Abstract
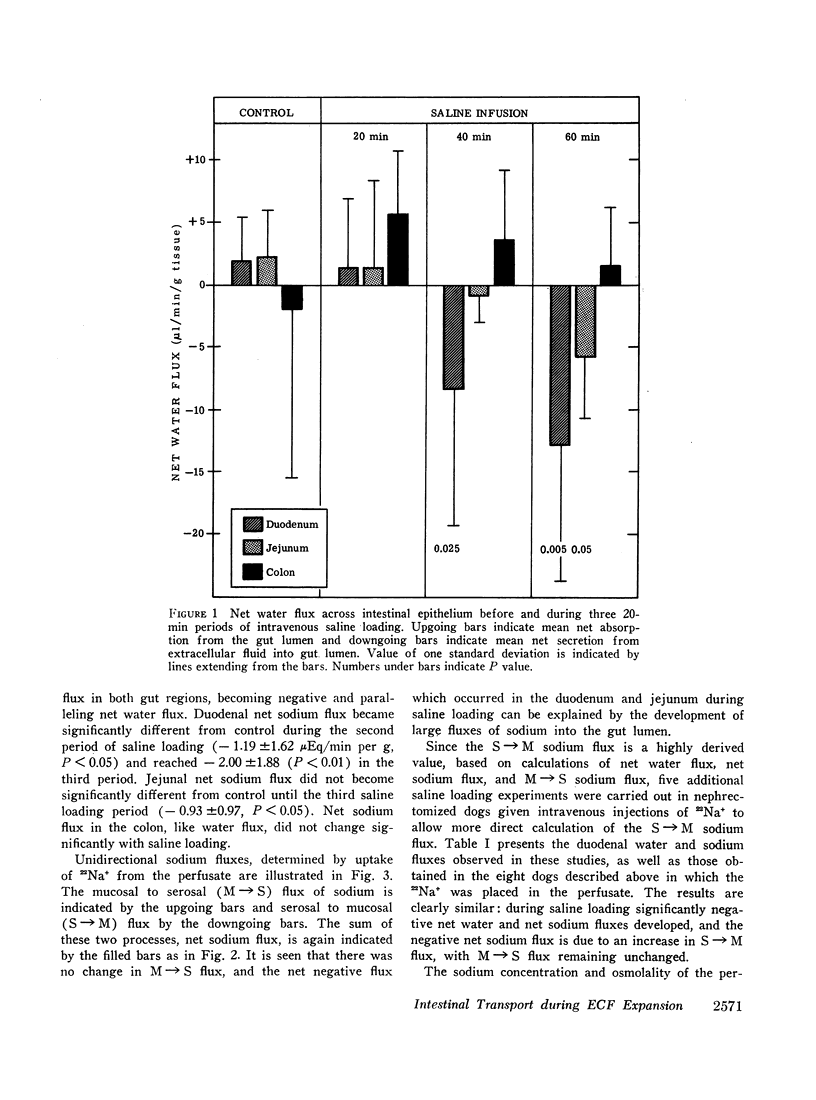
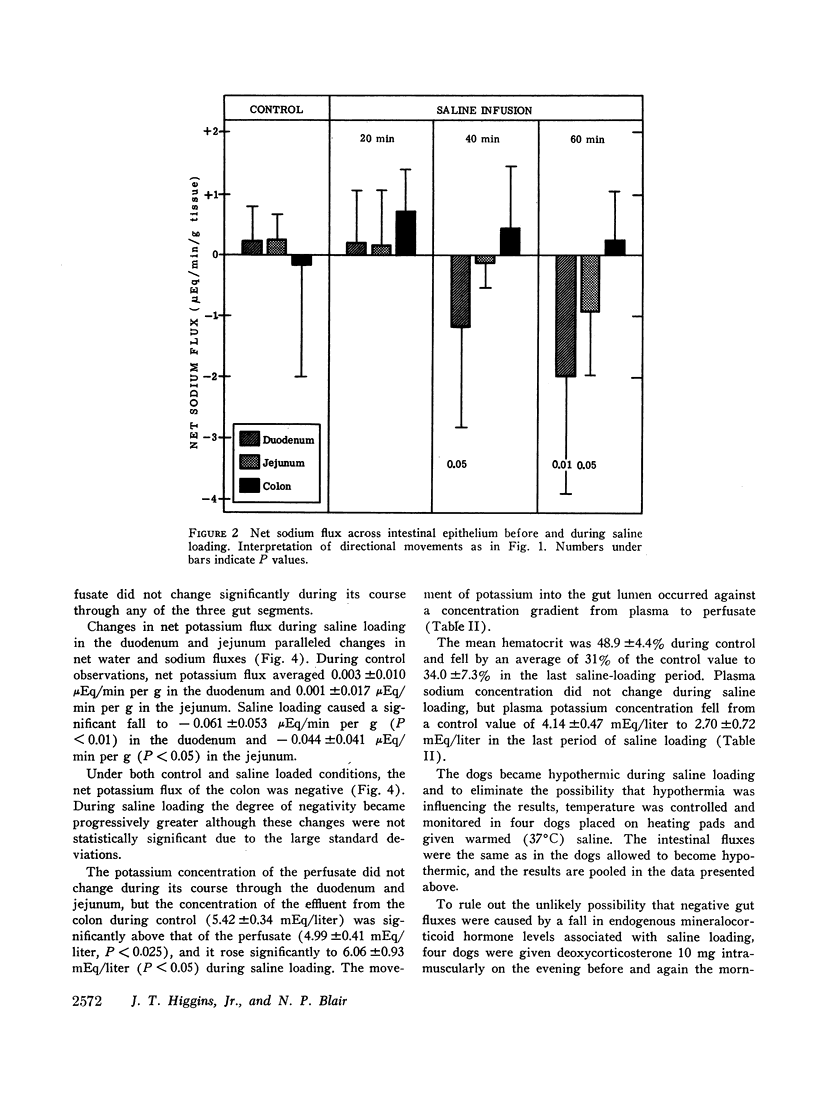
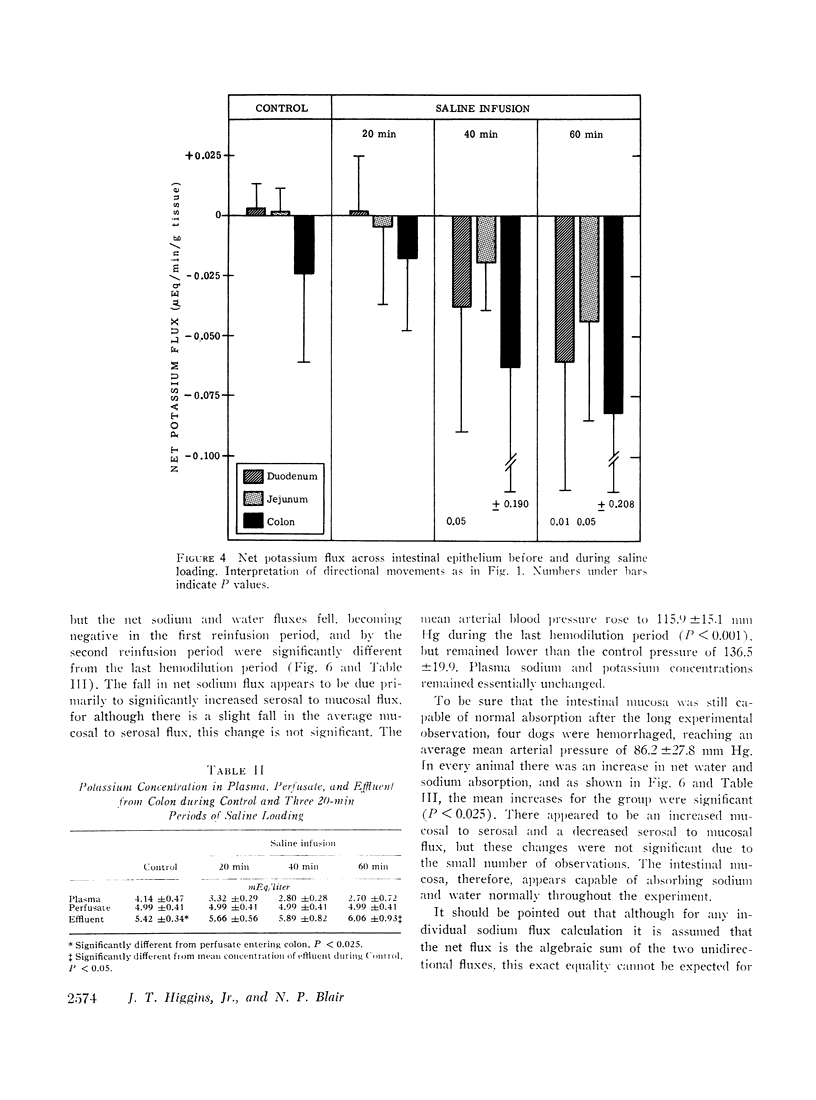
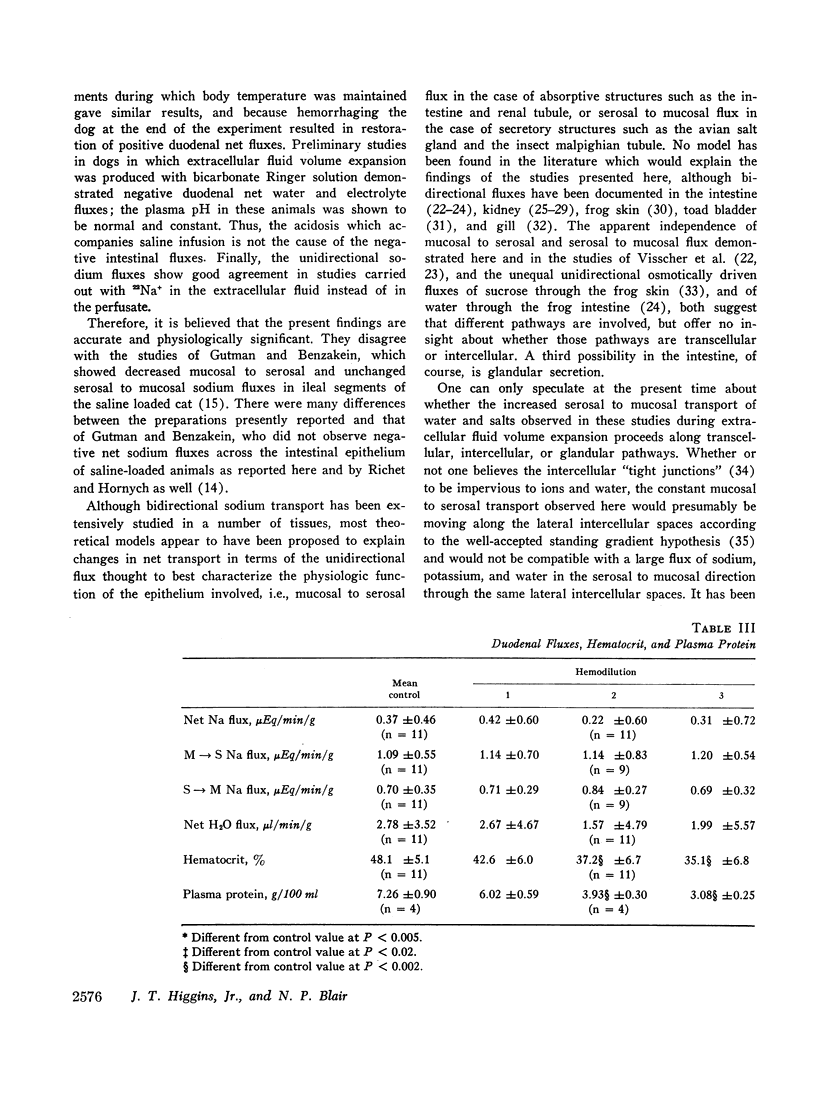
The effects of extracellular fluid volume expansion on intestinal transport of salts and water were studied in dogs by perfusing loops of bowel in vivo. Saline loading caused depression of duodenal and jejunal absorption with net secretion of salt and water into the lumen. Studies of unidirectional transport of 22Na+ revealed that the negative net sodium flux was due primarily, and perhaps exclusively, to increased serosal to mucosal transport, for mucosal to serosal sodium transport was not changed during volume expansion. Net transport of water and potassium paralleled net sodium flux. Administration of deoxycorticosterone did not affect the intestinal response to saline loading. Hemodilution, accomplished by equilibrating the dogs' blood with a reservoir of saline, did not affect intestinal absorption, but isotonic, iso-oncotic expansion of the extracellular fluid produced by reinfusing the saline-blood mixture from the reservoir resulted in negative net transport of water, sodium, and potassium by the duodenum. It is suggested that the small bowel is capable of secreting salts and water through intercellular spaces, and that this process is stimulated by extracellular fluid volume expansion.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentzel C. J., Parsa B., Hare D. K. Osmotic flow across proximal tubule of Necturus: correlation of physiologic and anatomic studies. Am J Physiol. 1969 Aug;217(2):570–580. doi: 10.1152/ajplegacy.1969.217.2.570. [DOI] [PubMed] [Google Scholar]
- Bricker N. S., Klahr S., Purkerson M., Schultze R. G., Avioli L. V., Birge S. J. In vitro assay for a humoral substance present during volume expansion and uraemia. Nature. 1968 Sep 7;219(5158):1058–1059. doi: 10.1038/2191058a0. [DOI] [PubMed] [Google Scholar]
- Buckalew V. M., Jr, Martinez F. J., Green W. E. The effect of dialysates and ultrafiltrates of plasma of saline-loaded dogs on toad bladder sodium transport. J Clin Invest. 1970 May;49(5):926–935. doi: 10.1172/JCI106312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHINARD F. P., ENNS T. Relative renal excretion patterns of sodium ion, chloride ion, urea, water and glomerular substances. Am J Physiol. 1955 Aug;182(2):247–250. doi: 10.1152/ajplegacy.1955.182.2.247. [DOI] [PubMed] [Google Scholar]
- DAVIS J. O., HOLMAN J. E., CARPENTER C. C., URQUHART J., HIGGINS J. T., Jr AN EXTRA-ADRENAL FACTOR ESSENTIAL FOR CHRONIC RENAL SODIUM RETENTION IN PRESENCE OF INCREASED SODIUM-RETAINING HORMONE. Circ Res. 1964 Jan;14:17–31. doi: 10.1161/01.res.14.1.17. [DOI] [PubMed] [Google Scholar]
- Davis B. B., Jr, Knox F. G. Current concepts of the regulation of urinary sodium excretion--a review. Am J Med Sci. 1970 Jun;259(6):373–382. doi: 10.1097/00000441-197006000-00002. [DOI] [PubMed] [Google Scholar]
- Diamond J. M. Standing-gradient model of fluid transport in epithelia. Fed Proc. 1971 Jan-Feb;30(1):6–13. [PubMed] [Google Scholar]
- Earley L. E., Daugharty T. M. Sodium metabolism. N Engl J Med. 1969 Jul 10;281(2):72–86. doi: 10.1056/NEJM196907102810205. [DOI] [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. Junctional complexes in various epithelia. J Cell Biol. 1963 May;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordtran J. S. Marker perfusion techniques for measuring intestinal absorption in man. Gastroenterology. 1966 Dec;51(6):1089–1093. [PubMed] [Google Scholar]
- Gutman Y., Benzakein F. Effect of saline loading on absorption from the cat ileum in vivo. Isr J Med Sci. 1970 Mar-Apr;6(2):195–200. [PubMed] [Google Scholar]
- Hayslett J. P., Kashgarian M., Epstein F. H. Changes in proximal and distal tubular reabsorption produced by rapid expansion of extracellular fluid. J Clin Invest. 1967 Jul;46(7):1254–1263. doi: 10.1172/JCI105618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C. I., Davis J. O. Evidence from cross circulation studies for a humoral mechanism in the natriuresis of saline loading. Proc Soc Exp Biol Med. 1966 Apr;121(4):1058–1063. doi: 10.3181/00379727-121-30965. [DOI] [PubMed] [Google Scholar]
- LEAF A., ANDERSON J., PAGE L. B. Active sodium transport by the isolated toad bladder. J Gen Physiol. 1958 Mar 20;41(4):657–668. doi: 10.1085/jgp.41.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINSKY N. G., LALONE R. C. THE MECHANISM OF SODIUM DURESIS AFTER SALINE INFUSION IN THE DOG. J Clin Invest. 1963 Aug;42:1261–1276. doi: 10.1172/JCI104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson R. A., Schedl H. P. Absorption of sodium, chloride, water, and simple sugars in rat small intestine. Am J Physiol. 1966 Oct;211(4):939–942. doi: 10.1152/ajplegacy.1966.211.4.939. [DOI] [PubMed] [Google Scholar]
- Lewy J. E., Windhager E. E. Peritubular control of proximal tubular fluid reabsorption in the rat kidney. Am J Physiol. 1968 May;214(5):943–954. doi: 10.1152/ajplegacy.1968.214.5.943. [DOI] [PubMed] [Google Scholar]
- Loeschke K., Bentzel C. J., Csáky T. Z. Asymmetry of osmotic flow in frog intestine: functional and structural correlation. Am J Physiol. 1970 Jun;218(6):1723–1731. doi: 10.1152/ajplegacy.1970.218.6.1723. [DOI] [PubMed] [Google Scholar]
- Maetz J. Seawater teleosts: evidence for a sodium-potassium exchange in the branchial sodium-excreting pump. Science. 1969 Oct 31;166(3905):613–615. doi: 10.1126/science.166.3905.613. [DOI] [PubMed] [Google Scholar]
- Maude D. L. Mechanism of salt transport and some permeability properties of rat proximal tubule. Am J Physiol. 1970 Jun;218(6):1590–1595. doi: 10.1152/ajplegacy.1970.218.6.1590. [DOI] [PubMed] [Google Scholar]
- Nutbourne D. M., Howse J. D., Schrier R. W., Talner L. B., Ventom M. G., Verroust P. J., De Wardener H. E. The effect of expanding the blood volume of a dog on the short-circuit current across an isolated frog skin incorporated in the dog's circulation. Clin Sci. 1970 Jun;38(6):629–648. doi: 10.1042/cs0380629. [DOI] [PubMed] [Google Scholar]
- Oschman J. L., Berridge M. J. The structural basis of fluid secretion. Fed Proc. 1971 Jan-Feb;30(1):49–56. [PubMed] [Google Scholar]
- Richet G., Hornych A. The effect of an expansion of extracellular fluids on net Na flux in the jejunum of rats. An experimental model for the study of the third factor. Nephron. 1969;6(3):365–378. doi: 10.1159/000179739. [DOI] [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Ussing H. H. Anomalous transport of electrolytes and sucrose through the isolated frog skin induced by hypertonicity of the outside bathing solution. Ann N Y Acad Sci. 1966 Jul 14;137(2):543–555. doi: 10.1111/j.1749-6632.1966.tb50180.x. [DOI] [PubMed] [Google Scholar]
- Watson J. F. Effect of saline loading on sodium reabsorption in the dog proximal tubule. Am J Physiol. 1966 Apr;210(4):781–785. doi: 10.1152/ajplegacy.1966.210.4.781. [DOI] [PubMed] [Google Scholar]
- Wright F. S., Knox F. G., Howards S. S., Berliner R. W. Reduced sodium reabsorption by the proximal tubule of Doca-escaped dogs. Am J Physiol. 1969 Apr;216(4):869–875. doi: 10.1152/ajplegacy.1969.216.4.869. [DOI] [PubMed] [Google Scholar]
- de Rouffignac C., Morel F. Micropuncture study of water, electrolytes, and urea movements along the loops of henle in psammomys. J Clin Invest. 1969 Mar;48(3):474–486. doi: 10.1172/JCI106005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wardener H. E. Control of sodium reabsorption. Br Med J. 1969 Sep 20;3(5672):676–683. doi: 10.1136/bmj.3.5672.676. [DOI] [PMC free article] [PubMed] [Google Scholar]


