Abstract
The development of the notochord involves a complex set of cellular behaviors. While these morphogenic behaviors are common to all chordates, the ascidian provides a particularly attractive experimental model because of its relative simplicity. In particular, all notochord morphogenesis in ascidians takes place with only 40 cells, as opposed to the hundreds of cells in vertebrate models systems. Initial steps in ascidian notochord development convert a monolayer of epithelial-like cells in the pre-gastrula embryo to a cylindrical rod of single-cell diameter. Convergent extension is responsible for the intercalation of notochord cells and some degree of notochord elongation, while a second phase of elongation is observed as the notochord narrows medially and increases in volume. The mechanism by which the volume of the notochord increases differs between ascidian species. Some ascidian species produce extracellular pockets that will eventually coalesce to form a lumen running the length of the notochord, while others appear to make intercellular vacuoles. By either mechanism, the resulting notochord serves as a hydrostatic skeleton allowing for the locomotion of the swimming larva. Several basic cell behaviors, such as cell shape changes, cell rearrangement, establishment of cell polarity, and alteration of extracellular environment, are displayed in the process of notochord morphogenesis. Modern analysis of ascidian notochord morphogenesis promises to contribute to our understanding of these fundamental biological processes.
Introduction
The notochord is one the defining characteristics of the Chordates (and is the root of the name chordate). In extant chordate classes the precise functions of the notochord differ, but the unifying role of the notochord is that of an axial scaffold which acts to both physically shape and elongate the embryo, and, in vertebrates, through the secretion of various inducing factors, to pattern axial structures such as the neural tube and somites (Christ et al., 2004; Danos and Yost, 1995; Fouquet et al., 1997; Goldstein and Fishman, 1998; Lohr et al., 1997; Munsterberg and Lassar, 1995; Pourquie et al., 1993; Stemple, 2005; Yamada et al., 1993; Yamada et al., 1991). The ascidian notochord does not appear to have inductive activity. For example, the secreted factor shh is expressed in vertebrates in the ventral neural tube and notochord, while its ortholog in Ciona is expressed only in the ventral neural tube (Takatori et al., 2002). One possibility is that inductive activity of the notochord co-evolved with new vertebrate tissues such as sclerotome, whose induction from the somite requires the notochord. However, it is also possible that ascidians (and the larger group of animals known as tunicates, to which ascidians belong) lost the inductive activities of the notochord. It is becoming clear that the tunicates should not be viewed as ‘basal” chordates, but rather as animals that had a more complicated chordate ancestor that became simplified. Emphasizing this simplification are results from phylogenetic analyses indicating that the tunicates, and not the cephalochordates, are the sister-group of the vertebrates (Philippe et al., 2005; Delsuc et al., 2006). Cephalochordates share a number of structures with vertebrates, such as somites, that are not present in tunicates, suggesting a simplification of the tunicate body. It will be informative to discern whether the cephalochordate notochord has inductive activity.
One role of the notochord that is often overlooked is its role as a support for locomotion in chordates with free-swimming larvae (tunicates, fish and amphibians). In fish and amphibian embryos the notochord provides a rigid support within the tail before the vertebral column has formed. To aide in swimming, the notochord forms a stiff but flexible rod running between the flanks of the trunk skeletal muscle. Thus as the opposing muscles contract and relax in an alternating pattern, the notochord acts as a spring to recoil the trunk and tail. While the roles of the notochord in gastrulation movements (Keller et al., 2003; Keller, 2006) and subsequent patterning of the axial structures are often emphasized, it is likely the original role of the notochord was as a structural element for swimming in the larva (Romer, 1966). In amniotes, whose larvae are not free swimming, the relative size of the notochord in cross-section to the total cross-sectional area is greatly reduced, perhaps reflecting the loss of this function of the notochord.
Despite the differing roles of the notochord in different species, the core morphogenic processes, such as convergent extension (Keller 2002, 2006; Keller et al., 2003), appear to be well conserved. In studying these conserved processes, the ascidian provides a unique model due to its relative simplicity in comparison to vertebrate models. The simplicity of the ascidian is reflected both at the level of the whole embryo (fewer cell types, and fewer total cells), and at the molecular/genetic level (smaller genome with fewer genes). Whether ascidians are more simple due to a basal position or to a secondary simplification (or, most likely, as combination of the two, depending on the particular trait) is of less importance than the conservation of basic cellular processes. For the notochord in particular, all morphogenetic process takes place in a group of 40 cells that appear to be terminally differentiated and mitotically inactive, a feature which isolates morphogenesis from other processes.
Notochord differentiation and development before cell rearrangement
The ascidian notochord arises from two distinct lineages: a primary lineage which derives from anterior blastomeres A7.3 and A7.7 (and their bilateral partners) producing the anterior 32 cells, and a secondary lineage which derives from the posterior blastomere B8.6 (and its bilateral partner) producing posterior 8 cells (Nishida, 1987). Specification of the notochord requires an inductive signal from the endoderm, which has been identified as fibroblast growth factor (FGF) (Kim et al., 2000; Minokawa et al., 2001; Shimauchi et al., 2001b). In the primary lineage this induction occurs at the 32-cell stage, and notochord precursors acquire developmental autonomy at the 64-cell stage. The secondary lineage differentiates slightly later and becomes restricted to notochord fate at the 110-cell stage. The gene network underlying the induction and competence of notochord precursors has recently emerged. It involves the MAP kinase cascade (Kim and Nishida, 2001; Minokawa et al., 2001; Nakatani and Nishida, 1997; Nishida, 2003), the Notch-Su(H) pathway (Corbo et al., 1998; Hudson and Yasuo, 2006), the nodal signaling pathway (Hudson and Yasuo, 2006; Imai et al., 2006), BMP pathway (Darras and Nishida, 2001), and the transcriptional factors HNF-3, ZicL, Ets, FoxA-a, and FoxD (Imai et al., 2006; Imai et al., 2002a; Imai et al., 2002b; Miya and Nishida, 2003; Shimauchi et al., 2001a).
At the 110-cell stage, coinciding with the onset of gastrulation, ten presumptive notochord cells (8 from the primary lineage and 2 from the secondary lineage) form a semicircular arc around the anterior lip of the blastopore (Fig. 1A). All cells undergo two more divisions along the anterior-posterior (A/P) axis of the embryo which occur in the ensuing 75 minutes, by which the 40 notochord cells are generated (Fig. 1B, and C). Little is known about the regulation of these two final mitotic events in the notochord lineage, although they occur immediately preceding the onset of notochord morphogenesis, making this a potentially interesting area of study. At the end of the final two mitoses, the cells of the developing notochord form a monolayer epithelium with the basal side coming in contact with the neural plate on the dorsal side, and with the apical side facing ventrally towards the blastopore and archenteron (Fig. 1 AC) (Miyamoto and Crowther, 1985; Munro and Odell, 2002b).
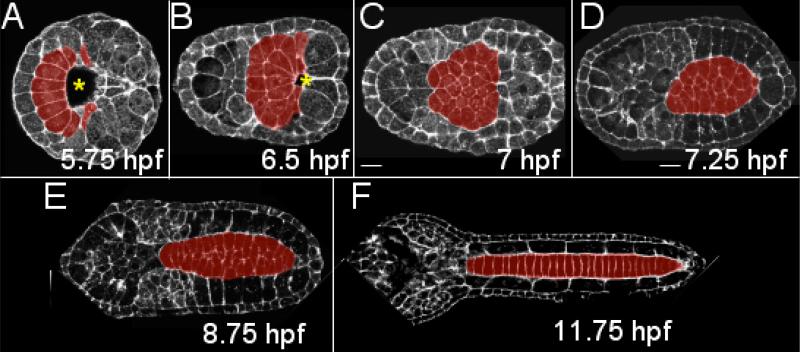
Figure 1.
Ascidian notochord development from the onset of gastrulation to the completion of convergent extension. (A) Ten presumptive notochord cells (red) forms a semicircular arc anterior to the blastopore (*). Two rounds of cell division generates 20 (B) and finally 40 notochord cells that form a monolayer epithelia (C). Infolding (not shown here) and convergent extension transform the notochord precursor into a column of 40 stacked cells (D-F). To collect these images Ciona savignyi embryos were stained with bodipy-phalloidin and imaged with a laser scanning confocal microscope. hpf: hours post fertilization at 18°C. All images are dorsal view, with anterior to the left.
Infolding and convergent extension
The transformation of the notochord precursor from a sheet of cells to a rod of single-cell diameter proceeds as an infolding (also called an invagination) of the sheet at the apical side followed by medial intercalation of the individual cells. In the process the basal surface expands to maximize contacts between notochord and the surrounding tissues. Individual notochord cells elongate in the mediolateral axis and intercalate in a process that appears identical to convergent extension as described in axial mesoderm and nervous tissue of vertebrates (Fig. 1D and E) (Keller, 2002). The convergent extension movements continue after the infolding is complete. As a result of the infolding, the cells assume a wedge-shape, and subsequently each cell's basal end extends around the cross-sectional circumference of the notochord making each cell a perfect disk. By the end of convergent extension, all of the disk-shaped cells will have intercalated into single file along the A/P axis (Fig. 1F).
These morphogenetic movements have been described by Munro and Odell in two seminal papers (Munro and Odell, 2002a; Munro and Odell, 2002b). In the beginning of convergence and extension, notochord cells become motile (Miyamoto and Crowther, 1985; Munro and Odell, 2002b) and extend actin-rich lamellipodia. This cellular behavior is intrinsic to notochord cells, and can be observed when notochord cells are cultured in isolation (Jiang et al., 2005; Munro and Odell, 2002a). Basal contact between notochord cells and their neighboring tissues provides a cue which directs notochord lamellipodia to extend preferentially along the mediolateral axis of the embryo. As a result, notochord cells converge mediolaterally and the notochord as a whole extends anterior-posteriorly (A/P). Ascidian notochord convergence is very robust in the sense that no particular neighboring tissue is required for this movement (Munro and Odell, 2002a). Munro and Odell's observations reveal the regulative nature of ascidian notochord morphogenesis and suggest a complex interplay between morphogenetic behaviors intrinsic to notochord cells and instructive interactions with surrounding tissues. Their results also highlight the similarity between the morphogenesis of ascidian notochord and axial mesoderm in vertebrates.
The molecular mechanism underlying ascidian convergence and extension movements has begun to emerge recently. It appears that the planar cell polarity (PCP) pathway which regulates convergence and extension of axial mesoderm and neural plate in vertebrates (Keller, 2002) is also essential for ascidian notochord formation. Disheveled (dsh), a central player in the PCP pathway, is required for proper notochord cell intercalation in the ascidian Ciona (Keys et al., 2002). Embryos transgenic for mutant dsh fail to elongate their tails, due to defects in notochord intercalation. We have analyzed a Ciona line in which the PCP pathway gene Prickle (pk) carries an apparent null mutation (Jiang et al., 2005). This pk mutation, known as aimless (aim), severely disrupts notochord morphogenesis. Detailed analysis showed that notochord cells in embryos homozygous for the aim mutation extended motile actin-based protrusions (as do cells from wild-type embryos), but the protrusions failed to assume the mediolateral bias that is required for convergence. Examination of the subcellular localization of Dsh protein in wildtype and homozygous aim embryos revealed a potentially important difference (Jiang et al., 2005). In wildtype embryos Dsh is membrane associated except at the notochord-muscle boundary, where it is excluded. Thus, Dsh localization displays a mediolateral polarization (Fig. 2A). In homozygous aim embryos Dsh membrane localization is disrupted (Fig. 2B), and mediolateral polarization of the notochord cells is lost. Whether this loss of Dsh polarization is the root cause of the failure of the notochord cells to converge, or is instead a down-stream consequence, remains to be determined.
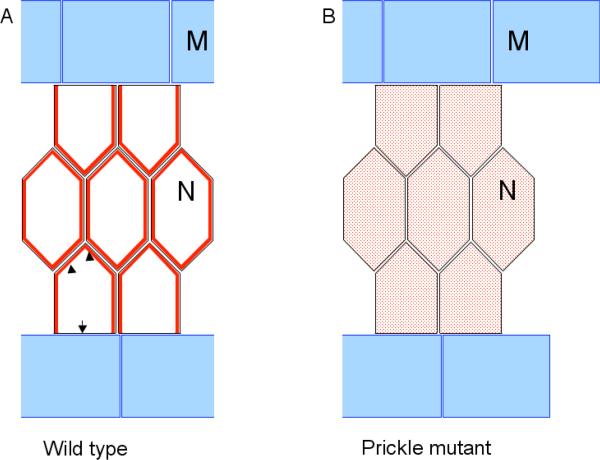
Figure 2.
Model of mediolateral polarity establishment in notochord. (A) Disheveled (red) is localized at notochord cell membrane (arrowhead) but is excluded from the portion that borders lateral muscle cells (arrow). This polarized Disheveled localization may be the result of signaling from the muscle tissue to initiate notochord intercalation along mediolateral axis. In the absence of normal Prickle activity, Disheveled membrane localization is lost and becomes cytoplasmic (red shading) (B). N: notochord; M: muscle. Dorsal view; anterior to the left.
The localization pattern of Dsh in wild type Ciona embryos may indicate a mediolateral polarizing signal from the flanking muscles. Munro and Odell found an effect by neighboring tissues on the polarization of notochord cell protrusions in the ascidian Boltenia villosa (Munro and Odell, 2002a; Munro and Odell, 2002b). In Ciona the muscles express the ortholog of vertebrate wnt-5 (Imai et al., 2004; http://hoya.zool.kyoto-u.ac.jp/download.html), which in vertebrates, along with wnt-11, has been shown to activate the PCP pathway (Heisenberg et al., 2000; Matsui et al., 2005; Moon et al., 1993; Rauch et al., 1997; Tada and Smith, 2000). Furthermore, overexpression of wnt-5 has been shown to disrupt notochord development in the ascidian Halocynthia roretzi (Sasakura and Makabe, 2001). Complicating this picture, however, it appears that H. roretzi may have more than one wnt-5 like gene (Miya and Nishida, 2002; Sasakura, et al. 1998), one of which is expressed in the notochord itself.
Despite the importance of the PCP pathway to notochord morphogenesis it should be pointed out that in embryos overexpressing mutant dsh, and in homozygous aim embryos, the field of notochord cells do not simply remain as the broad sheet of cells as is seen in the gastrula/early neural embryo (Fig. 1 A-C), but rather shows extensive mediolateral narrowing and elongation along the A/P axis, albeit with defective intercalation. This suggests that other forces besides PCP activity play important roles in shaping the notochord. These could include passive stretching of the notochord field as other tissues, such as muscle, elongate. In addition, it has been shown in vertebrates that the boundary between the notochord and neighboring tissues has apparent axis-organizing activity. In Xenopus, notochord cells that come into contact with the notochord/somites boundary assume polarized protrusive activity (Domingo and Keller, 1995). While this boundary phenomenon may be related to the PCP pathway, models suggest that boundaries can have organizing activity on mesoderm independent of other spatial cues (Green et al., 2004).
A/P regionalization of the ascidian notochord
The intercalation of cells at the midline from two rows of converging cells is stochastic (i.e., notochord cells from the left and the right sides of the embryo mix randomly at the midline) (Miyamoto and Crowther, 1985; Munro and Odell, 2002b; Nishida, 1987). However, the 32 cells derived from the primary cell lineage are never observed to mix with the 8 cells of the secondary notochord lineage. Beyond this distinction between the anterior primary and posterior secondary lineages, there appears to be no A/P regionalization of the notochord in ascidians in terms of morphology or gene expression. It is interesting to compare the notochord development in ascidians to Oikopleura dioica, which belongs to the appendicularians, a sister group to ascidians. The notochord in Oikopleura dioica consists of just 20 cells in a single file along the A/P axis. A recent study uncovered the differential expression of five hox genes in the Oikopleura notochord (Seo et al., 2004). No hox gene has been found to express in Ciona intestinalis notochord (Ikuta et al., 2004). The fact that vertebrates also show hox gene expression in the notochord (Prince et al., 1998), suggests that the absence of hox expression in the ascidian notochord may be a derived feature. The significance of hox-gene expression in the Oikopleura notochord is not known, although it could conceivably result in regional specification of the notochord itself or of neighboring tissues. It is also not known if the hox gene expression in Oikopleura precedes notochord morphogenesis, and thus may control the A/P lineage, or if expression follows notochord morphogenesis.
Roles of notochord morphogenesis in shaping of ascidian embryo
One of the mechanical functions of notochord is to elongate the embryonic A/P axis. Several studies in amphibian or chick embryos have shown that removal or disruption of the notochord greatly reduces the elongation of embryos during development (Adams et al., 1990; Keller, 2006). Analysis of the Ciona notochord mutant aim demonstrates the requirement of the notochord for ascidian embryo elongation. The phenotype of this mutant is in accordance with the observation by Reverberi et al. showing that the tail of ascidian tadpoles from which the notochord is removed surgically is greatly reduced in length (Reverberi, 1960). It is not known if the notochord is the only driving force for tail elongation, although the phenotype of another mutant, Chongmague, suggests at that posterior tail epidermis can elongate to certain extent even when notochord elongation is disrupted (Nakatani et al., 1999).
Using a different approach to study the role of the notochord in morphogenesis, Di Gregorio et al. interrupted notochord terminal differentiation by mis-expressing a Xenopus homologue of homeobox gene bix in notochord precursors via transient transgenesis (Di Gregorio et al., 2002). Cells expressing this transgene failed to differentiate into notochord and convergence/extension was interrupted. Consequently, muscle and endodermal strand failed to organize properly. The authors also investigated the requirement for proper muscle morphogenesis in notochord development, and showed that convergent extension of the notochord was greatly compromised in embryos in which muscle differentiation and morphogenesis were impaired. They concluded that morphogenesis of each tissue, notochord or muscle, is dependent on the proper formation of the other. However, this study cannot rule out the possibility that each tissue merely plays a passive role in supporting the morphogenesis of the other. We have noticed that in the absence of notochord convergent extension, the muscle can still assume its stereotypic arrangement, although the muscle cells do not extend to their proper length along the A/P axis (Jiang et al., 2005). The extent to which the notochord influences the arrangement of muscle remains to be explored in detail.
Post-convergent extension elongation
Convergence and extension alone lengthen the notochord by approximately three-fold (compare Fig. 1C and 1F). However, the elongation of the notochord and ascidian tail continues long after convergent extension is complete. This post-convergent extension morphogenesis brings about an additional three-fold elongation (compare Fig. 3A and 3H). While interest in ascidian notochord morphogenesis has focused on convergent extension, the post-convergent extension elongation phase has not been extensively studied. However, this process is fundamental in ascidian morphogenesis and has parallels in vertebrate notochord development. More than half of the notochord elongation after convergent extension is achieved by medial narrowing with accompanying A/P elongation of individual notochord cells. In this process, each cell transforms from a coin-shaped disk (Fig. 3A) into a drum-shaped disk (Fig. 3C). According to Miyamoto and Crowther, there is little increase in cell volume in this period and the elongation of the notochord is brought about mainly by cell-shape changes. Although the cell-shape changes occurring during this period appear simple, very little is known about how they are achieved (Miyamoto and Crowther, 1985).
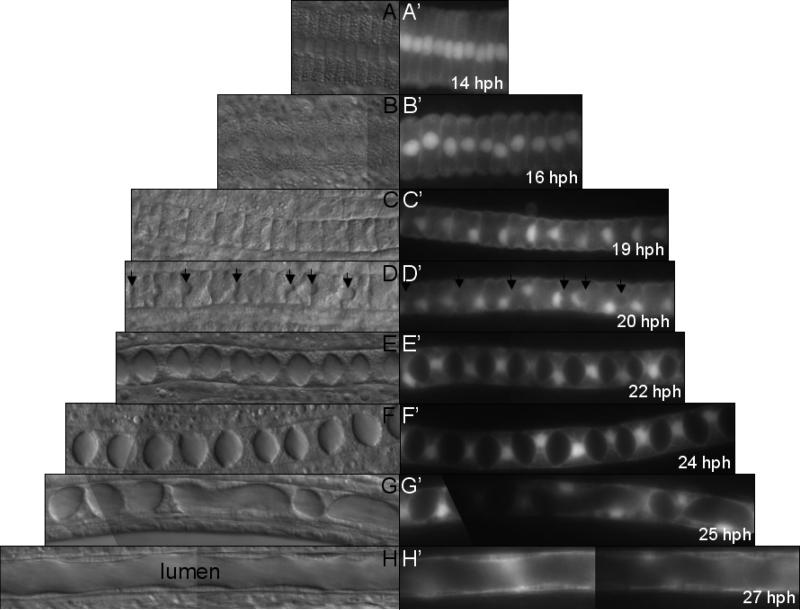
Figure 3.
Post convergent extension notochord morphogenesis. Normaski (A-H) and fluorescent (A’-H’) images of notochord in stable transgenic Ciona savignyi embryos expressing GFP under the Ciona intestinalis brachyury promoter. In these cells the GFP tends to accumulate in nuclei. Arrows indicate emergence of intercellular pockets (D and D’). Approximately 10 cells from the second quarter of notochord column are imaged. hpf: hours post fertilization at 16°C. Dorsal view, anterior to the left.
A/P polarity in Ciona notochord cells
Examination of the localization of Pk and Strabismus (Stbm, another PCP protein) after convergent extension revealed, unexpectedly, an A/P polarity in individual notochord cells in Ciona embryos (Jiang et al., 2005). Both Pk and Stbm proteins are localized to the anterior end of each notochord cell. Morphologically, notochord cell nuclei are invariably located at the posterior end (Fig. 3C’ and D’), except in the posterior-most cell where it is at the anterior end. This asymmetrical positioning of nuclei in Ciona notochord cells was captured by electron microscopy previously and went unnoticed by the authors (Miyamoto and Crowther, 1985). Using a genetic approach, we showed that normal activity of Pk is required for this A/P polarity. These observations led to three interesting questions. First, the reiterated A/P polarity in the first 39 notochord cells is reminiscent of the segmental polarity of Drosophila embryos and vertebrate somites, but in ascidian the repeated element is the single cell. Thus, is this arrangement a vestige of an ancestral segmentation that was simplified in ascidian lineage, or it is unrelated to other forms of morphological reiteration? Second, how is the A/P polarity of the cells is established and maintained? Our initial observation suggests a recruitment of PCP proteins in the process, but the interplay among PCP proteins and other pathways is unknown. However, it is worth noting that Ciona wnt-5, which is expressed laterally in the muscles during convergent extension, is expressed strongly in the posterior pole of the embryo by the tailbud stage (Imai et al., 2004; http://hoya.zool.kyoto-u.ac.jp/download.html). Third, what is the importance of the polarity for the morphogenesis or function of the notochord? Anterior localization of Pk is not a unique feature of Ciona because it is also found in the developing notochord cells (and neural plate cells) of zebrafish embryos (Ciruna et al., 2006). Despite the apparent conservation of Pk localization between Ciona and zebrafish, comparison of notochords from different ascidian species indicates that A/P polarization of the notochord cells is not universal. Although A/P polarity of the notochord cells, as indicated by nuclei position, is present in the majority of ascidians examined (Table 1 and Fig. 4), there are exceptions. The positions of nuclei in notochord cells in Botrylloides violaceus, Aplidium californicum, Clavelina huntsmani (Fig. 4 C, F, and H) and Halocynthia roretzi (Nishida and Kumano, personal communication) remain variable throughout the development. Whether PCP proteins are polarized in the A/P axis of notochord cells from all species has not been determined.
Table 1.
Morphological variations in ascidian notochords
| Order | Suborder | Family | Species | Life style | A/P polarity | Lumenzation |
|---|---|---|---|---|---|---|
| Enterogona | Phlebobranchia | Cionidae | Ciona savignyi | Solitary | Yes | Yes |
| Enterogona | Phlebobranchia | Cionidae | Ciona intestinalis | Solitary | Yes | Yes |
| Enterogona | Phlebobranchia | Ascidiidae | Ascidia zara | Solitary | Yes | Yes |
| Enterogona | Phlebobranchia | Ascidiidae | Ascidia ceratodes | Solitary | Yes | Yes |
| Enterogona | Phlebobranchia | Ascidiidae | Ascidiella aspersa | Solitary | Yes | Yes |
| Enterogona | Aplousobranchia | Clavelinid | Clavelina huntsmani | Colonial | No | No |
| Enterogona | Aplousobranchia | Polyclinid | Aplidium californicum | Colonial | No | Yes |
| Pleurogona | Stolidobranchia | Styelidae | Styela clava | Colonial | Yes | Yes |
| Pleurogona | Stolidobranchia | Styelidae | Styela montereyensis | Colonial | Yes | Yes |
| Pleurogona | Stolidobranchia | Styelidae | Styela plicata | Colonial | Yes | No |
| Pleurogona | Stolidobranchia | Styelidae | Botryllus schlosseri | Colonial | Yes | Yes |
| Pleurogona | Stolidobranchia | Styelidae | Botrylloides violaceus | Colonial | No | No |
| Pleurogona | Stolidobranchia | Pyuridae | Pyura haustor | Solitary | Yes | Yes |
| Pleurogona | Stolidobranchia | Pyuridae | Boltenia villosa | Solitary | Yes | Yes |
| Pleurogona | Stolidobranchia | Pyuridae | Halocynthia roretzi | Solitary | No | No |
| Pleurogona | Stolidobranchia | Molgulida | Molgula manhattensis | Solitary | Yes | Yes |
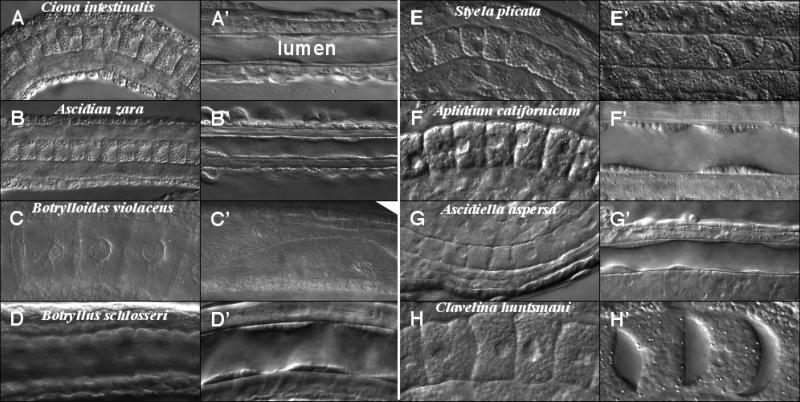
Figure 4.
Notochord morphology at post convergent extension elongation stage (A-H) and swimming larva stage (A’-H’) for various ascidian species. Images collected by Normaski microscopy.
In Ciona intestinalis, the notochord eventually forms a cell-free lumen at its core that runs the length of the A/P axis (see Fig. 4A’ and below). There is striking diversity among ascidians in notochord morphology at this final stage of development (Fig. 4, Table 1) (Burighel and Cloney, 1997). One hypothesis is that the A/P polarity is important for subsequent lumen formation. However, this has turned out not to be the case. Notochords without apparent A/P polarity can either develop lumens, like in Aplidium californicum (Fig. 4 F’), or can remain as a solid rod, as in Botrylloides violaceus (Fig. 4 C’). Thus, the presence of A/P polarity does not correlate with the formation of a luminal notochord, and the function of A/P polarity in ascidian notochords remains elusive. Our comparative analysis also shows that the absence of A/P polarity occurs in both the Enterogona and Pleurogona, and in both solitary and colonial ascidians. Hence we don't know if this morphological character is ancestral in ascidians or is convergent.
Transformation from a solid rod-shape notochord to a luminal notochord
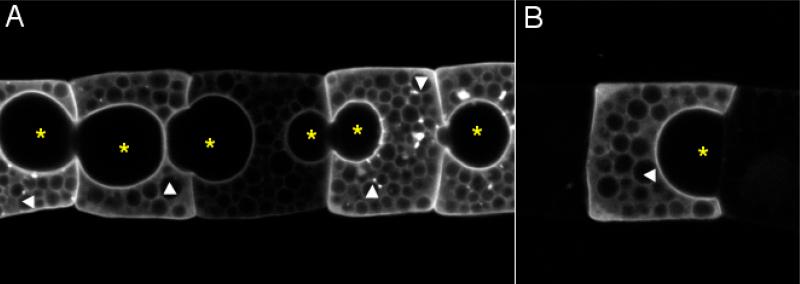
The formation of the tubular lumen in the Ciona notochord begins with the appearance of small luminal spaces between adjacent cells (see arrow in Fig. 3D and D’). The location of these structures has been a subject of contention. Some authors considered these spaces to be intracellular inclusions and refer to them as vacuoles (Berrill, 1947; Conklin, 1931; Miyamoto and Crowther, 1985; Sebastian, 1953), while others believed them to be in extracellular space (Burighel and Cloney, 1997; Cloney, 1964; Mancuso and Dolcemascolo, 1977). We investigated this question by tracing the outlines of individual notochord cells. For this a membrane-targeted GFP was expressed specifically in notochord cells by use of the Brachyury promoter (Corbo et al., 1997). The transgene was introduced by electroporation, resulting in mosaic expression. Fig. 5 shows a group of notochord cells expressing varying levels of GFP (Fig. 5A), as well as an isolated GFP-expressing cell (Fig. 5B). The contours of GFP indicate these structures are outside of the notochord cells. We suggest that these pockets should not be called vacuoles, since a vacuole is an intercellular inclusion.
Figure 5.
Extracellular pockets in notochord column. A membrane-targeted GFP is expressed in notochord cells in mosaic pattern by electroporation. (A) A string of notochord cells expressing membrane-bound GFP at different levels. (B) An isolated GFP-positive cell. * indicates extracellular pockets. Arrowheads indicate intracellular yolk granules. Anterior to the left.
In Ciona, the pockets gradually increases in size (Fig. 3 D-F and D’-F) and form biconvex lenticular pockets along the notochord. Simultaneously the bordering notochord cells become biconcave to accommodate the enlarging pockets at the anterior and posterior ends. Over time, the lenticular pockets shift in position and tilt toward each other. Because the direction of the tilts alternate along the notochord the string of pockets appears in a zigzag pattern (Cloney, 1964). As the pockets grow larger they began to fuse, eventually becoming confluent, forming a single lumen (Fig. 3 G, H and G’, H’). The notochord cells themselves are pushed aside and become endothelial-like.
Little is know about the composition of the lumen or the dynamic transformation of cylindrical cells to “endothelial” cells. In Ciona intestinalis, several intercellular pockets usually form simultaneously in the middle of notochord column and the rest follow. The formation of pockets in the posterior ten or so notochord cells usually lags, giving an overall impression of an anterior to posterior progression. Sometimes no pocket is observed between the posterior-most cells. In the ascidian Ascidia callosa, in which the notochord cells do not appear to be polarized along the A/P axis, Golgi complexes associated with small vesicles that accumulate near the anterior and posterior surfaces of each cell at the time intercellular pockets are forming (Cloney, 1964). This suggests that some protein-containing product could be produced and secreted by notochord cells to facilitate osmotic inflation, resulting in the formation of extracellular pockets. As matrix continues to accumulate, adjacent notochord cells would remain in contact only around the periphery of the notochord. The plasma membranes of adjacent cells lie in very close contact and form zonula occuludens in order to seal the lumen (Cloney, 1964). These tight junctions have to recede further to the periphery of notochord to accommodate the increase of extracellular matrix. In the mean time, notochord cells begin to change into an endothelial-like shape. Passive response to the matrix accumulation could be the cause of this cell shape change, although no evidence for this exists.
Ascidian notochord as a hydrostatic skeleton
The notochord in the fully-formed free swimming Ciona larva consists of a central lumen of extra-cellular matrix of unknown composition, surrounded by a monolayer of notochord cells which are in turn wrapped by a sheath of connective matrix consisting of a basal lamina and a layer of subjacent fibrils (Fig. 6) (Cloney, 1964; Cloney, 1969). In the ascidian larva the notochord possibly serves two functions, one in tail morphogenesis, the other in the biomechanics of the tail. The tension-resisting notochord sheath allows the notochord to become rigid as the lumen enlarges, most likely by osmotic pressure. Osmotic inflation of notochord lumen is possibly used also as a mechanism of force-generation which results in further elongation of the tail. Support for this hypothesis comes from the observation that tail length continues to increase as the extracellular lumen emerges and expands (Compare Fig. 3C, C’ with H, H’) (Miyamoto and Crowther, 1985). However because the authors believed that vacuoles are intracellular structures, they concluded that the cell volume changes are responsible for this further elongation. If the increase of the lumen is responsible for the further elongation of the tail in Ciona, this mechanism must not be viewed as universal because in many ascidian species the notochord does not form an extracellular lumen, or the extracellular lumen is minimal (Fig. 4 and table 1). Whether the notochord ceases to lengthen after the cell shape changes following convergent extension in species lacking a luminal notochord, or instead continues to elongate via some other mechanism, is not known.
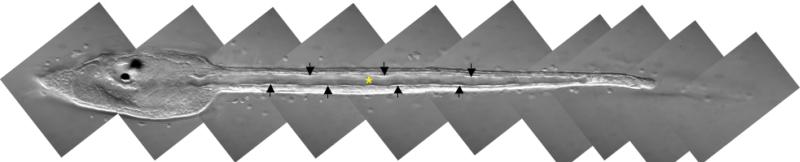
Figure 6.
A Ciona savignyi swimming tadpole imaged by Normarski microscopy. At this stage, notochord is a tubular lumen is at the center (*) encircled by endothelial-like notochord cells (arrows) in the periphery. The notochord at this stage plays an important role in locomotion by serving as a hydrostatic skeleton in the larval tail. Lateral view; anterior to the left.
It is interesting to note the wide diversity in notochord morphology, both among the ascidians, and among the chordate classes. As mentioned above, not all ascidians develop a luminal notochord. Whether the luminal notochord is associated with stronger swimming, and/or with a longer-lasting swimming period before settlement, remains to be determined. The presence of tension resisting fluid in extracellular space appears to be unique in tunicates (Fenaux, 1998). In contrast, notochords of cephalochordates (Flood, 1975), and lampreys, sturgeons, lungfishes (Schmitz, 1998a; Schmitz, 1998b), hagfishes (Koob and Long, 2000), larval trout (Symmons, 1979), frog embryos and tadpoles (Adams et al., 1990; Bruns and Gross, 1970; Koehl et al., 1990) contain intracellular vacuoles. With the exception of cephalochordate notochord, which contains striated muscle fibers (Flood, 1975; Ruppert, 1997), notochords of chordates are considered hydrostatic skeletons without active contractile ability. Thus two basic notochord types are observed to fulfill the notochord's biomechanical function in chordates: the extracellular hydrostatic skeleton of most tunicates, and intracellular hydrostatic skeleton of other chordates. It is not known which of these two notochord forms is ancestral, although the transition between the two forms may be as simple as alternatively trafficking the matrix-filled vacuoles to the secretory pathway versus retaining them within the cytoplasm.
Fate of the notochord
In ascidians the larval stage is transient and is thought to serve primarily in dispersal. Although there is great variety in dispersal mechanisms among ascidian species, including many species that have lost their tails, and hence their ability to swim (Jeffery et al., 1999), the larval stage is invariably followed by a period of metamorphosis during which the adult organs develop (Cloney, 1982). Among the dramatic changes taking place during ascidian metamorphosis is the resorption of the tail. Although the tail test (or tunic) is sloughed off, the other components of the tail, including notochord (Deschet et al., 2003) and muscle, are withdrawn into the body where they undergo programmed cell death (Chambon et al., 2002; Bates et al., 2004). There is no evidence for the persistence of any of the notochord lineage in the adult ascidian.
Summary
Several cell behaviors important in development and morphogenesis are displayed during the formation of the ascidian notochord. Cell proliferation, growth, rearrangement, shape change, restructuring of the intercellular adhesion apparatus, and alteration of the extracellular space occur as the notochord is transformed from an epithelial plate to an elongated rod. Modern molecular analysis, aimed at understanding not only how the ascidian notochord is formed, but also the underlying principles of these cellular behaviors, should shed new light on these processes.
Acknowledgments
This work is supported by a grant from the NIH (HD38701) to WCS. We wish to thank Matthew Kourakis and Carin Ezal for their comments on this manuscript.
References
- Adams DS, Keller R, Koehl MA. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development. 1990;110:115–30. doi: 10.1242/dev.110.1.115. [DOI] [PubMed] [Google Scholar]
- Bates WR. Cellular features of an apoptotic form of programmed cell death during the development of the ascidian, Boltenia villosa. Zoolog Sci. 2004;21:553–563. doi: 10.2108/zsj.21.553. [DOI] [PubMed] [Google Scholar]
- Berrill NJ. Metamorphosis in ascidians. J. Morphology. 1947;81:249–267. doi: 10.1002/jmor.1050810207. [DOI] [PubMed] [Google Scholar]
- Bruns RR, Gross J. Studies on the tadpole tail. I. Structure and organization of the notochord and its covering layers in Rana catesbeiana. Am J Anat. 1970;128:193–233. doi: 10.1002/aja.1001280206. [DOI] [PubMed] [Google Scholar]
- Burighel P, Cloney RA. Urochordate: Ascidiacea. In: Ruppert EE, editor. Hemichordata, Chaetognatha, and the invertebrate chordates. Vol. 15. John Wiley & Sons, Inc.; New York: 1997. pp. 221–347. Microscopic anatomy of in vertebrates. [Google Scholar]
- Chambon JP, Soule J, Pomies P, Fort P, Sahuquet A, Alexandre D, Mangeat PH, Baghdiguian S. Tail regression in Ciona intestinalis (Prochordate) involves a Caspase-dependent apoptosis event associated with ERK activation. Development. 2002;129:3105–3114. doi: 10.1242/dev.129.13.3105. [DOI] [PubMed] [Google Scholar]
- Christ B, Huang R, Scaal M. Formation and differentiation of the avian sclerotome. Anat Embryol (Berl) 2004;208:333–50. doi: 10.1007/s00429-004-0408-z. [DOI] [PubMed] [Google Scholar]
- Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature. 2006;439:220–4. doi: 10.1038/nature04375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloney RA. Development of the ascidian notochord. Acta Embryol Morphol Exp. 1964;7:111–130. [Google Scholar]
- Cloney RA. Cytoplasmic filaments and morphogenesis: the role of the notochord in ascidian metamorphosis. Z Zellforsch Mikrosk Anat. 1969;100:31–53. doi: 10.1007/BF00343819. [DOI] [PubMed] [Google Scholar]
- Cloney RA. Ascidian Larvae and the Events of Metamorphosis. Amer. Zool. 1982;22:817–826. [Google Scholar]
- Conklin EG. The development of centrifuged eggs of ascidians. Journal of Experimental Zoology. 1931;60:1–119. [Google Scholar]
- Corbo JC, Fujiwara S, Levine M, Di Gregorio A. Suppressor of hairless activates brachyury expression in the Ciona embryo. Dev Biol. 1998;203:358–68. doi: 10.1006/dbio.1998.9067. [DOI] [PubMed] [Google Scholar]
- Corbo JC, Levine M, Zeller RW. Characterization of a notochord-specific enhancer from the Brachyury promoter region of the ascidian, Ciona intestinalis. Development. 1997;124:589–602. doi: 10.1242/dev.124.3.589. [DOI] [PubMed] [Google Scholar]
- Danos MC, Yost HJ. Linkage of cardiac left-right asymmetry and dorsal-anterior development in Xenopus. Development. 1995;121:1467–74. doi: 10.1242/dev.121.5.1467. [DOI] [PubMed] [Google Scholar]
- Darras S, Nishida H. The BMP signaling pathway is required together with the FGF pathway for notochord induction in the ascidian embryo. Development. 2001;128:2629–38. doi: 10.1242/dev.128.14.2629. [DOI] [PubMed] [Google Scholar]
- Deschet K, Nakatani Y, Smith WC. Generation of Ci-Brachyury-GFP stable transgenic lines in the ascidian Ciona savignyi. Genesis. 2003;35:248–259. doi: 10.1002/gene.10195. [DOI] [PubMed] [Google Scholar]
- Delsuc F, Brinkmann H, Chourrout D, Philippe H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 2006;439:965–8. doi: 10.1038/nature04336. [DOI] [PubMed] [Google Scholar]
- Di Gregorio A, Harland RM, Levine M, Casey ES. Tail morphogenesis in the ascidian, Ciona intestinalis, requires cooperation between notochord and muscle. Dev Biol. 2002;244:385–95. doi: 10.1006/dbio.2002.0582. [DOI] [PubMed] [Google Scholar]
- Domingo C, Keller R. Induction of notochord cell intercalation behavior and differentiation by progressive signals in the gastrula of Xenopus laevis. Development. 1995;121:3311–3321. doi: 10.1242/dev.121.10.3311. [DOI] [PubMed] [Google Scholar]
- Fenaux R. Anatomy and functional morphology of the Appendicularia. In: Bone Q, editor. The biology of pelagic tunicates. Oxford University Press; New York: 1998. pp. 25–34. [Google Scholar]
- Flood PR. Fine structure of the notochord of amphioxus. Symp. Zool. Soc. Lond. 1975:81–104. [Google Scholar]
- Fouquet B, Weinstein BM, Serluca FC, Fishman MC. Vessel patterning in the embryo of the zebrafish: guidance by notochord. Dev Biol. 1997;183:37–48. doi: 10.1006/dbio.1996.8495. [DOI] [PubMed] [Google Scholar]
- Green JB, Dominguez I, Davidson LA. Self-organization of vertebrate mesoderm based on simple boundary conditions. Dev Dyn. 2004;231:576–581. doi: 10.1002/dvdy.20163. [DOI] [PubMed] [Google Scholar]
- Goldstein AM, Fishman MC. Notochord regulates cardiac lineage in zebrafish embryos. Dev Biol. 1998;201:247–52. doi: 10.1006/dbio.1998.8976. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Hudson C, Yasuo H. A signalling relay involving Nodal and Delta ligands acts during secondary notochord induction in Ciona embryos. Development. 2006;133:2855–64. doi: 10.1242/dev.02466. [DOI] [PubMed] [Google Scholar]
- Ikuta T, Yoshida N, Satoh N, Saiga H. Ciona intestinalis Hox gene cluster: Its dispersed structure and residual colinear expression in development. Proc Natl Acad Sci U S A. 2004;101:15118–23. doi: 10.1073/pnas.0401389101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai KS, Levine M, Satoh N, Satou Y. Regulatory blueprint for a chordate embryo. Science. 2006;312:1183–7. doi: 10.1126/science.1123404. [DOI] [PubMed] [Google Scholar]
- Imai KS, Satoh N, Satou Y. An essential role of a FoxD gene in notochord induction in Ciona embryos. Development. 2002a;129:3441–53. doi: 10.1242/dev.129.14.3441. [DOI] [PubMed] [Google Scholar]
- Imai KS, Satou Y, Satoh N. Multiple functions of a Zic-like gene in the differentiation of notochord, central nervous system and muscle in Ciona savignyi embryos. Development. 2002b;129:2723–32. doi: 10.1242/dev.129.11.2723. [DOI] [PubMed] [Google Scholar]
- Imai KS, Hino K, Yagi K, Satoh N, Satou Y. Gene expression profiles of transcription factors and signaling molecules in the ascidian embryo: towards a comprehensive understanding of gene networks. Development. 2004;131:4047–4058. doi: 10.1242/dev.01270. [DOI] [PubMed] [Google Scholar]
- Jeffery WR, Swalla BJ, Ewing N, Kusakabe T. Evolution of the ascidian anural larva: evidence from embryos and molecules. Mol Biol Evol. 1999;16:646–654. doi: 10.1093/oxfordjournals.molbev.a026147. [DOI] [PubMed] [Google Scholar]
- Jiang D, Munro EM, Smith WC. Ascidian prickle regulates both mediolateral and anterior-posterior cell polarity of notochord cells. Curr Biol. 2005;15:79–85. doi: 10.1016/j.cub.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–4. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- Keller R, Davidson LA, Shook DR. How we are shaped: The biomechanics of gastrulation. Differentiation. 2003;71:171–205. doi: 10.1046/j.1432-0436.2003.710301.x. [DOI] [PubMed] [Google Scholar]
- Keller R. Mechanisms of elongation in embryogenesis. Development. 2006;133:2291–302. doi: 10.1242/dev.02406. [DOI] [PubMed] [Google Scholar]
- Keys DN, Levine M, Harland RM, Wallingford JB. Control of intercalation is cell-autonomous in the notochord of Ciona intestinalis. Dev Biol. 2002;246:329–40. doi: 10.1006/dbio.2002.0656. [DOI] [PubMed] [Google Scholar]
- Kim GJ, Nishida H. Role of the FGF and MEK signaling pathway in the ascidian embryo. Dev Growth Differ. 2001;43:521–33. doi: 10.1046/j.1440-169x.2001.00594.x. [DOI] [PubMed] [Google Scholar]
- Kim GJ, Yamada A, Nishida H. An FGF signal from endoderm and localized factors in the posterior-vegetal egg cytoplasm pattern the mesodermal tissues in the ascidian embryo. Development. 2000;127:2853–62. doi: 10.1242/dev.127.13.2853. [DOI] [PubMed] [Google Scholar]
- Koehl MAR, Adams DS, Keller RE. Mechanical development of the notochord in early tail-bud amphibian embryos. In: Akkas N, editor. Biomechanics of active movement and deformation of cells. Springer-Verlag; Heidelberg: 1990. pp. 471–485. [Google Scholar]
- Koob TJ, Long JH., Jr. The vertebrate body axis: evolution and mechanical function. American Zoologist. 2000:40. [Google Scholar]
- Lohr JL, Danos MC, Yost HJ. Left-right asymmetry of a nodal-related gene is regulated by dorsoanterior midline structures during Xenopus development. Development. 1997;124:1465–72. doi: 10.1242/dev.124.8.1465. [DOI] [PubMed] [Google Scholar]
- Mancuso V, Dolcemascolo G. Ultrastructure of the chord in larvae of Ciona intestinalis during the tail development stage. Acta Embryol Exp (Palermo) 1977:207–20. [PubMed] [Google Scholar]
- Matsui T, Raya A, Kawakami Y, Callol-Massot C, Capdevila J, Rodriguez-Esteban C, Izpisua Belmonte JC. Noncanonical Wnt signaling regulates midline convergence of organ primordia during zebrafish development. Genes Dev. 2005;19:164–75. doi: 10.1101/gad.1253605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokawa T, Yagi K, Makabe KW, Nishida H. Binary specification of nerve cord and notochord cell fates in ascidian embryos. Development. 2001;128:2007–17. doi: 10.1242/dev.128.11.2007. [DOI] [PubMed] [Google Scholar]
- Miya T, Nishida H. An Ets transcription factor, HrEts, is target of FGF signaling and involved in induction of notochord, mesenchyme, and brain in ascidian embryos. Dev Biol. 2003;261:25–38. doi: 10.1016/s0012-1606(03)00246-x. [DOI] [PubMed] [Google Scholar]
- Miyamoto DM, Crowther RJ. Formation of the notochord in living ascidian embryos. J Embryol Exp Morphol. 1985;86:1–17. [PubMed] [Google Scholar]
- Moon RT, Campbell RM, Christian JL, McGrew LL, Shih J, Fraser S. Xwnt-5A: a maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis. Development. 1993;119:97–111. doi: 10.1242/dev.119.1.97. [DOI] [PubMed] [Google Scholar]
- Munro EM, Odell G. Morphogenetic pattern formation during ascidian notochord formation is regulative and highly robust. Development. 2002a;129:1–12. doi: 10.1242/dev.129.1.1. [DOI] [PubMed] [Google Scholar]
- Munro EM, Odell GM. Polarized basolateral cell motility underlies invagination and convergent extension of the ascidian notochord. Development. 2002b;129:13–24. doi: 10.1242/dev.129.1.13. [DOI] [PubMed] [Google Scholar]
- Munsterberg AE, Lassar AB. Combinatorial signals from the neural tube, floor plate and notochord induce myogenic bHLH gene expression in the somite. Development. 1995;121:651–60. doi: 10.1242/dev.121.3.651. [DOI] [PubMed] [Google Scholar]
- Nakatani Y, Moody R, Smith WC. Mutations affecting tail and notochord development in the ascidian Ciona savignyi. Development. 1999;126:3293–301. doi: 10.1242/dev.126.15.3293. [DOI] [PubMed] [Google Scholar]
- Nakatani Y, Nishida H. Ras is an essential component for notochord formation during ascidian embryogenesis. Mech Dev. 1997;68:81–9. doi: 10.1016/s0925-4773(97)00131-7. [DOI] [PubMed] [Google Scholar]
- Nishida H. Cell lineage analysis in ascidian embryos by intracellular injection of a tracer enzyme. III. Up to the tissue restricted stage. Dev Biol. 1987;121:526–41. doi: 10.1016/0012-1606(87)90188-6. [DOI] [PubMed] [Google Scholar]
- Nishida H. Spatio-temporal pattern of MAP kinase activation in embryos of the ascidian Halocynthia roretzi. Dev Growth Differ. 2003;45:27–37. doi: 10.1046/j.1440-169x.2003.00672.x. [DOI] [PubMed] [Google Scholar]
- Philippe H, Lartillot N, Brinkmann H. Multigene analyses of bilaterian animals corroborate the monophyly of Ecdysozoa, Lophotrochozoa, and Protostomia. Mol Biol Evol. 2005;22:1246. doi: 10.1093/molbev/msi111. [DOI] [PubMed] [Google Scholar]
- Pourquie O, Coltey M, Teillet MA, Ordahl C, Le Douarin NM. Control of dorsoventral patterning of somitic derivatives by notochord and floor plate. Proc Natl Acad Sci U S A. 1993;90:5242–6. doi: 10.1073/pnas.90.11.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince VE, Price AL, Ho RK. Hox gene expression reveals regionalization along the anteroposterior axis of the zebrafish notochord. Dev Genes Evol. 1998;208:517–522. doi: 10.1007/s004270050210. [DOI] [PubMed] [Google Scholar]
- Rauch GJ, Hammerschmidt M, Blader P, Schauerte HE, Strahle U, Ingham PW, McMahon AP, Haffter P. Wnt5 is required for tail formation in the zebrafish embryo. Cold Spring Harb Symp Quant Biol. 1997;62:227–34. [PubMed] [Google Scholar]
- Reverberi G. The causal formation of the brain in the ascidian larva. Acta. Embryol. Morph. Exp. 1960;3:296–336. [Google Scholar]
- Romer AS. Vertebrate Paleontology. University of Chicago Press; Chicago: 1966. [Google Scholar]
- Ruppert EE. Cephalochordata (Acrania). In: Ruppert EE, editor. Hemichordata, Chaetognatha, and the invertebrate chordates. Vol. 15. John Wiley & Sons, Inc.; New York: 1997. pp. 349–504. Microscopic anatomy of in vertebrates. [Google Scholar]
- Sasakura Y, Ogasawara M, Makabe KW. HrWnt-5: A maternally expressed ascidian Wnt gene with posterior localization in early embryos. Int. J. Dev. Biol. 1998;42:573–579. [PubMed] [Google Scholar]
- Sasakura Y, Makabe KW. Ascidian Wnt-5 gene is involved in the morphogenetic movement of notochord cells. Dev Growth Differ. 2001;43:573–82. doi: 10.1046/j.1440-169x.2001.00602.x. [DOI] [PubMed] [Google Scholar]
- Schmitz RJ. Comparative ultrastructure of the cellular components of the unconstricted notochord in the sturgeon and the lungfish. Journal of Morphology. 1998a;236:75–104. doi: 10.1002/(SICI)1097-4687(199805)236:2<75::AID-JMOR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Schmitz RJ. Immunohistochemical identification of the cytoskeletal elements in the notochord cells of bony fishes. J Morphol. 1998b;236:105–16. doi: 10.1002/(SICI)1097-4687(199805)236:2<105::AID-JMOR2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Sebastian VO. The development of Herdmania pallida (Heller). Proc. Indian Acad. Sci. 1953;37B:174–187. [Google Scholar]
- Seo HC, Edvardsen RB, Maeland AD, Bjordal M, Jensen MF, Hansen A, Flaat M, Weissenbach J, Lehrach H, Wincker P, Reinhardt R, Chourrout D. Hox cluster disintegration with persistent anteroposterior order of expression in Oikopleura dioica. Nature. 2004;431:67–71. doi: 10.1038/nature02709. [DOI] [PubMed] [Google Scholar]
- Shimauchi Y, Chiba S, Satoh N. Synergistic action of HNF-3 and Brachyury in the notochord differentiation of ascidian embryos. Int J Dev Biol. 2001a;45:643–52. [PubMed] [Google Scholar]
- Shimauchi Y, Murakami SD, Satoh N. FGF signals are involved in the differentiation of notochord cells and mesenchyme cells of the ascidian Halocynthia roretzi. Development. 2001b;128:2711–21. doi: 10.1242/dev.128.14.2711. [DOI] [PubMed] [Google Scholar]
- Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development. 2005;132:2503–12. doi: 10.1242/dev.01812. [DOI] [PubMed] [Google Scholar]
- Symmons S. Journal of Zoology. Vol. 189. London: 1979. Notochordal and elastic components of the axial skeleton of fishes and their functions in locomotion. pp. 157–206. [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–38. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Takatori N, Satou Y, Satoh N. Expression of hedgehog genes in Ciona intestinalis embryos. Mech Dev. 2002;116:235–8. doi: 10.1016/s0925-4773(02)00150-8. [DOI] [PubMed] [Google Scholar]
- Yamada T, Pfaff SL, Edlund T, Jessell TM. Control of cell pattern in the neural tube: motor neuron induction by diffusible factors from notochord and floor plate. Cell. 1993;73:673–86. doi: 10.1016/0092-8674(93)90248-o. [DOI] [PubMed] [Google Scholar]
- Yamada T, Placzek M, Tanaka H, Dodd J, Jessell TM. Control of cell pattern in the developing nervous system: polarizing activity of the floor plate and notochord. Cell. 1991;64:635–47. doi: 10.1016/0092-8674(91)90247-v. [DOI] [PubMed] [Google Scholar]