Abstract
Translocator protein (TSPO) is an 18-kDa high affinity cholesterol- and drug-binding protein found primarily in the outer mitochondrial membrane. Although TSPO is found in many tissue types, it is expressed at the highest levels under normal conditions in tissues that synthesize steroids. TSPO has been associated with cholesterol import into mitochondria, a key function in steroidogenesis, and directly or indirectly with multiple other cellular functions including apoptosis, cell proliferation, differentiation, anion transport, porphyrin transport, heme synthesis, and regulation of mitochondrial function. Aberrant expression of TSPO has been linked to multiple diseases, including cancer, brain injury, neurodegeneration, and ischemia reperfusion injury. There has been an effort during the last decade to understand the mechanisms regulating tissue- and disease-specific TSPO expression and to identify pharmacological means to control its expression. This review focuses on the current knowledge regarding the chemicals, hormones, and molecular mechanisms regulating Tspo gene expression under physiological conditions in a tissue- and disease-specific manner. The results described here provide evidence that the PKCε-ERK1/2-AP1/Stat3 signal transduction pathway is the primary regulator of Tspo gene expression in normal and pathological tissues expressing high levels of TSPO.
Keywords: steroidogenic cells, cancer, hormones, environmental factors, peripheral benzodiazepine receptor, gene expression
1-Introduction
Translocator protein (18 kDa; TSPO), formerly known as peripheral-type benzodiazepine receptor, represents a conserved family of ubiquitous proteins (Papadopoulos et al., 2006a). TSPO was first identified in 1977 as an alternative binding site in the kidney for the benzodiazepine diazepam (Braestrup and Squires, 1977; Papadopoulos et al., 2006a). TSPO was then characterized by its ability to bind small molecule drugs, cholesterol, and porphyrins with diverse affinities (Papadopoulos et al., 2006a). In mammals, the biological significance of TSPO has been studied for decades and has been shown to be involved in a variety of cellular functions, including cholesterol transport and steroid hormone synthesis, mitochondrial respiration, mitochondrial permeability transition pore (MPTP) opening, apoptosis, and cell proliferation (Fan et al., 2009). Although some cellular functions of Tspo are conserved, such as cholesterol-binding and transport, their biological significance seems to have adapted to serve specific functions critical for various tissues. Nevertheless, the conservation of TSPO throughout evolution highlights the significance of this protein for proper cellular function and development. In fact, functional inactivation of Tspo induces an early embryonic-lethal phenotype in mouse (Papadopoulos et al., 1997).
Tspo is considered a housekeeping gene that is expected to remain permanently activated, but the complex mechanism by which its expression is regulated is not completely understood. Although TSPO has been extensively studied at the level of binding to drugs and other proteins, its genetic regulation and mRNA expression patterns remain elusive. Function, pharmacology, and expression of TSPO on the protein level have been comprehensively reviewed (Chen and Guilarte, 2008; Corsi et al., 2008; Gavish et al., 1999; Han et al., 2003; Papadopoulos et al., 2006a; Papadopoulos and Lecanu, 2009; Veenman et al., 2007). As such, this review will focus on our current knowledge of the mechanisms that regulate tissue- and disease-specific TSPO expression. We hope that by understanding these mechanisms we may better appreciate the function of TSPO in normal cells, as well as its role in evolution of the various disease states where this protein has been implicated.
2-Molecular identity
TSPO is a nuclear-encoded protein localized primarily to the outer mitochondrial membrane in all tissues tested. The Tspo gene is localized to the 22q13.31 chromosome in the human genome as a single copy and encodes 169 amino acids (Chang et al., 1992; Riond et al., 1991). TSPO is conserved throughout evolution, from bacteria to humans, emphasizing the functional importance of the protein. It should be noted that a second family of TSPO proteins, TSPO2, was recently identified. Tspo2 encodes a conserved family of proteins that act as mediators of cholesterol redistribution-dependent erythroblast maturation during mammalian erythropoiesis (Fan et al., 2009), though information regarding the regulation TSPO2 expression is currently unavailable. The cDNA for Tspo has been cloned from multiple species, including human, cow, pig, rat, and mouse. Cloning and characterization of the Tspo gene in human and rat revealed that it is composed of four exons, with a large intron containing repetitive sequences separating the first and second exons. The first exon and parts of the second and fourth exons remain untranslated. Multiple transcription start sites have been identified in the Tspo promoter in different species, including multiple internal transcription initiation sites (Giatzakis and Papadopoulos, 2004) (and Barlow, Batarseh, and Papadopoulos, unpublished data).
Sequence analysis has shown that the human Tspo gene is regulated by a TATA-less, GC-rich promoter that contains five tandem putative binding sites for Sp factors. While this promoter architecture is commonly associated with housekeeping genes, a similar structure is found in the promoters of several growth factor receptor genes that have been implicated in the regulation of cell proliferation and carcinogenesis (Dimario, 2002). Typical of housekeeping genes, this could also explain the presence of more than one transcription initiation site in the human sequence. The mouse Tspo promoter harbors additional putative binding sites for transcription factors including v-ets erythroblastosis virus E26 oncogene homolog (Ets), AP-1, AP-2, Ik2, GATA, Sf-1, SOX, and SRY (Giatzakis and Papadopoulos, 2004). Extensive promoter characterization in multiple cellular models will be required to elucidate the roles of each of the transcription factors binding at the Tspo promoter. Among the identified transcription factor binding sites, it is likely that those that are conserved between mouse and human sequences serve important functions in expression of the gene (Table 1).
Table 1.
Conserved putative transcription factor binding sites between human and mouse TSPO promoters. Sites were predicted using the Promo virtual laboratory (www.alggen.lsi.upc.es) with a 0 matrix dissimilarity rate between binding site sequences in the database and those in the promoter (only 100% matches are predicted).
| ABF1 | GR | POU3F2 |
| AGL3 | HELIOS | PR |
| Alfin1 | HNF-3beta | R2 |
| Antp | HOXA 3/8/9/10 | RAR-beta |
| AP1 | LIM1 | RAR-gamma |
| AP-2 | LVc | RC2 |
| AR | MafG | Sox2 |
| BR-C Z2 | MF3 | Sp1/ Sp3 |
| BTEB4 | MNB1a | STAT members |
| C/EBP α & δ; muEBP-C2 | Msx-1 | TCF-2 |
| CAC-binding protein | MYB2 | TGA1a |
| Cart-1 | MYBAS1 | TMF |
| Cdx-1 | Myf-3 | USF-1 |
| c-Ets-2 | MyoD | USF2 |
| c-Myc | Myogenin | USF2b |
| CREMtau | Ncx | VDR |
| CREMtau1 | NF-1 | YY1 |
| CREMtau2 | Nkx2-1 | YY1 |
| Crx | N-Myc | Zeste |
| DBP | Nrf2:MafK | ZF5 |
| Elk-1 | Ovo-B | Zic1 |
| ETF | p300 | Zic2 |
| FACB | Pax-2, 4, 5, 6 | Zic3 |
| GAGA factor | PEA3 | |
| GATA-1 | POU1F1a | |
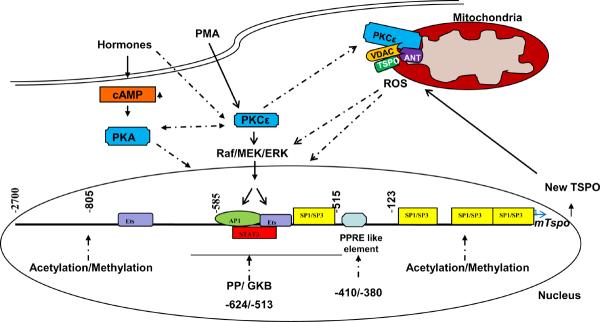
The functionality of the cloned Tspo promoter was demonstrated in vitro (Batarseh et al., 2008; Giatzakis et al., 2007; Giatzakis and Papadopoulos, 2004) and in vivo (Giatzakis et al., 2007). Transgenic mice expressing GFP fused to the full length 2.7-kb promoter, as well as to partial 0.8- and 0.1-kb promoters, were generated and evaluated for Tspo expression. GFP expression driven by the Tspo promoter was expressed to high levels in steroidogenic cells with a specificity related to the length of the promoter used, suggesting the presence of tissue-specific regulatory elements (Giatzakis et al., 2007). The detailed temporal and spatial analysis of GFP expression by the various lengths of the Tspo promoter is under investigation in our laboratory. A schematic representation of the Tspo gene promoter and the location of functional transcription sites identified within this region in the mouse and human sequences are shown in Figure 1.
Figure 1.
Hypothetical model for transcriptional regulation of Tspo in hormone- and cAMP- responsive MA-10 Leydig cells, and non-responsive NIH/3T3 fibroblasts. Data presented in this review identified PKCε as the key regulator of Tspo expression. Activation of PKCε, mediated through a MAPK-ERK pathway, targets the AP-1, Ets, and STAT3 transcription factors, whose binding sites are localized to the −805/−515-bp region upstream of the Tspo transcription start site. Ets and Sp1/Sp3 transcription factors play a major role in basal Tspo transcription, and epigenetic modifications of Tspo may also play a role in its expression. Upon activation, PKCε is translocated to the outer mitochondrial membrane, where it interacts with the two primary TSPO-associated proteins VDAC and ANT. Cross-talk between PKC and cAMP-dependent protein kinase (PKA) is possible. Known interactions are denoted by solid lines and hypothetical pathways are denoted by dotted lines.
3-TSPO distribution in healthy tissues
TSPO is found in most tissues, although its expression within each varies considerably (Casellas et al., 2002; Gavish et al., 1999; Papadopoulos et al., 2006a). Interestingly, TSPO distribution in rodent tissues generally parallels that seen in human tissues (Bribes et al., 2004; Galiegue et al., 2004; Gavish et al., 1999; Han et al., 2003; Papadopoulos et al., 2006a; Roivainen et al., 2009; Veenman and Gavish, 2006). Secretory and glandular tissues, such as adrenal glands, pineal gland, salivary glands, olfactory epithelium, ependyma, and gonads, are particularly rich in TSPO (Papadopoulos et al., 2006a). Intermediate levels of this protein are found in renal and myocardial tissues, and lower levels are present in the brain and liver (Giatzakis and Papadopoulos, 2004; Papadopoulos et al., 2006a). Heterogeneous cell type-specific TSPO expression has been characterized in several tissues. For example, distinct regional expression is present in adrenal tissue, the medulla is virtually devoid of TSPO, and TSPO is expressed at high levels in the cortex (Anholt et al., 1986). Similarly, TSPO is selectively localized in Leydig cells of the testis, and in the distal convoluted tubules and the thick ascending lip of the loop of Henle in the kidney (De Souza et al., 1985). Subcellular localization studies demonstrated that TSPO is primarily an outer mitochondrial protein (Anholt et al., 1986; Basile and Skolnick, 1986; ntkiewicz-Michaluk et al., 1988). In addition, it has been reported to be present in Golgi apparatus, lysosomes and peroxisomes (O'Beirne et al., 1990), and on plasma membrane (Oke et al., 1992). Surprisingly, TSPO is present in mature human erythrocytes, which lack mitochondria and nuclei (Olson et al., 1988), and perinuclear and nuclear localization of TSPO has been observed in breast cancer cells (Hardwick et al., 1999).
TSPO expression has been primarily studied at the protein level. However, a steady-state mRNA profile established in our laboratory showed that Tspo mRNA is present in all tissues, and correlates with reported protein expression levels. Steady-state mRNA levels are high in adrenal gland, kidney, spleen, skeletal muscle, and lung, are intermediate in heart and testis, and are low in liver and brain. These data suggest that differential TSPO expression in tissues may be due, at least in part, to differences in transcriptional regulation (Giatzakis and Papadopoulos, 2004).
4-TSPO expression in pathological specimens
TSPO expression levels have been correlated with certain disease states. It is highly expressed in steroidogenic cells such as testicular, adrenocortical, and brain glial tumor cells, whose proliferation can be regulated by TSPO drug ligands such as benzodiazepines (Garnier et al., 1993). At the same time, TSPO levels are elevated in cancerous tissues of the breast, ovary, colon, prostate, and brain, compared to normal human tissues, suggesting a role for TSPO in carcinogenesis (Batarseh et al., 2010; Katz et al., 1988; Katz et al., 1990b; Katz et al., 1990a). A positive correlation between TSPO levels and the metastatic potential of human breast and brain gliomas, as well as astrocytomas, has also been shown (Batarseh et al., 2010; Benavides et al., 1988; Miettinen et al., 1995). The role of TSPO in tumor growth may be supported by the concerted effects of the receptor itself on cell proliferation and survival. Indeed, both proliferative and antiapoptotic properties have been ascribed to TSPO based on extensive data using TSPO drug ligands (Veenman et al., 2007).
In human breast cancer, the link between cancer progression and TSPO expression was demonstrated in a series of studies in which it was shown that (i) TSPO is overexpressed in highly invasive human breast cancer cells and biopsies as compared to non-invasive cells and control biopsies (Galiegue et al., 2004; Han et al., 2003; Hardwick et al., 1999), (ii) the ability of aggressive cancer cells to form tumors depends on the amount of TSPO present in the cells, (iii) TSPO expression is directly involved in regulating cell proliferation, (iv) TSPO drug ligands control cell proliferation, and (v) knocking down TSPO expression is associated with decreased cell proliferation in vitro and tumor growth in vivo (Han et al., 2003; Hardwick et al., 1999; Hardwick et al., 2001; Hardwick et al., 2002; Papadopoulos et al., 2000). Additionally, the ability of highly aggressive human breast cancer cells to form tumors in vivo is associated with the amount of TSPO present in the cells (Brown et al., 2000; Garnier et al., 1993; Hardwick et al., 1999; Li and Papadopoulos, 1998; Papadopoulos et al., 2000).
The specific role of TSPO in cancer cells is unclear. In 1909, White first reported that cholesterol accumulates in solid tumors (White RM, 1909). Since then, numerous studies have indicated that cholesterol accumulation might be a general property of tumor cells (Dessi et al., 1992; Dessi et al., 1994; Kolanjiappan et al., 2003; Rudling and Collins, 1996; Yoshioka et al., 2000). Cholesterol has been shown to play a certain but controversial role in the advancement of a variety of pathologies including breast cancer (Coleman et al., 1997; Kokoglu et al., 1994; Subbaiah et al., 1997), and epidemiological studies have indicated the possibility that prolonged consumption of foods rich in cholesterol might promote cancer growth and progression (Freeman and Solomon, 2004; Graaf et al., 2004). Cholesterol synthesis inhibitors, such as statins, are selective for tumor as opposed to normal cells, where they inhibit cancer cell growth and motility (Chan et al., 2003; Freeman and Solomon, 2004), exert anti-cancer effects in animal models (Alonso et al., 1998; Farina et al., 2002; Jani et al., 1993; Narisawa et al., 1994), and impart clinical benefit in chemoprevention (Lishner et al., 2001; Wachtershauser et al., 2001). Together, these data suggest that intracellular cholesterol accumulation in organelles is critical for maintaining the tumor cell phenotype. It is our hypothesis that in tumor cells, increased cholesterol levels in mitochondria and nuclei are needed for the increased energy needs of these cells, increased membrane biogenesis, and specific gene expression during mitosis. We propose that higher levels of TSPO might be the driving force behind the transport of lipids to mitochondria and nuclei. Because the physiopathologic consequences of cholesterol accumulation on carcinogenesis have not been defined, it is also possible that TSPO-mediated cholesterol accumulation in the membranes of tumor cells might influence the degree of membrane fluidity shown to activate signaling mechanisms involved in cell proliferation (Singleton and Bourguignon, 2004).
TSPO expression is upregulated in the brain at sites of injury and inflammation, as well as following a number of neuropathological conditions including stroke, herpes and HIV encephalitis, and neurodegenerative disorders such as Alzheimer's disease, multiple sclerosis, amyotrophic lateral sclerosis, Parkinson's disease, and Huntington's disease (Batarseh et al., 2010). The molecular mechanisms underlying these diseases are unknown, although some factors identified to play a role in the regulation of TSPO expression, such as reactive oxygen species (ROS), interleukin 1 and tumor necrosis factor (TNF) α (see below), may play a role.
A role for TSPO in inflammation has also been documented. For example, astrocyte swelling, an important event in hepatic encephalopathy that triggers ROS production, increases nuclear accumulation of the Sp1 transcription factor, which upregulates Tspo mRNA and may augment hepatic encephalopathy (Kruczek et al., 2009). This induction is reduced when Sp1 siRNA was utilized, confirming that Sp1 regulates Tspo expression. Moreover, elevation of Zn2+ levels in hepatic encephalopathy could activate protein kinase C (PKC), which has been reported to activate Sp1 (Kruczek et al., 2009). This hypothesis fits in well with our findings (see below), where we demonstrate the role of Sp1/Sp3 transcription factors in regulating Tspo expression, as well as the role of the PKC pathway. However, the relationship between Sp1 and PKCε remains to be determined.
Hauet and colleagues demonstrated that TSPO expression is modulated during ischemia-reperfusion injury, indicating a role for TSPO in maintaining kidney function and renal protection (Favreau et al., 2009). Since ischemia-reperfusion injury is also linked to ROS, a feedback loop between TSPO levels and ROS may exist, in which TSPO could induce ROS production under conditions of stress, thereby further elevating TSPO levels and supporting cell survival. ROS could also be induced by other cellular stressors that induce TSPO levels required for cell survival, including activation of multiple signaling pathways responsible for diverse cellular processes. For example, pathways involved in cellular proliferation, cell growth and survival, and metabolism and angiogenesis, such as the ERK1/2 pathway, are regulated at least in part by ROS (Weinberg and Chandel, 2009). The role of ROS in regulating multiple transcription factor family members, including AP-1, AP-2, and NFκB, which are involved in proliferation, differentiation, and morphogenesis, is well-documented (Dalton et al., 1996; Dalton et al., 1999). Importantly, the Tspo promoter contains putative binding sites for these transcription factors, suggesting that ROS may potentially regulate TSPO expression through these sites.
Other examples of the role of TSPO during inflammation have been described. For example, upregulation of TSPO has been observed in different cell types after exposure to phorbol PMA, interleukin-1, or TNF (Table 2). This increase in TSPO levels may impart cellular protection against ROS damage. Other studies have implicated TSPO in pro-apoptotic mechanisms due to ROS (Carayon et al., 1996). An important distinction has to be drawn regarding TSPO expression during ROS production. It is likely that low levels of ROS induce TSPO expression, thus playing an important role in membrane biogenesis and cholesterol transport. This confers protective characteristics on the mitochondrial membrane and prevents cytochrome c leakage. On the other hand, high levels of ROS lead to cytotoxicity and reduced TSPO expression, which may perturb the mitochondrial membrane due to lack of cholesterol transport. This would induce cytochrome c leakage and deregulation of the mitochondrial permeability transition pore (MTTP), resulting in cell death. Interestingly, ROS has been shown to induce the 18-kDa TSPO monomeric form under ischemia (62), where high levels of ROS are present. It was also shown to induce the formation of TSPO homopolymers through dityrosine formation (Delavoie et al., 2003).
Table 2.
Effect of various pharmacological agents on Tspo gene expression and protein synthesis.
| Cell type or tissue | Species | Transcription factor/protein | Stimulus or process | Target | Effect | Refs |
|---|---|---|---|---|---|---|
| MA-10 Leydig | Mouse | PPARα | Peroxisome proliferators | Promoter mRNA Protein | ↓ | (Boujrad et al., 2000; Gazouli et al., 2002) |
| Testis, adrenals and ovaries | Mouse | PPARα | Peroxisome proliferators | mRNA | ↓ | (Gazouli et al., 2002) |
| MA-10 Leydig | Mouse | Predicted Sp1/Sp3 CG boxes | TSA | Promoter | ↑ | Not shown |
| HepG2 liver | Human | Predicted Sp1/Sp3 CG boxes | TSA | Promoter | ↑ | Not shown |
| COS-7 kidney | Monkey | Predicted Sp1/Sp3 CG boxes | TSA | Promoter | ↑ | Not shown |
| MA-10 Leydig | Mouse | Predicted Sp1/Sp3 CG boxes | 5-Azacytidine | Promoter | No change | Not shown |
| MDA-MB-231 breast cancer | Human | Predicted Sp1/Sp3 CG boxes | 5-Azacytidine | Promoter | No change | Not shown |
| MA-10 Leydig | Mouse | - | PMA | Promoter mRNA | No change | (Batarseh et al., 2008) |
| NIH-3T3 fibroblasts | Mouse | AP1 Ets | PMA | Promoter mRNA Protein | ↑ | (Batarseh et al., 2008) |
| COS-7 kidney | Monkey | AP1 Ets | PMA | Promoter mRNA Protein | ↑ | (Batarseh et al., 2008) |
| MA-10 Leydig | Mouse | PD98059 | Promoter mRNA | ↑ | Not shown | |
| NIH-3T3 fibroblasts | Mouse | PD98059 | Promoter | ↑ | ||
| Y1 adrenocortical | Mouse | GKB-Activated receptor | Ginkgo biloba | Promoter Protein | ↓ | (Amri et al., 2003) |
| Y1 adrenocortical | Mouse | Vitam A/E/D; Cortico; Testo | Promoter | No change | (Amri et al., 2003) | |
| Polygonal astrocytes | Rat | Interleukin-1 | Protein | ↑ | (Oh et al., 1992) | |
| Thymoma EL4.NOB-1 | Mouse | Interleukin-1 | Protein | ↓ | (Moynagh et al., 1993) | |
| Primary Leydig | Pig | TNF-α | mRNA Protein | ↑ | (Rey et al., 2000) | |
| MDA-MB-231 | Human | PPARα | Ginkgolide B | Protein | ↓ | (Hardwick et al., 2001) |
| MCF-7 breast cancer | Human | PPARα | Ginkgolide B | Protein | No change | (Hardwick et al., 2001) |
Bacterial TSPO was shown to act as oxygen sensor regulating photosynthetic membrane complex formation (Yeliseev et al., 1997; Yeliseev and Kaplan, 1995). Carayon and colleagues have proposed that TSPO expression may participate in an antioxidant pathway, since TSPO was found to be regulated in mitochondria from hematopoietic cells (Carayon et al., 1996). It is possible that TSPO protects hematopoietic cells from death induced by oxygen radical damage, when the ability of the cell to resist hydrogen peroxide damage correlates with the high levels of TSPO (Carayon et al., 1996). Moreover, Jurkat cells, which contain low levels of TSPO, can be transfected with Tspo cDNA to improve their resistance to hydrogen peroxide damage. This observation can be attributed to the endogenous TSPO ligand, dicarboxylic porphyrin, which may contribute to mitochondrial protection against ROS damage.
5-Tissue-specific transcriptional regulation of Tspo gene expression
As noted above, TSPO is expressed at the highest level in testis, and in Leydig, adrenal cortical, ovarian granulose, and luteal cells, as well as in placenta and brain glia cells, which are all know to form steroids de novo. Even within the same tissue, there are up to 40-fold differences in the amount of TSPO present in adrenal cortical and Leydig cells compared to chromaffin cells of the adrenal and seminiferous tubule Sertoli cells of the testis, respectively (Garnier et al., 1993). Detailed studies using TSPO-rich steroidogenic MA-10 mouse Leydig and Y-1 mouse adrenal cortical cells, compared to TSPO-poor NIH-3T3 mouse fibroblasts, indicated that the large differences seen in protein expression and drug ligand binding correlate with differences in steady-state Tspo mRNA levels. This observation suggests that this difference is regulated at least in part at the transcriptional level. A subsequent extensive characterization of the Tspo promoter revealed a differential utilization of the promoter in steroidogenic MA-10 and Y1 cells compared to non-steroidogenic NIH-3T3 cells, with the former requiring 585 bp of the promoter to maintain full activity, while the latter requires an area extending to 805 bp (Giatzakis and Papadopoulos, 2004). The finding that basal Tspo activity is significantly higher in MA-10 compared to NIH-3T3 cells suggests that an endogenous factor linked to the steroidogenic phenotype of the MA-10 cells drives the constitutive expression of Tspo. Analysis of the mechanisms underlying differential Tspo transcription in Leydig and fibroblasts unveals the importance of two proximal Sp1/Sp3 sites, and members of the Ets family of transcription factors for basal transcriptional activity (Giatzakis et al., 2007). Further studies have demonstrated that separate regions of the promoter drive Tspo transcription in steroidogenic and non-steroidogenic cells, suggesting that tissue-specific transcriptional regulation accounts for differences in Tspo expression between these cell types. Although Sp1/Sp3 factors are ubiquitously expressed, their levels differ in several tissues. For example, expression of Sp1 is similar in both steroidogenic and non-steroidogenic tissues, while Sp3 levels are higher in steroidogenic cells, suggesting that the difference in Tspo mRNA levels could be partially attributed to differences in Sp3 expression (Giatzakis et al., 2007; Giatzakis and Papadopoulos, 2004).
The ability of various compounds to affect Tspo expression was used as a means to investigate the transcriptional regulation of the gene. Among the compounds tested was the PKC activator and tumor promoter phorbol-12-myristate 13-acetate (PMA). In NIH-3T3 and SV-40 transformed COS-7 monkey kidney cells, which both express TSPO to low levels, PMA induced Tspo promoter activity, mRNA and protein expression, and cell proliferation (Batarseh et al., 2008). In contrast, TSPO-rich MA-10 cells failed to respond to PMA treatment (Batarseh et al., 2008). The effect of PMA on NIH-3T3 cells was localized at the 585-515-bp region of the Tspo promoter, and additional point mutations and electrophoretic mobility shift assays (EMSA) revealed that transcription factors binding at the AP-1 and Ets sites of the Tspo promoter mediated the stimulatory effect of PMA.
Subsequent studies have shown that PMA exerts its effects in TSPO-poor NIH-3T3 cells by activating protein kinase C epsilon (PKCε). However, the presence of high levels of constitutively-active, where PMA is not needed to activate the kinase, endogenous PKCε regulates Tspo expression in TSPO-rich steroidogenic cells, where it targets AP-1 and Ets, whose binding sites are localized to the 805-515-bp region upstream of the Tspo transcription start site (Figure 1). As noted previously, transgenic mice expressing GFP under the control of different fragments of the Tspo promoter allowed some insights regarding the in vivo localization of the area responsible for specificity in TSPO expression, and confirmed the critical role of the 805-bp promoter region for TSPO expression (Giatzakis et al., 2007).
PKCε is a serine/threonine-specific protein kinase and a member of the typical group of PKC isoforms abundant in endocrine, neuronal, and immune cells (Griner and Kazanietz, 2007; Ono et al., 1988). PKCε plays a role in a variety of signaling events involved in cell proliferation, differentiation, apoptosis, nervous functions, and secretory vesicle trafficking (Garczarczyk et al., 2009). Interestingly, the profile of TSPO expression in endocrine tissues and various cancers, as well as the role of TSPO in cell proliferation, tumor invasion, and metastasis (see below), parallels that of PKCε (Batarseh et al., 2008). Moreover, PKCε levels are induced in gliosis in a manner parallel to the induction of TSPO (Fields and Gustafson, 2003; Sharif and Sharif, 1999). Considering that TSPO controls the rate of adrenal cortical steroid formation (Lacapere and Papadopoulos, 2003), and that this profile is shared between PKCε and TSPO, it is not surprising that PKCε-null mice have dramatically reduced circulating corticosterone levels (Hodge et al., 2002). Indeed, this phenotype may be attributable to reduced adrenal cortical levels of TSPO.
PKCε exerts its effects through multiple downstream signaling pathways, including the mitogen-activated protein kinase MAPK (Raf-ERK1/2), and by acting on various targets such as the signal transducer and activator of transcription 3 (STAT3) (Aziz et al., 2007a; Aziz et al., 2007b; Garczarczyk et al., 2009). Using specific pathway inhibitors, and gene overexpression and knockdown by gene specific siRNAs or shRNAs in both steroidogenic and non-steroidogenic cells, we demonstrated the MAPK (Raf-1-MEK1/2-ERK1/2) signaling pathway is the downstream target through which PKCε regulates Tspo gene transcription (Batarseh et al., 2010). MAPK was also found to exert its effect, at least in part, through the c-Jun and STAT3 transcription factors. A schematic representation of the transcriptional regulation of Tspo is shown in Figure 1. It is important to note that although the studies presented above focused exclusively on comparing steroidogenic and non-steroidogenic cells, Mazurika et al. (2009) provided evidence that such differential regulation of TSPO expression may also exist in other tissues, such as the uterus and the kidney, before and after ovariectomy (Mazurika et al., 2009).
6-Regulation of Tspo gene expression in cancer cells
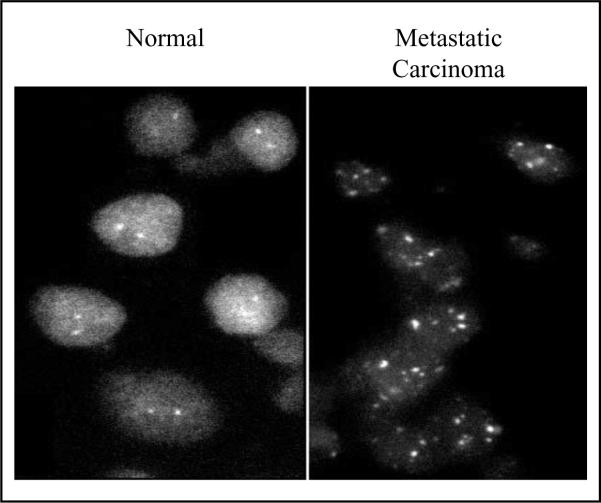
In search of the mechanisms underlying increased expression of TSPO in cancer tissues, both DNA (Southern) blot and fluorescence in situ hybridization analyses indicated that the Tspo gene was amplified in aggressive metastatic MDA-MB-231 relative to non-aggressive MCF-7 human breast cancer cells (Hardwick et al., 2002). These observations were confirmed in samples from a breast tumor metastasis biopsy, where the observed increased TSPO protein levels (Han et al., 2003) correlated with Tspo gene amplification (Figure 2). These data suggest that Tspo gene amplification may be partially responsible for the increased TSPO expression in cancer cells, and could be an important indicator of breast cancer progression.
Figure 2.
Tspo gene amplification observed in samples from normal and breast tumor metastasis biopsies. DNA was labeled with biotin-16-dUTP and hybridized to interphase nuclei prepared from human biopsies as previously described (Hardwick et al., 2002). Biotin-labeled DNA was detected using fluorescein-avidin DCS (FITC). Samples from normal and metastatic carcinoma specimens showing two and multiple fluorescence in situ hybridization, respectively, are depicted.
We also investigated the mechanism(s) regulating transcription of the Tspo gene in aggressive metastatic MDA-MB-231 breast cancer cells compared to non-aggressive MCF-7 cells. RLM-5'RACE analysis of MCF-7, MDA-MB-231, and human mammary epithelial cells indicated that transcription of the Tspo gene initiates from a common promoter at multiple sites within an approximate 40–50-bp window mapping within and adjacent to the predicted first exon (Barlow and Papadopoulos, unpublished data). Transient transfection analysis of a set of nested 5' deletion mutants of the promoter in MDA-MB-231 cells showed that activity was approximately 140-fold greater than in cells expressing the promoter-less vector (data not shown). Interestingly, while deletion analysis in low-passage MCF-7 cells exhibited approximately 7-fold less activity for most constructs, analysis of proximal promoter constructs containing all five putative GC boxes exhibited near maximal activity in both cell lines (Barlow, Batarseh, and Papadopoulos, unpublished data). To investigate whether unique combinatorial regulation at the proximal promoter contributes to the increased expression of TSPO in MDA-MB-231 cells, we analyzed a series of nested 3' deletion mutations through the putative first exon of the human Tspo gene (data not shown). While the −121/+66 promoter construct showed 7-fold higher activity in MDA-MB-231 cells compared to MCF7 cells, deletion of 45 bases from exon 1 (−121/+21) reduced the activity of the Tspo promoter to nearly equivalent levels in both cell lines. Based on these data, we hypothesize that a downstream promoter element located between +21/+66 is necessary for increased expression of TSPO in aggressive metastatic human breast cancer cells, which remains to be investigated (Barlow, Batarseh, and Papadopoulos, unpublished observations).
The possibility that Tspo gene expression is also modulated by epigenetic mechanisms has also been investigated. Methylation is one of the best-characterized mechanisms of epigenetic regulation, where a methyl group is added to the fifth carbon of the cytosine pyrimidine ring in a CpG island, reducing gene expression (Jaenisch and Bird, 2003). Computer analysis of the cloned human Tspo promoter sequence revealed high GC content in the proximal region of the promoter, and further analysis showed that the Tspo gene is situated within a CpG island (67% C+G, 0.73 Obs./Exp) that extends approximately 470 bp upstream and 615 bp downstream of the transcription initiation window. To investigate whether methylation plays a role in the differential expression of Tspo in different tissues, we treated various cell lines transfected with the Tspo promoter with 5-Azacytidine. This compound when added to cells incorporates into DNA and inhibits DNA methylation, thus providing a functional approach to investigate loss of methylation in specific gene regions and activation of the associated genes (Christman, 2002). We did not observe any changes in Tspo promoter activity following treatment (Batarseh and Papadopoulos, unpublished observations), which could be explained by the possibility that demethylation is not sufficient to activate the promoter. The deacetylation inhibitor Trichostatin A (TSA) prevents histone deacetylation, leaving the promoter open for transcription factors to bind and induce transcription (Jaenisch and Bird, 2003). TSA could also affect gene transcription through other mechanisms, including transcription factor acetylation, which may activate or repress target gene activity. Treatment of different cell lines with TSA induced a dramatic increase in Tspo promoter activity (Batarseh and Papadopoulos, unpublished observations), and the specificity of the effect of TSA and the role of epigenetic regulation on Tspo expression continues to be explored.
7-Regulation of TSPO expression by hormones, chemicals, and environmental factors
One of the most well-studied functions of TSPO is in the regulation of steroid hormone biosynthesis (Papadopoulos et al., 2006a). In steroidogenesis, TSPO is part of a multiprotein mitochondrial complex (Papadopoulos et al., 2007) that mediates import of substrate cholesterol from intracellular stores into mitochondria (Lacapere and Papadopoulos, 2003), which is the hormone-sensitive and rate-determining step in the hormonal regulation of steroidogenesis. Acute treatment of Leydig and adrenal cells with human choriogonadotropin or adrenocorticotropin, respectively, did not affect Tspo mRNA or protein levels, although the conformation of the protein changed and a higher affinity binding site appeared (Boujrad et al., 1994). However, the presence of hormones is critical for constitutive TSPO expression, as dramatic decreases of TSPO in adrenal glands, testis, and ovary were observed in hypophysectomy experiments (Anholt et al., 1985). Steroid hormones likely regulate TSPO expression, as administration of aldosterone rescues decreases in renal TSPO levels following removal of the adrenal gland (Basile et al., 1987). Continuous administration of a gonadotropin releasing hormone (GnRH)-agonist decreased TSPO expression and progesterone formation by corpora lutea of pregnant rats (Papadopoulos et al., 1999; Sridaran et al., 1999). Forced swimming, a stress-inducing condition, increases the density of TSPO ligand binding in the rat cerebral cortex and kidney (Rago et al., 1989), whereas inescapable shock reduces TSPO ligand binding in the cerebral cortex, kidney, heart, and pituitary gland (Drugan et al., 1986). Angiotensin II (ANG II) has been suggested to be an endogenous regulator of TSPO in several tissues during stress, as acute administration of ANG II reduced TSPO binding in kidney, heart, and cerebral cortex (Holmes and Drugan, 1994). TSPO levels were also shown to be modulated in a tissue- and brain region-specific manner in response to stressors and steroid hormone exposure (Bitran et al., 1998).
Among the various steroids tested, long-term treatment of rats with estradiol reduces TSPO ligand binding in the testis, and increases it in the kidney; TSPO levels in other tissues are not affected (Mazurika et al., 2009). Steroid hormone changes during the rat oestrous cycle affect TSPO levels in various genital organs (Fares et al., 1988), and ovarian and synthetic steroids significantly increase TSPO levels in the ovary, oviduct, and uterus (Bar-Ami et al., 1994). Thus, estradiol is likely a tissue-specific regulator of TSPO expression (Bar-Ami et al., 1994; Bitran et al., 1998; Fares et al., 1988; Gavish et al., 1986; Mazurika et al., 2009; Veenman and Gavish, 2006; Weizman et al., 1997).
Most of the studies described above are based on radioligand binding assays, and not on direct protein or mRNA measurements. Thus, it is possible that the observed effects are due to conformational changes of TSPO, rather than changes in Tspo mRNA and protein levels. It is also possible that protein-protein interactions at the outer mitochondrial membrane between the various proteins shown to interact with TSPO, such as the voltage-dependent anion channel (VDAC), anion nucleotide translocator (ANT), and the TSPO associated proteins, peripheral benzodiazepine receptor-associated protein 1 (PRAX-1) and peripheral benzodiazepine receptor- associated protein (PAP7), may be regulated by hormones, resulting in changes in TSPO drug ligand binding (Golani et al., 2001; Papadopoulos et al., 2007). However, the effect of estradiol and other steroids on TSPO expression is intriguing. For example, Mazurika and colleagues (Mazurika et al., 2009) reported that ovariectomy, in the absence of treatment with estradiol, did not significantly affect kidney Tspo steady-state mRNA levels (Mazurika et al., 2009), likely due to a compensatory post-transcriptional mechanism induced to maintain such levels. On the other hand, ovariectomy combined with estradiol treatment increases Tspo mRNA levels in the uterus, due to the possible release of a transcriptional attenuation site. Thus, it is highly likely that Tspo gene transcription is regulated by estrogen, further supporting the idea that Tspo expression is regulated by differential hormonal signals in different tissues. Indeed, steroid hormone response elements have been identified in both the mouse and human Tspo promoter (Table 1), and a response element specific for estrogen receptor ERβ is present in the mouse Tspo promoter (www.alggen.lsi.upc.es). It is also possible that steroid receptors may act as co-regulators, either directly or indirectly, via cross-talk of the AP-1, Ets, Sp1/Sp3, and STAT3 sites that regulate Tspo expression (see below).
GnRH, PMA, and a number of other compounds have been used to study TSPO function and regulation (Table 2). Below we will discuss a few such compounds that have been best-characterized for their effect on regulation of the Tspo promoter.
Peroxisome proliferators (PPs) comprise a large class of industrial and pharmaceutical chemicals, and include the widely-used fibrate hypolipidemic drugs, bezafibrate and clofibrate, used in industry as lubricants, surfactants, wetting agents, corrosion inhibitors, and phthalate ester plasticizers (Gazouli et al., 2002). In rodents, PPs are reported to have major effects on the expression of several genes implicated in lipid metabolism (Reddy et al., 1980; Schoonjans et al., 1993; Staels and Auwerx, 1992), where they activate specific receptors, called PP-activated receptors (PPARs), that belong to the nuclear hormone receptor gene superfamily (Corton et al., 2000; Desvergne and Wahli, 1999). Leydig cells express PPARα and PPARβ/δ proteins (Braissant et al., 1996; Gazouli et al., 2002; Schultz et al., 1999), and PPARγ has been recently identified in MA-10 cells (Kowalewski et al., 2009). PPs regulate TSPO expression through distinct mechanisms of action by decreasing the level of Tspo mRNA, including inhibition of either transcriptional or post-transcriptional events (Gazouli et al., 2002). While mechanisms that positively regulate gene expression by PPs via interaction between PPAR-RXR and positive PP response element (PPRE) sequences are well understood (Corton et al., 2000; Desvergne and Wahli, 1999), negative gene regulatory mechanisms remain to be thoroughly resolved. Due to the capacity of these receptors to be activated by pharmacological agents and industrial compounds, they may play a pivotal role in both pharmacological and chemical control of gene expression.
We examined the mechanism underlying transcriptional regulation of the Tspo gene by the PP bezafibrate and methyl-hexyl phthalate. Transient transfection of Tspo promoter constructs into MA-10 cells, followed by measurement of reporter activity, identified the −1,219/−420 Tspo promoter region as that which mediates the inhibitory effect of bezafibrate and MEHP on Tspo transcription (Gazouli and Papadopoulos, unpublished data). Subsequent deletion and mutation analysis, together with DNase I footprinting and EMSAs, indicated that PPs negatively regulate the interaction between nuclear proteins and a −595/−565-bp element of the Tspo promoter (Gazouli and Papadopoulos, unpublished observations). This element competed for binding of MA-10 nuclear proteins to a −410/−380 PPRE-like element found in the Tspo promoter, and contained a putative AP-1 binding site. These results suggest that the inhibitory effect of PPs on Tspo expression is due to a PPARα-mediated indirect transrepression of AP-1 activity. Interestingly, MEHP and bezafibrate reduced the binding of AP-1 to its cognate site responsible for mediating the effect of PKCε on the Tspo promoter, suggesting that PPs may reduce TSPO expression through antagonizing the binding of the AP1 family member to their binding site on the DNA and thus reducing Tspo promoter activity and expression.
The putative interaction between PPARs and members of the AP-1 family of transcription factors corresponds to a previously reported mechanism of gene transcriptional regulation (Delerive et al., 1999; Sakai et al., 1995). In these reports, nuclear receptors were shown to complex with c-Jun, leading to decreased AP-1 activity. In addition, PPARs interfere with AP-1, STATs, and NF-kB signaling pathways via competition with cofactors (Delerive et al., 1999). Thus, a PPARα-mediated indirect transrepression of Tspo gene transcription is possible and is consistent with published data.
In contrast to the observations presented above regarding the effect of PPs on Tspo mRNA and protein levels in Leydig cells (Gazouli et al., 2002), liver Tspo mRNA levels were increased under the same conditions, in agreement with the proposed role of TSPO in cell growth/tumor formation in non-steroidogenic tissues (Gazouli et al., 2002). The detailed mechanism responsible for Tspo induction in liver cells is unknown. However, considering that exposure of liver cells to PPs (i) induced the expression of c-Jun, JunB, c-fos, c-myc, and egr-1 (Pauley et al., 2002), (ii) increased ERK phosphorylation (Pauley et al., 2002), (iii) induced the formation of high levels of H2O2 (Carayon et al., 1996; Yeldandi et al., 2000), and (iv) reduced the levels of glutathione peroxidase and glutathione S-transferase, one can hypothesize that the resulting increased oxidative environment may be responsible for changes in gene transcription, including that of Tspo.
It has been reported that flavonoids induce increased Tspo mRNA transcription in human neuroblastoma and SNU-CF colorectal adenocarcinoma cells, increasing apoptosis rates and cytotoxicity, respectively (Lee et al., 2009). As some flavonoids may cause extensive oxidative stress, while others reduce free radical levels, it is difficult to deduce the reason behind the increased Tspo expression. While investigating the role of MAPK in mediating the effect of PKCε in the regulation of Tspo gene transcription, we used the well-described MEK inhibitor 2-(2-Amino-3-methoxyphenyl)-4H-1-benzopyran-4-one (PD98059), which contrary to results obtained with other MAPK inhibitors, induced Tspo expression (Batarseh and Papadopoulos, unpublished observations). We performed detailed experiments to understand the function of PD98059 in our model system, and found that low doses of PD898059 induced oxidative stress sufficient to drive Tspo expression. This effect most likely is due to its action on the homeostasis of the cellular antioxidant defense system, a finding that can be explained by the fact the PD898059 is a flavonoid previously shown to reduce antioxidant molecule levels in various cell types (Kirkland and Franklin, 2003). Nevertheless, these serendipitous data suggest that ROS are regulators of Tspo expression, a finding currently under investigation in our laboratory.
Treatment of rats and adrenocortical cells with the standardized Gingko biloba extract EGb 761 reduced TSPO ligand binding and protein levels in adrenal cells in stressed animals (Amri et al., 1996; Amri et al., 1997). The effect of this extract was primarily due to the presence of bioactive terpene trilactone ginkgolide B. Isolated ginkgolide B reduced adrenal cortical Tspo mRNA and protein expression, as well as Tspo promoter activity, likely through a transcription factor that binds the promoter to decrease Tspo gene expression (Amri et al., 2002; Amri et al., 2003; Papadopoulos, 2003). A regulatory element was identified in the area of −624/−513 bp of the Tspo promoter, which was confirmed by EMSA. This suggests that a transcription factor normally binds to the promoter, and that ginkgolide B could either inhibit the transcription factor to prevent its binding to DNA, or confer conformational changes on its receptor to induce dissociation of the transcription factor from the promoter and reduce Tspo transcription. EGb 761 and ginkgolide B also reduced the amount of TSPO ligand binding and protein in MBA-MB-231 aggressive metastatic, but not MCF-7 non-aggressive, breast cancer cells, both in vitro and in vivo (Amri et al., 2002; Amri et al., 2003; Papadopoulos et al., 2000). Further studies should clarify the anticancer role of Ginkgo, and modulation of TSPO expression through transcription machinery regulation.
Soy-rich diets have been linked to the low incidence of breast cancer in women. Mukhopadhyay et al. (2008) demonstrated that casein induced the capacity of TSPO ligand binding, as well as TSPO expression and protein levels in breast tumors formed in female Sprague Dawley rats induced by a single administration of the carcinogen 7,12-dimethylbenz(a)anthracene. Replacing casein with soy reduced all of these effects. Since induction of TSPO has been linked to different cancers and correlated with cancer aggressiveness and tumor prognosis, it is possible that reducing TSPO levels by soy administration could slow breast tumor development. Interestingly, soy protein has been shown to exert its effect on target genes through inhibiting both AP-1- and NFκB-dependent and independent signaling pathways (Valachovicova et al., 2004). We have previously demonstrated that AP-1 regulates Tspo expression, and it would be of great interest to demonstrate whether soy proteins act on the Tspo promoter through the same transcription factors.
8-Post-transcriptional modifications
An alternatively spliced mRNA product of the authentic full-length Tspo gene, which lacks exon 2, has been observed in all tissues to amounts 10-fold that of full-length Tspo (Lin et al., 1993). This spliced variant, named S-TSPO, contains a typical initiator sequence with a different open reading frame; interestingly, the protein encoded by this variant has not been observed to date. Similarly, transcription of Tspo from mouse testis and MA-10 cells initiates at a number of sites at the 5' end of the gene, making exon 1 variable in length (Giatzakis and Papadopoulos, 2004). Additionally, we have reported internal transcription initiation of Tspo, though to very low rates compared to initiation at the 5' end of the gene. At least two internal initiation sites exist, including one at the 3' end of exon 2 and one at the 5' end of exon 3. Since many potential translation start sites located downstream of the internal transcription start sites are in-frame with the translation start site in exon 2, and because these mRNAs are capped and could therefore be translated, the alternative transcripts could give rise to short proteins, partly identical to TSPO. Such alternative behavior is usually observed in genes that lack TATA and CCAAT boxes with the proximal promoter, as is the case with Tspo. Indeed, previous studies using antibodies and photolabeled ligands for TSPO have reported smaller proteins that are recognized by these reagents, suggesting that alternative transcripts encoding short proteins, partly identical to TSPO, may exist (Garnier et al., 1994).
Zhang et al. (2006) reported three different Tspo transcripts from the porcine gene (long, middle, and short), suggesting post-transcriptional modification of Tspo RNA by splicing. Only the long transcript corresponded to the authentic Tspo found in other mammalian species, and was the only form detected to significant levels. Similarly, we observed at least three different transcripts of Tspo in human breast cancer cells, including the full-length transcript and two shorter forms that lacked all or some of exon 2 compared to the full-length Tspo transcript (data not shown). Importantly, similar results were reported in clones generated from a testes cDNA library, indicating that this observation is not cancer-specific. Moreover, Mazurika et al. (2009) reported that adrenal Tspo mRNA was alternatively spliced after ovariectomy. The physiological relevance of the alternatively spliced forms has not yet determined, but their presence could be attributed to a possible shift in the open reading frame or errors in the splicing machinery.
Additional post-transcriptional mechanisms have been described for Tspo, where the PP perfluorodecanoic acid inhibits Leydig cell steroid formation by inhibiting TSPO ligand binding and protein expression (Boujrad et al., 2000). Perfluorodecanoic acid exerts its effect on TSPO by accelerating Tspo mRNA decay, which could influence down-stream protein synthesis rates (Boujrad et al., 2000).
9-Post-translational modifications
We previously identified putative phosphorylation motifs in the C-terminal domain of the cloned rat, bovine, and murine TSPO protein, and PKA, but not other kinases, was able to phosphorylate TSPO in mitochondrial preparations (Papadopoulos, 2003; Whalin et al., 1994). In digitonin-permeabilized MA-10 Leydig cells, TSPO was also phosphorylated in a cAMP-dependent manner, confirming TSPO is a Protein Kinase A (PKA) substrate. This phosphorylation site was not present in human TSPO, suggesting that phosphorylation may not be a ubiquitous mechanism regulating TSPO function. Further studies are required to unveil any possible direct or indirect role for PKA in the regulation of TSPO expression (Figure 1).
10-Conclusion
Our goal herein was to bring together data generated over the last two decades concerning the expression of TSPO in healthy and pathological tissues, as well as on the agents and mechanisms regulating TSPO expression. Although considered a housekeeping gene, TSPO can be regulated by hormones, various chemicals, environmental agents, stress, and pathological conditions. The majority of these factors regulate Tspo gene expression, though post-transcriptional and post-translational modifications of TSPO, as well as regulation at the protein level have been reported. A close analysis of the data presented above unveils that under basal conditions in TSPO-rich cells, and inducible conditions in TSPO-poor cells, Tspo expression is under the control of the PKCε-ERK1/2-AP1/STAT3 pathways, and that Ets and Sp1/Sp3 transcription factors play a major role in basal Tspo transcription. Table 3 shows the parallelism between the expression levels of TSPO, PKCε, ERK, and STAT3 in various cancers and brain injury. With the exception of melanoma, and squamous and renal carcinoma cells, where it was previously shown that TSPO did not correlate with tumor growth (Han et al., 2003; Katz et al., 1989), all other tumor and brain injury data suggest a definite correlation between PKCε, ERK, STAT3, and TSPO expression. These data together suggest that PKCε is the master regulator of TSPO expression and function.
Table 3.
Correlation of TSPO, PKCε, and STAT3 expression in multiple cancers and diseases. Arrows indicate increased and reduced expression/activity, respectively. Arrowheads denote a perturbation of ERK1/2 pathway activity. (N/S) not studied.
PKCε is one of the best-characterized PKC isoforms that has gained notoriety for its role as an oncogene (Akita, 2002; Basu and Sivaprasad, 2007; Gorin and Pan, 2009). The reported oncogenic roles it plays span from participating in tumor development, metastasis, and invasion in many tissues (Gorin and Pan, 2009). Indeed, high-grade tumors frequently have high levels of PKCε that correlate with high TSPO expression, as well as activity of TSPO with respect to cell proliferation, tumor invasion, and tumor metastasis. Knockdown of Tspo in breast cancer cells lowers their metastatic potential and proliferation rates, whereas PKCε inhibition reduces tumor growth and metastasis of MDA-MB-231 breast cancer cells, and sensitizes breast cancer cells to TNF-induced death (Basu et al., 2001).
Similar to TSPO, reports are emerging implicating PKCε in conferring protection to cardiac cells against ischemia reperfusion injury. Furthermore, immunoprecipitation experiments pulled down PKCε together with VDAC and ANT (Budas and Mochly-Rosen, 2007). Considering the reported physical and functional interaction between TSPO, VDAC and ANT (Budas and Mochly-Rosen, 2007; McEnery et al., 1992) these results indicate a close and possibly physical interaction between PKCε and TSPO. In addition, there are several studies linking PKCε to oxidative stress, where PKCε participates in ROS generation, either directly or by inducing the expression of genes involved in ROS generation. Likewise, PKCε is activated by ROS and is translocated to the mitochondria, where it is involved in MPTP regulation (Ardehali, 2006; Wang and Zheng, 2009). Finally, the finding that PKCε null mice have dramatically reduced circulating corticosterone levels provides a direct link between it and TSPO expression and steroidogenesis (Hodge et al., 2002).
Because TSPO is associated with numerous physiological functions and diseases, understanding regulation of its expression during health and disease may prove beneficial for preventive, therapeutic, diagnostic, and prognostic purposes. Linking Tspo gene regulation to a specific pathway, as well as identifying important factors and cofactors participating in regulating its expression, provide new insights for understanding TSPO regulation in both steroidogenic and non-steroidogenic cells. Further identification of effectors, transcription factors, and their activation states and interactions in regulating TSPO in different pathophysiological conditions should allow us to target and control TSPO levels.
Acknowledgments
This work was supported by Grant R01 ES07747 from the National Institutes of Health (to V.P.). V.P. was also supported by a Canada Research Chair in Biochemical Pharmacology.
Abbreviations
- ANT
adenine nucleotide translocator
- AP1
activator protein 1
- DBI
diazepam binding inhibitor
- DMSO
dimethyl-sulfoxide
- EMSA
electrophoretic mobility shift assay
- ER
estrogen receptor
- ERK1/2
extracellular-signal-regulated kinases 1/2
- Ets
v-ets erythroblastosis virus E26 oncogene homolog
- GnRH
gonadotropin releasing hormone
- GR
glucocorticoid receptor
- MAPK
mitogen-activated protein kinase
- MEHP
mono (2-ethylhexyl) phthalate
- MPTP
mitochondrial permeability transition pore
- PAP7
peripheral benzodiazepine receptor- associated protein 7
- PKCε
protein kinase C epsilon
- PMA
phorbol-12-myristate 13-acetate
- PP
peroxisome proliferator
- PPRE
PP response element
- PR
progesterone receptor
- PRAX-1
peripheral benzodiazepine receptor-associated protein 1
- RLM-5'RACE
RNA ligase-mediated 5' rapid amplification of complementary ends
- RXR
retinoid X receptor or 9-cis retinoic acid receptor
- Sp1
specificity protein 1/Sp1 transcription factor
- Sp3
specificity protein 3/Sp3 transcription factor
- STAT3
signal transducer and activator of transcription 3
- TSA
trichostatin A
- TSPO
translocator protein (18 kDa)
- VDAC
voltage-dependent anion channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Akita Y. Protein kinase C-epsilon (PKC-epsilon): its unique structure and function. J. Biochem. 2002;132:847–852. doi: 10.1093/oxfordjournals.jbchem.a003296. [DOI] [PubMed] [Google Scholar]
- Alonso DF, Farina HG, Skilton G, Gabri MR, De Lorenzo MS, Gomez DE. Reduction of mouse mammary tumor formation and metastasis by lovastatin, an inhibitor of the mevalonate pathway of cholesterol synthesis. Breast Cancer Res. Treat. 1998;50:83–93. doi: 10.1023/a:1006058409974. [DOI] [PubMed] [Google Scholar]
- Amri H, Ogwuegbu SO, Boujrad N, Drieu K, Papadopoulos V. In vivo regulation of peripheral-type benzodiazepine receptor and glucocorticoid synthesis by Ginkgo biloba extract EGb 761 and isolated ginkgolides. Endocrinol. 1996;137:5707–5718. doi: 10.1210/endo.137.12.8940403. [DOI] [PubMed] [Google Scholar]
- Amri H, Drieu K, Papadopoulos V. Ex vivo regulation of adrenal cortical cell steroid and protein synthesis, in response to adrenocorticotropic hormone stimulation, by the Ginkgo biloba extract EGb 761 and isolated ginkgolide B. Endocrinol. 1997;138:5415–5426. doi: 10.1210/endo.138.12.5604. [DOI] [PubMed] [Google Scholar]
- Amri H, Drieu K, Papadopoulos V. Use of ginkgolide B and a ginkgolide-activated response element to control gene transcription: example of the adrenocortical peripheral-type benzodiazepine receptor. Cell Mol. Biol. (Noisy. -le-grand) 2002;48:633–639. [PubMed] [Google Scholar]
- Amri H, Drieu K, Papadopoulos V. Transcriptional suppression of the adrenal cortical peripheral-type benzodiazepine receptor gene and inhibition of steroid synthesis by ginkgolide B. Biochem. Pharmacol. 2003;65:717–729. doi: 10.1016/s0006-2952(02)01603-9. [DOI] [PubMed] [Google Scholar]
- Anholt RR, De Souza EB, Kuhar MJ, Snyder SH. Depletion of peripheral-type benzodiazepine receptors after hypophysectomy in rat adrenal gland and testis. Eur. J. Pharmacol. 1985;110:41–46. doi: 10.1016/0014-2999(85)90026-3. [DOI] [PubMed] [Google Scholar]
- Anholt RR, Pedersen PL, De Souza EB, Snyder SH. The peripheral-type benzodiazepine receptor. Localization to the mitochondrial outer membrane. J. Biol. Chem. 1986;261:576–583. [PubMed] [Google Scholar]
- Ardehali H. Signaling mechanisms in ischemic preconditioning: interaction of PKCepsilon and MitoK(ATP) in the inner membrane of mitochondria. Circ. Res. 2006;99:798–800. doi: 10.1161/01.RES.0000247029.31997.a4. [DOI] [PubMed] [Google Scholar]
- Aziz MH, Manoharan HT, Church DR, Dreckschmidt NE, Zhong W, Oberley TD, Wilding G, Verma AK. Protein kinase Cepsilon interacts with signal transducers and activators of transcription 3 (Stat3), phosphorylates Stat3Ser727, and regulates its constitutive activation in prostate cancer. Cancer Res. 2007a;67:8828–8838. doi: 10.1158/0008-5472.CAN-07-1604. [DOI] [PubMed] [Google Scholar]
- Aziz MH, Manoharan HT, Sand JM, Verma AK. Protein kinase Cepsilon interacts with Stat3 and regulates its activation that is essential for the development of skin cancer. Mol. Carcinog. 2007b;46:646–653. doi: 10.1002/mc.20356. [DOI] [PubMed] [Google Scholar]
- Bar-Ami S, Amiri Z, Fares F, Gavish M. Modulation of peripheral benzodiazepine receptors in female rat genital organs by various gonadal steroids. Life Sci. 1994;54:1965–1975. doi: 10.1016/0024-3205(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Basile AS, Skolnick P. Subcellular localization of “peripheral-type” binding sites for benzodiazepines in rat brain. J. Neurochem. 1986;46:305–308. doi: 10.1111/j.1471-4159.1986.tb12965.x. [DOI] [PubMed] [Google Scholar]
- Basile AS, Ostrowski NL, Skolnick P. Aldosterone-reversible decrease in the density of renal peripheral benzodiazepine receptors in the rat after adrenalectomy. J. Pharmacol. Exp. Ther. 1987;240:1006–1013. [PubMed] [Google Scholar]
- Basu A, Mohanty S, Sun B. Differential sensitivity of breast cancer cells to tumor necrosis factor-alpha: involvement of protein kinase C. Biochem. Biophys. Res. Commun. 2001;280:883–891. doi: 10.1006/bbrc.2000.4209. [DOI] [PubMed] [Google Scholar]
- Basu A, Sivaprasad U. Protein kinase Cepsilon makes the life and death decision. Cell Signal. 2007;19:1633–1642. doi: 10.1016/j.cellsig.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batarseh A, Giatzakis C, Papadopoulos V. Phorbol-12-myristate 13-acetate acting through protein kinase Cepsilon induces translocator protein (18-kDa) TSPO gene expression. Biochem. 2008;47:12886–12899. doi: 10.1021/bi8012643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batarseh A, Li J, Papadopoulos V. Protein kinase Cε regulation of translocator protein (18 kDa) Tspo gene expression is mediated through a MAPK pathway targeting STAT3 and c-Jun transcription factors. Biochem. 2010 doi: 10.1021/bi100020e. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides J, Cornu P, Dennis T, Dubois A, Hauw JJ, MacKenzie ET, Sazdovitch V, Scatton B. Imaging of human brain lesions with an omega 3 site radioligand. Ann. Neurol. 1988;24:708–712. doi: 10.1002/ana.410240603. [DOI] [PubMed] [Google Scholar]
- Bitran D, Carlson D, Leschiner S, Gavish M. Ovarian steroids and stress produce changes in peripheral benzodiazepine receptor density. Eur. J Pharmacol. 1998;361:235–242. doi: 10.1016/s0014-2999(98)00708-0. [DOI] [PubMed] [Google Scholar]
- Boujrad N, Gaillard JL, Garnier M, Papadopoulos V. Acute action of choriogonadotropin on Leydig tumor cells: induction of a higher affinity benzodiazepine-binding site related to steroid biosynthesis. Endocrinol. 1994;135:1576–1583. doi: 10.1210/endo.135.4.7925120. [DOI] [PubMed] [Google Scholar]
- Boujrad N, Vidic B, Gazouli M, Culty M, Papadopoulos V. The peroxisome proliferator perfluorodecanoic acid inhibits the peripheral-type benzodiazepine receptor (PBR) expression and hormone-stimulated mitochondrial cholesterol transport and steroid formation in Leydig cells. Endocrinol. 2000;141:3137–3148. doi: 10.1210/endo.141.9.7678. [DOI] [PubMed] [Google Scholar]
- Braestrup C, Squires RF. Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H)diazepam binding. Proc. Natl. Acad. Sci. U. S. A. 1977;74:3805–3809. doi: 10.1073/pnas.74.9.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinol. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- Bribes E, Carriere D, Goubet C, Galiegue S, Casellas P, Simony-Lafontaine J. Immunohistochemical assessment of the peripheral benzodiazepine receptor in human tissues. J. Histochem. Cytochem. 2004;52:19–28. doi: 10.1177/002215540405200103. [DOI] [PubMed] [Google Scholar]
- Bromberg J. Stat proteins and oncogenesis. J. Clin. Invest. 2002;109:1139–1142. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RC, Degenhardt B, Kotoula M, Papadopoulous V. Location-dependent role of the human glioma cell peripheral-type benzodiazepine receptor in proliferation and steroid biosynthesis. Cancer Lett. 2000;156:125–132. doi: 10.1016/s0304-3835(00)00451-1. [DOI] [PubMed] [Google Scholar]
- Budas GR, Mochly-Rosen D. Mitochondrial protein kinase Cepsilon (PKCepsilon): emerging role in cardiac protection from ischaemic damage. Biochem. Soc. Trans. 2007;35:1052–1054. doi: 10.1042/BST0351052. [DOI] [PubMed] [Google Scholar]
- Carayon P, Portier M, Dussossoy D, Bord A, Petitpretre G, Canat X, Le FG, Casellas P. Involvement of peripheral benzodiazepine receptors in the protection of hematopoietic cells against oxygen radical damage. Blood. 1996;87:3170–3178. [PubMed] [Google Scholar]
- Casellas P, Galiegue S, Basile AS. Peripheral benzodiazepine receptors and mitochondrial function. Neurochem. Int. 2002;40:475–486. doi: 10.1016/s0197-0186(01)00118-8. [DOI] [PubMed] [Google Scholar]
- Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin. Cancer Res. 2003;9:10–19. [PubMed] [Google Scholar]
- Chang YJ, McCabe RT, Rennert H, Budarf ML, Sayegh R, Emanuel BS, Skolnick P, Strauss JF., III The human “peripheral-type” benzodiazepine receptor: regional mapping of the gene and characterization of the receptor expressed from cDNA. DNA Cell Biol. 1992;11:471–480. doi: 10.1089/dna.1992.11.471. [DOI] [PubMed] [Google Scholar]
- Chen MK, Guilarte TR. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol. Ther. 2008;118:1–17. doi: 10.1016/j.pharmthera.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman JK. 5-Azacytidine and 5-aza-2'-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene. 2002;21:5483–5495. doi: 10.1038/sj.onc.1205699. [DOI] [PubMed] [Google Scholar]
- Coleman PS, Chen LC, Sepp-Lorenzino L. Cholesterol metabolism and tumor cell proliferation. Subcell. Biochem. 1997;28:363–435. doi: 10.1007/978-1-4615-5901-6_13. [DOI] [PubMed] [Google Scholar]
- Corsi L, Geminiani E, Baraldi M. Peripheral benzodiazepine receptor (PBR) new insight in cell proliferation and cell differentiation review. Curr. Clin. Pharmacol. 2008;3:38–45. doi: 10.2174/157488408783329878. [DOI] [PubMed] [Google Scholar]
- Corton JC, Anderson SP, Stauber A. Central role of peroxisome proliferator-activated receptors in the actions of peroxisome proliferators. Annu. Rev. Pharmacol. Toxicol. 2000;40:491–518. doi: 10.1146/annurev.pharmtox.40.1.491. [DOI] [PubMed] [Google Scholar]
- Corvinus FM, Orth C, Moriggl R, Tsareva SA, Wagner S, Pfitzner EB, Baus D, Kaufmann R, Huber LA, Zatloukal K, Beug H, Ohlschlager P, Schutz A, Halbhuber KJ, Friedrich K. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia. 2005;7:545–555. doi: 10.1593/neo.04571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton TP, Li Q, Bittel D, Liang L, Andrews GK. Oxidative stress activates metal-responsive transcription factor-1 binding activity. Occupancy in vivo of metal response elements in the metallothionein-I gene promoter. J Biol. Chem. 1996;271:26233–26241. doi: 10.1074/jbc.271.42.26233. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Shertzer HG, Puga A. Regulation of gene expression by reactive oxygen. Annu. Rev. Pharmacol. Toxicol. 1999;39:67–101. doi: 10.1146/annurev.pharmtox.39.1.67. [DOI] [PubMed] [Google Scholar]
- De Souza EB, Anholt RR, Murphy KM, Snyder SH, Kuhar MJ. Peripheral-type benzodiazepine receptors in endocrine organs: autoradiographic localization in rat pituitary, adrenal, and testis. Endocrinol. 1985;116:567–573. doi: 10.1210/endo-116-2-567. [DOI] [PubMed] [Google Scholar]
- Delavoie F, Li H, Hardwick M, Robert JC, Giatzakis C, Peranzi G, Yao ZX, Maccario J, Lacapere JJ, Papadopoulos V. In vivo and in vitro peripheral-type benzodiazepine receptor polymerization: functional significance in drug ligand and cholesterol binding. Biochem. 2003;42:4506–4519. doi: 10.1021/bi0267487. [DOI] [PubMed] [Google Scholar]
- Delerive P, De BK, Besnard S, Vanden BW, Peters JM, Gonzalez FJ, Fruchart JC, Tedgui A, Haegeman G, Staels B. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J. Biol. Chem. 1999;274:32048–32054. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- Dessi S, Batetta B, Anchisi C, Pani P, Costelli P, Tessitore L, Baccino FM. Cholesterol metabolism during the growth of a rat ascites hepatoma (Yoshida AH-130) Br. J. Cancer. 1992;66:787–793. doi: 10.1038/bjc.1992.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessi S, Batetta B, Pulisci D, Spano O, Anchisi C, Tessitore L, Costelli P, Baccino FM, Aroasio E, Pani P. Cholesterol content in tumor tissues is inversely associated with high-density lipoprotein cholesterol in serum in patients with gastrointestinal cancer. Cancer. 1994;73:253–258. doi: 10.1002/1097-0142(19940115)73:2<253::aid-cncr2820730204>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- Dimario JX. Activation and repression of growth factor receptor gene transcription (Review) Int. J. Mol. Med. 2002;10:65–71. [PubMed] [Google Scholar]
- Drugan RC, Basile AS, Crawley JN, Paul SM, Skolnick P. Inescapable shock reduces [3H]Ro 5–4864 binding to “peripheral-type” benzodiazepine receptors in the rat. Pharmacol. Biochem. Behav. 1986;24:1673–1677. doi: 10.1016/0091-3057(86)90504-6. [DOI] [PubMed] [Google Scholar]
- Fafalios A, Akhavan A, Parwani AV, Bies RR, McHugh KJ, Pflug BR. Translocator protein blockade reduces prostate tumor growth. Clin. Cancer Res. 2009;15:6177–6184. doi: 10.1158/1078-0432.CCR-09-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Rone MB, Papadopoulos V. Translocator protein 2 is involved in cholesterol redistribution during erythropoiesis. J. Biol. Chem. 2009;284:30484–30497. doi: 10.1074/jbc.M109.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares F, Bar-Ami S, Brandes JM, Gavish M. Changes in the density of peripheral benzodiazepine binding sites in genital organs of the female rat during the oestrous cycle. J Reprod. Fertil. 1988;83:619–625. doi: 10.1530/jrf.0.0830619. [DOI] [PubMed] [Google Scholar]
- Farina HG, Bublik DR, Alonso DF, Gomez DE. Lovastatin alters cytoskeleton organization and inhibits experimental metastasis of mammary carcinoma cells. Clin. Exp. Metastasis. 2002;19:551–559. doi: 10.1023/a:1020355621043. [DOI] [PubMed] [Google Scholar]
- Favreau F, Rossard L, Zhang K, Desurmont T, Manguy E, Belliard A, Fabre S, Liu J, Han Z, Thuillier R, Papadopoulos V, Hauet T. Expression and modulation of translocator protein and its partners by hypoxia reoxygenation or ischemia and reperfusion in porcine renal models. Am. J. Physiol Renal Physiol. 2009;297:F177–F190. doi: 10.1152/ajprenal.90422.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields AP, Gustafson WC. Protein kinase C in disease: cancer. Methods Mol. Biol. 2003;233:519–537. doi: 10.1385/1-59259-397-6:519. [DOI] [PubMed] [Google Scholar]
- Freeman MR, Solomon KR. Cholesterol and prostate cancer. J. Cell Biochem. 2004;91:54–69. doi: 10.1002/jcb.10724. [DOI] [PubMed] [Google Scholar]
- Frisullo G, Angelucci F, Caggiula M, Nociti V, Iorio R, Patanella AK, Sancricca C, Mirabella M, Tonali PA, Batocchi AP. pSTAT1, pSTAT3, and T-bet expression in peripheral blood mononuclear cells from relapsing-remitting multiple sclerosis patients correlates with disease activity. J. Neurosci. Res. 2006;84:1027–1036. doi: 10.1002/jnr.20995. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Casellas P, Kramar A, Tinel N, Simony-Lafontaine J. Immunohistochemical assessment of the peripheral benzodiazepine receptor in breast cancer and its relationship with survival. Clin. Cancer Res. 2004;10:2058–2064. doi: 10.1158/1078-0432.ccr-03-0988. [DOI] [PubMed] [Google Scholar]
- Garczarczyk D, Toton E, Biedermann V, Rosivatz E, Rechfeld F, Rybczynska M, Hofmann J. Signal transduction of constitutively active protein kinase C epsilon. Cell. Signal. 2009;21:745–752. doi: 10.1016/j.cellsig.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Garnier M, Boujrad N, Oke BO, Brown AS, Riond J, Ferrara P, Shoyab M, Suarez-Quian CA, Papadopoulos V. Diazepam binding inhibitor is a paracrine/autocrine regulator of Leydig cell proliferation and steroidogenesis: action via peripheral-type benzodiazepine receptor and independent mechanisms. Endocrinol. 1993;132:444–458. doi: 10.1210/endo.132.1.8380386. [DOI] [PubMed] [Google Scholar]
- Garnier M, Boujrad N, Ogwuegbu SO, Hudson JR, Jr., Papadopoulos V. The polypeptide diazepam-binding inhibitor and a higher affinity mitochondrial peripheral-type benzodiazepine receptor sustain constitutive steroidogenesis in the R2C Leydig tumor cell line. J Biol. Chem. 1994;269:22105–22112. [PubMed] [Google Scholar]
- Gavish M, Okun F, Weizman A, Youdim MB. Modulation of peripheral benzodiazepine binding sites following chronic estradiol treatment. Eur. J Pharmacol. 1986;127:147–151. doi: 10.1016/0014-2999(86)90218-9. [DOI] [PubMed] [Google Scholar]
- Gavish M, Bachman I, Shoukrun R, Katz Y, Veenman L, Weisinger G, Weizman A. Enigma of the peripheral benzodiazepine receptor. Pharmacol. Rev. 1999;51:629–650. [PubMed] [Google Scholar]
- Gazouli M, Yao ZX, Boujrad N, Corton JC, Culty M, Papadopoulos V. Effect of peroxisome proliferators on Leydig cell peripheral-type benzodiazepine receptor gene expression, hormone-stimulated cholesterol transport, and steroidogenesis: role of the peroxisome proliferator-activator receptor alpha. Endocrinol. 2002;143:2571–2583. doi: 10.1210/endo.143.7.8895. [DOI] [PubMed] [Google Scholar]
- Giatzakis C, Papadopoulos V. Differential utilization of the promoter of peripheral-type benzodiazepine receptor by steroidogenic versus nonsteroidogenic cell lines and the role of Sp1 and Sp3 in the regulation of basal activity. Endocrinol. 2004;145:1113–1123. doi: 10.1210/en.2003-1330. [DOI] [PubMed] [Google Scholar]
- Giatzakis C, Batarseh A, Dettin L, Papadopoulos V. The role of Ets transcription factors in the basal transcription of the translocator protein (18 kDa) Biochem. 2007;46:4763–4774. doi: 10.1021/bi062208o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusti L, Costa B, Viacava P, Castagna M, Iacconi P, Ricci RE, Zaccagnini M, Miccoli P, Lucacchini A. Peripheral type benzodiazepine receptor in human parathyroid glands: up-regulation in adenoma. J. Endocrinol. Invest. 2004;27:826–831. doi: 10.1007/BF03346276. [DOI] [PubMed] [Google Scholar]
- Golani I, Weizman A, Leschiner S, Spanier I, Eckstein N, Limor R, Yanai J, Maaser K, Scherubl H, Weisinger G, Gavish M. Hormonal regulation of peripheral benzodiazepine receptor binding properties is mediated by subunit interaction. Biochem. 2001;40:10213–10222. doi: 10.1021/bi010431+. [DOI] [PubMed] [Google Scholar]
- Gorin MA, Pan Q. Protein kinase C epsilon: an oncogene and emerging tumor biomarker. Mol. Cancer. 2009;8:9. doi: 10.1186/1476-4598-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ. The risk of cancer in users of statins. J. Clin. Oncol. 2004;22:2388–2394. doi: 10.1200/JCO.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat. Rev. Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- Han Z, Slack RS, Li W, Papadopoulos V. Expression of peripheral benzodiazepine receptor (PBR) in human tumors: relationship to breast, colorectal, and prostate tumor progression. J. Recept. Signal. Transduct. Res. 2003;23:225–238. doi: 10.1081/rrs-120025210. [DOI] [PubMed] [Google Scholar]
- Hardwick M, Fertikh D, Culty M, Li H, Vidic B, Papadopoulos V. Peripheral-type benzodiazepine receptor (PBR) in human breast cancer: correlation of breast cancer cell aggressive phenotype with PBR expression, nuclear localization, and PBR-mediated cell proliferation and nuclear transport of cholesterol. Cancer Res. 1999;59:831–842. [PubMed] [Google Scholar]
- Hardwick M, Rone J, Han Z, Haddad B, Papadopoulos V. Peripheral-type benzodiazepine receptor levels correlate with the ability of human breast cancer MDA-MB-231 cell line to grow in SCID mice. Int. J. Cancer. 2001;94:322–327. doi: 10.1002/ijc.1472. [DOI] [PubMed] [Google Scholar]
- Hardwick M, Cavalli LR, Barlow KD, Haddad BR, Papadopoulos V. Peripheral-type benzodiazepine receptor (PBR) gene amplification in MDA-MB-231 aggressive breast cancer cells. Cancer Genet. Cytogenet. 2002;139:48–51. doi: 10.1016/s0165-4608(02)00604-0. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Raber J, McMahon T, Walter H, Sanchez-Perez AM, Olive MF, Mehmert K, Morrow AL, Messing RO. Decreased anxiety-like behavior, reduced stress hormones, and neurosteroid supersensitivity in mice lacking protein kinase C epsilon. J. Clin. Invest. 2002;110:1003–1010. doi: 10.1172/JCI15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes PV, Drugan RC. Stress-induced regulation of the renal peripheral benzodiazepine receptor: possible role of the renin-angiotensin system. Psychoneuroendocrinology. 1994;19:43–54. doi: 10.1016/0306-4530(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Churchill E, Mochly-Rosen D. Epsilon protein kinase C as a potential therapeutic target for the ischemic heart. Cardiovasc. Res. 2006;70:222–230. doi: 10.1016/j.cardiores.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jani JP, Specht S, Stemmler N, Blanock K, Singh SV, Gupta V, Katoh A. Metastasis of B16F10 mouse melanoma inhibited by lovastatin, an inhibitor of cholesterol biosynthesis. Invasion Metastasis. 1993;13:314–324. [PubMed] [Google Scholar]
- Katz Y, Eitan A, Amiri Z, Gavish M. Dramatic increase in peripheral benzodiazepine binding sites in human colonic adenocarcinoma as compared to normal colon. Eur. J. Pharmacol. 1988;148:483–484. doi: 10.1016/0014-2999(88)90135-5. [DOI] [PubMed] [Google Scholar]
- Katz Y, Moskovitz B, Levin DR, Gavish M. Absence of peripheral-type benzodiazepine binding sites in renal carcinoma: a potential biochemical marker. Br. J Urol. 1989;63:124–127. doi: 10.1111/j.1464-410x.1989.tb05146.x. [DOI] [PubMed] [Google Scholar]
- Katz Y, Ben-Baruch G, Kloog Y, Menczer J, Gavish M. Increased density of peripheral benzodiazepine-binding sites in ovarian carcinomas as compared with benign ovarian tumours and normal ovaries. Clin. Sci. (Lond) 1990a;78:155–158. doi: 10.1042/cs0780155. [DOI] [PubMed] [Google Scholar]
- Katz Y, Eitan A, Gavish M. Increase in peripheral benzodiazepine binding sites in colonic adenocarcinoma. Oncol. 1990b;47:139–142. doi: 10.1159/000226806. [DOI] [PubMed] [Google Scholar]
- Kirkland RA, Franklin JL. Prooxidant Effects of NGF Withdrawal and MEK Inhibition in Sympathetic Neurons. Antioxid. & Redox Signal. 2003;5:635–639. doi: 10.1089/152308603770310301. [DOI] [PubMed] [Google Scholar]
- Kokoglu E, Karaarslan I, Karaarslan HM, Baloglu H. Alterations of serum lipids and lipoproteins in breast cancer. Cancer Lett. 1994;82:175–178. doi: 10.1016/0304-3835(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Kolanjiappan K, Ramachandran CR, Manoharan S. Biochemical changes in tumor tissues of oral cancer patients. Clin. Biochem. 2003;36:61–65. doi: 10.1016/s0009-9120(02)00421-6. [DOI] [PubMed] [Google Scholar]
- Kowalewski MP, Dyson MT, Manna PR, Stocco DM. Involvement of peroxisome proliferator-activated receptor gamma in gonadal steroidogenesis and steroidogenic acute regulatory protein expression. Reprod. Fertil. Dev. 2009;21:909–922. doi: 10.1071/RD09027. [DOI] [PubMed] [Google Scholar]
- Kruczek C, Gorg B, Keitel V, Pirev E, Kroncke KD, Schliess F, Haussinger D. Hypoosmotic swelling affects zinc homeostasis in cultured rat astrocytes. Glia. 2009;57:79–92. doi: 10.1002/glia.20737. [DOI] [PubMed] [Google Scholar]
- Lacapere JJ, Papadopoulos V. Peripheral-type benzodiazepine receptor: structure and function of a cholesterol-binding protein in steroid and bile acid biosynthesis. Steroids. 2003;68:569–585. doi: 10.1016/s0039-128x(03)00101-6. [DOI] [PubMed] [Google Scholar]
- Lee SW, Lee JT, Lee MG, Lee HW, Ahn SJ, Lee YJ, Lee YL, Yoo J, Ahn BC, Ha JH. In vitro antiproliferative characteristics of flavonoids and diazepam on SNU-C4 colorectal adenocarcinoma cells. J. Nat. Med. 2009;63:124–129. doi: 10.1007/s11418-008-0300-x. [DOI] [PubMed] [Google Scholar]
- Li H, Papadopoulos V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinol. 1998;139:4991–4997. doi: 10.1210/endo.139.12.6390. [DOI] [PubMed] [Google Scholar]
- Lin D, Chang YJ, Strauss JF, III, Miller WL. The human peripheral benzodiazepine receptor gene: cloning and characterization of alternative splicing in normal tissues and in a patient with congenital lipoid adrenal hyperplasia. Genomics. 1993;18:643–650. doi: 10.1016/s0888-7543(05)80367-2. [DOI] [PubMed] [Google Scholar]
- Lishner M, Bar-Sef A, Elis A, Fabian I. Effect of simvastatin alone and in combination with cytosine arabinoside on the proliferation of myeloid leukemia cell lines. J. Investig. Med. 2001;49:319–324. doi: 10.2310/6650.2001.33896. [DOI] [PubMed] [Google Scholar]
- Mazurika C, Veenman L, Weizman R, Bidder M, Leschiner S, Golani I, Spanier I, Weisinger G, Gavish M. Estradiol modulates uterine 18 kDa translocator protein gene expression in uterus and kidney of rats. Mol. Cell Endocrinol. 2009;307:43–49. doi: 10.1016/j.mce.2009.04.001. [DOI] [PubMed] [Google Scholar]
- McEnery MW, Snowman AM, Trifiletti RR, Snyder SH. Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc. Natl. Acad. Sci. U. S. A. 1992;89:3170–3174. doi: 10.1073/pnas.89.8.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen H, Kononen J, Haapasalo H, Helen P, Sallinen P, Harjuntausta T, Helin H, Alho H. Expression of peripheral-type benzodiazepine receptor and diazepam binding inhibitor in human astrocytomas: relationship to cell proliferation. Cancer Res. 1995;55:2691–2695. [PubMed] [Google Scholar]
- Moynagh PN, O'Neill LA, Williams DC. Interleukin-1 and phorbol myristate acetate modulate the peripheral-type benzodiazepine receptor in lymphocytes and glial cells. Biochem. Pharmacol. 1993;46:821–827. doi: 10.1016/0006-2952(93)90490-n. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Rajaratnam V, Mukherjee S, Das SK. Control of peripheral benzodiazepine receptor-mediated breast cancer in rats by soy protein. Mol. Carcinog. 2008;47:310–319. doi: 10.1002/mc.20387. [DOI] [PubMed] [Google Scholar]
- Narisawa T, Fukaura Y, Terada K, Umezawa A, Tanida N, Yazawa K, Ishikawa C. Prevention of 1,2-dimethylhydrazine-induced colon tumorigenesis by HMG-CoA reductase inhibitors, pravastatin and simvastatin, in ICR mice. Carcinog. 1994;15:2045–2048. doi: 10.1093/carcin/15.9.2045. [DOI] [PubMed] [Google Scholar]
- ntkiewicz-Michaluk L, Guidotti A, Krueger KE. Molecular characterization and mitochondrial density of a recognition site for peripheral-type benzodiazepine ligands. Mol. Pharmacol. 1988;34:272–278. [PubMed] [Google Scholar]
- O'Beirne GB, Woods MJ, Williams DC. Two subcellular locations for peripheral-type benzodiazepine acceptors in rat liver. Eur. J. Biochem. 1990;188:131–138. doi: 10.1111/j.1432-1033.1990.tb15380.x. [DOI] [PubMed] [Google Scholar]
- Oh YJ, Francis JW, Markelonis GJ, Oh TH. Interleukin-1-beta and tumor necrosis factor-alpha increase peripheral-type benzodiazepine binding sites in cultured polygonal astrocytes. J. Neurochem. 1992;58:2131–2138. doi: 10.1111/j.1471-4159.1992.tb10955.x. [DOI] [PubMed] [Google Scholar]
- Oke BO, Suarez-Quian CA, Riond J, Ferrara P, Papadopoulos V. Cell surface localization of the peripheral-type benzodiazepine receptor (PBR) in adrenal cortex. Mol. Cell Endocrinol. 1992;87:R1–R6. doi: 10.1016/0303-7207(92)90248-5. [DOI] [PubMed] [Google Scholar]
- Olson JM, Ciliax BJ, Mancini WR, Young AB. Presence of peripheral-type benzodiazepine binding sites on human erythrocyte membranes. Eur. J. Pharmacol. 1988;152:47–53. doi: 10.1016/0014-2999(88)90834-5. [DOI] [PubMed] [Google Scholar]
- Ono Y, Fujii T, Ogita K, Kikkawa U, Igarashi K, Nishizuka Y. The structure, expression, and properties of additional members of the protein kinase C family. J. Biol. Chem. 1988;263:6927–6932. [PubMed] [Google Scholar]
- Papadopoulos V, Amri H, Boujrad N, Cascio C, Culty M, Garnier M, Hardwick M, Li H, Vidic B, Brown AS, Reversa JL, Bernassau JM, Drieu K. Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids. 1997;62:21–28. doi: 10.1016/s0039-128x(96)00154-7. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Dharmarajan AM, Li H, Culty M, Lemay M, Sridaran R. Mitochondrial peripheral-type benzodiazepine receptor expression. Correlation with gonadotropin-releasing hormone (GnRH) agonist-induced apoptosis in the corpus luteum. Biochem. Pharmacol. 1999;58:1389–1393. doi: 10.1016/s0006-2952(99)00215-4. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Kapsis A, Li H, Amri H, Hardwick M, Culty M, Kasprzyk PG, Carlson M, Moreau JP, Drieu K. Drug-induced inhibition of the peripheral-type benzodiazepine receptor expression and cell proliferation in human breast cancer cells. Anticancer Res. 2000;20:2835–2847. [PubMed] [Google Scholar]
- Papadopoulos V. Peripheral benzodiazepine receptor: structure and function in health and disease. Ann. Pharm. Fr. 2003;61:30–50. [PubMed] [Google Scholar]
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006a;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Lecanu L, Brown RC, Han Z, Yao ZX. Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neurosci. 2006b;138:749–756. doi: 10.1016/j.neuroscience.2005.05.063. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Liu J, Culty M. Is there a mitochondrial signaling complex facilitating cholesterol import? Mol. Cell Endocrinol. 2007;265–266:59–64. doi: 10.1016/j.mce.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Lecanu L. Translocator protein (18 kDa) TSPO: an emerging therapeutic target in neurotrauma. Exp. Neurol. 2009;219:53–57. doi: 10.1016/j.expneurol.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley CJ, Ledwith BJ, Kaplanski C. Peroxisome proliferators activate growth regulatory pathways largely via peroxisome proliferator-activated receptor alpha-independent mechanisms. Cell Signal. 2002;14:351–358. doi: 10.1016/s0898-6568(01)00260-1. [DOI] [PubMed] [Google Scholar]
- Perletti G, Tessitore L, Sesca E, Pani P, Dianzani MU, Piccinini F. Epsilon PKC acts like a marker of progressive malignancy in rat liver, but fails to enhance tumorigenesis in rat hepatoma cells in culture. Biochem. Biophys. Res. Commun. 1996;221:688–691. doi: 10.1006/bbrc.1996.0657. [DOI] [PubMed] [Google Scholar]
- Pongracz J, Clark P, Neoptolemos JP, Lord JM. Expression of protein kinase C isoenzymes in colorectal cancer tissue and their differential activation by different bile acids. Int. J. Cancer. 1995;61:35–39. doi: 10.1002/ijc.2910610107. [DOI] [PubMed] [Google Scholar]
- Rago L, Kiivet RA, Harro J, Pold M. Central- and peripheral-type benzodiazepine receptors: similar regulation by stress and GABA receptor agonists. Pharmacol. Biochem. Behav. 1989;32:879–883. doi: 10.1016/0091-3057(89)90052-x. [DOI] [PubMed] [Google Scholar]
- Reddy JK, Azarnoff DL, Hignite CE. Hypolipidaemic hepatic peroxisome proliferators form a novel class of chemical carcinogens. Nat. 1980;283:397–398. doi: 10.1038/283397a0. [DOI] [PubMed] [Google Scholar]
- Rey C, Mauduit C, Naureils O, Benahmed M, Louisot P, Gasnier F. Up-regulation of mitochondrial peripheral benzodiazepine receptor expression by tumor necrosis factor alpha in testicular leydig cells. Possible involvement in cell survival. Biochem. Pharmacol. 2000;60:1639–1646. doi: 10.1016/s0006-2952(00)00500-1. [DOI] [PubMed] [Google Scholar]
- Riond J, Mattei MG, Kaghad M, Dumont X, Guillemot JC, Le FG, Caput D, Ferrara P. Molecular cloning and chromosomal localization of a human peripheral-type benzodiazepine receptor. Eur. J. Biochem. 1991;195:305–311. doi: 10.1111/j.1432-1033.1991.tb15707.x. [DOI] [PubMed] [Google Scholar]
- Roivainen A, Nagren K, Hirvonen J, Oikonen V, Virsu P, Tolvanen T, Rinne JO. Whole-body distribution and metabolism of [N-methyl-11C](R)-1-(2-chlorophenyl)-N-(1-methylpropyl)-3-isoquinolinecarb oxamide in humans; an imaging agent for in vivo assessment of peripheral benzodiazepine receptor activity with positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging. 2009;36:671–682. doi: 10.1007/s00259-008-1000-1. [DOI] [PubMed] [Google Scholar]
- Rudling M, Collins VP. Low density lipoprotein receptor and 3-hydroxy-3-methylglutaryl coenzyme A reductase mRNA levels are coordinately reduced in human renal cell carcinoma. Biochim. Biophys. Acta. 1996;1299:75–79. doi: 10.1016/0005-2760(95)00195-6. [DOI] [PubMed] [Google Scholar]
- Sakai M, Matsushima-Hibiya Y, Nishizawa M, Nishi S. Suppression of rat glutathione transferase P expression by peroxisome proliferators: interaction between Jun and peroxisome proliferator-activated receptor alpha. Cancer Res. 1995;55:5370–5376. [PubMed] [Google Scholar]
- Schoonjans K, Staels B, Grimaldi P, Auwerx J. Acyl-CoA synthetase mRNA expression is controlled by fibric-acid derivatives, feeding and liver proliferation. Eur. J Biochem. 1993;216:615–622. doi: 10.1111/j.1432-1033.1993.tb18181.x. [DOI] [PubMed] [Google Scholar]
- Schultz R, Yan W, Toppari J, Volkl A, Gustafsson JA, Pelto-Huikko M. Expression of peroxisome proliferator-activated receptor alpha messenger ribonucleic acid and protein in human and rat testis. Endocrinol. 1999;140:2968–2975. doi: 10.1210/endo.140.7.6858. [DOI] [PubMed] [Google Scholar]
- Sharif TR, Sharif M. Overexpression of protein kinase C epsilon in astroglial brain tumor derived cell lines and primary tumor samples. Int. J. Oncol. 1999;15:237–243. [PubMed] [Google Scholar]
- Singleton PA, Bourguignon LY. CD44 interaction with ankyrin and IP3 receptor in lipid rafts promotes hyaluronan-mediated Ca2+ signaling leading to nitric oxide production and endothelial cell adhesion and proliferation. Exp. Cell Res. 2004;295:102–118. doi: 10.1016/j.yexcr.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Sridaran R, Philip GH, Li H, Culty M, Liu Z, Stocco DM, Papadopoulos V. GnRH agonist treatment decreases progesterone synthesis, luteal peripheral benzodiazepine receptor mRNA, ligand binding and steroidogenic acute regulatory protein expression during pregnancy. J. Mol. Endocrinol. 1999;22:45–54. doi: 10.1677/jme.0.0220045. [DOI] [PubMed] [Google Scholar]
- Staels B, Auwerx J. Perturbation of developmental gene expression in rat liver by fibric acid derivatives: lipoprotein lipase and alpha-fetoprotein as models. Dev. 1992;115:1035–1043. doi: 10.1242/dev.115.4.1035. [DOI] [PubMed] [Google Scholar]
- Subbaiah PV, Liu M, Witt TR. Impaired cholesterol esterification in the plasma in patients with breast cancer. Lipids. 1997;32:157–162. doi: 10.1007/s11745-997-0020-5. [DOI] [PubMed] [Google Scholar]
- Valachovicova T, Slivova V, Bergman H, Shuherk J, Sliva D. Soy isoflavones suppress invasiveness of breast cancer cells by the inhibition of NF-kappaB/AP-1-dependent and -independent pathways. Int. J. Oncol. 2004;25:1389–1395. [PubMed] [Google Scholar]
- Veenman L, Gavish M. The peripheral-type benzodiazepine receptor and the cardiovascular system. Implications for drug development. Pharmacol. Ther. 2006;110:503–524. doi: 10.1016/j.pharmthera.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Veenman L, Papadopoulos V, Gavish M. Channel-like functions of the 18-kDa translocator protein (TSPO): regulation of apoptosis and steroidogenesis as part of the host-defense response. Curr. Pharm. Des. 2007;13:2385–2405. doi: 10.2174/138161207781368710. [DOI] [PubMed] [Google Scholar]
- Venturini I, Zeneroli ML, Corsi L, Avallone R, Farina F, Alho H, Baraldi C, Ferrarese C, Pecora N, Frigo M, Ardizzone G, Arrigo A, Pellicci R, Baraldi M. Up-regulation of peripheral benzodiazepine receptor system in hepatocellular carcinoma. Life Sci. 1998;63:1269–1280. doi: 10.1016/s0024-3205(98)00388-9. [DOI] [PubMed] [Google Scholar]
- Wachtershauser A, Akoglu B, Stein J. HMG-CoA reductase inhibitor mevastatin enhances the growth inhibitory effect of butyrate in the colorectal carcinoma cell line Caco-2. Carcinog. 2001;22:1061–1067. doi: 10.1093/carcin/22.7.1061. [DOI] [PubMed] [Google Scholar]
- Wang YX, Zheng YM. ROS-dependent signaling mechanisms for hypoxic Ca2+ responses in pulmonary artery myocytes. Antioxid. Redox. Signal. 2009 doi: 10.1089/ars.2009.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg F, Chandel NS. Reactive oxygen species-dependent signaling regulates cancer. Cell Mol. Life Sci. 2009;66:3663–3673. doi: 10.1007/s00018-009-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weizman R, Leschiner S, Schlegel W, Gavish M. Peripheral-type benzodiazepine receptor ligands and serum steroid hormones. Brain Res. 1997;772:203–208. doi: 10.1016/s0006-8993(97)00815-9. [DOI] [PubMed] [Google Scholar]
- Wen TC, Peng H, Hata R, Desaki J, Sakanaka M. Induction of phosphorylated-Stat3 following focal cerebral ischemia in mice. Neurosci. Lett. 2001;303:153–156. doi: 10.1016/s0304-3940(01)01711-6. [DOI] [PubMed] [Google Scholar]
- Whalin ME, Boujrad N, Papadopoulos V, Krueger KE. Studies on the phosphorylation of the 18 kDa mitochondrial benzodiazepine receptor protein. J. Recept. Res. 1994;14:217–228. doi: 10.3109/10799899409066033. [DOI] [PubMed] [Google Scholar]
- White RM. On the occurrence of crystals in tumours. J Pathol Bacteriol. 1909;13:3–10. [Google Scholar]
- Yeldandi AV, Rao MS, Reddy JK. Hydrogen peroxide generation in peroxisome proliferator-induced oncogenesis. Mutat. Res. 2000;448:159–177. doi: 10.1016/s0027-5107(99)00234-1. [DOI] [PubMed] [Google Scholar]
- Yeliseev AA, Kaplan S. A sensory transducer homologous to the mammalian peripheral-type benzodiazepine receptor regulates photosynthetic membrane complex formation in Rhodobacter sphaeroides 2.4.1. J Biol. Chem. 1995;270:21167–21175. doi: 10.1074/jbc.270.36.21167. [DOI] [PubMed] [Google Scholar]
- Yeliseev AA, Krueger KE, Kaplan S. A mammalian mitochondrial drug receptor functions as a bacterial “oxygen” sensor. Proc. Natl. Acad. Sci. U. S. A. 1997;94:5101–5106. doi: 10.1073/pnas.94.10.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeon SW, Jung MW, Ha MJ, Kim SU, Huh K, Savage MJ, Masliah E, Mook-Jung I. Blockade of PKC epsilon activation attenuates phorbol ester-induced increase of alpha-secretase-derived secreted form of amyloid precursor protein. Biochem. Biophys. Res. Commun. 2001;280:782–787. doi: 10.1006/bbrc.2000.4181. [DOI] [PubMed] [Google Scholar]
- Yoshioka Y, Sasaki J, Yamamoto M, Saitoh K, Nakaya S, Kubokawa M. Quantitation by (1)H-NMR of dolichol, cholesterol and choline-containing lipids in extracts of normal and phathological thyroid tissue. NMR Biomed. 2000;13:377–383. doi: 10.1002/1099-1492(200011)13:7<377::aid-nbm658>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Ravindranath L, Mohamed AS, Zohar O, Chen GH, Lyketsos CG, Etcheberrigaray R, Alkon DL. MAP kinase signaling cascade dysfunction specific to Alzheimer's disease in fibroblasts. Neurobiol. Dis. 2002;11:166–183. doi: 10.1006/nbdi.2002.0520. [DOI] [PubMed] [Google Scholar]