Previous studies have shown that blood clotting factor X binds to adenovirus serotype 5 hexon protein and targets virus to liver hepatocytes. In this study, the authors exploit the strong binding interactions between factor X and hexon to retarget replication-defective and replication competent Ad5 vectors in vitro and in vivo to new receptors.
Abstract
It has been shown that blood clotting factors, including factor X (FX), bind to the adenovirus serotype 5 (Ad5) hexon protein and target the virus to liver hepatocytes after intravenous injection. These factors bind to hexon via their conserved vitamin K-dependent γ-carboxyglutamic acid (GLA) domains with subnanomolar affinity. In this work, we have used this strong interaction to retarget Ad to new receptors, using the GLA domain of FX fused to single-chain antibody variable fragment (ScFv). We demonstrate that fusion of the GLA domain of human FX to receptor-specific ScFvs will target Ad5 vectors to cells expressing these receptors. Fusion of an αHer2 ScFv to GLA increased in vitro transduction of Her2-positive versus Her2-negative cells when compared with untargeted virus. Similar results were obtained with ScFvs against the epidermal growth factor receptor (EGFR) and against the stem cell marker ATP-binding cassette protein G2 (ABCG2). Direct expression of GLA fusion protein from replication-defective or replication-competent Ad increased infection and killing of cancer cells in vitro and in vivo. These data demonstrate the potential of using GLA domains to bridge secreted ligands with intracellularly produced Ad5 vectors for vector targeting.
Introduction
It was shown that blood coagulation factor X (FX) is a predominant mediator in delivering adenovirus serotype 5 (Ad5) viruses to hepatocytes in vivo (Parker et al., 2006; Kalyuzhniy et al., 2008; Waddington et al., 2008; Alba et al., 2009). FX is a vitamin K-dependent clotting factor bearing a GLA (γ-carboxylated glutamic acid) at its N terminus and a serine protease at its C terminus (see Fig. 1A). These domains are separated by two epidermal growth factor (EGF)-like domains. The GLA domain of FX binds the hypervariable regions on the hexon protein of Ad5 with nanomolar affinity. Binding of FX to the virus retargets the virus to heparan sulfate proteoglycans on hepatocytes via the heparan sulfate exosite in the serine protease domain of FX. Inhibiting FX binding to virus can be achieved by pretreating mice with warfarin (Parker et al., 2006; Shashkova et al., 2008; Waddington et al., 2008) or by inserting proteins in hexon to block GLA binding (Kalyuzhniy et al., 2008; Vigant et al., 2008; Shashkova et al., 2009).
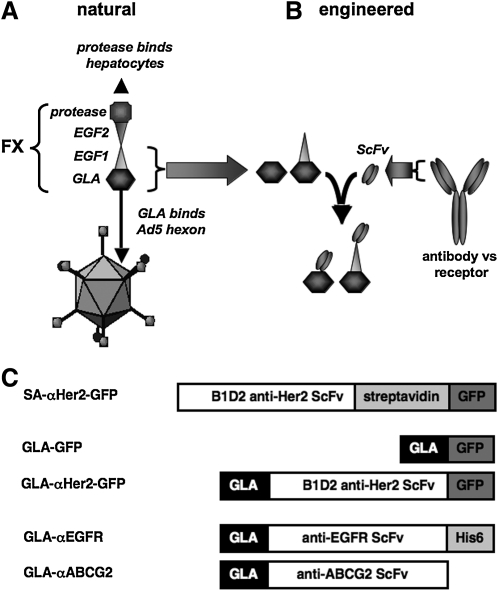
FIG. 1.
(A) Schematic of natural binding of FX to the adenovirus hexon. (B) Schematic of the engineered GLA–ScFv fusion protein strategy for generating targeting ligands. (C) GLA fusion protein constructs and controls designed for Her2, EGFR, and ABCG2 targeting. ABCG2, ATP-binding cassette protein G2; Ad5, adenovirus serotype 5; EGF, epidermal growth factor; EGFR, EGF receptor; GFP, green fluorescent protein; His6, hexahistidine; SA, streptavidin; ScFv, single-chain antibody variable fragment.
One of the challenges to retargeting Ad5 concerns the addition of new ligands to the virus to target new receptors. In particular, it has been challenging to genetically engineer ligands into Ad that normally traffic through the secretory pathway, because these require disulfide formation and glycosylation for function. In contrast, Ad is built in the nucleus, where neither disulfides nor glycosylation can occur. Therefore, secreted ligands genetically engineered into the virus generally fail because of the inability to modify the ligands appropriately. Efforts to circumvent this problem have involved the fusion of secreted ligands to soluble coxsackievirus–adenovirus receptor (CAR) or leucine zipper fusion proteins (Pereboev et al., 2002; Li et al., 2007; Glasgow et al., 2009), bridging them by metabolic biotinylation (Campos et al., 2004), by fusion of receptor and virus binding antibodies (Noureddini et al., 2006), and reengineering single-chain antibody variable fragments (ScFvs) for stability in the absence of disulfide formation and glycosylation (Hedley et al., 2006)
Given the high affinity of FX for Ad5 hexon we hypothesized that fusing the secreted GLA domain to ligands might enable high-affinity “bridging” of these secreted ligands to Ad5 for vector targeting.
In this work, we have fused the GLA and GLA-EGF1 domains from FX to ScFvs directed against Her2, epidermal growth factor receptor (EGFR), and the ATP-binding cassette protein G2 (ABCG2). We demonstrate that these GLA fusion proteins can retarget replication-defective and replication-competent Ad5 vectors in vitro and in vivo to new receptors. Retargeting was mediated by addition of exogenous GLA fusion proteins to Ad5 or by expression of the GLA proteins from replication-defective or replication-competent Ad5 viruses.
Materials and Methods
Cell culture
Human cancer cell line SKOV-3 (ovarian carcinoma) and human cancer cell lines SK-BR-3, MDA-MB-435, and MDA-MB-468 (breast carcinoma) were purchased from the American Type Culture Collection (Manassas, VA). 293 human embryonic kidney cells were purchased from Microbix (Toronto, ON, Canada). All cell lines were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT) and penicillin–streptomycin (HyClone).
αHer2 ScFv fusion protein construct
The B1D2 ScFv was generously provided by J.D. Marks (University of California at San Francisco, San Francisco, CA). B1D2 has subnanomolar affinity for the Her2 receptor (Schier et al., 1996). The human FX gene was purchased from Origene (Rockville, MD). Primers were designed for polymerase chain reaction (PCR) of either the GLA domain alone (FX 5 prime H3, CTCACTAAGCTTACCATGGGCGCCCACTGCAC; FX GLA 3 prime, CTCTGGGATCCGAACCGCCACGGTACCCCACCGGTTTGTAT) or the GLA-EGF1 domains (FX 5 prime H3; FX GLA-EGF1 3 prime, CTCTGGGATCCGAACCGCCACGGTACCCCACCGGTAATTCA) from factor X and to insert a 5′ HindIII site and 3′ AgeI, Acc65I, and BamHI sites. PCR products were cloned into the HindIII and BamHI sites of B1D2/pEFGP-N1-AAT plasmid. This plasmid is derived from pEGFP and contains a secretion signal peptide followed by the B1D2 single-chain antibody (ScFv) fused to the gene encoding green fluorescent protein (GFP). Expression is from the human cytomegalovirus immediate-early [hCMV(IE)] promoter. AgeI deletion and religation of the plasmid backbone yielded a control construct with B1D2 deleted. Acc65I–BsrG1 deletion and religation followed by insertion of ligated primers bearing a stop codon (Age-Not stop NC, GGCCGCTTACTAGTCACTCACA; Age-Not stop c, CCGGTGTGAGTGATGACTAG) yielded a control construct lacking both the B1D2 and GFP proteins. Another control plasmid used was B1D2/pEGFP-N1-AAT with streptavidin (SA) cloned into the multiple cloning site.
αEGFR ScFv fusion protein construct
The EGFR ScFv was amplified by PCR out of plasmid pTNHaa αEGFR, generously provided by K.W. Peng (Mayo Clinic, Rochester, MN), using primers pTNH6-Haa ScFv 5 prime (GGTTCGGATCCATGGGCCCTAATCGAGGGAAGGGCGGCC) and pTNH6-Haa ScFv 3 prime (CTCCACCAATTGGAGTGTACACTAGTGATGGTGATGGTG). The 3′ primer contains a hexahistidine (His6) tag for purification purposes. The single chain was cloned into pCR 2.1 TOPO (Invitrogen, Carlsbad, CA) by standard TA cloning methods. EGFR ScFv was then cloned into B1D2/pEGFP-N1-AAT between the ApaI and BsrGI sites, replacing the B1D2 ScFv.
αABCG2 ScFv fusion protein construct
5D3, a hybridoma cell line expressing an antibody against the stem cell marker ABCG2, was generously provided by B. Sorrentino (St. Jude Childrens' Cancer Center, Memphis, TN). The 5D3 ScFv was generated using previously published primers and methods (Barbas et al., 2001). Restriction sites on the amino and carboxy termini of the 5D3 ScFv were added by PCR, using primer 5D3 5prime into pEGFP (GGTTCGGATCCATGGGCCCTAGGCCGAGCTCGATATTCAGATG) and primer 5D3 3prime into pEGFP (CACATGCGGCCGCTTAGGTGGCGACCGGTATACCTTCCTGGCCGGCCTGGCC). The single chain was TA cloned into pCR 2.1 TOPO. The 5D3 ScFv was then cloned into B1D2/pEGFP-N1-AAT, using the restriction sites BamHI and BstZ17I without GFP fusion protein.
Viruses
Ad-Red is a first-generation Ad5 vector that has been rendered replication incompetent due to deletions in E1 and E3. It expresses the dsRed2 transgene from the CMV promoter (Campos et al., 2004). Ad-LacZ-RD is a similar virus with the lacZ gene in place of the dsRed2 transgene. Ad-GL-RC virus is a replication-competent virus derived from Ad5 with E3 deleted, but overexpressing the adenovirus death protein (ADP) (Shashkova et al., 2008). The EGFP-luciferase fusion gene expresses the enhanced green fluorescent protein fused to firefly luciferase off the CMV promoter. This virus was produced by insertion of the SnaBI–HpaI CMV-EGFP-Luciferase-SV40 poly(A) cassette from pEGFP-Luc (Clontech, Mountain View, CA) into the HpaI site between E1A and E1B in Ad5 (Shashkova et al., 2008). This insertion into the E3-deleted virus generates a genome of 103% of wild-type Ad5 and has no effect on virus efficacy relative to unmodified Ad5. Ad-GL-FB-RC is identical to Ad-GL-RC with the exception that a large 75-amino acid biotin acceptor domain (BAP) has been added to the C terminus of the fiber protein (Parrott et al., 2003; Campos and Barry, 2006). This modification prevents Ad-GL-FB-RC from binding CAR. This same BAP was inserted into hypervariable region 5 (HVR5) of hexon to generate Ad-GL-HB-RC (Shashkova et al., 2009). To generate replication-defective Ad-GLA-αEGFR-RD, the EGFR/pEGFP-N1-AAT plasmid was digested with BglII and NotI to remove the GLA-EGF1-αEGFR-ScFv transgene. This fragment was cloned into the multiple cloning site of pShuttle-CMV (Stratagene/Agilent Technologies, La Jolla, CA). This plasmid was recombined with AdEasy vector and transfected into 293 cells to produce virus. To generate Ad-GLA-αEGFR-RC, EGFR/pEGFP-N1-AAT plasmid was digested with SnaBI and NotI to isolate the CMV-GLA-EGF1–αEGFR-SV40polyA expression cassette. This was inserted into the HpaI site between Ad5 E1A and E1B in pXC1 plasmid (Microbix) at the same site as GFPLuc in Ad-GL-RC (Shashkova et al., 2008). pXC1 GLA-αEGFR plasmid was cotransfected with pBHGKD3E3 (Habib et al., 2002) into 293 cells. Homologous recombination resulted in the replication-competent Ad GLA-αEGFR-RC. Viruses were purified by double cesium chloride banding and viral concentrations were determined on the basis of absorbance at 260 nm (A260).
Generation and analysis of GLA fusion proteins
Fusion protein construct plasmids were transfected into 293 cells, using Lipofectamine 2000 (Invitrogen). Three days posttransfection, cell-free medium was collected and 100-μl aliquots were analyzed for GFP fluorescence, using a Multimode DTX 880 plate reader (Beckman Coulter, Fullerton, CA). All the cells produced GFP in the medium, indicating that proteins were being secreted. To establish stable cell lines, the cells were trypsinized 3 days after transfection, seeded into a larger plate, and were selected with G418 (1000 μg/ml).
Testing fusion proteins for binding to Her2-positive cells
Five milliliters of supernatant from transfected 293 cells expressing the GLA fusion proteins or control proteins were incubated with 106 SK-BR-3 or MDA-MB-435 cells on ice for 1 hr. Cells were then washed four times with 4 ml of phosphate-buffered saline (PBS) solution and then analyzed for green fluorescence, using a FACScan (BD Biosciences, San Jose, CA). Ten thousand cells were analyzed in each of three replicate tests.
Targeting Ad5 to Her2-positive cells, ABCG2-positive cells, or EGFR-overexpressing cells
Supernatants from transfected 293 cells expressing the GLA fusion proteins or control proteins were incubated with purified Ad-Red virus at a concentration of 1 × 107 viral particles (VP)/ml for 1 hr. One milliliter of supernatant containing virus was applied to 106 SK-BR-3, SKOV-3, MDA-MB-435, Chinese hamster ovary (CHO)-ABCG2, or CHO cells for 30 min at 37°C. Cells were washed four times with 4 ml of PBS and plated onto tissue culture-treated plastic. After 24 hr, cells were removed from plates with cell dissociation buffer (GIBCO; Invitrogen). Cells were washed three times with PBS and analyzed by flow cytometry, using a FACScan, for red fluorescence. Ten thousand cells were analyzed in each of three replicate tests.
In vivo targeting of adenovirus to Her2 cells
Nude mice were injected intraperitoneally with 4 × 106 SKOV-3 cells in PBS. After 28 days, GLA-αHer2 supernatants or control 293 supernatants were mixed for 1 hr with replication-competent oncolytic Ad5 expressing GFP-luciferase, and these were injected intraperitoneally into the mice. Luciferase imaging was performed 24 hr after viral treatment. Ten days later, two animals from each group were killed and their abdomens were opened to reveal the peritoneum for imaging.
Survival of mice bearing SKOV-3 tumors after treatment with targeted oncolytic viruses
SKOV-3 cells (4 × 106) were injected intraperitoneally into nude mice. Viral particles (3 × 1011) of Ad-GL-RC and Ad-GL-FB-RC were mixed with or without GLA-αHer2 supernatants for 1 hr at 4°C. Viral particles (3 × 1010) were then injected intraperitoneally into each mouse with SKOV-3 tumors 21 days after tumor implantation. Mice were monitored every other day and were killed if they showed severe abdominal swelling or other signs of distress.
Purification of GLA-αEGFR-ScFv protein from cell supernatants
293 cells were transfected with EGFR/pEGFP-N1-AAT plasmid. The cells were incubated in serum-free medium supplemented with synthetic vitamin K (menadione) at 10 ng/ml and cell supernatants were collected 5 days after transfection. Guanidine hydrochloride, sodium phosphate, and NaCl were added to a final concentration of 6 M guanidine hydrochloride, 20 mM sodium phosphate (95% dibasic and 5% monobasic), and 500 mM NaCl. The solution was then adjusted to pH 7.8 with 1 N HCl or 1 N NaOH. This solution was added to ProBond nickel beads (Invitrogen) that had been washed with water and denaturing binding buffer at a ratio of 1:100 (beads to medium). Binding was done at room temperature for 1 hr or 4°C overnight with constant rotation. Purification was then completed under denaturing conditions according to the ProBond protocol. Bulk purification was done in conical tubes instead of by column purification when purifying from large volumes of medium. Purified protein was validated by Coomassie staining and Western blotting. Final concentrations were determined by bicinchoninic acid (BCA) assay (Pierce Biotechnology/Thermo Fisher Scientific, Rockford, IL).
In vitro targeting of adenovirus with purified GLA fusion protein
Purified GLA-αEGFR ScFv protein was mixed with 2 × 109 VP of Ad-GL-RC or Ad-GL-HB-RC at molar ratios between 0 and 100:1 GLA:hexon for 1 hr at 4°C. Viral particles (4 × 108) coated with GLA-αEGFR ScFv were then added to 4 × 104 SKOV-3 cells seeded on a 96-well black-walled plate. After 24 hr transgene expression was measured by luciferase assay (n = 4; Promega, Madison, WI).
Intravascular injections
Mice were injected intravenously with 3 × 1010 VP of Ad-GL-RC that had been preincubated with a 100-fold molar excess of purified GLA-αEGFR protein, bovine serum albumin (BSA), or buffer in 100 μl of Hanks' buffered salt solution (HBSS) for 1 hr at 4°C. Warfarinized mice were injected subcutaneously with 133 μg of warfarin (Sigma-Aldrich, St. Louis, MO) in 100 μl of peanut oil 3 and 1 days before Ad injection as described by Shashkova and colleagues (2008) to inactivate endogenous FX.
Surface plasmon resonance analysis of hexon binding to purified GLA-αEGFR protein
Analysis was performed with a Biacore 3000 device (Biacore/GE Healthcare Life Sciences, Piscataway, NJ). Purified GLA-EGF-αEGFR-ScFv protein was covalently linked to Biacore CM5 chips via amine coupling (1500 response units) according to the manufacturer's instructions. Seven 2-fold serially diluted aliquots of empty adenoviral capsid were injected into the flowcells at 30 μl/min for 1 min in triplicate. Protein concentrations ranged from 200 to 3.125 ng/μl. Adenovirus was suspended in HBSS (GIBCO; Invitrogen). Dissociation was monitored for 10 min. Flowcells were regenerated in 5 mM EDTA in HBSS for 1 min. Sensorgrams were analyzed by Biacore evaluation kinetic analysis.
Transwell targeting of Ad-GL to SKOV-3 cells with Ad-GLA-EGF-EGFR
A549 cells were seeded onto the upper well of a 24-well Transwell plate containing a permeable membrane for cell adhesion. These wells were infected with Ad-LacZ-RD or Ad-GLA-αEGFR at a multiplicity of infection (MOI) of 1000. Twenty-four hours later, the A549 cells were washed to remove uninfected virus before these upper wells were added to Transwell plates with SKOV-3 cells in the bottom wells. Twenty-four hours before addition of the upper wells, SKOV-3 cells were plated onto the lower standard plastic wells of the Transwell plate. Each lower well was infected with Ad-GL virus at an MOI of 10 and the A549 upper wells were inserted into these SKOV-3 wells to provide the virus with secreted targeting protein. After 24 hr, cells were analyzed for luciferase activity (n = 4).
In vitro cell-killing assay for Ad GLA-αEGFR-RC virus
SKOV-3 cells plated on a 96-well plate were infected with serially diluted Ad GLA-αEGFR-RC or control Ad-GL virus. Two weeks after infection, cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (Sigma-Aldrich) (n = 4).
In vivo targeting of subcutaneous SKOV-3 tumors
Nude mice were injected subcutaneously with 3 × 106 SKOV-3 cells. Eight days later, the mice were injected intratumorally with buffer, or with a mixture of 1.5 × 1010 VP of Ad-EL-RC plus 1.5 × 1010 VP of Ad-LacZ-RD, 1.5 × 1010 VP of Ad-EL-RC plus 1.5 × 1010 VP of Ad-GLA-αEGFR-RD, 1.5 × 1010 VP of Ad-EL-RC plus 1.5 × 1010 VP of Ad-RC, or 1.5 × 1010 VP of Ad-EL-RC plus 1.5 × 1010 VP of Ad-GLA-αEGFR-RC. Mice were injected six times over 11 days and luciferase expression from Ad-EL was monitored on the indicated days. Tumor size and survival were also monitored over time. Tumor volume was determined by the following formula: V = (1/2) × width × length × length. Mice were killed when tumor volumes reached 2 ml or the tumors became ulcerated. For imaging studies, mice were anesthetized with intraperitoneally injected ketamine–xylazine and then injected intraperitoneally with 100 μl of luciferin (20 mg/ml; Molecular Imaging Products, Bend, OR). Mice were imaged with the Lumazone imaging system (Photometrics, Tucson, AZ), using 1-min exposures.
Statistical analysis
Data are presented as mean values of triplicate measurements unless otherwise noted. Error bars represent the standard deviation. Statistical analysis was done with PRISM software. Statistical significance was evaluated by one-way analysis of variance (ANOVA) followed by the Bonferroni post test. p < 0.05 was considered significant. Survival analysis was performed by log-rank analysis.
Animal studies
All animal studies were approved by the Mayo Clinic Institutional Review Board and were conducted in accordance with the approved animal protocols.
Results
Generation of GLA and GLA-EGF1 ScFv fusion proteins
FX has a GLA-EGF1-EGF2-SP structure in which GLA binds the Ad5 hexon and the serine protease (SP) binds cell surfaces (Fig. 1A and Waddington et al., 2008). To use GLA to retarget Ad5, plasmids were constructed that removed the SP domain and fused the GLA domain of FX to ScFvs (Fig. 1B and C). FX binding to hexon is calcium dependent, with the GLA domain binding seven Ca2+ atoms, the EGF1 domain binding a single Ca2+, and EGF2 binding no Ca2+ (Waddington et al., 2008). Given this, and that GLA and EGF1 are naturally separated by a flexible linker, we generated GLA fusion proteins with and without EGF1 (Fig. 1C and data not shown). Although both fusions bound to target cells and increased viral transduction, the GLA fusion with the EGF1 domain was more efficient (data not shown). Therefore all subsequent fusion proteins were made with the GLA–EGF1 domains and are designated simply as “GLA”. GLA was fused to ScFvs with a 13-amino acid flexible linker between the proteins to increase the likelihood that the different domains could function in the fusion. Each construct was cloned into expression plasmids bearing an N-terminal secretion signal peptide. This allowed for secretion of the fusion proteins into the extracellular environment. ScFvs directed against Her2 and epidermal growth factor receptor (αHer2 and αEGFR, respectively) were used as positive control targeting ligands as these same ScFvs were previously used to retarget measles virus (Nakamura et al., 2005; Hasegawa et al., 2007).
GLA-αHer2 fusion proteins bind to Her2-positive, but not Her2-negative cells
We first tested fusion of GLA to the B1D2 ScFv that binds the Her2 receptor with 1.6 × 10–11 M affinity (Tang et al., 2007). Fusion of B1D2 to measles virus was demonstrated to mediate receptor-specific targeting to Her2 (Hasegawa et al., 2007). Her2 is overexpressed on 15–20% of breast cancers and its expression is correlated with more aggressive tumors (Bosher et al., 1995). In ovarian carcinoma, Her2 has been shown to be overexpressed in 20 of 20 tumor cell lines derived from stage III and stage IV tumors (Hellstrom et al., 2001). GLA-αHer2 was also fused to GFP to detect binding of the ligand to cells (Fig. 1C). Control fusion proteins were produced lacking the αHer2 ScFv or substituting an irrelevant protein, streptavidin (SA), fused to B1D2.
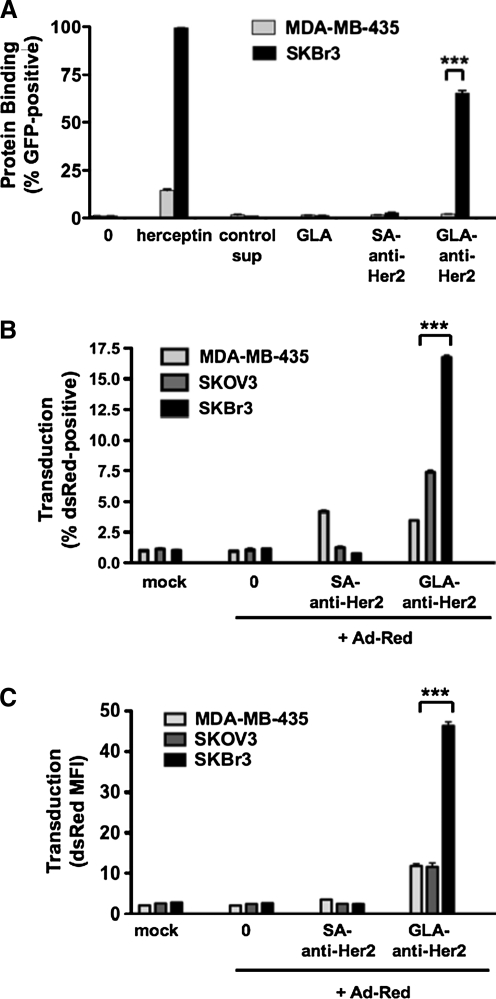
Each of the control and GLA-αHer2 fusion protein plasmids was transfected into 293 cells. After 3 days, medium from these cells was collected and these cell supernatants were applied to breast cancer cells that express high (SK-BR-3) or low (MDA-MB-435) amounts of Her2. To verify Her2 levels, SK-BR-3 and MDA-MB-435 breast cancer cells were stained with Herceptin, a monoclonal antibody against the Her2 receptor. Flow cytometry demonstrated that SK-BR-3 cells were nearly 100% positive for the Her2 receptor, whereas MDA-MB-435 cells were 15% positive (Fig. 2A). SK-BR-3 and MDA-MB-435 cells were incubated with cell supernatants containing the GLA fusion proteins for 1 hr and were analyzed for green fluorescence from GFP by flow cytometry. This assay showed that incubation of SK-BR-3 cells with supernatants from GLA-αHer2-GFP generated approximately 60% GFP-positive cells, whereas less than 2% of MDA-MB-435 cells stained positively for GFP (Fig. 2A and data not shown; p < 0.001 for both). In contrast, GLA and streptavidin-αHer2 did not produce GFP-positive cells (Fig. 2A). The lack of binding by streptavidin-αHer2 might be due to the C-terminal display of the ScFv on this tetrameric protein. These data suggested that GLA-αHer2 fusion proteins could target GFP to cells in a Her2-dependent fashion.
FIG. 2.
(A) Secreted GLA-αHer2-GFP fusion proteins bind to Her2-positive SK-BR-3 cells in vitro. Supernatant from 293 cells stably transfected to express various GLA fusion proteins or controls was incubated with 1 × 106 MDA-MB-435 or SK-BR-3 cells. For Her2 expression analysis, cells were incubated with biotinylated Herceptin followed by streptavidin Alexa Fluor 488 incubation. After 1 hr of incubation on ice, cells were washed and analyzed by fluorescence-activated cell sorting (FACS) for green fluorescence. Incubation of Ad-Red virus with GLA-αHer2-GFP protein before infection increased the transduction of Her2-positive cells. Ad-Red viral particles (1 × 107) were incubated with supernatant from cells expressing GLA-αHer2-GFP or control protein for 1 hr and then applied to cells for 30 min at 37°C. Cells were then washed and plated overnight. After 24 hr, cells were trypsinized and analyzed by FACS. (B) Percent dsRed positive. (C) Mean fluorescence index (MFI). ***p < 0.001.
GLA-αHer2 fusion protein retargets Ad5 to Her2-positive cells in vitro
To determine whether the B1D2 fusion protein could retarget Ad5 to Her2-expressing cells, targeting was tested on Her2-positive breast and ovarian cancer cells (SK-BR-3 and SKOV-3) and on Her2-negative MDA-MB-435 breast cancer cells. Replication-defective Ad5 expressing red fluorescent protein (Ad-Red) was incubated with GLA and control cell supernatants for 1 hr. This mixture was then applied to cells at an MOI of 10 VP/cell (0.2 plaque-forming units [PFU]/cell) for 30 min at 37°C. Cells were washed and were analyzed by flow cytometry for red fluorescence 24 hr later (Fig. 2B and C). GLA-αHer2-GFP fusion proteins increased transduction 15-fold on Her2-expressing cells.
GLA-αHer2 fusion proteins retarget Ad5 to Her2-positive cells in vivo
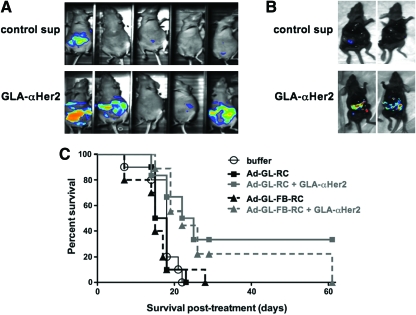
Nude mice were injected intraperitoneally with 4 × 106 SKOV-3 cells 28 days before intraperitoneal virus injection. GLA-αHer2 supernatants or control 293 supernatants were mixed for 1 hr with replication-competent oncolytic Ad5 expressing GFP-luciferase (Ad-GL-RC; Shashkova et al., 2008) and these were injected intraperitoneally into the mice. Luciferase imaging, 24 hr later, revealed large areas of luciferase activity in three of the five mice that received the GLA targeting protein. In contrast, only one mouse that received control 293 supernatant had moderate luciferase signal (Fig. 3A). Because of high variation amongst the mice, these differences in luciferase activity did not reach statistical significance (p = 0.25; GLA-αHer2 vs. control supernatant). Ten days later, two animals from each group were killed and their abdomens were opened to reveal the peritoneum for imaging (Fig. 3B). This revealed that most of the luciferase activity in the mice was confined to tumor sites and that this activity was higher in the GLA-treated animals.
FIG. 3.
In vivo effects of GLA-αHer2 on SKOV-3 infection by Ad5. (A and B) Nude mice injected intraperitoneally with 4 × 106 SKOV-3 cells 28 days before treatment were injected with 1 × 109 Ad-GL-RC virus that had been mixed with control 293 cell supernatant or with the supernatant from GLA-αHer2-expressing cells. (A) Luciferase images were taken on day 24 after virus injection. (B) Two mice from each group in (A) were imaged after the abdominal cavity was opened to facilitate viewing 10 days after viral injection. (C) Nude mice injected intraperitoneally with 4 × 106 SKOV-3 cells 21 days before virus treatment were injected with 1 × 109 Ad-GL-RC or Ad-GL-FB-RC virus with or without prior incubation with GLA-αHer2 supernatant. Survival was monitored over time after this single treatment and was analyzed by Kaplan–Meier survival analysis. Statistical comparisons described in text were performed by log-rank analysis. Color images available online at www.liebertonline.com/hum.
The oncolytic effects of the GLA protein were next tested with a CAR-binding standard oncolytic Ad5 (Ad-GL-RC) and a CAR binding-ablated oncolytic Ad5 called Ad-GL-FB-RC. Ad-GL-FB-RC is identical to Ad-GL-RC with the exception that a large 75-amino acid biotin acceptor peptide (BAP) has been added to the C terminus of the fiber protein. We have previously shown that addition of this BAP to fiber inhibits binding of fiber to CAR (Parrott et al., 2003; Campos and Barry, 2006). Therefore Ad-GL-FB-RC is a non-CAR-binding analog of Ad-GL-RC that allows comparison of the role of CAR binding in oncolysis.
Ad-GL-RC and Ad-GL-FB-RC were mixed with or without GLA-αHer2 supernatant for 1 hr and then were injected intraperitoneally into groups of 10 nude mice 21 days after intraperitoneal SKOV-3 tumor initiation and Kaplan–Meier survival curves were generated (Fig. 3C). Most untreated animals had to be killed within 20 days of single treatment. Animals receiving Ad-GL-RC or Ad-GL-FB-RC died with similar kinetics to buffer-treated mice (p = 0.69 and 0.19, respectively, when compared with the buffer group by log-rank analysis). In contrast, survival was extended for groups receiving either virus when combined with GLA-αHer2 (p = 0.026 for Ad-GL-RC vs. buffer, p = 0.007 for Ad-GL-FB-RC vs. buffer, p = 0.29 for Ad-GL-RC vs. Ad-GL-RC plus GLA-αHer2, p = 0.0065 for Ad-GL-FB-RC vs. Ad-GL-FB-RC plus GLA-αHer2 by log-rank). These data suggest that retargeting by GLA-αHer2 at initial intraperitoneal injection can improve oncolytic effects, and this targeting does not require CAR binding.
ScFv targeting EGFR
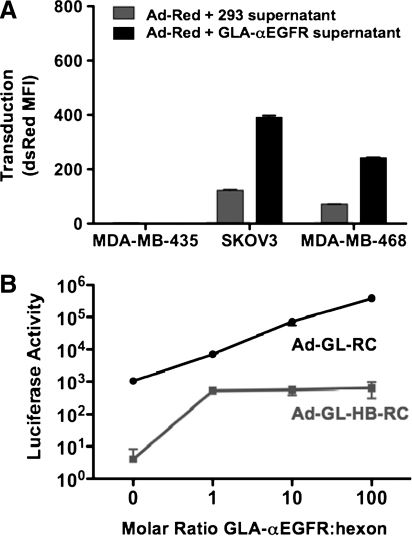
EGFR is overexpressed in several cancers including lung, breast, colon, pancreas, and brain. Overexpression of EGFR is associated with higher grade, increased proliferation, and poorer survival. An ScFv against EGFR was used previously to retarget measles virus to EGFR-positive cells (Nakamura et al., 2005). A plasmid coding for this ScFv with a His6 tag fused to GLA (Fig. 1C), was transfected into cells to produce cell supernatants containing secreted GLA fusion proteins. Ad-Red was incubated with supernatants from GLA-αEGFR-transfected cells and from control 293 cells and was applied to cells at an MOI of 1000 VP/cell (20 PFU/cell). Under these conditions, GLA-αEGFR supernatant increased transduction of EGFR-expressing SKOV-3 and MDA-MB-468 cancer cells 4-fold when compared with cells treated with virus incubated with control 293 cell supernatant (Fig. 4A). In contrast, GLA-αEGFR did not enhance transduction of MDA-MB-435 cells, which do not express EGFR.
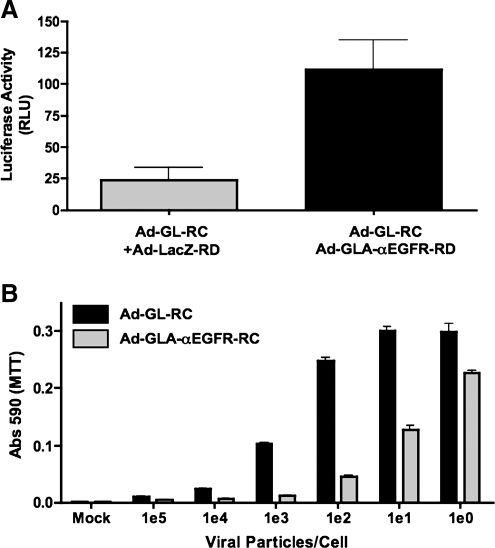
FIG. 4.
GLA can be fused to other targeting ScFvs and used to target Ad5. (A) αEGFR ScFv fused to GLA in cell supernatant was used to target Ad-Red to EGFR-overexpressing cells lines SKOV-3 and MDA-MD-468. Supernatant from 293 cells not secreting the targeting protein was incubated with Ad-Red and used as a negative control. dsRed expression was analyzed after 24 hr by FACS. (B) Purified GLA-αEGFR protein was incubated with Ad-GL-RC or Ad-GL-HB-RC at the indicated GLA-to-hexon molar ratios for 1 hr at 4°C before infection of EGFR-expressing SKOV-3 cells. After 24 hr, in vitro luciferase expression was measured.
GLA-ScFv targeting ABCG2
ABCG2 is an efflux protein that is believed to be responsible for the side-population (SP) phenotype of many adult stem cells and tumor stem cells (Hirschmann-Jax et al., 2004). In addition to effluxing Hoechst 33342 dye, ABCG2 also effluxes many anticancer drugs such as mitoxantrone and doxorubicin. Putative stem cells expressing ABCG2 are also resistant to chemotherapy. For this reason, these cells may be important targets for cancer therapy. We generated an ScFv against ABCG2 from the αABCG2 hybridoma 5D3 (Fig. 1C). Preincubation of Ad-Red virus with supernatants containing GLA-αABCG2 before infection of CHO cells stably expressing ABCG2 resulted in greater than 3-fold improvement in dsRed fluorescence when compared with cells infected with virus preincubated with 293 supernatant (p < 0.001 by ANOVA; see Supplementary Fig. 1 at www.liebertonline.com/hum). Considering that this ScFv has not been optimized for affinity, this increased transduction suggests that improved ScFvs versus ABCG2 may mediate more robust effects.
Retargeting by purified GLA-αEGFR
The His6-tagged GLA-αEGFR ScFv was purified from 293 cell supernatants by nickel resin affinity and quantitated by Western blotting, using FX as a standard curve (see Supplementary Fig. 2 at www.liebertonline.com/hum). The purified protein was mixed at various molar ratios of GLA to hexon to Ad-GL-RC. After 1 hr of binding at 4°C, the virus–protein mixtures were added to SKOV-3 cells and luciferase activity was measured 24 hr later (Fig. 4B). Increasing amounts of purified GLA-αEGFR produced increasing amounts of transduction.
GLA targeting is blocked by hexon modification of Ad5
We previously demonstrated that insertion of a large 75-amino acid biotin acceptor protein (BAP) into hypervariable region 5 (HVR5) of the hexon of Ad5 reduces binding by FX 10,000-fold (Kalyuzhniy et al., 2008). When this hexon–BAP was previously inserted into oncolytic Ad-GL-RC to create Ad-GL-HB-RC, it markedly reduced liver hepatocyte infection in vivo (Shashkova et al., 2009). Given its lower affinity for FX, we used Ad-GL-HB-RC as a control for GLA protein binding (Fig. 4B). In this case, addition of increasing amounts of GLA-αEGFR ScFv to Ad-GL-HB-RC produced markedly reduced increases in transduction of SKOV-3 cells as compared with unmodified Ad-GL-RC. These data show that blocking binding of the GLA fusion protein to hexon ablates the retargeting effects of the GLA fusion protein.
Affinity of GLA-αEGFR ScFv binding to Ad5
The His6-tagged GLA-αEGFR protein was affinity purified and used for surface plasmon resonance (SPR) analysis of its binding to Ad5 hexon. GLA-αEGFR ScFv was bound in Biacore sensor chips and Ad5 virions were then flowed across the cells; their binding to the proteins was quantitated by SPR (see Supplementary Fig. 3 at www.liebertonline.com/hum). Kinetic analysis of the sensorgrams for purified GLA-αEGFR ScFv demonstrated a KD for the protein of 2.39 × 10–8. This was consistent with previously reported KD of Ad5 binding to immobilized FX of 1.94 × 10–8 (Waddington et al., 2008). This suggests that fusion of this ScFv directly to the GLA domain of FX retains much of the binding affinity of FX for Ad5 hexon.
Purified GLA-ScFv does not detarget Ad5 from liver after intravenous injection
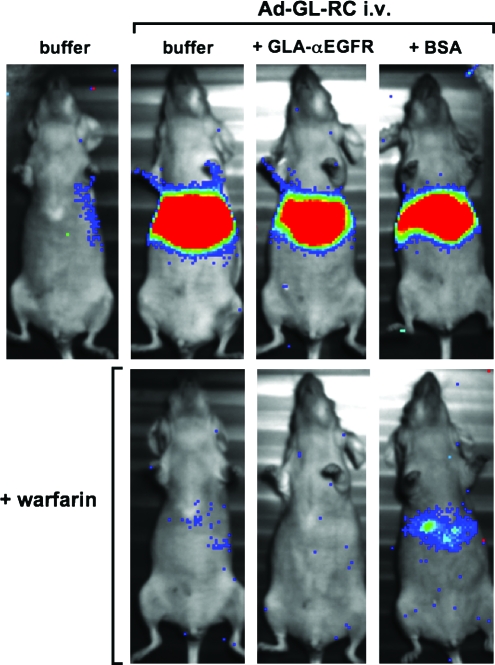
Given that the GLA fusion protein retained much of its binding affinity for Ad5 hexon, we hypothesized that binding of the GLA fusion protein to Ad5 before intravenous injection might block binding of native FX to the virus and reduce hepatocyte transduction. To test this, Ad-GL-RC was incubated with purified GLA-αEGFR at a 100-fold molar ratio of GLA to hexon for 1 hr at 4°C, as in Fig. 4B. In parallel, control viruses were incubated in buffer alone or with BSA as a negative control protein. After incubation, the mixtures were injected intravenously into nude mice and luciferase imaging was performed 24 hr later (Fig. 5). Under these conditions, liver infection by Ad5 was unaffected by addition of BSA or GLA-αEGFR. Notably, when mice were pretreated with warfarin to inactivate FX before injection, liver transduction was substantially reduced. This suggests that the observed hepatocyte transduction is mediated by endogenous FX.
FIG. 5.
Luciferase imaging of mice 24 hr after injection with Ad-GL virus. Warfarin was administered to the indicated animals on days 1 and 3 before viral injection. Ad-GL was mixed with either buffer, bovine serum albumin, or purified GLA-αEGFR protein 1 hr before injection. Color images available online at www.liebertonline.com/hum.
Using a replication-defective Ad expressing GLA fusion protein to target oncolytic Ad5 in trans
Although a combination of purified protein with Ad5 could be used for targeting, it would be simpler to have the virus express its own targeting ligand. To test this, we generated a replication-defective E1/E3-deleted Ad5 expressing GLA-αEGFR ScFv (Ad-GLA-αEGFR-RD). To test the ability of this virus to secrete its ligand and retarget Ad5, we tested it in a paracrine model using Transwell culture plates. Ad5-permissive A549 cells were seeded into the upper wells of a 24-well Transwell plate containing a permeable membrane for cell adhesion. These wells were infected with Ad-GLA-αEGFR-RD or control virus Ad-LacZ-RD at an MOI of 1000. Twenty-four hours after infection, the cells were washed to remove free virions before these upper wells were added to Transwell plates with SKOV-3 cells in the bottom wells. Before addition of the upper well, each lower well was infected with Ad-GL-RC virus at an MOI of 10. Under these conditions, untargeted Ad5 would be expected to poorly infect the SKOV-3 cells in the lower wells. If Ad-GLA-αEGFR-RD could provide secreted GLA-αEGFR protein in trans from the upper wells, then SKOV-3 transduction might be increased. Under these conditions, infection of A549 cells by Ad-GLA-αEGFR-RD in the upper wells increased Ad-GL infection of SKOV-3 cells in the lower wells by 470% when compared with Ad-LacZ-RD control virus (Fig. 6A).
FIG. 6.
(A) In vitro luciferase expression from SKOV-3 cells infected with Ad-GL. SKOV-3 cells have low CAR expression and are not readily infected by Ad at low MOI. A549 cells infected with either Ad-GLA-αEGFR-RD or Ad-LacZ-RD control virus provided secreted protein via a permeable Transwell. Protein secreted by Ad-GLA-αEGFR-RD-infected A549 cells enhanced the infection of SKOV-3 cells by Ad-GL-RC. n = 4. (B) In vitro cell-killing assay for Ad-GLA-αEGFR-RC virus. SKOV-3 cells plated on a 96-well plate were infected with serially diluted Ad-GLA-αEGFR-RC or control Ad-GL-RC virus. Two weeks after infection, cell viability was assessed by MTT assay (n = 4).
Self-targeting by oncolytic Ad5 expressing a GLA-targeting protein in vitro
The simplest way to use this system to treat tumors would be to have the oncolytic virus express its own GLA fusion protein. This would allow a self-targeting approach in which progeny virus would be targeted by the transgene proteins expressed by the parental viruses. To test this, we generated replication-competent Ad5 expressing GLA-αEGFR (Ad-GLA-αEGFR-RC) by insertion of the CMV-GLA-αEGFR-SV40 cassette into the HpaI site between E1A and E1B in Ad5. This insertion site is the same as was used to generate Ad-GL expressing GFP-luciferase. To test its oncolytic ability, oncolytic Ad-GL-RC and Ad-GLA-αEGFR-RC were incubated with SKOV-3 cells at various MOIs (Fig. 6B). Determination of cell viability after 14 days by MTT assay showed that Ad-GLA-αEGFR-RC was nearly 100-fold improved in killing of SKOV-3 cells after 14 days.
In vivo oncolytic activity of GLA-expressing viruses
Ad-GLA-αEGFR-RD could retarget itself for gene delivery, but not mediate oncolytic effects because it is replication defective. For oncolytic therapy, Ad-GLA-αEGFR-RD would have to be used in combination with another replication-competent Ad5 to provide targeting ligand in trans as in Fig. 6A. This targeting approach would have the safety advantage of separating the targeting moiety from the replication-competent virus, but would require the introduction of two separate viruses. In contrast, Ad-GLA-αEGFR-RC carries its own targeting protein, allowing one virus to be used for therapy.
To perform a direct comparison, Ad-GLA-αEGFR-RD had to be paired with an oncolytic Ad. Therefore, Ad-GLA-αEGFR-RD was combined with Ad-GL-RC to provide oncolysis as well as the ability to image infection with luciferase. The negative control virus group used Ad-LacZ-RD combined with Ad-GL-RC. Ad-GL-RC was also paired with Ad-GLA-αEGFR-RC to remove it as a variable between groups and also to allow for luciferase imaging. The negative control virus for Ad-GLA-αEGFR-RC was Ad-RC lacking any transgene.
The SKOV-3 subcutaneous tumor model was used to allow precise measurement of tumor size that is not feasible in the peritoneal model. Intratumoral virus injections were started 8 days after tumor initiation. One group of eight mice was injected intratumorally with buffer, one group with Ad-GL-RC plus Ad-LacZ-RD, one group with Ad-GL-RC plus Ad-GLA-αEGFR-RD, one group with Ad-GL-RC plus Ad-RC, and one group with Ad-GL-RC plus Ad-GLA-αEGFR-RC. Each group of eight mice received six virus injections over 11 days to mimic a clinical treatment course. A total of 3 × 1010 VP was injected during each injection, with 1.5 × 1010 VP of Ad-GL-RC and 1.5 × 1010 VP of the GLA or control viruses.
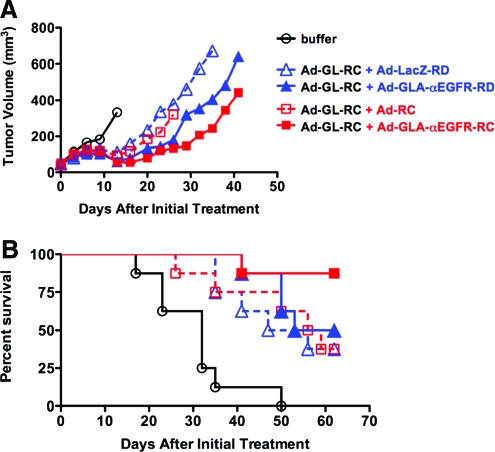
Luciferase imaging after 5 and 11 days of treatment showed expression due to Ad-GL-RC infection was observed in most of the mice, but was somewhat higher in the Ad-GLA-RD group (see Supplementary Fig. 4 at www.liebertonline.com/hum). The tumors grew most rapidly in buffer-treated animals (Fig. 7A), with the first animal having to be killed by day 16 after the start of treatment. (Note: Each line in Fig. 7 terminates when the first animal is removed from the group, because the tumor size average no longer applied.) Tumor growth was slowed relative to the buffer group for all virus groups, with the greatest delay being observed in the Ad-GLA-αEGFR-RD and Ad-GLA-αEGFR-RC groups. Paired two-tailed t test through day 26 (the last day when all virus groups still retained all mice) demonstrated that the Ad-GLA-αEGFR-RD group had smaller tumor sizes than its control Ad-LacZ-RD group (p = 0.0235). Likewise, the Ad-GLA-αEGFR-RC group tumors were smaller than the tumors in its control group with Ad-RC (p = 0.0272). These data indicated that expression of GLA protein reduced tumor sizes relative to matched control vectors.
FIG. 7.
Treatment of SKOV-3 ovarian cancer xenografts with GLA-expressing viruses. Subcutaneous tumors were initiated and treated six times with the indicated virus combinations described in text. Black lines represent mice treated with buffer. Blue lines represent mice treated with oncolytic Ad-GL-RC in combination with replication-defective gene-expressing viruses. Ad-LacZ-RD was the negative control replication-defective virus for Ad-GLA-αEGFR-RD in these groups. Red lines represent mice treated with oncolytic Ad-GL-RC in combination with oncolytic replication-competent viruses. Ad-RC was the negative control replication-competent virus for replication-competent Ad-GLA-αEGFR-RC. Dashed lines represent negative control viruses. Solid lines represent GLA-expressing viruses. (A) Mean tumor volumes. Note: Lines terminate when the first mouse was killed, because tumor averages after this point are skewed from the beginning averages. (B) Kaplan–Meier survival analysis through 60 days posttreatment. Color images available online at www.liebertonline.com/hum.
Kaplan–Meier survival curves demonstrated that all of the control mice died by day 50 (Fig. 7B). Death was delayed in all of the Ad-treated groups as compared with the buffer group. Fifty to 40% of the mice in the Ad-GLA-αEGFR-RD, Ad-LacZ, and Ad-RC groups survived through day 60, with few differences between the groups. Median survival times were 53, 47, and 56 days for Ad-GLA-αEGFR-RD, Ad-LacZ-RD, and Ad-RC, respectively. Therefore, even though Ad-GLA-αEGFR-RD gave smaller tumor sizes than its control Ad-LacZ-RD, this only slightly shifted survival time. This limited response is likely due to the loss of expression of the GLA fusion protein from dying cells. In contrast to the other groups, the Ad-GLA-αEGFR-RC group had significantly better survival with only one of eight animals dying over 60 days (Fig. 7B). Log-rank comparison of the survival curves between Ad-GLA-RC and its control Ad-RC group showed that the GLA-expressing virus mediated significantly improved survival (p = 0.05). These data suggest that an oncolytic adenovirus carrying a GLA targeting protein may mediate better antitumor effects than untargeted Ads.
Discussion
The discovery that binding of the FX GLA domain to the hexon protein on Ad5 played a role in liver transduction has opened the door to developing new ways to reduce viral uptake in the liver. In this study, we have taken a different approach and have tested an alternative use for FX with Ad5. We hypothesized that fusing the hexon-binding GLA domain of FX to ligands might be able to retarget the virus to new receptors. We also hoped that this would enable the use of potent ScFv ligands with the virus by bypassing the compatibility problem between secreted ligands and intracellularly assembled Ad.
Our data show that both the GLA and GLA-EGF1 domains of human coagulation FX can bind to Ad5 and redirect the virus to new receptors when they are fused to separate ScFvs. It is interesting to note that the GLA protein retargets virus more efficiently when coupled with the EGF1 domain from FX. These data suggest that binding of the GLA domain to Ad5 may be dependent on binding of calcium to the adjacent EGF1 domain or may simply be due to increasing the flexibility of the fusion protein. We show that three separate ScFvs targeting three different receptors worked with GLA-EGF1, suggesting that it should be a generally useful platform for many ligands.
First proof of principle was demonstrated with cell supernatants from transfected cells. This demonstrated that the GLA fusion could be combined with three different ScFvs to mediate targeting. Testing with purified GLA-αEGFR demonstrated that increasing amounts of the fusion protein mediated increased transduction. Importantly, this failed when GLA-αEGFR was combined with Ad5 whose hexons were modified with a large BAP, demonstrating that the GLA–hexon interaction is pivotal to the retargeting effect.
We hypothesized that the GLA affinity for hexon might be sufficient to block binding of native FX to the virus after intravenous injection and reduce hepatocyte infection. Unfortunately, preincubation of purified GLA-αEGFR failed to mediate this type of hepatocyte detargeting after intravenous injection. This failure could simply be due to the exceptionally high levels of FX in the blood (2 × 10–4 M) that may still rapidly replace GLA protein on the virus after injection. This is supported by the observation that inactivation of endogenous FX with warfarin before injection ablated liver infection. Regardless of the explanation, GLA fusions do not appear to detarget liver, so they are likely to be most useful for retargeting to new receptors.
To sidestep any need for protein purification and to allow for multiple generations of targeting by viruses in vivo, GLA fusion proteins were inserted into replication-defective and replication-competent oncolytic Ad to allow these vectors to retarget vectors in situ. Expression from a replication-defective Ad allows this virus to provide targeting protein in trans to an oncolytic Ad or another Ad gene therapy vector. Expression directly from an oncolytic Ad allows the virus to produce its own cell-targeting proteins. Under these conditions, we show in vivo that both replication-defective and replication-competent Ads expressing the EGFR-targeting ScFv fused to GLA reduce tumor size in an ovarian cancer model and that the replication-competent virus has markedly stronger effects on improving survival of mice bearing these tumors. These data suggest that GLA targeting with ScFvs may be an effective Ad-retargeting scheme for oncolytic therapy, cancer gene therapy, or other applications.
Unlike other methods that have been used to target adenovirus via fiber conjugates, the GLA fusion is conjugated to hexon. Evidence suggests that release of the overproduced fiber protein after lysis of adenovirus-infected cells inhibits viral spread. The proposed mechanism is that free-floating fiber binds to and blocks fiber receptors, like CAR, on uninfected cells (Rebetz et al., 2009). This implies that most fiber-targeted adenoviruses (including wild type) will spread poorly. In fact, this has been a major problem for adenoviral therapy, as exemplified by oncolytics that fail to spread beyond the needle track. Interestingly, free GLA fusion protein should not block adenovirus binding after binding to its ScFv receptor. Rather, receptor-bound GLA fusion protein could act as an attachment site for any adenovirus not saturated with GLA proteins. Because each adenovirus has 720 hexon proteins, it is not likely to become saturated with GLA fusion protein in an extracellular environment. Thus, GLA fusion-targeted adenovirus may be superior to other targeted adenovirus in its ability to spread to uninfected cells.
ScFvs have arguably been the most difficult ligand to use with Ad5 because of their need for disulfide formation and glycosylation. Therefore, successful retargeting with ScFvs suggests that the use of GLA or other hexon interacting domains with other ligands will likely mediate retargeting. High-affinity peptides, proteins, full-length antibodies, drug compounds, or magnetic particles could also be linked to the GLA domain. Because GLA can bind to any Ad5 or serotype that binds FX, this targeting approach can be applied to an array of adenoviruses to modify tropism. For those that do not bind FX, the development of other hexon display approaches may also enable retargeting of other Ads.
Previous attempts to target with high affinity antibodies bound to hexon via the high affinity avidin-biotin interaction (10−15M) failed (Campos and Barry 2006). In contrast, antibody targeting using the lower affinity GLA-hexon interaction (10−9M) succeeded here. This suggests that ligand-receptor and ligand-virus interactions must be sufficiently stable to target in vivo, but that if any of these interactions are too strong, the virus may become trapped on the receptor or fail in downstream interactions. This paradigm appears to apply for targeting via complex formation and will likely apply when inserting ligands genetically into Ad and other viral capsids (i.e., AAV). In these cases, insertion of a low affinity peptide ligand into the capsid of hexon on Ad or VP on AAV will likely fail to target in vivo. In contrast, insertion of too strong of a ligand or complex forming domain will likely fail due to the virus being trapped on receptors.
Supplementary Material
Acknowledgments
The authors thank Mary Barry and Shannon May for technical assistance. We thank Drs. Doronin and Shashkova for helpful discussions and assistance with reagents. The authors thank Dr. Marks for supplying the B1D2 ScFv, Dr. Sorrentino for supplying the ABCG2 ScFv, Dr. Peng for supplying the EGFR ScFv, and Dr. Bill Wold for supplying the plasmid pBHGKD3E3. This work was supported by a grant to M.A.B. from the Ralph C. Wilson Foundation, by an NIH P50 CA91956 Prostate Cancer SPORE grant at the Mayo Clinic, and by NIH P50CA097274 to the University of Iowa and the Mayo Clinic.
Author Disclosure Statement
M.A.B. and C.Y.C. are inventors on a provisional patent filed on GLA targeting technology.
References
- Alba R. Bradshaw A.C. Parker A.L. Bhella D. Waddington S.N. Nicklin S.A. Van Rooijen N. Custers J. Goudsmit J. Barouch D.H. McVey J.H. Baker A.H. Identification of coagulation factor (F)X binding sites on the adenovirus serotype 5 hexon: Effect of mutagenesis on FX interactions and gene transfer. Blood. 2009;114:965–971. doi: 10.1182/blood-2009-03-208835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas C.F., III Burton D.R. Scott J.K. Silverman G.J. Phage Display: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2001. pp. 953–964. [Google Scholar]
- Bosher J.M. Williams T. Hurst H.C. The developmentally regulated transcription factor AP-2 is involved in c-erbB-2 overexpression in human mammary carcinoma. Proc. Natl. Acad. Sci. U.S.A. 1995;92:744–747. doi: 10.1073/pnas.92.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos S.K. Barry M.A. Comparison of adenovirus fiber, protein IX, and hexon capsomeres as scaffolds for vector purification and cell targeting. Virology. 2006;349:453–462. doi: 10.1016/j.virol.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Campos S.K. Parrott M.B. Barry M.A. Avidin-based targeting and purification of a protein IX-modified, metabolically biotinylated adenoviral vector. Mol. Ther. 2004;9:943–955. doi: 10.1016/j.ymthe.2004.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow J.N. Mikheeva G. Krasnykh V. Curiel D.T. A strategy for adenovirus vector targeting with a secreted single chain antibody. PLoS One. 2009;4:e8355. doi: 10.1371/journal.pone.0008355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib N.A. Mitry R. Seth P. Kuppuswamy M. Doronin K. Toth K. Krajcsi P. Tollefson A.E. Wold W.S. Adenovirus replication-competent vectors (KD1, KD3) complement the cytotoxicity and transgene expression from replication-defective vectors (Ad-GFP, Ad-Luc) Cancer Gene Ther. 2002;9:651–654. doi: 10.1038/sj.cgt.7700481. [DOI] [PubMed] [Google Scholar]
- Hasegawa K. Hu C. Nakamura T. Marks J.D. Russell S.J. Peng K.W. Affinity thresholds for membrane fusion triggering by viral glycoproteins. J. Virol. 2007;81:13149–13157. doi: 10.1128/JVI.01415-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley S.J. Auf Der Maur A. Hohn S. Escher D. Barberis A. Glasgow J.N. Douglas J.T. Korokhov N. Curiel D.T. An adenovirus vector with a chimeric fiber incorporating stabilized single chain antibody achieves targeted gene delivery. Gene Ther. 2006;13:88–94. doi: 10.1038/sj.gt.3302603. [DOI] [PubMed] [Google Scholar]
- Hellstrom I. Goodman G. Pullman J. Yang Y. Hellstrom K.E. Overexpression of HER-2 in ovarian carcinomas. Cancer Res. 2001;61:2420–2423. [PubMed] [Google Scholar]
- Hirschmann-Jax C. Foster A.E. Wulf G.G. Nuchtern J.G. Jax T.W. Gobel U. Goodell M.A. Brenner M.K. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc. Natl. Acad. Sci. U.S.A. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyuzhniy O. Di Paolo N.C. Silvestry M. Hofherr S.E. Barry M.A. Stewart P.L. Shayakhmetov D.M. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc. Natl. Acad. Sci. U.S.A. 2008;105:5483–5488. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.J. Everts M. Pereboeva L. Komarova S. Idan A. Curiel D.T. Herschman H.R. Adenovirus tumor targeting and hepatic untargeting by a coxsackie/adenovirus receptor ectodomain anti-carcinoembryonic antigen bispecific adapter. Cancer Res. 2007;67:5354–5361. doi: 10.1158/0008-5472.CAN-06-4679. [DOI] [PubMed] [Google Scholar]
- Nakamura T. Peng K.W. Harvey M. Greiner S. Lorimer I.A. James C.D. Russell S.J. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat. Biotechnol. 2005;23:209–214. doi: 10.1038/nbt1060. [DOI] [PubMed] [Google Scholar]
- Noureddini S.C. Krendelshchikov A. Simonenko V. Hedley S.J. Douglas J.T. Curiel D.T. Korokhov N. Generation and selection of targeted adenoviruses embodying optimized vector properties. Virus Res. 2006;116:185–195. doi: 10.1016/j.virusres.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Parker A.L. Waddington S.N. Nicol C.G. Shayakhmetov D.M. Buckley S.M. Denby L. Kemball-Cook G. Ni S. Lieber A. McVey J.H. Nicklin S.A. Baker A.H. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood. 2006;108:2554–2561. doi: 10.1182/blood-2006-04-008532. [DOI] [PubMed] [Google Scholar]
- Parrott M.B. Adams K.E. Mercier G.T. Mok H. Campos S.K. Barry M.A. Metabolically biotinylated adenovirus for cell-targeting, ligand screening, and vector purification. Mol. Ther. 2003;8:689–702. doi: 10.1016/s1525-0016(03)00213-2. [DOI] [PubMed] [Google Scholar]
- Pereboev A.V. Asiedu C.K. Kawakami Y. Dong S.S. Blackwell J.L. Kashentseva E.A. Triozzi P.L. Aldrich W.A. Curiel D.T. Thomas J.M. Dmitriev I.P. Coxsackievirus–adenovirus receptor genetically fused to anti-human CD40 scFv enhances adenoviral transduction of dendritic cells. Gene Ther. 2002;9:1189–1193. doi: 10.1038/sj.gt.3301767. [DOI] [PubMed] [Google Scholar]
- Rebetz J. Na M. Su C. Holmqvist B. Edqvist A. Nyberg C. Widegren B. Salford L.G. Sjogren H.O. Arnberg N. Qian Q. Fan X. Fiber mediated receptor masking in non-infected bystander cells restricts adenovirus cell killing effect but promotes adenovirus host co-existence. PLoS One. 2009;4:e8484. doi: 10.1371/journal.pone.0008484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier R. Bye J. Apell G. McCall A. Adams G.P. Malmqvist M. Weiner L.M. Marks J.D. Isolation of high-affinity monomeric human anti-c-erbB-2 single chain Fv using affinity-driven selection. J. Mol. Biol. 1996;255:28–43. doi: 10.1006/jmbi.1996.0004. [DOI] [PubMed] [Google Scholar]
- Shashkova E.V. Doronin K. Senac J.S. Barry M.A. Macrophage depletion combined with anticoagulant therapy increases therapeutic window of systemic treatment with oncolytic adenovirus. Cancer Res. 2008;68:5896–5904. doi: 10.1158/0008-5472.CAN-08-0488. [DOI] [PubMed] [Google Scholar]
- Shashkova E.V. May S.M. Doronin K. Barry M.A. Expanded anticancer therapeutic window of hexon-modified oncolytic adenovirus. Mol. Ther. 2009;17:2121–2130. doi: 10.1038/mt.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y. Lou J. Alpaugh R.K. Robinson M.K. Marks J.D. Weiner L.M. Regulation of antibody-dependent cellular cytotoxicity by IgG intrinsic and apparent affinity for target antigen. J. Immunol. 2007;179:2815–2823. doi: 10.4049/jimmunol.179.5.2815. [DOI] [PubMed] [Google Scholar]
- Vigant F. Descamps D. Jullienne B. Esselin S. Connault E. Opolon P. Tordjmann T. Vigne E. Perricaudet M. Benihoud K. Substitution of hexon hypervariable region 5 of adenovirus serotype 5 abrogates blood factor binding and limits gene transfer to liver. Mol. Ther. 2008;16:1474–1480. doi: 10.1038/mt.2008.132. [DOI] [PubMed] [Google Scholar]
- Waddington S.N. McVey J.H. Bhella D. Parker A.L. Barker K. Atoda H. Pink R. Buckley S.M. Greig J.A. Denby L. Custers J. Morita T. Francischetti I.M. Monteiro R.Q. Barouch D.H. van Rooijen N. Napoli C. Havenga M.J. Nicklin S.A. Baker A.H. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.