Abstract
In neonatal rats, strychnine-sensitive glycine receptors are widely expressed in the spinal cord, brainstem, and forebrain. During development, these 'neonatal' receptors are replaced by an adult isoform whose expression becomes restricted primarily to brain stem and spinal cord. Unlike most forebrain regions, functional strychnine-sensitive glycine receptors appear to persist within adult rat amygdala. However, the subunit composition of glycine receptors expressed by amygdala neurons and its relationship to the adult isoform in brain stem/spinal cord has not been precisely defined. In this report, we have utilized RT-PCR and single-cell RT-PCR to demonstrate that the ‘neonatal’ α2 subunit mRNA persists in adult rat amygdala neurons and is the predominant α subunit. We further demonstrate that native amygdala glycine receptors are relatively insensitive to the receptor antagonist picrotoxin, suggesting that α2 and β subunits may be present together in the same multi-subunit complex. We further demonstrate that α2 and β subunits cloned from adult rat amygdala can form functional channels when expressed in a heterologous system. Together, these studies highlight both the unique characteristics of strychnine-sensitive glycine receptors in the adult rat amygdala as well as the possibility that α2/β channels may represent the adult forebrain isoform of the strychnine-sensitive glycine receptor.
Keywords: single-cell RT-PCR, heterologous expression, limbic forebrain, picrotoxin
INTRODUCTION
The amygdala is crucial for the learning/memory aspects of fear/anxiety (Wilensky et al., 2000; Buchel et al., 1999; Dickson-Anson & McGaugh 1997) and is an important contributor to risk-assessment during this emotion (Bechara et al., 1999; Adolphs et al., 1998). This brain region is a complex collection of highly integrated subdivisions, with each serving a unique role in the sense-memory-behavior pathway. For example, the lateral and basolateral amygdala receive highly processed sensory information from numerous sensory systems and project heavily to regions intimately associated with the cognitive and autonomic aspects of anxiety/fear behaviors (reviewed in De Olmos et al., 1985). Neurons within these subdivisions can generally be characterized as either pyramidal glutamatergic projection neurons or non-pyramidal GABAergic interneurons (McDonald 1992, 1985, 1982). We refer to the lateral and basolateral subdivisions collectively as the ‘basolateral complex’ (BLC) throughout to emphasize their similarities and close anatomical association, recognizing that this over-simplified consolidation may not be representative under all circumstances.
In addition to GABAA receptors, strychnine-sensitive glycine receptors can also act as inhibitory, chloride-conducting ion channels during brief activation in adult neurons. Native glycine receptors are composed of five distinct integral membrane protein subunits (Langosch et al., 1988) that are, in the adult spinal cord, derived from two separate gene families, α and β (Grenningloh et al., 1990; Grenningloh et al., 1987). This complex association of five distinct subunit proteins in a functional complex is further complicated by the existence of multiple α subunit isoforms. Four different α subunit genes are currently known (α1-α4: Matzenbach et al., 1994; Kuhse et al., 1991; Kuhse et al., 1990; Grenningloh et al., 1987); and, α subunit mRNAs can undergo alternative splicing to generate novel subunit proteins (e.g. α3K/α3L, Nikolic et al. 1998; α1/α1ins, Malosio et al., 1991a; α2A/α2B, Kuhse et al., 1991). Finally, individual subunit or splice variant mRNAs can be developmentally regulated. For example, the α2 subunit mRNA predominates in neonatal forebrain and brain stem/spinal cord (Kuhse et al., 1991; Malosio et al., 1991b). During brain stem/spinal cord development, the embryonic/neonatal α2-homomeric ‘neonatal isoform’ is largely replaced by an ‘adult isoform’ consisting of the α1 and β subunits (Watanabe & Akagi 1995; Malosio et al., 1991b; Becker et al., 1988). In contrast, α2 mRNA present embryonic/neonatal forebrain drops precipitously during development but is generally not replaced by α1 mRNA, although the β subunit is also widely expressed throughout the adult forebrain (Fujita et al., 1991). Since the β subunit does not form functional channels itself (Grenningloh et al., 1990), it is currently believed that strychnine-sensitive glycine receptors are absent from most adult forebrain regions. However, recent reports (McCool & Botting 2000; Danober & Pape 1998) have described functional strychnine-sensitive glycine-gated chloride channels within the lateral/basolateral amygdala. Given their restricted expression in the forebrain (Malosio et al., 1991b) and the central role of ligand-gated chloride channels in the regulation of anxiety/fear, strychnine-sensitive glycine receptors may represent a novel target for future anti-anxiety therapies. The studies described below represent our initial attempts to understand the molecular characteristics of strychnine-sensitive glycine receptors expressed in the BLC.
MATERIALS & METHODS
Total RNA Preparation
Total RNA was prepared from adult rat nervous tissue using Trizol reagent (Life Technologies, Rockville, Maryland, U.S.A.) according to the manufacturer’s instructions. Basolateral amygdala was dissected from coronal brain slices that were prepared as previously described (McCool & Botting, 2000). Spinal cord from approximately T5 to T12 or intact BLC was used for the preparation of total RNA.
Reverse Transcription
RT-PCR was performed as previously described (Yan & Surmeier, 1996). Unless noted, all reagents were obtained from Life Technologies. Briefly, RNA was mixed with random hexanucleotides (0.05µg/µl final concentration), dithiothreitol (10mM), acetylated-bovine serum albumin (0.7µg/µl; Promega Corp., Madison, Wisconsin, U.S.A.), and 0.25U/µl ribonuclease inhibitor, heated at 70°C for 10 minutes, and cooled on ice for 1–2 minutes. Following addition of ice-cold dNTPs (0.5mM each), ribonuclease inhibitor (0.25U/µl), and Superscript II reverse transcriptase (10U/µl), samples were incubated at room temperature (~23°C) for 10 minutes then at 42°C for 50 minutes. This reaction was terminated by heat inactivation (70°C for 10 minutes) then chilled briefly on ice. The RNA strand of any RNA/DNA duplexes was removed by incubation with ribonuclease H (0.05U/ml) at 37°C for 20 minutes. Samples were immediately used for polymerase chain reaction (see below).
For single-cell RT-PCR, dissociated basolateral amygdala neurons were prepared from coronal brain slices as previously described (McCool & Botting, 2000). Individual neurons were harvested by aspiration into a borosilicate glass pipette containing ~5µl of ribonuclease-free water containing ribonulcease inhibitor (0.1U/µl). Neuronal debris was subsequently ejected into the random primers/dithiothreitol/bovine serum albumin mixture (above) and stored on dry ice or at −80°C (<2 days). Harvested neuronal debris was heat denatured and subjected to reverse transcription as described above.
Thermal Cycling
3–5µl of the reverse transcription reaction was combined with Taq buffer (1X, Promega Corp., Madison, Wisconsin, U.S.A.), MgCl2 (2mM final), deoxynulceotides (0.4mM), and gene-specific ‘forward’ and ‘reverse’ primers (1µM each), and was overlaid with molecular grade mineral oil (Sigma Chemical Co., St. Louis, Missouri, U.S.A.). Following heat denaturation at 94° C for 2 minutes, Taq DNA polymerase (0.05U/µl; Promega Corp., Madison, Wisconsin, U.S.A.) was added. Samples were subjected to 40 cycles of 94° C for 1 minute, 50° C for 1 minute, and 72° C for 1.5 minutes in a PTC-100 thermal cycler (MJ Research, Inc., Waltham, Massachusetts, U.S.A.). Amplicons were analyzed by agarose gel electrophoresis using ethidium bromide fluorescence. Negative controls were included with every reaction to insure that amplicons were derived from reverse transcription products. In some experiments, amplicons were cloned using the pGEM-T system (Promega Corp., Madison, Wisconsin, U.S.A.) and their identity confirmed by restriction analysis and by sequence analysis (DNA Technologies Lab, Dept. Veterinary Pathobiology, Texas A&M University).
Oligonucleotides
Primers were derived from cDNA sequences for rat strychnine-sensitive glycine receptor subunits as they appear in public databases (GenBank, NCBI website, www.ncbi.nlm.nih.gov/Entrez/) or in the literature. For comparisons of α subunit expression level in total RNA, degenerate oligonucleotide primers were designed from conserved regions identified using sequence comparisons (BLAST; NCBI website, www.ncbi.nlm.nih.gov/BLAST/) between the different cloned rat α subunit cDNAs (α1-α3; see Figure 1). These degenerate oligos do not share significant similarity with glycine receptor β subunit coding sequences.
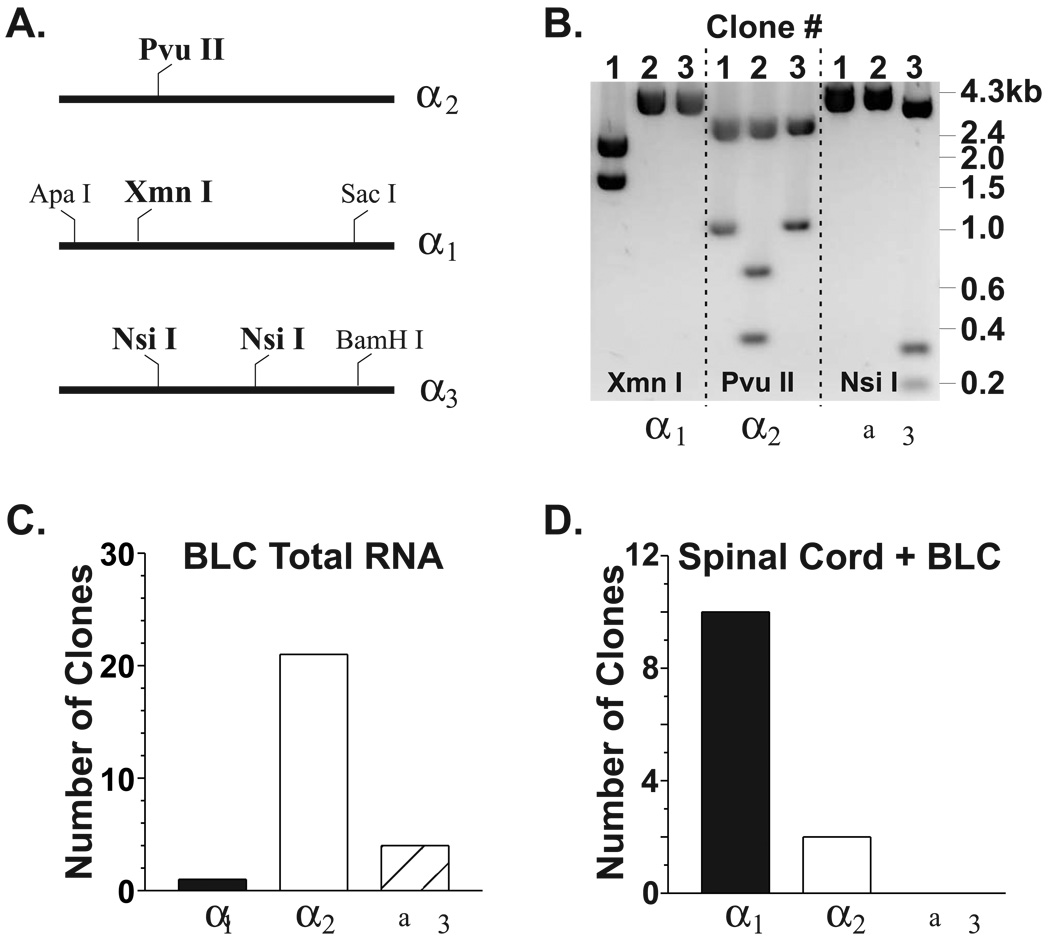
FIGURE 1.
Degenerate oligonucleotides that amplify strychnine-sensitive, glycine receptor α1-α3 cDNAs. Sequences in public databases were compared using standard procedures (see Methods) to define highly conserved regions between the various α isoforms. Sequences for degenerate oligos were: upper primer, 5' TTG TTC TTT GCC/T AAT GAG AAG/A GG; and, lower primer, 5’ GCC ATC CAG/A ATG TCA ATA/T GC. Numbers indicate the relative nucleotide positions within the α1(accession # D00833; Grenningloh et al., 1987), α2 (accession # X61159, Kuhse et al., 1991), and α3 cDNAs (accession # M55250, Kuhse et al., 1990). “y” = pyrimidine nucleotide; “r” = purine nucleotide; “w” = A or T nucleotide.
For single-cell analysis of subunit mRNA expression, oligonucleotide primers were derived from unique sequences to specifically amplify individual subunit cDNAs. All oligonucleotides were obtained from commercial sources (Sigma Genosys, The Woodlands, Texas, U.S.A.). Primers to GAD67 (Ceranik et al., 1997) and GAPDH (Salazar et al., 1999) were previously described. For the α1 subunit of the strychnine-sensitive glycine receptor, the forward primer was 5’ AAC TCG TGG ACT TTA CAG CA (nt#151–170 of the α1 cDNA); and the reverse primer was 5’ ATT CAT TGT AGG CGA GAC GG (reverse compliment of nt#481–500). For the α2 subunit, the primers were 5’ ATC AAT GGG AAG GAC ATC AGG A (forward primer, nt#701–722, accession # X61159, Kuhse et al., 1991) and 5’ GTT ATC AGT GGT GAC ATC ATG G (reverse compliment of nt#998-977). For the α3 subunit, the primers were 5’ CCA AGC GTT TGC CTC TAG C (forward primer, nt#281–299, accession # M55250, Kuhse et al., 1990) and 5’ AGG ATC ATT CCA CTT CTG ACG (reverse compliment of nt#691–711).
Cloning of rat strychnine-sensitive glycine receptor subunits
For the heterologous expression experiments, full-length cDNAs corresponding to sequences of the α2 and β subunits were reverse transcribed from total RNA isolated from adult rat basolateral amygdala as described above. The following oligonucleotides were used to amplify the appropriate cDNAs: for the α2 subunit, 5’ tag cct cga gaa ttc ATG AAC CGG CAG CTA GTG AA (forward primer, nt#552–571 in capital letters) and 5’ gcc gcc cgg gtc gac CTA TTT CTT GTG GAC ATC TT (reverse compliment of nt#1891–1910 in capital letters); for the β subunit, 5’ tag cct cga gaa ttc ATG AAG TTT TCG TTG GCA GT (forward primer, nt#225–234 in capital letters; from Grenningloh et al., 1990) and 5’ gcc gcc cgg gtc gac TCA TAA ATA TAT AGA CCA AT (reverse compliment of nt#1685–1714). Amplicons were gel isolated, cut with Eco RI/Xho I, and subcloned into EcoRI/XhoI digested expression vector (pCI; Promega Corp., Madison, Wisconsin, U.S.A.). Expression constructs were fully sequenced to confirm the integrity of the cDNA. For the α2 subunit clone, no sequence differences were noted between our isolate and the cDNA sequence previously reported (Kuhse et al., 1991). For the β subunit clone, the following nucleotide differences between our isolate and the GenBank cDNA sequence (Grenningloh et al., 1990) were noted: nucleotide #433, G®A; nucleotide #1378, C®T; and nucleotide #1447, T®C. In no case did these nucleotide differences encode alterations in the predicted polypeptide sequence.
Tissue Culture/Transfections
Mouse L-cell fibroblasts (NCTC-929; American Type Culture Collection, Manassas, Virginia, U.S.A.) were grown in Dulbecco’s modified Eagle’s Medium (Sigma Chemical Company, St. Louis, Missouri, U.S.A.) containing 10% fetal bovine serum (HyClone Laboratories, Logan, Utah, U.S.A.) and 100unit/ml penicillin:100µg/ml streptomycin (Life Technologies, Rockville, Maryland, U.S.A.). For transfections, cells were plated onto plastic coverslips (Electron Microscopy Sciences, Fort Washington, Pennsylvania, U.S.A.) placed in 6-well plates. Sub-confluent cultures (~20–40%) were transfected using a calcium phosphate-mediated procedure as previously described (McCool et al., 1998). For transfection of α2 subunit cDNA, 0.5µg/well of the expression construct was used. However, when α and β subunits were co-transfected, 2–2.5µg/well of the β subunit construct was combined with the 0.5µg α2 construct. Transfected cells were identified using epifluorescence by co-transfection with 0.1ug/well of a ‘green fluorescent protein’ expression plasmid (pEGFP-N1; ClonTech, Palo Alto, California, U.S.A.).
Electrophysiology
The whole cell patch clamp technique was performed on transfected L-cells that were continuously perfused with a HEPES-buffered saline solution (containing in mM: 150 NaCl, 10 HEPES, 2.5 KCl, 2.5 CaCl2, 1.0 MgCl2, 10 D-glucose; pH 7.4 with NaOH, osmolality 320mmol/kg adjusted with sucrose). Drugs were applied within 100µm of the cell using two silica tubes (150mm I.D.; Hewlett Packard Analytical Direct, Wilmington, Delaware, U.S.A.) mounted on a manipulator. Switching between ‘drug’ and ‘wash’ tubes was accomplished using a solenoid controlled by a S88 stimulator (Asto-Med Inc., West Warwick, Rhode Island, U.S.A.). A Cs+-based internal pipette solution (in mM: 130 CsCl, 10 HEPES, 10 EGTA, 1 CaCl2, 4 Mg-ATP; pH 7.2 with CsOH, osmolality 305mmol/kg adjusted with sucrose) was used.
Recordings were done at room temperature according to published procedures (Hamill et al., 1981; McCool & Botting, 2000) using an Axopatch ID amplifier (Axon Instruments Inc., Foster City, California, U.S.A.) in voltage-clamp mode. Whole-cell and series resistance were determined by mathematical fits of capacitive transients during square-wave voltage steps using the pClamp 7.0 software package (Axon Instruments Inc., Foster City, California, U.S.A.) and were compensated manually. Data was passed through a three-pole low-pass Bessel filter (1 kHz), digitized at 5kHz, and stored on a microcomputer until analyzed. Data analysis was performed off-line using the Clampfit (Axon Instruments Inc., Foster City, California, U.S.A.) and Prism (GraphPad Software Inc., San Diego, California, U.S.A.) software packages.
For concentration-response relationships, peak current responses at different concentrations of picrotoxin were normalized to a response from an ~EC50 concentration of glycine. Normalized data were plotted versus the logarithm of the concentration of antagonist; and IC50/Hill co-efficient determined from fits to a standard >four parameter logistic equation’.
Adult rat basolateral complex neurons were isolated from coronal brain sections as previously described (McCool & Botting, 2000). Rats were euthanized according to an ULACC-approved protocol in accordance with NIH Guidelines.
RESULTS
Glycine Receptor α Subunit Expression in Total Basolateral Amygdala RNA
To provide information on the types of glycine receptor subunits expressed in the basolateral complex, both immunological and molecular biological approaches can be utilized. Unfortunately, subunit-specific immunological reagents are not readily available for many strychnine-sensitive glycine receptor subunits. Furthermore, the small anatomical size of the amygdala’s various subdivisions precludes many types of molecular biological analyses. We therefore performed reverse transcription-polymerase chain reaction (RT-PCR) analysis of total RNA from basolateral amygdala. Degenerate PCR primers (‘pan’ oligos, see Figure 1) were used to indiscriminately amplify cDNAs corresponding to rat glycine receptor α1-α3 subunits. Since sequences for the rat α4 gene product are not available in public databases, we did not examine BLC total RNA or neurons for expression of this subunit. Agarose gel electrophoresis confirmed that our degenerate primers produced a single population of amplicons (not shown). However, these amplicons should consist of up to three independent populations corresponding to each of the three rat α subunits recognized by our degenerate oligonucleotides. Because each specific α subunit population would contain sequences unique from the other populations, we cloned and analyzed the amplicons produced by our ‘pan’ oligos using restriction sites specific for the individual subunits (Figure 2A; Flint et al, 1997). Digestion of these clones with a specific restriction endonuclease yielded a restriction pattern that was unique for each subunit (Figure 2B) and thus identified the subunit from which each cDNA clone was derived. In two separate experiments, 81% (21/26) of the BLC clones corresponded to the α2 subunit; while, 15% (4/26) and 4% (1/26) were derived from the α3 and α1 subunit, respectively (Figure 2C). When similar experiments (n=3) were performed on adult rat spinal cord total RNA, 100% (27/27) clones were derived from α1 cDNAs (not shown). Finally, equivalent masses of BLC and spinal cord total RNA (2.5µg each) were mixed together and subjected to RT-PCR with the degenerate primers (Figure 2D). cDNA clones from both the α1 (10/12 clones) and the α2 (2/12 clones) subunits were identified. Together, these results indicate that our degenerate oligonucleotides could synthesize amplicons from all the known glycine receptor α subunits and that the α2 subunit was the predominant form represented in total basolateral amygdala RNA.
FIGURE 2.
The glycine receptor α2 subunit mRNA is the predominant isoform found in total RNA from adult rat basolateral amygdala. (A) Using the degenerate oligonucleotides defined in Figure 1, the relative abundance of PCR products corresponding to individual subunit mRNAs was assayed by cloning and subsequent restriction analysis with enzymes diagnostic for each subunit (indicated in bold). (B) RT-PCR products generated from total adult rat BLC RNA using degenerate oligonucleotides (Figure 1) were cloned and subjected to restriction analysis. Examples of the unique restriction sites within each subunit are shown here. For clone #1, digestion with Xmn I yielded fragments indicative of the α1 subunit. Unique restriction patterns for clone #2 (Pvu II) and clone #3 (Nsi I) indicate they are derived from the α2 and α3 subunits, respectively. (C) In two separate experiments, the ‘neonatal’ α2 subunit is the predominant cDNA generated from total adult basolateral complex RNA with the rank-order of expression being α2 ≫ α3 β α1. (D) Mixing equal masses of total RNA from adult spinal cord and adult basolateral complex yielded a mixture of PCR products similar to those obtained from each source independently (see text).
We also analyzed the population of PCR products produced with the ‘pan’ oligos using dot-blot hybridization with subunit-specific oligonucleotide probes (not shown; McCool & Farroni 2000) according to standard procedures (Flint et al., 1997). Hybridization intensities were standardized to know amounts of cloned cDNAs corresponding to each of the rat glycine receptor α subunit cDNAs placed on the same blot. For PCR products derived from total BLC RNA, 71%, 17%, and 12% of the hybridization intensity corresponded to the α2, α1, and α3 subunits, respectively. Similar experiments on total spinal cord RNA revealed that the relative abundance of α1, α2, and α3 subunit PCR products was 87%, 4%, and 9%, respectively. Together, these values are very similar to those obtained by restriction analysis of cDNA clones, strongly suggesting that α2 is the most abundant α subunit mRNA expressed in the adult rat basolateral amygdala.
Glycine receptor α subunit mRNA expression in single, dissociated BLA neurons
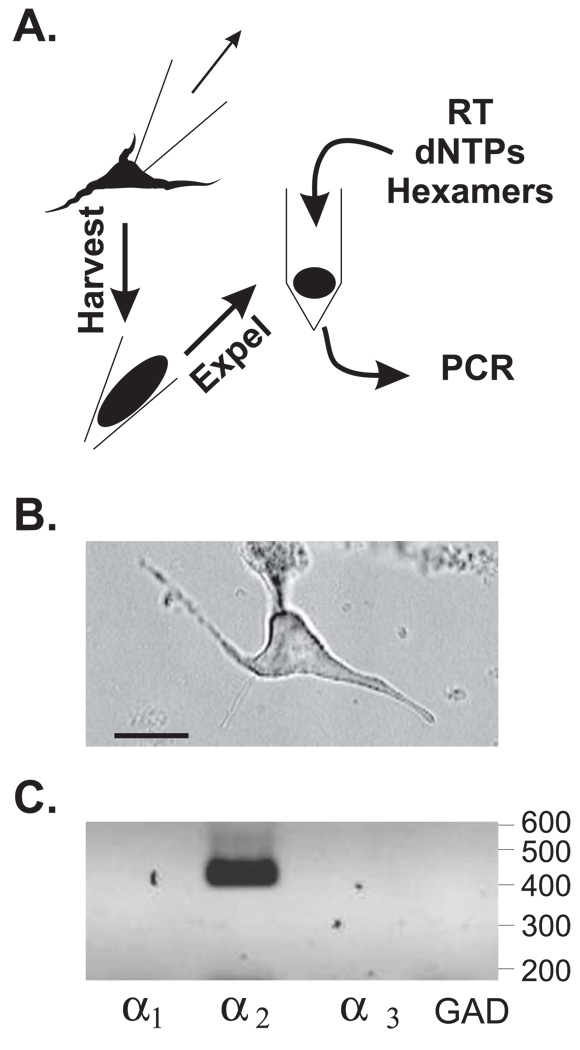
While the RT-PCR results from total basolateral amygdala RNA suggests that the α2 subunit mRNA is the predominant isoform in this brain region, they do not identify the specific population of cells which express this subunit. Although acutely dissociated BLC neurons express glycine-gated currents (McCool & Botting 2000), numerous non-neuronal cell types can also express strychnine-sensitive glycine receptors (Wheeler et al., 2000; Spittler et al., 1999; Belachew et al., 1998; Pastor et al., 1995), potentially complicating the interpretation of RT-PCR using total RNA. To identify the strychnine-sensitive glycine receptor subunit mRNAs expressed in neuronal phenotypes, acutely dissociated BLC neurons were subjected to single-cell RT-PCR (Yan & Surmeier 1996; Fig. 3A). Note that the subunit expression profile for each neuron-like cell can be determined using this method (Figure 3B & C). We also measured the expression of the GABA synthetic enzyme, glutamic acid decarboxylase (GAD-67) that is primarily expressed in relatively small population of GABAergic interneurons within the lateral and basolateral amygdala (McDonald 1985). GAD is a phenotypic marker allowing us to differentiate between this class of neurons and the more prevalent GAD−, presumably glutamatergic projection neurons. The housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was used to confirm the integrity of the RNA harvested from each neuron.
FIGURE 3.
Glycine receptor α subunit mRNAs can be detected in single neurons with RT-PCR. (A) Acutely dissociated neurons were harvested and subjected to single-cell RT-PCR using the method summarized here (cf. Yan & Surmeier 1996). (B) A typical BLC neuron selected from analysis was generally large and pyramidal-shaped. Bar = 20µm. This neuron was harvested as shown in A and subjected to RT-PCR. (C) Results from RT-PCR analysis of the neuron in B. PCR products were separated by agarose gel electrophoresis and detected with ethidium bromide staining. A ‘negative’ of the original image is shown for clarity. Numbers at right indicate that relative migration of molecular weight markers expressed in base pairs (not shown). These results indicate that the neuron in B was GAD and expressed the glycine receptor α2 subunit mRNA.
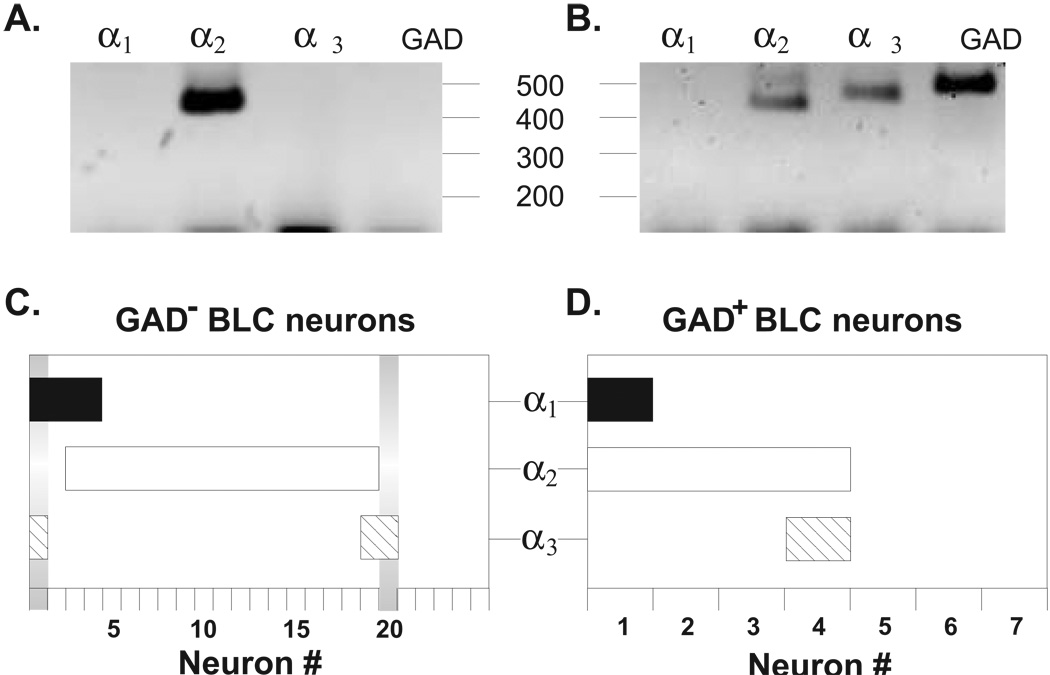
For the GAD− population of BLA neurons that were GAPDH+ (n = 25), 20 out of 25 neurons appeared to express at least one isoform of the glycine receptor α subunit (e.g. GlyR+/GAD−; Figure 4A). Among these GlyR+/GAD− neurons, a majority (16 out of 20) appeared to express only a single α subunit isoform, with the α2 subunit being the sole subunit expressed by 14 neurons and α1 or α3 being found in 1 neuron each. A minority of GlyR+/GAD−neurons (4 out of 20) expressed more than a single α subunit and appeared rather heterogeneous. For example, two GlyR+/GAD− neurons expressed both the α1 and α2 subunits; and α1 + α3 and α2 + α3 combinations were each expressed in one neuron, respectively. In addition to GAD−neurons, we also measured glycine receptor α subunit expression in several GAD+, presumably GABAergic neurons (Figure 4B). However, only 4 out of 7 GAD+ neurons examined appeared to express strychnine-sensitive glycine receptor α subunit mRNAs. Similar to the GAD−population of neurons, 4 out of 4 of these GlyR+/GAD+ neurons expressed the α2 subunit mRNA; although, in two neurons, this subunit was expressed in combination with the α1or α3 subunits, respectively. Thus, regardless of GAD phenotype, the α2, α1, and α3 subunits were expressed by 88% (17 out of 20), by 21% (5 out of 24), and by 17% (4 out of 24) of the BLC neurons, respectively. Furthermore, 25% (6 out of 24) GlyR+ neurons expressed more than one glycine receptor α subunit.
FIGURE 4.
Single cell RT-PCR indicates that the α2 subunit mRNA is the predominant species expressed in dissociated adult rat basolateral complex neurons. (A) & (B) Examples of single cell RT-PCR results from a GAD (A) and a GAD+ (B) neuron. (C) Summary of single cell RT-PCR data from the GAD population of neurons. The expression profile of an individual neuron is indicated along the y-axis, with coincident detection of multiple subunits being represented by overlapping areas of the different bars. For example, neuron #1 expressed both the α1 and α3 subunits while neuron #20 expressed only the α3 subunit (vertical gray bars). A majority of the GAD neurons expressed a single α subunit, with the α2 subunit being the most frequently detected. A substantial fraction of cells also appeared to express multiple α subunit mRNAs. (D) Summary of single cell RT-PCR results form the GAD+ population of presumed interneurons. While the α2 subunit was detected most frequently in this class of neurons, α1 and α3 subunits were also detected in neurons expressing multiple α subunits.
Comparisons of native BLC glycine receptors with heterologous α2/β receptors
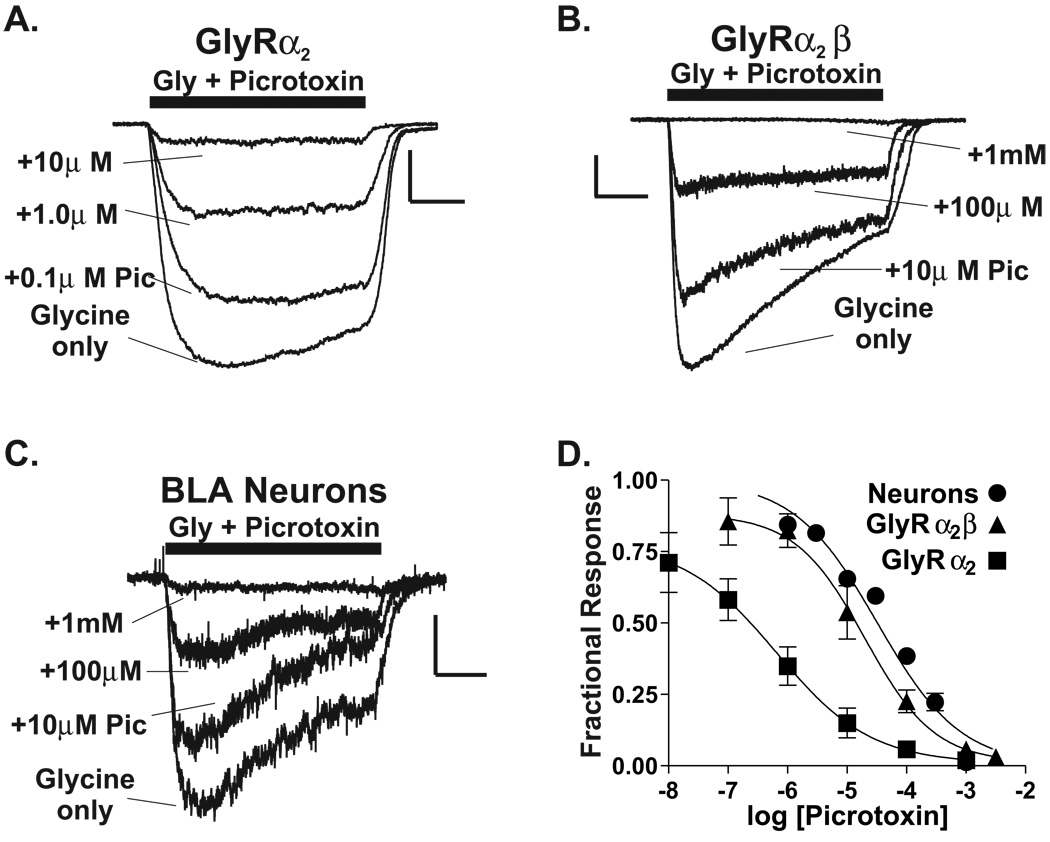
Previous in situ hybridization studies (Fujita et al, 1991) have demonstrated the expression of the glycine receptor β subunit mRNA in numerous forebrain areas, including the basolateral complex. Using RT-PCR of total RNA, we have confirmed that β subunit mRNA is expressed in this brain region (data not shown). Given that the glycine receptor α2 subunit is the most predominant α subunit expressed by neurons in this region, α2/β heteromers may be present in the same neurons. Indeed, the glycine-induced currents of acutely isolated basolateral amygdala neurons are relatively insensitive to picrotoxin (Figure 5C & D), with an IC50 (32µM) indicative of native receptors containing the β subunit (Shan et al, 2001; Handford et al, 1996; Pribilla et al, 1992).
FIGURE 5.
The picrotoxin sensitivity of native BLC glycine receptors is similar to α2/β channels expressed in a heterologous system. (A) Picrotoxin sensitivity of glycine-gated currents from a L-cell transfected with rat GlyRα2 expression construct (see Methods). For all traces, an EC50 concentration of glycine was used. Note that co-application of 10µM picrotoxin with glycine inhibited the current >90% when compared to glycine alone. Calibration bars: x = 1 second; y = 0.3nA. (B) Picrotoxin sensitivity of glycine currents from a L-cell transfected with both rat GlyRα2 and rat GlyRβ expression constructs. Note that 10µM picrotoxin inhibited only ~25% of the current. Calibration bars: x = 1 second; y = 0.2nA. (C) Picrotoxin sensitivity of glycine currents in acutely dissociated adult rat BLC neurons. Note that 10mM picrotoxin inhibited ~24% of the glycine current. Calibration bars: x = 1 second; y = 0.15nA. (D) Picrotoxin concentration-response relationships for L-cells expressing rat GlyRα2, rat GlyRα2 + β, and native BLC glycine receptors. The IC50 for picrotoxin for a2/β-expressing cells was 200µM (Hillslope = −0.74, n = 4) and was similar to that determined for native receptors (IC50 = 320µM, Hillslope = −0.62, n = 3–9). In contrast, GlyRα2 homomeric channels were substantially more sensitive to picrotoxin with an IC50 = 0.7µM (Hillslope = −0.55, n = 4–8).
To test whether α2/β heteromers could be responsible for picrotoxin-insensitive glycine currents in acutely isolated neurons, cDNAs for these subunits were cloned from total basolateral amygdala RNA, sequenced, and subcloned into a mammalian expression vector. These constructs were introduced into L-cell fibroblasts (cf. Valenzuela et al., 1998). Glycine-gated currents in cells expressing α2 homomer channels were markedly more sensitive to picrotoxin than were native BLC neurons, with an IC50 = 0.6µM. In contrast, currents in L-cells expressing α2/β heteromeric channels had a lower apparent affinity for picrotoxin (IC50 = 20µM) and were more similar to BLC neurons. Importantly, untransfected cells did not respond to glycine (n = 4, data not shown). Thus, α2 and β subunits can form functional heteromeric channels; and, the picrotoxin sensitivity of α2/β heteromeric channels is similar to the native receptors expressed by adult rat BLC neurons.
DISCUSSION
One of the main findings of this work is that, while mRNAs for various strychnine-sensitive glycine receptor subunits can be detected in total RNA derived from adult rat basolateral amygdala, the α2 subunit appears to be highly expressed relative to the other α subunit isoforms. This conclusion requires that our degenerate oligonucleotides must detect all the known rat cDNAs without bias. This assumption is supported by our finding that the ‘pan’ oligonucleotides detect the α1subunit as the most predominant isoform in adult rat spinal cord RNA, in agreement with numerous studies. Furthermore, both α1and α2 subunits are detected when equal masses of total RNA from spinal cord and basolateral amygdala are mixed together, suggesting that these oligonucleotides reliably produce PCR products derived from all subunits. The second assumption made during these studies is that the relative numbers of PCR products corresponding to a given subunit cDNA would be faithfully represented by the relative numbers of isolates following cloning. This assumption is supported by results from direct dot-blot hybridization of PCR products with subunit-specific oligonucleotides which, like the cloning/restriction analysis study (Figure 2), indicate that the α2 subunit is the most abundant isoform in adult rat basolateral amygdala total RNA.
The expression of strychnine-sensitive glycine receptor α subunit mRNAs within total BLC RNA suggests that the glycine-gated currents expressed by isolated BLC neurons (McCool & Botting 2000) are mediated predominantly by α2 subunit-containing receptors. However, numerous glial subtypes can also express functional glycine receptors as well as corresponding subunit mRNAs (Belachew et al., 1998; Kirchhoff et al., 1996; Pastor et al., 1995), making definitive conclusions regarding neuronal isoforms from total RNA problematic. We therefore utilized single-cell reverse transcription/polymerase chain reaction to detect the glycine receptor α subunit mRNAs in individual neurons. Importantly, we were able to detect GlyR α subunit expression in 80% of cells of examined, a percentage that compares favorably with previous findings that >90% of cells have physiological responses to glycine (McCool & Botting, 2000; Danober & Pape, 1998). The slightly lower detection rate with single cell RT-PCR may highlight the exquisite sensitivity of electrophysiological measurements as well as the difficulties faced when measuring gene expression in individual neurons. Regardless, the most frequently detected isoform expressed by individual adult rat BLC neurons was the α2 subunit.
While we acknowledge that low levels of subunit expression might complicate interpretation of single cell RT-PCR results, the agreement of our single-cell data with the total RNA data strongly supports our proposition that the α2 subunit is the major strychnine-sensitive glycine receptor isoform expressed in this brain region. This is important because we (McCool & Botting, 2000) have recently demonstrated that the glycine-gated currents expressed by acutely isolated adult basolateral amygdala neurons are quite sensitive to inhibition by the antagonist strychnine (IC50 ~ 40nM) if one pretreats with the antagonist. In fact, their sensitivity is similar to that reported for the ‘adult’ α1 subunit expressed in Xenopus oocytes (IC50 = 16nM; Schmeiden et al., 1999). However, concentration response data for amygdala glycine receptors derived from co-incident application of strychnine and glycine yield variable IC50s, ranging from <100nM to ~1µM, depending upon the length of the application and the point in the current response where one chooses to measure amplitude. Similar findings have been reported by another laboratory using acutely isolated VTA neurons from neonatal rats (Ye et al., 1998). Because the relative amount of pretreatment with strychnine has varied in the literature, its slow, time-dependent inhibition may contribute to the apparently misconceived notion that α2-containing glycine receptors are strychnine-insensitive. For amygdala neurons, this clearly appears not be the case.
Our finding that a significant number of BLC neurons also contain more than one subunit is particularly interesting. Along with descriptions of α subunit co-expression in spinal cord (Kirchhoff et al. 1996; Watanabe & Akagi 1995), the presence of multiple α subunits in BLC neurons may substantially modify receptor pharmacology and function. It appears unlikely that we failed to detect expression of some subunits in a significant number of ‘single subunit’-expressing neurons because 1) each subunit was detected independently, and 2) the relative amount of PCR product did not appear to depend upon the number of subunits expressed by a given neuron. We propose then that neurons expressing more than one GlyR α subunit may represent a distinct population of cells; and, the expression of different strychnine-sensitive glycine receptor subunits could prove to be useful markers for different classes of GAD−projection neurons in the basolateral/lateral amygdala. It might also be interesting to correlate GlyR α subunit expression patterns with the distinct populations of basolateral neurons that project to unique brain regions (Shinonaga et al., 1993). We were surprised to identify a substantial number of GAD+, presumably GABAergic neurons since the morphological characteristics of most neurons we selected for examination were consistent with the pyramidal projection neurons. Large, pyramidal GABA+ neurons have also been identified in the BLC (McDonald 1985; McDonald 1982); and, the GAD+ population in our studies may correspond to this unique population of neurons.
Finally, due to the prominent expression of the GlyR α2 subunit reported here and the likelihood of coincident expression of the β subunit (Fujita et al., 1991; McCool unpublished observations) in the basolateral complex, we examined the α and β subunits for possible functional associations. In neonatal tissue, the α2 subunit most likely assembles as a homomeric receptor (e.g. the ‘neonatal isoform’; Becker et al., 1988) due to the absence of coincident β subunit expression during early forebrain and spinal cord development. However, when co-expressed in L-cell fibroblasts, the α2 and β subunits appear to form functional, picrotoxin-insensitive heteromeric receptors. Because BLC neurons also express the α2 subunit mRNA along with picrotoxin-insensitive glycine-gated currents, our findings support the hypothesis that the lateral/basolateral amygdala glycine receptor consists of the α2 and β subunits. This is also supported by studies that demonstrating BLC immunoreactivity that is not exclusively due to expression of the α1 subunit (Danober & Pape 1998).
Although we cannot yet remark on the functional significance of strychnine-sensitive glycine receptors expressed within the amygdala, numerous studies exemplify the functional role of ligand-gated chloride channels within the basolateral complex. Activation of GABAA receptors by direct injection of selective agonists can both inhibit fear learning (Wilensky et al., 1999) and reduce the expression of social anxiety (Sanders & Shekhar 1995). Conversely, GABAA receptor inhibition by direct injection of antagonists can produce anxiety (Sanders et al., 1995). Importantly, this anxiety-producing (anxiogenic) effect of GABAA receptor antagonists can be attenuated by concurrent inhibition of ionotropic glutamate receptors (Sajdyk & Shekhar 1997), suggesting that the tonic activity of ligand-gated chloride channels in the amygdala is an important determinant in the natural expression of anxiety-like behaviors. It is therefore possible that amygdala glycine receptors profoundly influence these behaviors in a similar fashion. Furthermore, it is clear that non-α1-containing glycine receptors may also be expressed in adult rat nucleus accumbens (McCool, unpublished observation), striatum (Darstein et al., 2000), and motor cortex (Becker et al., 1993). Due to the widespread distribution of the glycine receptor β subunit, we therefore suggest that the α2/β heteromeric channel may be the ‘adult forebrain isoform’ of the strychnine-sensitive glycine receptor.
ACKNOWLEDGEMENTS
This work was supported in part from a Pharmaceutical Research and Manufacturers of America Foundation Starter Grant and by a National Institute on Alcohol Abuse and Alcoholism Grant AA-13120 (B.A.M.).
ABBREVIATIONS
- BLC
lateral/basolateral amygdala
- cDNA
complimentary deoxyribonucleic acid
- GABAA
γ-aminobutyric acid receptor type-A
- GAD67
67 kilodalton isoform of glutamic acid decarboxylase
- GAPDH
glyceraldehydes phosphate dehydrogenase
- GlyR
glycine receptor
- mRNA
messenger ribonucleic acid
- RT-PCR
reverse transcription-polymerase chain reaction
REFERENCES
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:417–418. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Belachew S, Rogister B, Rigo JM, Malgrange B, Mazy-Servais C, Xhauflaire G, Coucke P, Moonen G. Cultured oligodendrocyte progenitors derived from cerebral cortex express a glycine receptor which is pharmacologically distinct from the neuronal isoform. Eur J. Neurosci. 1998;10:3556–3564. doi: 10.1046/j.1460-9568.1998.00369.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J. Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CM, Betz H, Schoder H. Expression of inhibitory glycine receptors in postnatal rat cerebral cortex. Brain Res. 1993;606:220–226. doi: 10.1016/0006-8993(93)90988-y. [DOI] [PubMed] [Google Scholar]
- Becker CM, Hoch W, Betz H. Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO J. 1988;7:3717–3726. doi: 10.1002/j.1460-2075.1988.tb03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel C, Dolan RJ, Armony JL, Friston KJ. Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. J. Neurosci. 1999;19:10869–10876. doi: 10.1523/JNEUROSCI.19-24-10869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceranik K, Bender R, Geiger JRP, Monyer H, Jonas P, Frotscher M, Lubke J. A novel type of GABAergic interneuron connecting the input and the output regions of the hippocampus. J. Neurosci. 1997;17:5380–5394. doi: 10.1523/JNEUROSCI.17-14-05380.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danober L, Pape HC. Strychnine-sensitive glycine responses in neurons of the lateral amygdala: an electrophysiological and immunocytochemical characterization. Neurosci. 1998;85:427–441. doi: 10.1016/s0306-4522(97)00648-9. [DOI] [PubMed] [Google Scholar]
- Darstein M, Landwehrmeyer GB, Kling C, Becker CM, Feuerstein TJ. Strychnine-sensitive glycine receptors in rat caudatoputamen are expressed by cholinergic interneurons. Neurosci. 2000;96:33–39. doi: 10.1016/s0306-4522(99)00535-7. [DOI] [PubMed] [Google Scholar]
- De Olmos J, Alheid GF, Beltramino CA. Amygdala. In: Paxinos G, editor. The Rat Nervous System. 2nd Edition. San Diego: Academic Press; 1985. pp. 223–334. [Google Scholar]
- Dickson-Anson H, McGaugh JL. Bicuculline administered into the amygdala after training blocks benzodiazepine-induced amnesia. Brain Res. 1997;752:197–202. doi: 10.1016/s0006-8993(96)01449-7. [DOI] [PubMed] [Google Scholar]
- Flint AC, Maisch US, Weishaupt JH, Kriegstein AR, Monyer H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J. Neurosci. 1997;17:2469–2476. doi: 10.1523/JNEUROSCI.17-07-02469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Sato K, Sato M, Inoue T, Kozuka T, Tohyama M. Regional distribution of the cells expressing glycine receptor beta subunit mRNA in the rat brain. Brain Res. 1991;560:23–37. doi: 10.1016/0006-8993(91)91210-r. [DOI] [PubMed] [Google Scholar]
- Grenningloh G, Pribilla I, Prior P, Multhaup G, Beyreuther K, Taleb O, Betz H. Cloning and expression of the 58 kD beta subunit of the inhibitory glycine receptor. Neuron. 1990;4:963–970. doi: 10.1016/0896-6273(90)90149-a. [DOI] [PubMed] [Google Scholar]
- Grenningloh G, Rienitz A, Schmitt B, Methfessel C, Zensen M, Beyreuther K, Gundelfinger ED, Betz H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987;328:215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Handford CA, Lynch JW, Baker E, Webb GC, Ford JH, Sutherland GR, Schofield PR. The human glycine receptor beta subunit: primary structure, functional characterization, and chromosomal localization of the human and murine genes. Brain Res. Mol. Brain Res. 1996;35:211–219. [PubMed] [Google Scholar]
- Kirchhoff F, Mulhardt C, Pastor A, Becker CM, Kettenmann H. Expression of glycine receptor subunits in glial cells of the rat spinal cord. J. Neurochem. 1996;66:1383–1390. doi: 10.1046/j.1471-4159.1996.66041383.x. [DOI] [PubMed] [Google Scholar]
- Kuhse J, Kuryatov A, Maulet Y, Malosio ML, Schmieden V, Betz H. Alternative splicing generates two isoforms of the alpha 2 subunit of the inhibitory glycine receptor. FEBS Lett. 1991;283:73–77. doi: 10.1016/0014-5793(91)80557-j. [DOI] [PubMed] [Google Scholar]
- Kuhse J, Schmieden V, Betz H. Identification and functional expression of a novel ligand binding subunit of the inhibitory glycine receptor. J. Biol. Chem. 1990;265:22317–22320. [PubMed] [Google Scholar]
- Langosch D, Thomas L, Betz H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc. Natl. Acad. Sci. USA. 1988;85:7394–7398. doi: 10.1073/pnas.85.19.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malosio ML, Grenningloh G, Kuhse J, Schmieden V, Schmitt B, Prior P, Betz H. Alternative splicing generates two variants of the alpha 1 subunit of the inhibitory glycine receptor. J. Biol. Chem. 1991a;266:2048–2053. [PubMed] [Google Scholar]
- Malosio ML, Marqueze-Pouey B, Kuhse J, Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J. 1991b;10:2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzenbach B, Maulet Y, Sefton L, Courtier B, Avner P, Guenet JL, Betz H. Structural analysis of mouse glycine receptor alpha subunit genes. Identification and chromosomal localization of a novel variant. J. Biol. Chem. 1994;269:2607–2612. [PubMed] [Google Scholar]
- McCool BA, Farroni JS. Subunit composition of strychnine-sensitive glycine receptors in adult rat basolateral amygdala neurons. Soc. Neurosci; 30th Annual Meeting Abstracts; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Botting SK. Characterization of strychnine-sensitive glycine receptors in acutely isolated adult rat basolateral amygdala neurons. Brain Res. 2000;859:341–351. doi: 10.1016/s0006-8993(00)02026-6. [DOI] [PubMed] [Google Scholar]
- McCool BA, Pin J-P, Harpold MM, Brust PF, Stauderman KA, Lovinger DM. Rat group I metabotropic glutamate receptors inhibit neuronal Ca2+ channels via multiple signal transduction pathways in HEK 293 cells. J. Neurophysiol. 1998;79:379–391. doi: 10.1152/jn.1998.79.1.379. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Projection neurons of the basolateral amygdala: a correlative Golgi and retrograde tract tracing study. Brain Res. Bull. 1992;28:179–185. doi: 10.1016/0361-9230(92)90177-y. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Immunohistochemical identification of gamma-aminobutyric acid-containing neurons in the rat basolateral amygdala. Neurosci. Lett. 1985;53:203–207. doi: 10.1016/0304-3940(85)90186-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: a golgi study in the rat. J. Comp. Neurol. 1982;212:293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- Nikolic Z, Laube B, Weber RC, Lichter P, Kioschis P, Poustka A, Mulhardt C, Becker CM. The human glycine receptor subunit alpha3. Glra3 gene structure, chromosomal localization, and functional characterization of alternative transcripts. J. Biol. Chem. 1998;273:19708–19714. doi: 10.1074/jbc.273.31.19708. [DOI] [PubMed] [Google Scholar]
- Pastor A, Chvatal A, Sykova E, Kettenmann H. Glycine- and GABA-activated currents in identified glial cells of the developing rat spinal cord slice. Eur. J. Neurosci. 1995;7:1188–1198. doi: 10.1111/j.1460-9568.1995.tb01109.x. [DOI] [PubMed] [Google Scholar]
- Pribilla I, Takagi T, Langosch D, Borrman J, Betz H. The atypical M2 segment of the beta subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. EMBO J. 1992;11:4305–4311. doi: 10.1002/j.1460-2075.1992.tb05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar AA. Excitatory amino acid receptor antagonists block the cardiovascular and anxiety responses elicited by γ-aminobutyric acidA receptor blockade in the basolateral amygdala of rats. J. Pharmacol. Exper. Therap. 1997;283:969–977. [PubMed] [Google Scholar]
- Salazar R, Bell SE, Davis GE. Coordinate induction of the actin cytoskeletal regulatory proteins gelsolin, vasodilator-stimulated phosphoprotein, and profilin during capillary morphogenesis in vitro. Exp. Cell Res. 1999;249:22–32. doi: 10.1006/excr.1999.4460. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Morzorati SL, Shekhar A. Priming of experimental anxiety by repeated subthreshold GABA blockade in the rat amygdala. Brain Res. 1995;699:250–259. doi: 10.1016/0006-8993(95)00915-d. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar AA. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol. Biochem. Behav. 1995;52:701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- Schmeiden V, Kuhse J, Betz H. A novel domain of the inhibitory glycine receptor determining antagonist efficacies: further evidence for partial agonism resulting from self-inhibition. Molec. Pharmacol. 1999;56:464–472. doi: 10.1124/mol.56.3.464. [DOI] [PubMed] [Google Scholar]
- Shan Q, Haddrill JL, Lynch JW. A single beta subunit M2 domain residue controls the picrotoxin sensitivity of alphabeta heteromeric glycine receptor chloride channels. J. Neurochem. 2001;76:1109–1120. doi: 10.1046/j.1471-4159.2001.00124.x. [DOI] [PubMed] [Google Scholar]
- Shinonaga Y, Takada M, Mizuno N. Topographic organization of collateral projections from basolateral amygdaloid nucleus to both the prefrontal cortex and nucleus accumbens in the rat. Neurosci. 1993;58:389–397. doi: 10.1016/0306-4522(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Spittler A, Reissner CM, Oehler R, Gornikiewicz A, Gruenberger T, Manhart N, Brodowicz T, Mittlboeck M, Boltz-Nitulescu G, Roth E. Immunomodulatory effects of glycine on LPS-treated monocytes: reduced TNF-alpha production and accelerated IL-10 expression. FASEB J. 1999;13:563–571. doi: 10.1096/fasebj.13.3.563. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Cardoso RA, Wick MJ, Weiner JL, Dunwiddie TV, Harris RA. Effects of ethanol on recombinant glycine receptors expressed in mammalian cell lines. Alcohol. Clin. Exper. Res. 1998;22:1132–1136. [PubMed] [Google Scholar]
- Watanabe E, Akagi H. Distribution patterns of mRNAs encoding glycine receptors channels in the developing rat spinal cord. Neurosci. Res. 1995;23:377–382. doi: 10.1016/0168-0102(95)00972-V. [DOI] [PubMed] [Google Scholar]
- Wheeler M, Stachlewitz RF, Yamashina S, Ikejima K, Morrow AL, Thurman RG. Glycine-gated chloride channels in neutrophils attenuate calcium influx and superoxide production. FASEB J. 2000;14:476–484. doi: 10.1096/fasebj.14.3.476. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. J. Neurosci. 2000;20:7059–7066. doi: 10.1523/JNEUROSCI.20-18-07059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J. Neurosci. 1999;19:RC48. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Surmeier DJ. Muscarinic (m2/m4). receptors reduce N - and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G-protein pathway. J. Neurosci. 1996;16:2592–2604. doi: 10.1523/JNEUROSCI.16-08-02592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JH, Ren J, Liu PL, McArdle JJ. Glycine-activated chloride currents of neurons freshly isolated from the ventral tegmental area of rats. Brain Res. 1998;796:53–62. doi: 10.1016/s0006-8993(98)00317-5. [DOI] [PubMed] [Google Scholar]