband QAT was highest in the multiplex group followed by the simplex and typical groups (significant differences present between each group). Similarly, the mean QAT of additional siblings was significantly higher in the multiplex group, followed by the simplex and typical groups. There were no significant differences in mother QAT across the groups. Histograms of raw SRS scores for each family member are provided in Figure 3.
Figure 3.
Histograms of father, mother, proband and additional sibling raw SRS scores across family risk group status.
Research Question 2
As predicted and consistent with previous studies (e.g., Zwaigenbaum et al., 2005), the present sample of infant siblings from families with an older child with ASD had elevated rates of ASD or other developmental concerns when compared to families of typically developing older siblings (Figure 2a; Table 3). Infant siblings from the multiplex group were significantly more likely to develop an ASD than siblings in the simplex or typical groups. Over half of the infant siblings from the multiplex group (7 of 11) developed an ASD compared to only 9% (11 of 124) of the simplex and 4% (3 of 82) of the typical group. Infant siblings from the simplex group had higher rates of other developmental concerns (n = 34, 27%) than infant siblings from the typical group (n = 9, 11%). A majority of infant siblings from the simplex group (64%, n = 79) and the typical group (85%, n = 70) were classified as typically developing.
Table 3.
Infant sibling outcomes by family risk group (n= 217).
| Infant Sibling Outcome Group |
Family RiskGroup n (%) |
Comparisons Χ2(df= 1) |
||||
|---|---|---|---|---|---|---|
| Typical | Simplex | Multiplex | Typical vs. Simplex |
Typical vs. Multiplex |
Simplex vs. Multiplex |
|
| TYP | 70(85%) | 79(64%) | 2(18%) | 11.57** | 25.04** | 8.73** |
| ASD | 3 (4%) | 11 (9%) | 7 (64%) | 2.12 | 36.36** | 26.22** |
| DC | 9 (11%) | 34 (27%) | 2 (18%) | 8.08** | .48 | .44 |
Note. TYP = typical, DC = developmental concerns, ASD = autism spectrum disorders, Bonferroni method used to control Type I error across multiple comparisons
p < .01
p < .05
Model 2
This model was specified to test the relations between family QAT and infant sibling outcomes (Figure 1b). As in Model 1, this model included additional expected relations between mother, father, and sibling QAT and gender.
Research Question 3
Neither father nor mother QAT helped inform infant sibling outcomes, but both proband and additional sibling QAT did predict outcomes (Figure 2b; Table 4). Infant siblings who developed ASD or other developmental concerns had a proband with elevated QAT. This was accounted for by significantly higher proband QAT in the AUT and DC groups when compared to the TYP group. Additional sibling QAT also informed infant sibling outcomes, with more affected additional siblings (higher QAT) predicting ASD group membership. Additional sibling QAT was significantly higher in the AUT group when compared to the DC and TYP groups, who did not differ from each other.
Table 4.
ANOVA results for proband and additional sibling QAT in infant sibling typical, developmental concerns and ASD groups.
| Mean Difference (SE) |
||||||
|---|---|---|---|---|---|---|
| F | Infant Sibling Outcome Group |
n | M (SD) | DC | ASD | |
| Proband QAT | F (2,165) = 4.69** | TYP | 116 | 71.40(42.11) | −18.43(7.82)* | −24.60(10.54)* |
| DC | 35 | 89.83(38.76) | - | −6.17(11.99) | ||
| ASD | 17 | 96.00(31.86) | - | |||
| Additional Siblings | F (2, 55) = 9.34** | TYP | 35 | 24.03(18.56) | −10.68(7.29) | −42.97(10.01)** |
| Mean QAT | DC | 16 | 34.71(31.17) | - | −32.29(10.95)** | |
| ASD | 7 | 67.00(31.19) | - | |||
Note. QAT = quantitative autistic traits, TYP = typical, DC = developmental concerns, ASD = autism spectrum disorders
p < .01,
p < .05
This model did not address whether the severity of autism in the proband helped predict outcomes, since the model included typically developing older siblings as well. To examine this additional question, a second post-hoc model was completed for only families of children with an ASD. Within this model (Χ2(4) = 2.34, p = .67; CFI = 1.00; RMSEA = .00), proband severity (z = .75, p = .45) did not predict infant sibling outcomes. Likewise, when assessing only families of children with an ASD, additional sibling severity (z = -1.40, p = .16) did not predict infant sibling outcomes.
Additional Relations
The additional relations described below were included in both Models 1 and 2. Consistent with assortative mating theories of autism, higher father QAT was associated with higher mother QAT. Given the undetermined genetic loading associated with social and communication difficulties and the previously reported generational transmission of such difficulties (e.g., Constantino & Todd, 2003, 2005), we specified paths between father, mother and sibling QAT. Additionally, we specified an association between siblings, under the assumption that they will share some undetermined genetic combination of their mother and father. Father QAT predicted proband QAT but not additional sibling QAT (Figure 2). Mother QAT did not predict proband or additional sibling QAT. As predicted, proband QAT was associated with additional sibling QAT. Thus, more severely affected probands had siblings with more social and communication problems. Consistent with previous research, gender was also significantly associated with infant sibling outcomes. A higher ratio of male children (n = 19, 16%) developed an ASD compared to female children (n = 2, 2%). Similarly, 29 (25%) of male children were classified in the DC group compared with 16 (16%) of female children.
Discussion
Replicating the findings of previous retrospective, cross-sectional, and family history investigations, the present prospective longitudinal study found elevated social and communication features of the BAP in siblings and fathers of children with an ASD but not in mothers (Bailey et al., 1998; Constantino et al., 2006; Virkud et al., 2008). Social and communication features of the BAP in mothers, fathers and undiagnosed siblings did not inform infant sibling outcomes. However, Infant siblings from multiplex families were at significantly higher risk for ASD than infant siblings from simplex families.
Our first research question assessed if quantitative autistic traits vary across categorical groupings of sibling affectedness (i.e., typical, simplex, multiplex). As predicted, QAT was highest in the multiplex group, followed by the simplex group, and lowest in the typical group. This replicates previous studies (Constantino et al., 2008; Losh et al., 2008; Virkud et al., 2008) but adds a novel piece to the literature by using prospective methods and a different type of sample (infant sibling). Group differences in communication and reciprocal social relationships were present in fathers and siblings, but not mothers, a finding which is also consistent with some previous reports (e.g., Virkud et al., 2008). To our knowledge this is the first infant sibling study to report significantly higher QAT in multiplex families. However, given the small number of multiplex families who participated in this study, caution is warranted in interpreting these results. Replication with larger samples is needed to assess if indeed social-communication elements of the BAP are elevated in multiplex families. If replicated, these findings could have implications for family counseling.
Our second research question examined whether categorical indices of family affectedness (i.e., typical, simplex, multiplex) informed infant sibling outcomes at 36 months. As predicted, being from a multiplex family conferred the greatest risk for ASD in the infant siblings, with over half of the children in the multiplex group developing an ASD. The present study is not the first to report elevated recurrence or affectedness rates in multiplex families (e.g., Ritvo et al., 1989; Szatmari et al., 2000) but is the first prospective study to report such. The ratio of children who developed ASD in this multiplex sample is considerably higher than reported in previous infant sibling investigations (e.g., Yoder, Stone, Walden, & Malesa, 2009; Zwaigenbaum et al., 2005), but previous studies did not stratify outcomes by simplex or multiplex family status. Again, the multiplex findings of this study need to be approached cautiously, but with replication in larger samples, these findings could inform genetic counseling approaches.
Although the rate of ASD outcomes was twice as high in the simplex families (9%) as the typical families (4%), this difference was not statically significant. However, both rates are much lower than the ASD rate in multiplex families (64%). ASD outcomes in the simplex families are also significantly lower than reported in other infant sibling studies (e.g., Zwaigenbaum et al., 2005). This is likely due to the fact that other published investigations did not separately examine outcome rates in simplex and multiplex families.
Adverse outcome rates would be higher in simplex families if ‘affectnedness’ was defined more broadly. Within this study, simplex families had a rate (27%) of other developmental concerns that was elevated relative to the typical families. These adverse developmental outcomes (e.g., speech and language delays, separation anxiety, mild social delays) may reflect elements of the broader autism phenotype and, as such, may represent an alternate form of “recurrence” in simplex families. The high rate of other developmental concerns in simplex families is also important from an early intervention standpoint. As the rate of ASD increases, so does the rate of infant siblings from ASD families. This growing population should be targeted for early screening to identify not only early signs of ASD but also speech and language delays, social difficulties, and other global developmental concerns that may require services.
Our final research question assessed if a dimensional definition of family affectedness could inform infant sibling outcomes. As with the categorical approach, more affected siblings predicted higher rates of ASD or other developmental concerns in infant siblings. This finding was, however, driven by the inclusion of typical families. When they were removed from the analysis, sibling affectedness in the high risk (simplex and multiplex) families no longer predicted infant outcomes. Therefore, we did not find evidence that the severity of autism in probands (or other affected siblings) was informative in identifying which infants would develop ASD.
Subclinical social and communication features in parents did not inform infant sibling outcomes. Differential levels of the BAP were identified in fathers (research question 1) but did not inform infant sibling outcomes (research question 3). This finding is difficult to interpret. It could be that the presence of social-communication features of the BAP in fathers confers greater risk for ASD when compared to the general population but does not contribute further to the number of affected children a family will ultimately have. Previous reports speculate that ASD in simplex and multiplex families may follow different genetic and physiological pathways (e.g., Losh et al., 2008). The finding that father QAT was associated with proband QAT but not additional sibling QAT or infant sibling outcomes is consistent with this hypothesis. Further research is needed to tease apart the relations between father social-communication difficulties and offspring risks in larger samples with both simplex and multiplex families.
In addition to the three specific research questions, several additional ‘paths’ were specified in our models to examine relations among mother, father, proband, and additional sibling QAT. Echoing the findings of Virkud and colleagues (2008), there was a robust association between mother and father QAT in all families, providing preliminary support for an assortative mating hypothesis. As illustrated in Constantino and Todd (2005) and Hoekstra et al (2007) an association provides only preliminary support. It is a necessary but not sufficient element toward establishing mating bias. Additionally, higher QAT in probands was associated with higher QAT in their fathers and their siblings.
The present study contains several strengths including a prospective-longitudinal design, a typically developing comparison group, and gold standard autism diagnostic and outcome measures. However, this study is not without a number of limitations. One is the relatively early age of outcome assessment (36 months). Although many studies have shown good reliability and validity of autism diagnosis at this age (Chawarska, Klin, Paul & Volkmar, 2007; Kleinman et al., 2008), it may not capture children yet to be diagnosed with Asperger syndrome or other developmental difficulties that emerge at later ages (e.g., learning disabilities, ADHD). Therefore, there are likely children in the typical outcome group whose developmental concerns are not yet apparent. This, however, presents a conservative bias and we were still able to find many significant relationships between family risk and infant outcome, despite some possible misclassification (false negatives) at this young age.
A second limitation is that the classification of some families as simplex may be inaccurate, as some of the simplex families may become multiplex with the addition of another child. Only 36 (29%) of the 124 simplex families we studied had at least one typically developing child (in addition to the proband with ASD) to provide clearer evidence of simplex status. An unknown proportion of these families could become multiplex with the addition of more children. Another limitation is the small number of multiplex families enrolled (n = 11); our results and their interpretation clearly require replication in larger multiplex samples.
Finally, it is worth reiterating that the rates of ASD and other adverse developmental outcomes in this study should not be thought of as recurrence risk estimates. Participating families were not a representative community sample and were more likely to refer themselves to the study if they already had concerns about their infant (Ozonoff et al., 2009). Relatedly, the present sample may have included a higher proportion of multiplex families than occur naturally in the ASD population. Either or both of these situations would serve to inflate the rates of ASD and developmental concerns reported here.
In summary, the present study used a prospective longitudinal design to replicate previous cross-sectional and family history studies, finding elevated social and communication features of the BAP in siblings and fathers of ASD families. Both categorical and dimensional measures of sibling affectedness helped inform infant sibling outcomes. This work may, if replicated, help researchers, clinicians, and families identify children at greatest risk for ASD or other developmental concerns.
Key Points.
It is not clear whether the “affectedness” of family members can help predict which infants are at increased risk for ASD or other developmental concerns.
Using a prospective design, we replicated previous findings that families of children with ASD demonstrate elevated rates of social and communication features associated with the broader autism phenotype (BAP).
Social and communication features of the BAP in siblings were significantly higher in multiplex than simplex families.
Subclinical social and communication features of the BAP in mothers, fathers and unaffected siblings did not predict ASD outcomes in infants. However, having multiple family members with ASD (e.g., being from a multiplex family) did confer significantly higher risk for ASD outcomes.
Acknowledgements
The work in this manuscript was supported by grants R01 MH068398 (Ozonoff) and U54 MH068172 (Sigman) from the National Institute of Mental Health. We are grateful to Diane Larzelere for preparation of the manuscript. A special thank you to the children and families who participated in this longitudinal study.
Footnotes
Disclosure Statement.
The authors have no conflicts of interest.
References
- Arbuckle JL. Full information estimation in the presence of incomplete data. In: Marcoulides GA, Schumacker RE, editors. Advanced structural equation modeling: Issues and techniques. Erlbaum; Hillsdale, NJ: 1996. pp. 243–277. [Google Scholar]
- Bagozzi R, Yi Y. On the evaluation of structural equation models. Journal of the Academy of Marketing Science. 1988;16:74–94. [Google Scholar]
- Bailey A, Palferman S, Heavey L, Le Couteur A. Autism: The phenotype in relatives. Journal of Autism and Developmental Disorders. 1998;28(5):369–392. doi: 10.1023/a:1026048320785. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. The hyper-systemizing, assortative, mating theory of autism. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2006;30(5):865–872. doi: 10.1016/j.pnpbp.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Brian J, Bryson SE, Garon N, Roberts W, Smith IM, Szatmari P, et al. Clinical assessment of autism in high-risk 18-month-olds. Autism. 2008;12(5):433–456. doi: 10.1177/1362361308094500. [DOI] [PubMed] [Google Scholar]
- Browne M, Cudeck R. Alternative methods of assessing model fit. Sociological Methods & Research. 1992;21:230–258. [Google Scholar]
- Constantino JN, Abbacchi AM, Lavesser PD, Reed H, Givens L, Chiang L, et al. Developmental course of autistic social impairment in males. Development and Psychopathology. 2009;21:127–138. doi: 10.1017/S095457940900008X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. Comparison of the Social Responsiveness Scale with the Autism Diagnostic Interview-Revised. Journal of Autism and Developmental Disorders. 2003;33(4):427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Lajonchere C, Lutz M, Gray T, Abbacchi A, McKenna K, Singh D, Todd RD. Autistic social impairment in the siblings of children with pervasive developmental disorder. American Journal of Psychiatry. 2006;163(2):294–296. doi: 10.1176/appi.ajp.163.2.294. [DOI] [PubMed] [Google Scholar]
- Constantino JN, LaVesser PD, Zhang Y, Abbacchi AM, Gray T, Todd RD. Rapid quantitative assessment of autistic social impairment by classroom teachers. Journal of American Academy of Child and Adolescent Psychiatry. 2007;46(12):1668–1676. doi: 10.1097/chi.0b013e318157cb23. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Przybeck T, Friesen D, Todd RD. Reciprocal social behavior in children with and without pervasive developmental disorders. Developmental and Behavioral Pediatrics. 2000;21(1):2–11. doi: 10.1097/00004703-200002000-00002. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Autistic traits in the general population. Archives of General Psychiatry. 2003;60:524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Constantino J, Todd R. Intergenerational transmission of subthreshold autistic traits in the general population. Biological Psychiatry. 2005;57(6):655–660. doi: 10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Elder LM, Dawson G, Toth K, Fein D, Munson J. Head circumference as an early predictor of autism symtpoms in younger siblings of children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38:1104–1111. doi: 10.1007/s10803-007-0495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamliel I, Yirmiya N, Sigman M. The development of young siblings of children with autism from 4 to 54 months. Journal of Autism and Developmental Disorders. 2007;37:171–183. doi: 10.1007/s10803-006-0341-5. [DOI] [PubMed] [Google Scholar]
- Hoekstra RA, Bartels M, Verweij CJH, Boomsma DI. Heritability of autistic traits in the general population. Archives of Pediatric and Adolescent Medicine. 2007;161:372–377. doi: 10.1001/archpedi.161.4.372. [DOI] [PubMed] [Google Scholar]
- Kaplan D. Structural Equation Modeling: Foundations and extensions. 2nd ed Thousand Oaks; Sage: 2009. [Google Scholar]
- Kenward MG, Molenberghs G. Likelihood based frequentist interference when data are missing at random. Statistical Science. 1998;13:236–247. [Google Scholar]
- Kleinman JM, Robins DL, Ventola PE, Pandey J, Boorstein HC, Esser EL, et al. The Modified Checklist for Autism in Toddlers: A follow-up study investigating the early detection of autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38(5):827–839. doi: 10.1007/s10803-007-0450-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: A prospective study. Journal of Child Psychology and Psychiatry. 2006;47(6):629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. The analysis of social science data with missing values. Sociological Methods and Research. 1989;18:292–326. [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Dilavore PC, Risi S. Autism Diagnostic Observation Schedule. Western Psychological Services; Los Angeles, CA: 2002. [Google Scholar]
- Losh M, Childress D, Lam K, Piven J. Defining key features of the broad autism phenotype: A comparison across parents of multiple- and single-incidence autism families. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2008;147B:424–433. doi: 10.1002/ajmg.b.30612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen M. Mullen Scales of Early Learning. AGS; Circle Pines, MN: 1995. [Google Scholar]
- Muthén LK, Muthén BO. Mplus. Version 5 Muthén & Muthén; Los Angeles, CA: 2007. [Google Scholar]
- Ozonoff S, Macari S, Young GS, Goldring S, Thompson M, Rogers SJ. Atypical object exploration at 12 months of age is associated with autism in a prospective sample. Autism. 2008;12(5):457–472. doi: 10.1177/1362361308096402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Steinfeld MB, Hill MM, Cook I, Hutman T, et al. How early do parent concerns predict later autism diagnosis? Journal of Developmental and Behavioral Pediatrics. doi: 10.1097/dbp.0b013e3181ba0fcf. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven J. The broad autism phenotype: A complementary strategy for molecular genetic studies of autism. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2001;105:34–35. [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Volk HE, Todd RD. Autistic traits in a population-based ADHD twin sample. Journal of Child Psychology and Psychiatry. 2007;48(5):464–472. doi: 10.1111/j.1469-7610.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- Rogers S. What are infant siblings teaching us about autism in infancy? Autism Research. 2009;1:1–13. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruser TF, Arin D, Dowd M, Putnam S, Winklosky B, Rosen-Sheidley B, et al. Communicative competence in parents of children with autism and parents of children wtih specific language impairment. Journal of Autism and Developmental Disorders. 2007;37:1323–1336. doi: 10.1007/s10803-006-0274-z. [DOI] [PubMed] [Google Scholar]
- Virkud YV, Todd RD, Abbacchi AM, Zhang Y, Constantino JN. Familial aggregation of quantitative autistic traits in multiplex versus simplex autism. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;150B(3):328–334. doi: 10.1002/ajmg.b.30810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder P, Stone WL, Walden T, Malesa E. Predicting social impairment and ASD diagnosis in younger siblings of children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2009 doi: 10.1007/s10803-009-0753-0. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GS, Merin N, Rogers SJ, Ozonoff S. Gaze behavior and affect at 6 months: predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Developmental Science. 2009 doi: 10.1111/j.1467-7687.2009.00833.x. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]