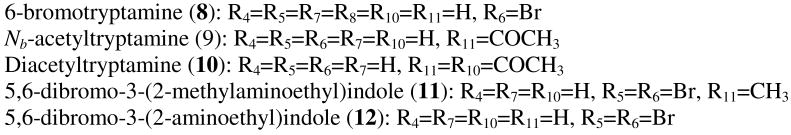1. Introduction
The marine environment has been explored in the search for new bioactive compounds over the last 50 years, becoming a highly important and rich source of potent molecules and drug leads reported to possess a wide scope of activities. Alkaloids constitute one of the largest classes of natural products and are synthesized by terrestrial and marine organisms on all evolutionary levels. Alkaloids are usually present in an organism as a mixture consisting of several major and a few minor compounds of the same biosynthetic origin and differing only in functional groups. This group of compounds has apparently evolved as a defense mechanism against predators and as a result alkaloids are often highly potent and toxic molecules.1 Marine invertebrates have proven to be an outstanding source of active molecules, one of the most promising being indole alkaloids. Although many of these marine alkaloids closely resemble the endogenous amines (serotonin, dopamine or histamine), their potential affinity to various neurological targets and consequential impact on animal behavior is virtually unexplored.
Indole alkaloids, their activity, synthesis and potential use in medicine have been already reviewed in several articles.2 In this review we provide information on current and potential pharmaceuticals including small molecule natural indole alkaloids, their biological properties, structure-activity relationship studies, and especially their potential for the treatment of neurological disorders.
1.1. The indole moiety in drugs
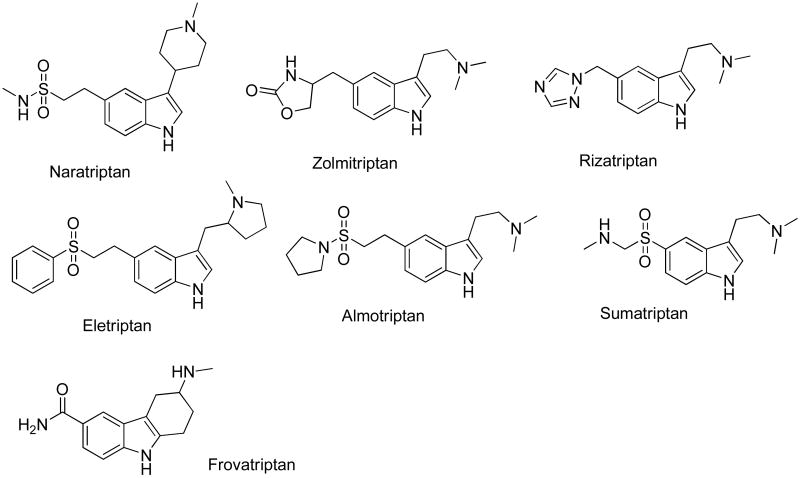
The indole moiety is present in a number of drugs currently on the market. Most of these belong to triptans which are used mainly in the treatment of migraine headaches (Fig. 1). All members of this group are agonists of migraine associated 5HT1B and 5HT1D serotonin receptors. Sumatriptan (Imitrex) was developed by Glaxo for the treatment of migraines and introduced into the market as the first member of the triptan family.3 Relative to the second generation triptans, sumatriptan has lower oral bioavailability and a shorter half-life. Frovatriptan (FROVA®) was developed by Vernalis for the treatment of menstruation associated headaches. Frovatriptan's affinity for migraine specific serotonin receptors 5HT1B is believed to be the highest among all triptans.4 In addition, frovatriptan binds to 5HT1D and 5HT7 receptor subtypes.5 Zolmitriptan marketed by AstraZeneca is used to treat acute migraine attacks and cluster headaches. GlaxoSmithKline's naratriptan (Amerge) is also used in the treatment of migraines and some of its side effects include dizziness, tiredness, tingling of the hands and feet and dry mouth. All available triptans are well tolerated and effective.6 The highest incidence of central nervous system (CNS) related side effects (dizziness, drowsiness) was reported for zolmitriptan (5 mg), rizatriptan (10 mg) and eletriptan (40 mg, 80 mg).7 The differences in side-effect profiles for triptans are not likely caused by their different affinity towards serotonin receptors or other neurological receptors in the CNS. There is a positive correlation between the lipophilicity coefficient and CNS side effects; these undesired effects are also dose-dependent.
Figure 1.
Currently available drugs from the triptan group.
1.2. Serotonin receptors – possible targets for neurologically active marine indole alkaloids
Given that depression affects approximately 18 million Americans annually,8 it is crucial to develop new effective treatments for this disorder. Intensive studies are being conducted in the area of new targets for antidepressant drugs,9,10 but most antidepressant drugs still target the neurotransmitter systems, mainly serotonin, dopamine and noradrenaline.
Serotonin is one of the neurotransmitters present in the central and peripheral nervous system which plays an important role in normal brain function and regulates sleep, mood, appetite, sexual function, memory, anxiety and many others.11 Serotonin exerts its effects through seven families of receptors (5-HT1 – 5-HT7) further divided into several subclasses. Except for 5-HT3 receptor which is a ligand-gated ion channel, the serotonin receptors belong to the G-protein coupled receptor family. Due to a lack of selective ligands, there is still little known about several 5-HT receptor subclasses.12 Marine monoindole alkaloids, sharing structure similarities with serotonin, are certain to become useful tools to facilitate the understanding of serotonin receptor function and generate new drug leads for the treatment of depression, anxiety, migraines and other 5HT receptor related disorders.
2. Natural indole alkaloids of marine origin
A growing number of indole alkaloids are being reported from various marine organisms. Due to the presence of specific enzymes, haloperoxidases, in the marine environment a large group of alkaloids isolated from sponges, seaweeds, ascidians and mollusks are halogenated.
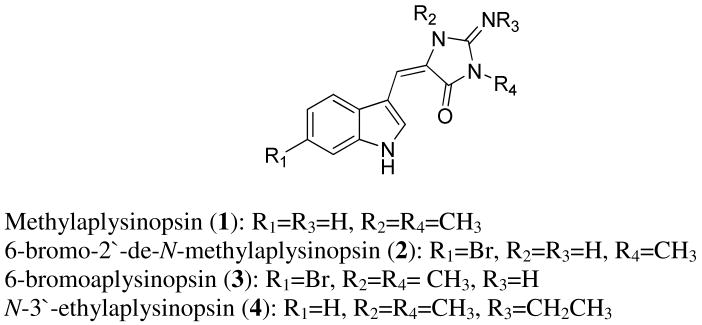
The structural similarity of indole alkaloids to endogenous amines and neurotransmitters has led researchers to postulate the possible neurological activity of these molecules. Several compounds carrying an indole moiety have been reported to possess affinity towards different serotonin receptors: barettin, 8,9-dihydrobarettin,13 tris-indole alkaloids gelliusine A and B,14 and σ-conotoxin.15 Methylaplysinopsin (1) (Fig. 2) isolated from Aplysinopsis reticulata by Baird-Lambert et al. was reported to inhibit monoamine oxidase (MAO) and to displace serotonin from its receptors.16 Other molecules from this group: 6-bromo-2′-de-N-methylaplysinopsin (2), 6-bromoaplysinopsin (3), and N-3′-ethylaplysinopsin (4) (Fig. 2) isolated from Smenospongia aurea were reported to displace high-affinity antagonist binding for human 5-HT2C and 5-HT2A receptors.17 N-3′-ethylaplysinopsin did not display selectivity to either of these two receptors (Ki of 3.5 μM and 1.7 μM for 5HT2C and 5HT2A receptor, respectively). 6-Bromoaplysinopsin showed only low selectivity towards 5HT2C receptors (Ki 0.33 μM and 2.0 μM for 5HT2C and 5HT2A receptor, respectively); however 6-bromo-2′-de-N-methylaplysinopsin exhibited strong (40 fold) selectivity to 5HT2C receptors (Ki 2.3 μM for 5HT2C and >100 μM for 5HT2A). Besides neurological activity, 6-bromoaplysinopsin also showed significant activity against Plasmodium falciparum.
Figure 2.
Aplysinopsin derivatives
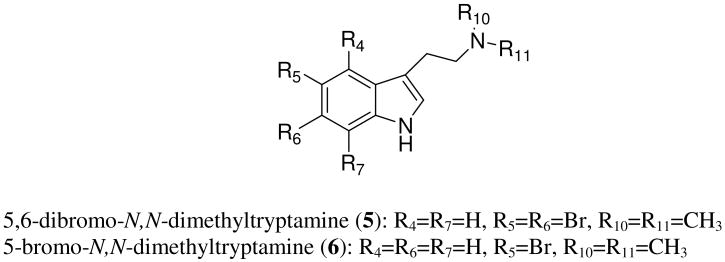
5,6-dibromo-N,N-dimethyltryptamine (5)18 and 5-bromo-N,N-dimethyltryptamine (6)19 (Fig. 3) exhibited antimicrobial activity as reported by Tymiak.20 The dibrominated compound was significantly more active over the monobromotryptamine. Both of the compounds were also found to possess neurological activity: 5,6-dibromo-N,N-dimethyltryptamine showed antidepressant action in forced swim test and tail suspension test;21,22 5-bromo-N,N-dimethyltryptamine exhibited strong sedative effect in the locomotor activity test.22
Figure 3.
Tryptamine derivatives
The dibromotryptamine (5) was also found to display significant activity in an MTT assay using HCT-116 colon carcinoma cell lines (IC50 values: p53+/+ 12.6 μM; p53-/- 85 μM; p21+/+ 85 μM; p21-/- 63 μM; where +/+ indicates parental cell line and -/- indicates knockouts).23
A new and interesting marine metabolite, sharing structure similarities with indoles and cannabinoids (Fig. 4) was recently reported to possess promising antidepressant activity in the forced swim test.22 Veranamine (7) isolated from Verongida rigida is an example of an unusual structure and supports the importance of isolation often being the only method that offers access to structurally new and unique molecules which could not be readily synthesized for a biological evaluation.
Figure 4.
Structure of veranamine (7)
Many naturally occurring indole alkaloids have not been tested for neurological activity. Their structures, however, indicate possible affinity to serotonin, dopamine or adrenergic receptors. As reported by Fahy et al.24 a fraction containing 6-bromotryptamine (8) showed antibacterial and antifungal actvity in vitro; however there is no data for the pure natural product. Another derivative of tryptamine, Nb-acetyltryptamine (9), was isolated from an unidentified fungus growing on the surface of the marine red alga Gracilaria verrucosa.25 The same compound, together with its diacetylated derivative (10), was reported from marine bacterium Roseivirga echinicomitans KMM6058T associated with the sea urchin Strongylocentrotus intermedius.26 Both compounds were found to be weakly cytotoxic towards Erlich carcinoma tumor cells; diacetyltryptamine exhibited higher helmolytic activity and caused 50% destruction of membrane of sperm and egg cells at the concentrations of 7.5 and 15 μg/ml, respectively. At the concentration of 50 μg/ml, compound 10 caused 100% inhibition of embryo development, while compound 9 did not show any inhibition. Neither of the compounds showed any activity towards yeast-like fungi and gram-positive or gram-negative bacteria. Dibrominated compounds 11 and 12 were reported for the first time by Van Lear et al. as antibacterial metabolites from a marine sponge Polyfibrospongia maynardii.27 The same alkaloids were later isolated from a Hyrtios erecta sponge and found to be selective inhibitors of neuronal isoform of nitric oxide synthase (nNOS).28
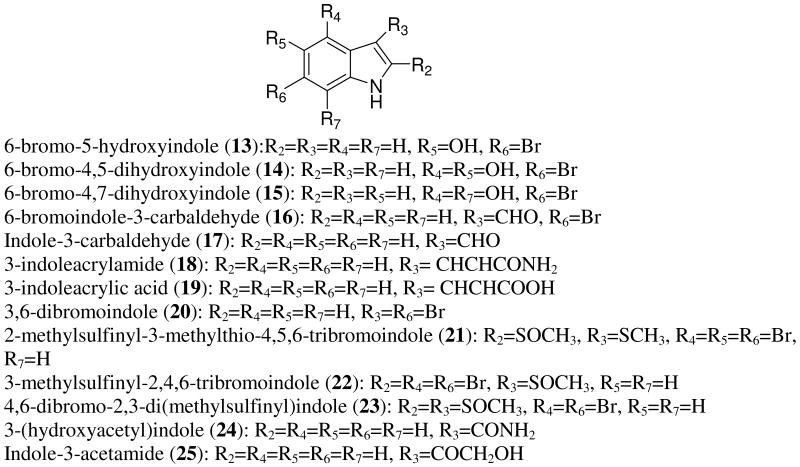
Three bromoindoles (13, 14, 15) isolated from the midintestinal gland of the gastropod Drupella fragum were reported to have antioxidative acitivites.29 6-Bromo-5-hydroxyindole (13) exhibited stable antioxidative activity higher than α-tocopherol. An additional two compounds, 6-bromoindole-3-carbaldehyde and its debrominated derivative, were obtained from an Acinetobacter sp. associated with the ascidian Stomozoa murrayi.30 The brominated metabolite (16) showed antimicrobial activity and inhibited the settlement of cyprid larvae of Balanus amphitrite with EC50 of 5 μg/mL.
Debrominated indole-3-carbaldehyde did not exhibit antimicrobial activity, and its antifouling effect was weaker (EC50 of 28 μg/mL). Davyt et al. reported a new indole derivative, 3-indoleacrylamide (18), possessing in vitro anthelmintic activity.31 The compound was isolated from the red alga Chondria atropurpurea together with several other known bisindole and indole alkaloids (19). Another antibacterial indole (20) was isolated from Distaplia regina, an ascidian collected from Palau.32 Interesting, sulfur containing polybrominated indoles were isolated from Laurencia brongniartii; these compounds did not show any cytotoxicity towards HT-29 and P-388 cell lines.33 Monoindole alkaloids have also been found to regulate the process of plant growth: this type of activity was reported for 3-(hydroxyacetyl)indole (24)34 and indole-3-acetamide (25).35
Table 1 lists other natural tryptamine derivatives isolated from marine organisms with no activity reported.
Table 1.
Natural marine tryptamine derivatives.
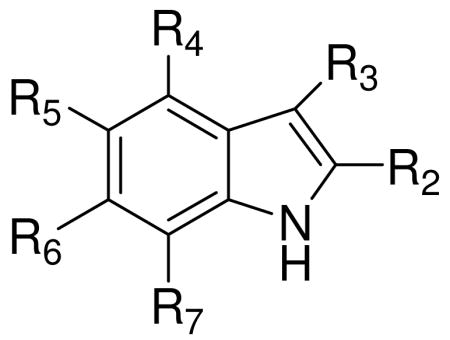 | |||||||
|---|---|---|---|---|---|---|---|
| Compound # | R2 | R3 | R4 | R5 | R6 | R7 | References, activity if reported |
| 26 | Cl | Cl | H | H | H | Cl | Brennan, M.R., Erickson, K.L.a Antifungal activity of crude extract. |
| 27 | Cl | Cl | H | H | H | Br | |
| 28 | Br | Cl | H | H | H | Br | |
| 29 | Br | Br | H | H | H | Br | |
| 30 | Cl | Cl | Cl | H | H | Cl | |
| 31 | Cl | Cl | Br | H | H | Br | |
| 32 | Br | Br | Br | H | H | Br | |
| 33 | Cl | Cl | Cl | H | H | H | |
| 34 | H | COCH2OH | H | H | Br | H | Guella, G. et al.b |
| 35 | H | H | Br | H | Br | H | Higa, T. et al.c |
| 36 | CH3 | H | Br | H | Br | H | |
| 37 | H | CHO | H | OH | Br | H | Cafieri, F. et al.d |
| 38 | Br | H | Br | H | Br | H | Tanaka, J., Higa, T.e |
| 39 | Br | Br | Br | H | Br | H | |
| 40 | SCH3 | H | Br | H | Br | H | |
| 41 | SOCH3 | SCH3 | Br | H | Br | H | |
| 42 | SCH3 | SOCH3 | Br | H | Br | H | |
| 43 | H | CH2CH2OH | H | OH | H | H | Salmoun, M.. et al.f |
| 44 | H | Br | H | Br | Br | H | Ji, N. et al.g |
| 45 | Br | Br | H | Br | Br | H | |
| 46 | Br | Br | H | H | Br | H | |
Brennan, M.R.; Erickson, K.L. Tetrahedron Lett. 1978, 19, 1637.
Guella, G.; Mancini, I.; Duhet, D.; Richer de Forges, B.; Pietra, F. Z. Naturforsch. C. 1989, 44, 914.
Higa, T.; Ichiba, T.; Okuda, R.K. Cell. Mol. Life Sci. 1985, 41, 1487.
Cafieri, F.; Fattorusso, E.; Mahajnah, Y.; Mangoni, A. Z. Naturforsch B. 1993, 48, 1408.
Tanaka, J.; Higa, T. Tetrahedron 1989, 45, 7301.
Salmoun, M.; Devijver, C.; Daloze, D.; Braekman, J-C.; van Soest, R. W.M. J. Nat. Prod. 2002, 65, 1173.
Ji, N-Y.; Li, X-M.; Ding, L-P.; Wang, B-G. Helv. Chim. Acta 2007, 90, 385.
3. Synthetic indole alkaloids
The literature reports numerous efforts to synthesize selective serotonin receptor ligands. Various structures have been reported as potent and selective agents for serotonin receptors; some of which share structural similarities with compounds isolated from sponges. EMDT (2-ethyl-5-methoxy-N,N-dimethyltryptamine) was synthesized as the first selective 5HT6 agonist.36 Tables 2-6 present the reported synthetic tryptamine related structures.
Table 2.
Oxindole derivatives.
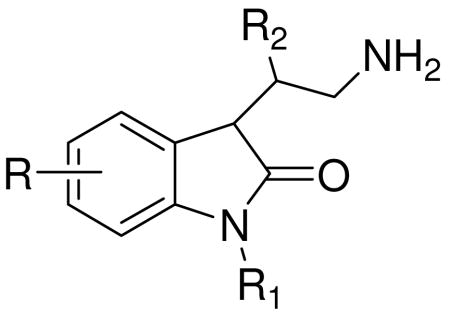 | ||||
|---|---|---|---|---|
| Compound # | R | R1 | R2 | Reference |
| 47 | 6-CH3O | H | H | Daisley and Walker40 |
| 48 | H | H | CH3 | |
| 49 | 5-CH3O | CH3 | CH3 | |
| 50 | H | CH3 | CH3 | |
| 51 | H | H | H | |
| 52 | 5-CH3O | H | H | |
| 53 | H | CH2CH3 | CH3 | |
| 54 | H | CH2CH2CH3 | CH3 | |
Table 6.
Arylthioether tryptamine derivatives.
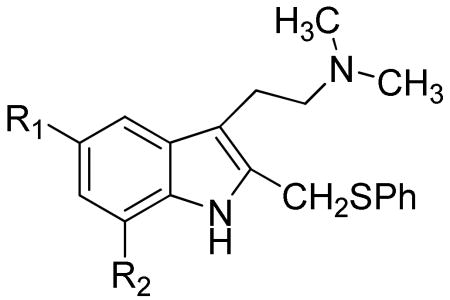 | |||
|---|---|---|---|
| Comp. # | R1 | R2 | References |
| 130 | F | H | Ramakrishna et al.54 |
| 131 | F | F | |
| 132 | Cl | H | |
| 133 | OCH3 | H | |
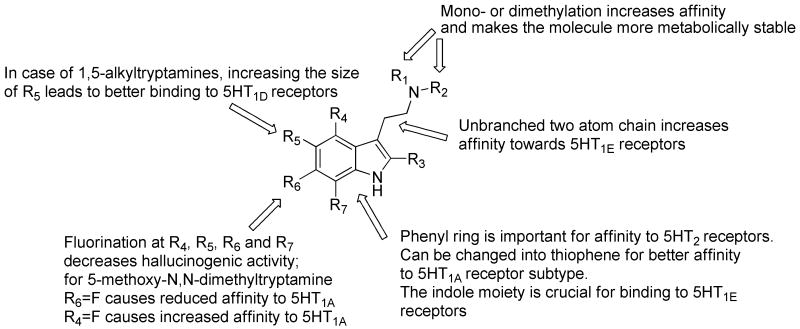
Several structure-activity relationship studies have been published outlining the best possible structures for agonists and antagonists of serotonin receptors. Dukat et al. reported structure-affinity relationship (SAFIR) and quantitative structure-activity relationship (QSAR) of several tryptamine derivatives towards the 5HT1E receptor subtype.37 According to these findings, the two-atom chain which separates the indole from terminal amine group is crucial for the binding of tryptamines to the receptor. Also, branching of this chain reduces the affinity. The indole moiety seems important for the affinity and any changes (benzene ring, replacement of NH by S) reduce the affinity. The substitution at the amine group, as long as the substituents remain small, does not affect affinity.
Agents binding to 5HT6 receptors were also extensively investigated.38 Glennon et al. found that N-mono- or N,N-dimethylation of serotonin derivatives resulted in a slight increase in affinity. The primary amine moiety of serotonin derivatives may be rapidly metabolized by oxidative deamination. This is likely to cause problems by reducing the ability of a molecule to cross the blood-brain barrier. Replacing a primary amine with secondary or tertiary amines increases the lipophilicity of the molecule and makes it less prone to metabolism, hence increasing its chance to become a useful drug.
Oxindoles have been reported to possess antidepressant activity in the early 1970s and according to the structure activity relationship studies, the optimal side chain for oxindoles was (CH2)3NHCH3 or any other group that could metabolize to this. Any branching of the side chain caused reduction in activity just like substitution of the indolinone aromatic ring. The substituent at heterocyclic nitrogen atom should be a phenyl and the group substituted at position 3 on indolinone should be small in order to keep the activity.39 Following these findings, a series of oxindole tryptamine derivatives, shown in Table 2, was prepared by Daisley and Walker.40 As shown in this study, compounds 47, 48, 49 and 50 given orally at the dose of 100 mg/kg caused hyperthermia in mice. The same dose (100 mg/kg) of compounds 47 and 50 delivered subcutaneously had moderate activity in the hot-plate assay, while 53 and 54 showed significant, but not consistent activity. Compound 54 caused slight suppression of appetite.
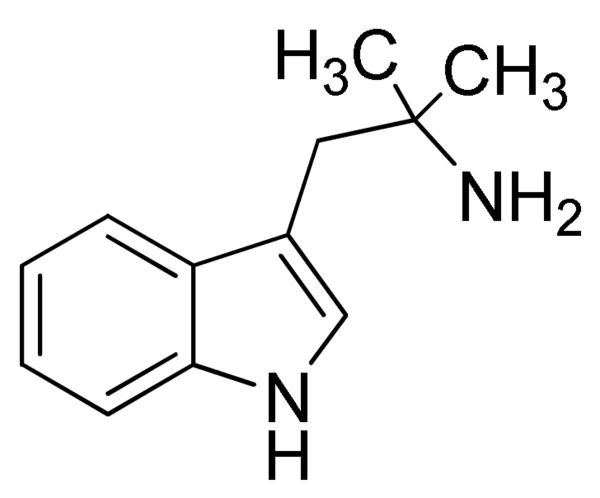
In 1967, Ostrovskaya published a report on pharmacological activity of 2-substituted tryptamines. Among tested compounds, 2-(2-methyl-2-amino)propylindole hydrochloride (55) was found to cause “motor excitation, tremor of the limbs and tail, stereotyped spasm of the head” when tested in mice and rats and administered i.v at doses 10-30 mg/kg.41
More recent synthetic and medicinal chemistry oriented research is focused on the preparation of ligands for specific types and subtypes of serotonin receptors. Xu et al.42 evaluated a series of 5-alkyltryptamine derivatives to find out which substituents were crucial for the affinity of the molecule towards 5HT1D receptors. In the case of 1,5-alkyltryptamines increasing the size of 5-alkyl substituent resulted in increased affinity towards 5-HT1D receptor. They also found that the substituent in position 5 did not have to possess hydrogen bonding properties in order to exhibit strong affinity towards this receptor type because it is the size of the group that dictates the affinity. 5-Tert-butyltryptamines showed the highest affinity for the 5HT1D receptor with compound 56 being the most potent agonist (Ki 0.45 nM).
Research completed by Blair and co-workers43 on thieno[3,2-b]- and thieno[2,3-b] pyrrole bioisosters of N,N-dimethyltryptamine revealed that thiophene cannot serve as a replacement for the phenyl ring in the indole moiety of tryptamines which bind to 5HT2 receptors. However, thiophene can be a suitable bioisostere for compounds possessing activity to 5HT1A receptor. Another paper by Blair et al. reports the effect of ring fluorination on the activity of hallucinogenic tryptamines.44 According to their findings, fluorination of tryptamines in positions 4, 5, 6, and 7 reduces hallucinogenic activity. Introducing fluorine at the 6 position of 5-methoxy-N,N-dimethyltryptamine decreases the 5HT1A receptor binding affinity. For example, compound 73 exhibited a Ki of 84.5 nM, while compound 70 exhibited a Ki of 1.7 nM. In the case of N,N-dimethyltryptamine, fluorination at position 6 caused a 5 fold decrease in affinity towards the 5HT1A receptor (compound 69 versus 75).
Fluorination of 5-methoxy-N,N-dimethyltryptamine at position 4 led to increased affinity towards the 5-HT1A receptor and resulted in potent and selective 4-fluoro-5-methoxy-N,N-dimethyltryptamine (74) with a Ki of 0.23 nM (compared to a Ki of 1.7 nM for compound 70). In the case of 5HT2A and 5HT2C receptors, fluorination at position 6 has only insignificant effects on the affinity to these receptors. Chen et al. described the process of preparation of N-(2-arylethyl)benzamines (compounds 76-82) as antagonists of 5HT6 receptors.45 Authors disclosed the use of these antagonists to treat cognitive dysfunction and any disorders associated with 5HT6 receptors: age-related cognitive disorders, anxiety, schizophrenia, Parkinson's disease, epilepsy, convulsions, migraine, and sleep disorders.
In 1998, Audia et al. presented several 8-substituted tetrahydro-beta-carboline compounds and tryptamine-like intermediates possessing high affinity towards all the subtypes of 5HT2 receptors.46 Compounds 83-89 were prepared to serve as molecular tools to develop selective therapeutic 5HT2A and 5HT2C agents, as well as to become effective drugs by themselves. Another patent publication by Audia et al. describes compounds 90-104 with affinity towards 5HT2A, 5HT2B and 5HT2C which could be useful in the treatment of various disorders associated with these receptors, including tachygastria, ichlasia, dyspepsia, schizophrenia, anxiety, depression and migraines.47 Indole derivatives 105, 106 and 107 and their affinity to 5HT2 receptors were the subject of a patent published in 1995 by Audia et al.48 This publication claimed the use of the aforementioned compounds to protect or to treat mammals suffering from 5HT2 receptor related disorders such as hypertension, depression and anxiety. Compounds 108, 109, 110, 112 and 113 were disclosed as inhibitors of angiogenesis, which plays a crucial role in the pathogenesis of cancer, immune and inflammatory disorders.49
Another subtype of serotonin receptors, 5-HT7, has been recently linked to psychiatric disorders like depression and schizophrenia, but their function still remains largely unknown. Vermeulen et al. presented a comprehensive study of inverse agonists of these receptors and determined that tryptamine derivatives like compound 111 without additional aromatic rings exhibit only poor affinity towards these receptors.50
A series of enantiomeric pairs of α-methyltryptamines was investigated by Nichols et al.51 The authors tested tryptamine analogues in 5HT1B and 5HT2 receptor binding assays and showed that enantioselectivity at both binding sites varied depending on the aromatic substituents. At both receptor subtypes, the order of affinity for the α-methyltryptamines was 5-substituted (116) > 4-substituted (115) > unsubstituted (114) > 6-substituted (117). In the case of 5-hydroxy-α-methyltryptamine (119) an S isomer had higher affinity to both receptors over the R isomer. For compounds 115 and 118, the R isomers exhibited higher affinity at the 5HT1B receptor but not at the 5HT2.
Three new indole alkaloids were produced by microbial transformation using Streptomyces staurosporeus. Yang and Cordell fed the bacterium with tryptamine hydrochloride, 5-fluorotryptamine hydrochloride and 6-fluorotryptamine, extracted the cultures and obtained β-hydroxy-Nb-acetyltryptamine (122), 5-fluoro- β-hydroxy-Nb-acetyltryptamine (123) and 6-fluoro- β-hydroxy-Nb-acetyltryptamine (124), respectively.52
Methods of preparation and insecticidal activity of several simple indole alkaloids were described in a patent by Young (compounds 126, 127, 128 and 129).53 Stereoisomers, salts, methods of preparation and medicines containing the arylthioether tryptamine derivatives (130 – 133) shown in Table 6 are claimed for possible use in central nervous system disorders related to the function of 5HT6 receptors (anxiety, depression, motor disorders).54
4. In the pipeline – novel tryptamine and indole derived structures
One of the new molecules reported in the last few years sharing structure similarities with indole alkaloids is the Wyeth compound, WAY-161503 (Fig. 7), a selective 5HT2C receptor agonist. According to the recent report, research conducted on the action of WAY-161503 confirms that 5HT2C receptors may play an inhibitory role in the regulation of reward-related behavior.55 WAY-161503 is covered by several patents and is claimed to be useful for the treatment or prevention of urinary incontinence,56,57,58 as well as for depressive disorders.59
Figure 7.
Structure of WAY-161503
Another compound, WAY-163909 (Fig. 8), a selective 5HT2C receptor agonist, was found to be of potential utility in obesity treatment. The same compound exhibited antidepressant and antipsychotic activity in preclinical animal models.60
Figure 8.
Anti-obesity, antidepressant and antipsychotic compound, WAY-163909.
A melatonin receptor agonist has recently completed Phase II Clinical Trials for sleep disorders in blind individuals, PD-6735 (Fig. 9), also contains an indole moiety. The drug was not only efficient in re-establishing the right day/night cycle but also displayed an excellent safety profile.
Figure 9.
PD-6735, a drug candidate for sleep disorders.
5. Conclusions
A summary of structure-activity relationship studies reported for monoindole and tryptamine derivatives is shown in Figure 10. Naturally occurring tryptamine derivatives possess a number of structural features not found in the reported synthetic molecules. For example, compounds 5, 11, 12, 26 – 46 possess two or more halogens. In addition halogen substituents at position 2 of the indole moiety (compounds 22, 26 – 33, 38, 39, 45, 46) are also unique to marine natural products. In marine tryptamine derivatives, the substituent at the amine nitrogen, if any, is usually methyl or acetyl (compounds 5, 6, 9 and 10), while in synthetic compounds this position is substituted with larger moieties (compounds 57, 62, 68, 76 – 82, 108 – 110). The indole nitrogen of marine monoindoles is usually not alkylated, in contrast to synthetic indole alkaloids (compounds 49, 50, 53, 54, 111, 112). Branching of the side chain is uncommon in natural marine indole alkaloids, while it is present quite frequently in synthetic molecules (compounds 48 - 50, 53, 54, 114 – 119, 122 – 128). Halogenation in position 4 of the indole ring is frequently reported in natural monoindole alkaloids (compounds 21, 22, 23, 30 – 42), while it is rather rare in the synthetic molecules. In this review we have discussed 46 natural indole alkaloids of marine origin and 87 synthetic molecules. The synthesis of monoindole alkaloids was in many cases inspired by the naturally occurring molecules and their similarity to serotonin. Some of the abovementioned natural indole alkaloids are not readily available by current synthetic methods, and as a result provide access to new and unusual chemotypes not previously investigated. When obtained in good yields, marine indole alkaloids can serve as starting materials for the preparation of a number of analogs providing preliminary structure activity relationship information. Marine natural products clearly provide valuable access to chemical diversity and regiochemistry that would otherwise require development of methodologies without preliminary evidence of biological activity. As a result there are interesting opportunities for marine natural products to inspire the development of novel ligands for neurological receptors.
Figure 10.
Summary of structure-activity relationship studies for tryptamine derivatives.
According to the World Health Organization, major depression will become the second leading cause of death by the year 2020 due to the complications arising from cardiovascular system and stress.61 There is a tremendous unmet need for new, safer and more effective antidepressant drugs since currently used antidepressants have significant side effects and about 30% of the population does not respond to these current treatments.62 Marine natural products have been overlooked as potent neurologically active molecules, considered primarily as anticancer and antimicrobial leads.
Marine indole alkaloids represent a rich group of natural compounds and have tremendous potential to become new drug leads for various psychiatric disorders as well as to provide better insights into the understanding of serotonin receptor function. These molecules are reasonable synthetic targets which further enhances their value as possible drug leads, however few if any have been prepared as part of synthetic or medicinal chemistry studies designed to generate optimized leads for depression and anxiety.
Figure 5.
Indole derivatives
Figure 6.
Structure of 2-(2-methyl-2-amino)propylindole (55).
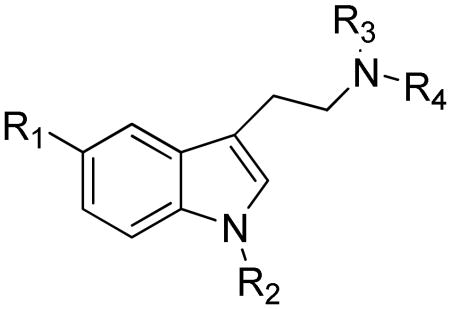
Table 3.
Synthetic tryptamine derivatives
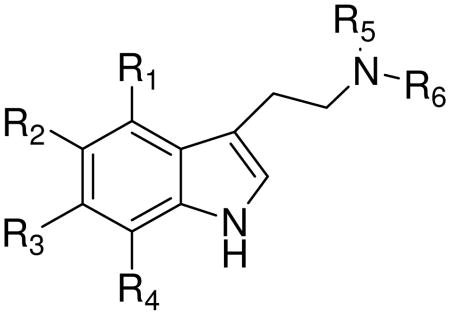 | |||||||
|---|---|---|---|---|---|---|---|
| Comp. # | R1 | R2 | R3 | R4 | R5 | R6 | References |
| 56 | H | C(CH3)3 | H | H | CH3 | H | Xu et al.42 |
| 57 | H | C(CH3)3 | H | H | CH2CH2CH3 | CH2CH2CH3 | |
| 58 | H | Br | H | H | H | H | |
| 59 | H | C(CH3)3 | H | H | CH2CH2CH3 | H | |
| 60 | H | C(CH3)3 | H | H | CH3 | CH3 | |
| 61 | H | c-pentane | H | H | H | H | |
| 62 | H | c-pentan-1-ene | H | H | Bn | Bn | |
| 63 | H | CH3 | H | H | H | H | |
| 64 | H | C2H5 | H | H | H | H | |
| 65 | H | i-Pr | H | H | H | H | |
| 66 | H | t-Bu | H | H | H | H | |
| 67 | H | c-hexyl | H | H | H | H | |
| 68 | H | Br | H | H | Bn | Bn | |
| 69 | H | H | F | H | CH2CH3 | CH2CH3 | Blair et al.44 |
| 70 | H | OCH3 | H | H | CH3 | CH3 | |
| 71 | OH | H | F | H | CH3 | CH3 | |
| 72 | OH | H | H | F | CH3 | CH3 | |
| 73 | H | OCH3 | F | H | CH3 | CH3 | |
| 74 | F | OCH3 | H | H | CH3 | CH3 | |
| 75 | H | H | H | H | CH2CH3 | CH2CH3 | |
| 76 | H | OCH3 | H | H | H | CH2PhOPh | Chen et al.45 |
| 77 | H | OPh | H | H | H | CH2PhOPh | |
| 78 | H | N2O | H | H | H | CH2PhOPh | |
| 79 | H | H | Br | H | H | H | |
| 80 | H | H | Cl | H | H | CH2PhOCH2CF2CHF2 | |
| 81 | H | Cl | H | H | H | CH2PhOCH2CF2CHF2 | |
| 82 | H | H | H | F | H | CH2PhOCH2CHF2 | |
| 83 | H | CH3 | H | Cl | H | H | Audia et al.46 |
| 84 | H | H | CH3 | Cl | H | H | |
| 85 | H | H | CH3 | H | H | H | |
| 86 | H | H | CH3 | CH3 | H | H | |
| 87 | H | H | H | Cl | H | H | |
| 88 | H | H | H | F | H | H | |
| 89 | H | H | CH3 | Br | H | H | |
| 90 | H | H | H | OCH3 | H | H | Audia et al.47 |
| 91 | H | CH3 | H | Br | H | H | |
| 92 | H | H | H | Cl | H | H | |
| 93 | H | H | H | F | H | H | |
| 94 | H | CH3 | H | Cl | H | H | |
| 95 | H | H | CH3 | Cl | H | H | |
| 96 | H | H | CH3 | H | H | H | |
| 97 | H | H | CH3 | CH3 | H | H | |
| 98 | H | H | CH3 | Br | H | H | |
| 99 | H | C2H5 | H | H | H | H | |
| 100 | H | iPr | H | H | H | H | |
| 101 | H | tBu | H | H | H | H | |
| 102 | H | Br | H | Br | H | H | |
| 103 | H | F | H | F | H | H | |
| 104 | H | CH3 | CH3 | H | H | H | |
| 105 | H | H | H | Cl | H | H | Audia et al.48 |
| 106 | H | H | H | Br | H | H | |
| 107 | H | H | CH3 | Br | H | H | |
| 108 | H | Cl | H | H | H | COPhOCH3 | Cao et al. 49 |
| 109 | H | H | H | H | H | COOC2H5 | |
| 110 | H | Cl | H | H | H | COPh | |
Table 4.
Substituted N-aryltryptamines
Table 5.
Tryptamine derivatives
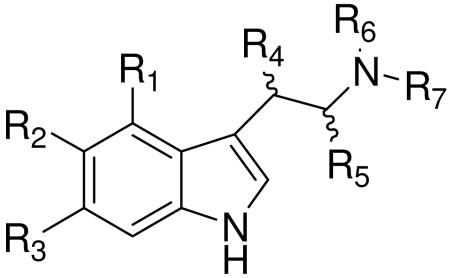 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Comp. # | R1 | R2 | R3 | R4 | R5 | R6 | R7 | References |
| 114 | H | H | H | H | CH3 | H | H | Nichols et al.51 |
| 115 | OCH3 | H | H | H | CH3 | H | H | |
| 116 | H | OCH3 | H | H | CH3 | H | H | |
| 117 | H | H | OCH3 | H | CH3 | H | H | |
| 118 | OH | H | H | H | CH3 | H | H | |
| 119 | H | OH | H | H | CH3 | H | H | |
| 120 | H | F | H | H | H | H | COCH3 | Yang et al.52 |
| 121 | H | H | F | H | H | H | COCH3 | |
| 122 | H | H | H | OH | H | H | COCH3 | |
| 123 | H | F | H | OH | H | H | COCH3 | |
| 124 | H | H | F | OH | H | H | COCH3 | |
| 125 | H | OH | H | H | H | H | COCH3 | |
| 126 | H | OH | H | H | CH3 | H | H | Young E.53 |
| 127 | H | OH | H | H | C2H5 | H | H | |
| 128 | H | OCH3 | H | H | CH3 | H | H | |
| 129 | H | H | H | H | C2H5 | H | H | |
Acknowledgments
The authors would like to thank Ms. Naomi Montalvo from the laboratory of Dr. Russell Hill, Center of Marine Biotechnology, University of Maryland Biotechnology Institute for the photograph used in the cover art and NIH for financial support.
Biographies

Mark T. Hamann is a Professor of Pharmacy, Chemistry and Biochemistry as well as a Research Professor with the Research Institute of Pharmaceutical Sciences at the University of Mississippi and an Adjunct Professor with the Center of Marine Biotechnology, University of Maryland Biotechnology Institute. He received his B.Sc. degree in Chemistry and Biology from Bemidji State University in Minnesota in 1985 and then started working in GMP pharmaceutical manufacturing at Solvay Pharmaceuticals in Baudette, Minnesota. Dr. Hamann completed his Ph.D. degree in Marine Natural Products Chemistry in 1992 at the University of Hawaii, under the guidance of the late Professor Paul Scheuer. During his research carrier, he has published over 120 scientific papers, reviews, and book chapters and currently serves as an editor for Biochimica et Biophysica Acta. Dr. Hamann's group is actively involved in the isolation, structure elucidation, and semisynthetic modification of marine natural products, with the emphasis on compounds possessing activity against infectious diseases, cancer and neuropsychiatric disorders.

Anna J. Kochanowska-Karamyan has received her M.Sc. in Pharmacognosy and PharmD. from the Skubiszewski Medical University of Lublin, Poland. In 2005 she joined the graduate program in the Department of Pharmacognosy, University of Mississippi, where she is currently completing her Ph.D. degree with Professor Mark Hamann. Her research interests are focused on isolation, characterization and synthesis of potential antidepressant drug leads from marine invertebrates.
Literature
- 1.Fattorusso E, Taglialatela-Scafati O, editors. Modern alkaloids. Structure, isolation, synthesis and biology. Wiley-VCH Verlag GmbH & Co KGaA; Weinheim: 2008. [Google Scholar]
- 2.(a) Aygun A, Pindur U. Curr Med Chem. 2003;10:1113. doi: 10.2174/0929867033457511. [DOI] [PubMed] [Google Scholar]; (b) Gupta L, Talwar A, Chauhan MS. Curr Med Chem. 2007;14:1789. doi: 10.2174/092986707781058904. [DOI] [PubMed] [Google Scholar]; (c) Gul W, Hamann MT. Life Sci. 2005;78:442. doi: 10.1016/j.lfs.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheftell FD, Bigal ME, Tepper SJ, Rapaport AM. Exp Rev Neurotherap. 2004;4:199. doi: 10.1586/14737175.4.2.199. [DOI] [PubMed] [Google Scholar]
- 4.Markus F, Mikko DK. Exp Opin Pharmacother. 2007;8:3029. doi: 10.1517/14656566.8.17.3029. [DOI] [PubMed] [Google Scholar]
- 5.Balbisi EA. Int J Clin Pract. 2004;58:695. doi: 10.1111/j.1368-5031.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari MD, Goadsby PJ, Roon KI, Lipton RB. Cephalgia. 2002;22:633. doi: 10.1046/j.1468-2982.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- 7.Dodick DW, Martin V. Cephalgia. 2004;24:417. doi: 10.1111/j.1468-2982.2004.00694.x. [DOI] [PubMed] [Google Scholar]
- 8.National Institutes of Health. Invisible disease: Depression. NIH publication 01-4591. 2001 www.nimh.nih.gov/publicat/invisible.cfm.
- 9.Holden C. Science. 2003;302:810. doi: 10.1126/science.302.5646.810. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan V, Nestler EJ. Nature. 2008;455:894. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichols DE, Nichols CD. Chem Rev. 2008;108:1614. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- 12.Fantegrossi WE, Murnane KS, Reissig CJ. Biochem Pharmacol. 2008;75:17. doi: 10.1016/j.bcp.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hedner E, Sjogren M, Frandberg PA, Johansson T, Goransson U, Dahlstrom M, Jonsson P, Nyberg F, Bohlin L. J Nat Prod. 2006;69:1421. doi: 10.1021/np0601760. [DOI] [PubMed] [Google Scholar]
- 14.Bifulco G, Bruno I, Minale L, Riccio R, Calignano A, Debitus C. J Nat Prod. 1994;57:1294. doi: 10.1021/np50111a020. [DOI] [PubMed] [Google Scholar]
- 15.England LJ, Imperial J, Jacobsen R, Craig AG, Gulyas J, Akhtar M, Rivier J, Julius D, Oliviera BM. Science. 1998;281:575. doi: 10.1126/science.281.5376.575. [DOI] [PubMed] [Google Scholar]
- 16.Baijrd-Lambert J, Davis PA, Taylor KM. Clin Exp Pharmacol Physiol. 1982;9:203. doi: 10.1111/j.1440-1681.1982.tb00798.x. [DOI] [PubMed] [Google Scholar]
- 17.Hu JF, Schetz JA, Kelly M, Peng J, Ang KKH, Flotow H, Leong CY, Ng SB, Buss AD, Wilkins SP, Hamann MT. J Nat Prod. 2002;65:476. doi: 10.1021/np010471e. [DOI] [PubMed] [Google Scholar]
- 18.Djura P, Stierle DB, Sullivan B, Faulkner DJ, Arnold E, Clardy J. J Org Chem. 1980;45:1435. [Google Scholar]
- 19.Debitus C, Laurent D, Pais M. J Nat Prod. 1988;51:799. [Google Scholar]
- 20.Tymiak AA, Rinehart KL, Jr, Bakus GJ. Tetrahedron. 1985;41:1039. [Google Scholar]
- 21.Diers JA, Ivey KD, El-Alfy A, Shaikh J, Wang J, Kochanowska AJ, Stocker JF, Hamann MT, Matsumoto RR. Pharm Biochem Behavior. 2008;89:46. doi: 10.1016/j.pbb.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.a Kochanowska AJ, Rao KV, Childress S, El-Alfy A, Matsumoto RR, Kelly M, Stewart GS, Sufka K, Hamann MT. J Nat Prod. 2008;71:186. doi: 10.1021/np070371u. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hamann MT, Kochanowska AJ, El-Alfy A, Matsumoto RR, Boujos A. WO 2009049030, 2009. Chem Abstr. 2009;150:414288.
- 23.Tasdemir D, Bugni TS, Mangalindan GC, Concepcion GP, Harper MK, Ireland CM. Z Naturforsch C. 2002;57:914. doi: 10.1515/znc-2002-9-1027. [DOI] [PubMed] [Google Scholar]
- 24.Fahy E, Potts BCM, Faulkner DJ, Smith K. J Nat Prod. 1991;54:564. [Google Scholar]
- 25.Li Y, Li XF, Kim DS, Choi HD, Son BW. Arch Pharm Res. 2003;26:21. doi: 10.1007/BF03179925. [DOI] [PubMed] [Google Scholar]
- 26.Oleinikova GK, Ivchuk OI, Denisenko VA, Chaikina EL, Menzorova NI, Nedashkovskaya OI, Kuznetsova TA. Chem Nat Comp. 2006;42:713. [Google Scholar]
- 27.Van Lear GE, Morton GO, Fulmor W. Tetrahedron Lett. 1973;14:299. doi: 10.1016/s0040-4039(00)89414-9. [DOI] [PubMed] [Google Scholar]
- 28.Aoki S, Ye Y, Higuchi K, Takashima A, Tanaka Y, Kitagawa I, Kobayashi M. Chem Pharm Bull. 2001;49:1372. doi: 10.1248/cpb.49.1372. [DOI] [PubMed] [Google Scholar]
- 29.Ochi M, Kataoka K, Ariki S, Iwatsuki C, Kodama M, Fukuyama Y. J Nat Prod. 1998;61:1043. doi: 10.1021/np980097r. [DOI] [PubMed] [Google Scholar]
- 30.Olguin-Uribe G, Abou-Mansour E, Boulander A, Debard H, Francisco C, Combaut G. J Chem Ecol. 1997;23:2507. [Google Scholar]
- 31.Davyt D, Entz W, Fernandez R, Mariezcurrena R, Mombru AW, Saldana J, Dominiguez L, Coll J, Manta E. J Nat Prod. 1998;61:1560. doi: 10.1021/np980114c. [DOI] [PubMed] [Google Scholar]
- 32.Quereshi A, Faulkner DJ. Nat Prod Lett. 1999;13:59. [Google Scholar]
- 33.El-Gamal AA, Wang WL, Duh CY. J Nat Prod. 2005;68:815. doi: 10.1021/np058001y. [DOI] [PubMed] [Google Scholar]
- 34.Bernart M, Gerwick WH. Phytochemistry. 1990;29:3697. [Google Scholar]
- 35.(a) Kishi Y, Goto T, Inoue S, Sugiura S, Kishimoto H. Tetrahedron Lett. 1966;7:3445. [Google Scholar]; (b) Cardellina JH, II, Nigh D, VanWagenen BC. J Nat Prod. 1986;49:1065. [Google Scholar]
- 36.Holenz J, Merce R, Diaz HL, Guitart X, Codony X, Dordal A, Romero G, Torrens A, Mas J, Andaluz B, Hernandez S, Monroy X, Sanchez E, Hernandez E, Perez R, Cubi R, Sanfeliu O, Buschmann H. J Med Chem. 2005;48:1781. doi: 10.1021/jm049615n. [DOI] [PubMed] [Google Scholar]
- 37.Dukat M, Smith C, Herrick-Dacis K, Teitler M, Glennon RA. Bioorg Med Chem. 2004;12:2545. doi: 10.1016/j.bmc.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 38.Glennon RA, Lee M, Rangisetty JB, Dukat M, Roth BL, Savage JE, McBride A, Rauser L, Hufeisen S, Lee DKH. J Med Chem. 2000;43:1011. doi: 10.1021/jm990550b. [DOI] [PubMed] [Google Scholar]
- 39.Canas-Rodriguez A, Leeming PR. J Med Chem. 1972;15:762. doi: 10.1021/jm00277a017. [DOI] [PubMed] [Google Scholar]
- 40.Daisley RW, Walker J. Eur J Med Chem. 1979;14:47. [Google Scholar]
- 41.Ostrovskaya RU. Bulletin Ex Biol Med. 1967;63:82. [Google Scholar]
- 42.Xu YC, Schaus JM, Walker C, Krushinski J, Adham N, Zgombick JM, Liang SX, Kohlman DT, Audia JE. J Med Chem. 1999;42:526. doi: 10.1021/jm9805945. [DOI] [PubMed] [Google Scholar]
- 43.Blair JB, Marona-Lewicka D, Kanthasamy A, Lucaites VL, Nelson DL, Nichols DE. J Med Chem. 1999;42:1106. doi: 10.1021/jm980692q. [DOI] [PubMed] [Google Scholar]
- 44.Blair JB, Kurrasch-Orbaugh D, Marona-Lewicka D, Cumbay MG, Watts VJ, Barker EL, Nichols DE. J Med Chem. 2000;43:4701. doi: 10.1021/jm000339w. [DOI] [PubMed] [Google Scholar]
- 45.Chen Z, Cohen MP, Fisher MJ, Giethlen B, Gillig JR, Ronald J, McCowan JR, Miller SC, Schaus JM. WO 2002078693, 2002. Chem Abstr. 2002;137:294763.
- 46.Audia JE, Droste JJ, Evrard DA, Fludzinski P, Murdoch GL, Nelson DL. US 5760051, 1998. Chem Abstr. 1998;129:54355.
- 47.Audia JE, Droste JJ, Evrard DA, Fludzinski P, Murdoch GL, Nelson D, Lloyd G. EP 620222, 1994. Chem Abstr. 1994;122:31501.
- 48.Audia JE, Cohen ML, Gidda JS, Nelson DLG, Baker SR, Ezquerra-Carrera J, Lamas-Peteira C, Pedregal-Tercero C. WO 9524200, 1995. Chem Abstr. 1995;128:39584.
- 49.Cao L, Choi S, Moon YC, Tamilarasu N, Qi H, Lennox WJ, Hwang S. WO 2006058088, 2006. Chem Abstr. 2006;145:27846.
- 50.Vermeulen ES, Van Smeden M, Schmidt AW, Sprouse JS, Wikstrom HV, Grol CJ. J Med Chem. 2004;47:5451. doi: 10.1021/jm049743b. [DOI] [PubMed] [Google Scholar]
- 51.Nichols DE, Lloyd DH, Johnson MP, Hoffman AJ. J Med Chem. 1988;31:1406. doi: 10.1021/jm00402a026. [DOI] [PubMed] [Google Scholar]
- 52.Yang SW, Cordell GA. J Nat Prod. 1997;60:230. doi: 10.1021/np960674g. [DOI] [PubMed] [Google Scholar]
- 53.Young E. Indole derivatives. GB 807877, 1959. Chem Abstr. 1959;53:67751.
- 54.Ramakrishna VSN, Kambhampati RS, Shirsath VS, Deshpande AD, Vishwakarma S, Jasti V. WO 2007046112, 2007. Chem Abstr. 2007;146:462134.
- 55.Hayes DJ, Clemens R, Greenshaw AJ. Psychopharmacology. 2008;203:579–588. doi: 10.1007/s00213-008-1404-4. [DOI] [PubMed] [Google Scholar]
- 56.Kamo I, Hashimoto T. WO 2006022420, 2006. Chem Abstr. 2006;144:226351.
- 57.Hildebrand KR. US 2007253995, 2007. Chem Abstr. 2007;147:474862.
- 58.McMurray G, Miner WD. WO 2004096196, 2004. Chem Abstr. 2004;141:388769.
- 59.Wolfgang CD, Polymeropoulos MH. WO 2007137227, 2007. Chem Abstr. 2007;148:1117.
- 60.Dunlop J, Marquis KL, Lim HK, Leung L, Kao J, Cheesman C, Rosenzweig – Lipson S. CNS Drug Rev. 2006;12:167. doi: 10.1111/j.1527-3458.2006.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. [February 9, 2009]; http://www.who.int/mental_health/management/depression/definition/en/
- 62.Isometsa ET, Henriksson MM, Aro HM, Heikkinen ME, Kuoppasalmi KI, Lonnqvist JK. Am J Psychiatry. 1994;151:530. doi: 10.1176/ajp.151.4.530. [DOI] [PubMed] [Google Scholar]