Abstract
Many individuals have been previously exposed to human adenovirus serotype 5 (Ad5). This prior immunity has long been known to hinder its use for gene therapy and as a gene-based vaccine. Given these immunogenicity problems, we have tested whether polyethylene glycol (PEG) can blunt immune effects against Ad5 during systemic and mucosal vaccination. Ad5 vectors were covalently modified with 5-, 20-, and 35-kDa linear PEG polymers and evaluated for their ability to produce immune responses against transgene antigen products and the vector itself. We show that shielding Ad5 with different-sized PEGs generally reduces transduction and primary antibody responses by the intramuscular or intranasal route. In contrast, PEGylated vectors generally appear better at boosting antibody responses in Ad-immune animals. Displaying either glucose or galactose on PEG mediated increased transduction and antibody responses by the intranasal, but not the intramuscular, route. In naive animals, PEGylated vectors generated T cell responses that were equal to or better than those by unmodified Ad. Priming by PEGylated vectors generally enabled better subsequent T cell responses after boost. Priming and boosting by PEGylated vectors produced T cell responses after boost that were equal to or higher than those produced by unmodified vectors. These data indicate that PEGylation can enable more effective application of Ad5 and perhaps other Ad serotype vaccines during prime–boost vaccination.
Overview Summary
Adenovirus is one of the most common viral vectors used in gene therapy and viral vector vaccine trials to date. However, there are considerable obstacles to the use of these vectors in vivo. One of these obstacles is the production of neutralizing antibody and cellular immune responses against the vector. These responses may not only reduce vector and vaccine efficacy, but may also perturb the immune system, making vaccinees more susceptible to infection by HIV-1. In this paper, we examined the ability of polyethylene glycol (PEG) to shield Ad5-based vectors from immune responses in the context of both systemic and mucosal vaccination. We found that although PEG blunted bulk transduction, it was particularly useful in prime–boost vaccination of mice.
Introduction
Adenoviral (Ad) vectors are arguably one of the most potent gene delivery vectors for systemic and mucosal immunization (Lubeck et al., 1997; Xiang et al., 1999; Barouch et al., 2004; Gomez-Roman et al., 2006). The attraction for the use of Ad viruses as gene delivery vectors stems from their ability to transduce a wide range of dividing and nondividing cell types in some cases to efficiencies of nearly 100%. Additional benefits include their stability, the ability to purify the vector to concentrations of up to 1013 particles/ml, and the fact that viral vectors self-assemble into particles of specific size (∼120 nm).
The immune system has evolved to repel infectious agents. It is therefore not surprising that immune responses are elicited against many of the gene-based vaccine vectors used to deliver antigens. Although Ad vectors are potent for mediating gene delivery, they also have the reputation for being one of the most immunogenic vectors for gene therapy (Fields et al., 2000). Both innate and adaptive immune responses can be elicited by Ad capsid proteins and against the transgene protein to attenuate antigen delivery and expression (Yang et al., 1994; Schnell et al., 2001).
Injection of naked Ad virions also produces robust neutralizing antibody responses against viral capsid proteins that can bind and inactivate the virus. These neutralizing antibodies are generally serotype specific and are generally directed against the hypervariable regions of the hexon protein (Crawford-Miksza and Schnurr, 1996; Roberts et al., 2006). Neutralizing antibodies are produced during natural infection or after one inoculation of the vector into a naive host. These preexisting or vector-induced antibodies are problematic for an Ad-based vaccine as they can attenuate the level of gene delivery if the same serotype of Ad is used (Croyle et al., 2001).
Whereas anti-Ad antibodies and/or T cell responses can attenuate vaccine efficacy, prior immunity to Ad (or any vaccine vector) may also have unexpected consequences. For example, in the STEP HIV vaccine trial, Ad serotype 5 (Ad5)-based vectors were used to vaccinate non-HIV-infected individuals. Rather than reducing HIV infection, preliminary analysis of vaccinees found a higher than expected rate of infection as compared with control subjects (Sekaly, 2008). More troubling was the preliminary correlation between increased HIV infection rates and higher levels of preexisting neutralizing antibodies against Ad5. These data suggest that prior immunity to Ad and any virus-vectored vaccine may not only attenuate efficacy, but may also create unexpected immunological consequences. Therefore, approaches to reduce the production of antivector immune responses and to shield vectors from existing immune responses may have utility for increasing vaccine efficacy and safety.
Polyethylene glycol (PEG) is a clinically approved polymer used to improve the pharmacokinetics of a variety of protein therapeutics. PEG has also been chemically conjugated to free amine groups on the Ad5 virion surface. PEG has been shown to reduce a number of dangerous side effects caused by intravenous injection of Ad5 (Croyle et al., 2005; Mok et al., 2005; Hofherr et al., 2007). For example, PEGylation of Ad5 reduces innate immune responses against the vector as evidenced by 75% reduction in interleukin (IL)-6 levels at 6 hr and 90% reduction in cumulative IL-6 over 48 hr (Mok et al., 2005). PEGylation also blunts thrombocytopenia, platelet activation, and endothelial activation after intravenous injection (Croyle et al., 2005; Hofherr et al., 2007). PEG also reduces uptake of the virus in macrophages and Kupffer cells (Mok et al., 2005), consistent with the ability of the polymer to reduce the production of new antibody and cellular immune responses against Ad (Croyle et al., 2001).
PEGylation of Ad has also been shown to protect the vector from preexisting neutralizing antibodies to allow multiple administration into Ad-immunized or passively immunized recipients (O'Riordan et al., 1999; Croyle et al., 2001, 2002). In mice previously exposed to Ad5, readministration of naked Ad resulted in up to a 10 million-fold reduction in transduction (O'Riordan et al., 1999; Croyle et al., 2002). When Ad was PEGylated with small (3- to 5-kDa) PEG, transduction was increased from 100- to 10,000-fold, suggesting this approach could be a viable alternative to serotype switching to allow Ad to be applied in the face of preexisting or vector-induced antibodies. Although promising, it should be noted that this rescue of transduction did not bring gene expression levels back to those occurring in naive mice. Instead, transduction levels by intravenous routes were still 100- to 10,000-fold lower than those in unimmunized mice. A more recent study compared the use of 5- and 20-kDa PEG for transduction and for immunization (Wortmann et al., 2008). Mice were passively immunized by transfer of serum from Ad-immunized mice and then injected with unmodified or 20-kDa-PEGylated Ad expressing hepatitis B surface antigen. In this case, passively transferred antibodies reduced immune responses against the hepatitis antigen by 84% as compared with unmodified vector. In contrast, the PEGylated vector produced only a 33% reduction in immune responses (Wortmann et al., 2008).
These data suggest that PEGylation of Ad may have some ability to shield Ad vaccines from preexisting immune responses. However, in the gene therapy realm, PEGylation has been shown to markedly reduce virus uptake into antigen-presenting cells (Croyle et al., 2005), making it uncertain whether this shielding modification will allow productive vaccine responses or will also reduce immune responses against transgene production. Previous work tested the effects of PEG only on immunization in the systemic compartment (Wortmann et al., 2008). It was therefore also unclear how different-sized PEGs might affect transduction by systemic and mucosal routes of vaccination. It was unclear how much protection would be mediated by different-sized PEGs in the context of a prime–boost regimen and by different routes of immunization.
This study has tested these parameters in mouse models, using reporter gene imaging combined with neutralizing antibodies and antibody and cellular immune assays against transgene products and HIV-1 antigens. We compare the effects of 5-, 20-, and 35-kDa PEG on transduction and immune responses mediated by Ad5 after systemic immunization by the intramuscular route and after mucosal immunization by the intranasal route. These tests have been performed in both naive and Ad-immunized mice to assess the actual utility of PEGylation as a means to shield Ad vaccines against preexisting and vector-induced immune responses. We also test the effects of appending carbohydrate ligands on the ends of PEG to determine whether targeting cell surface lectins may modulate vaccine effects.
Materials and Methods
Adenoviruses
First-generation replication-defective (E1/E3 deleted) Ad5 vectors were constructed with the AdEasy system (Statagene, La Jolla, CA) in 293A cells as described by Mok and coworkers (2005). Ad-LIG expresses a firefly luciferase IRES (internal ribosomal entry site) hrGFP (humanized Renilla reniformis green fluorescent protein) cassette from the cytomegalovirus (CMV) promoter. Ad-GL expresses an enhanced GFP (eGFP)–luciferase fusion gene and Ad-gag expresses the HIV-1 p55gag codon-optimized gene of strain HXB2 (Ad-gag). Both Ad-LIG and Ad-GL can be used for luciferase imaging. Neither the luciferase nor the hrGFP protein has useful T cell epitopes for enzyme-linked immunospot (ELISPOT) testing (data not shown). In contrast, both Ad-GL and Ad-gag have H-2Kd-restricted epitopes that can be used to monitor T cell responses in BALB/c mice. All adenoviruses were purified by CsCl banded and quantitated by determining the optical density at 260 nm (OD260).
Animals
Female BALB/c mice (6–8 weeks old) were purchased from Charles River Laboratories (Wilmington, MA) and housed in the Mayo Clinic (Rochester, MN) animal facility under the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC, Frederick, MD) guidelines with animal use protocols approved by the Mayo Clinic Animal Use and Care Committee. All animal experiments were carried out according to the provisions of the Animal Welfare Act, PHS Animal Welfare Policy, the principles of the NIH Guide for the Care and Use of Laboratory Animals, and the policies and procedures of Mayo Clinic.
Mice were immunized intramuscularly or intranasally. Mice immunized intramuscularly received 1 × 1010 viral particles (VP)/mouse in 25 μl injected into the right quadriceps. Mice immunized intranasally received 1 × 1010 VP/mouse in 20 μl (using 10 μl/nare) delivered under ketamine–xylazine anesthesia. Where indicated, the mice were boosted as described for priming. Two weeks after the boost, the mice were bled and killed, and spleens were collected.
Adenoviral PEGylation
For this study, we used succinimide-activated, amine-reactive PEG molecules of 5 kDa (Sunbright ME-050-HS; NOF, Tokyo, Japan) and 20 and 35 kDa (m-SCM-35K; JenKem Technology, Beijing, China). The 35-kDa PEG was used with and without galactose or glucose moieties as potential ligands for carbohydrate receptors. Ad-GL, Ad-LIG, and Ad-gag were modified with the 5-, 20-, and 35-kDa PEGs and with 35-kDa PEG-Gal and 35-kDa PEG-Glu polymers. All PEGylation was performed as previously described (Hofherr et al., 2007, 2008). Briefly, PEGylation was performed at room temperature for 1 hr and excess nonreactive PEG was removed by gel filtration with Sephadex G100 (GE Healthcare Life Sciences, Piscataway, NJ). The concentration of virus was determined by absorbance at 260 nm and confirmed by real-time polymerase chain reaction (PCR) as described in Mok and coworkers (2005).
Quantitation of free amines
Free amines were determined with a CBQCA protein quantitation kit (Invitrogen, Carlsbad, CA) as described in Hofherr and coworkers (2008). Briefly, viruses were diluted in phosphate-buffered saline (PBS), and KCN and 3-(4-carboxybenzoyl)quinoline-2-carboxaldehyde (CBQCA) were added for 1 hr. Fluorescence emission was detected at 550 nm with excitation at 465 nm, using a DTX 880 multimode detector (Beckman Coulter, Fullerton, CA). The percentage of free amines was determined relative to a standard curve of unmodified virus.
Western blot analysis
Ad-gag, modified and unmodified, was incubated with A549 cells at 25,000 VP/cell for 24 hr. Cells and supernatant were collected and lysed with lysing buffer (50 mM Tris-HCl, 1% Triton X-100, 0.15% sodium dodecyl sulfate, 150 mM NaCl, and 20 mM EDTA). Both supernatant and cell lysate were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred onto a nitrocellulose membrane (0.45-μm pore size; Bio-Rad, Hercules, CA). After blocking the membrane with 1.0% bovine serum albumin fraction V in DPBS (10 mM Tris-HCl [pH 7.5], 150 mM NaCl) for 1 hr at room temperature, the membranes were washed three times with TBS-T (TBS containing 0.1% Tween 20), reacted with an anti-HIV-1 Gag monoclonal serum at a dilution of 1:1000 for 1 hr at room temperature, and then incubated with horseradish peroxidase-conjugated goat anti-mouse (Pierce Biotechnology, Rockford, IL) for 1 hr at room temperature. Protein bands were detected with SuperSignal West Pico substrate (Pierce Biotechnology) and imaged with a Kodak In Vivo imaging system F (Carestream Health, Rochester, NY).
In vivo transduction
Cells were grown in a 96-well black plate (3603; Corning Life Sciences, Lowell, MA). Virus (each at 104 VP/cell) was added and incubated for 20 hr at 37°C and luminescence was measured with a Beckman Coulter DTX 880 multimode detector as described in Hofherr and coworkers (2008). The percent transduction was determined as compared with unmodified virus.
Particle sizing
Particle sizing was performed as described in Hofherr and coworkers (2008), using a 90Plus/BI-MAS multiangle particle sizer (Brookhaven Instruments, Holtsville, NY). Particle sizes are shown as the mean of three 3-min runs and standard error. Polydispersity was calculated and is proportional to the variance of the intensity-weighted diffusion coefficient distribution. Molecular weights were calculated according to the Mark–Houwink–Sakurada equation.
In vivo luciferase imaging
Luciferase-expressing viruses Ad-LIG or Ad-GL were administered as indicated elsewhere in text and the mice were imaged at various times with a MAG Biosystems Lumazone imaging system (Photometrics, Pleasanton, CA) as described by Hofherr and coworkers (2008). Mice were anesthetized with isoflurane and injected intraperitoneally with d-luciferin (20 mg/ml in PBS) in a volume of 200 μl; the mice were then immediately placed into the Lumazone imager and images were captured. All images were taken with a 10-min exposure and 2 × 2 binning, using no filters and no photomultiplication. Data analysis was performed on each image, using background subtracted mean intensities detected by the Lumazone imaging software at each time point, and graphed with Prism graphing software (GraphPad Software, San Diego, CA).
Adenovirus neutralization assay
Adenovirus-neutralizing antibodies (NAbs) were determined as previously described (Sprangers et al., 2003). Briefly, sera were heat inactivated at 56°C for 30 min before a serial doubling dilution was performed in a 96-well black plate (3603; Corning Life Sciences). Sera were diluted from 1:20 to 1:2560 in a volume of 50 μl of complete Dulbecco's modified Eagle's medium (DMEM) containing unmodified Ad-LIG or Ad-GL at 1 × 108 VP/ml. Naive mouse serum was used as negative control and was the maximal luciferase activity reference. The plates were incubated at 37°C for 1 hr. Fifty microliters of A549 cells at 1 × 105 cells/ml was added to each well. Plates were incubated for 20 hr at 37°C and 5% CO2 before readout. Luciferase activity was determined with 5× reporter lysis buffer and luciferase assay reagent (LAR) (Promega, Madison, WI) as described in the manufacturer's protocol. Briefly, 25 μl of 5× lysis buffer was added to each well, mixed, and frozen at −80C. The plates were thawed at room temperature and 50 μl of LAR was added. Each plate was shaken on an orbital shaker and luciferase activity was measured with a Beckman Coulter DTX 880 multimode detector. Neutralizing antibody titers were determined as the reciprocal dilution to inhibit 90% of luciferase activity and are expressed as the geometric mean titer (GMT).
Enzyme-linked immunospot assay
To measure cellular responses to eGFP and Gag, splenocytes were incubated in the presence of peptides at a concentration of 5 μg/ml. To measure GFP responses the HYLSTQSAL peptide was used. Gag cytotoxic T lymphocyte (CTL) and helper T cell responses were determined with the VGGHQAAMQMLKDTINEEAA peptide and peptide pool (ATLEEMMTACQGVGGPSHKA, TSNPPIPVGDIYKRWIILGL, and FKTLRAEQATQEVKNWMTDT), respectively. Spleens from individual mice were minced and then forced through a 40-μm Nylon cell strainer (BD Biosciences, San Jose, CA). Single-cell suspensions of splenocytes were plated in 96-well polyvinylidene difluoride-backed plates (MultiScreen-IP; Millipore, Billerica, MA) coated with 50 μl of anti-mouse interferon (IFN)-γ monoclonal antibody (mAb) (AN18, 5 μg/ml; Mabtech, Stockholm, Sweden) and kept overnight at 4°C. The plates were blocked with HEPES-buffered complete RPMI medium at 37°C for 2 hr. Equal volumes (50 μl) of each peptide pool and splenocytes (107 cells/ml) were added to the wells in duplicate. Plates were incubated overnight (14 to 16 hr) at 37°C with 5% CO2. After the plates were washed six times with PBS, 50 μl of 1:1000-diluted biotinylated anti-mouse IFN-γ mAb (Mabtech) was added to each well. Plates were incubated at room temperature for 2 hr and then washed three times with PBS. Fifty microliters of streptavidin–alkaline phosphatase conjugate (1:1000 dilution; Mabtech) was added to each well. After incubation at room temperature for 1 hr, the plates were washed five times with PBST. Finally, 100 μl of BCIP/NBT (5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium) Plus alkaline phosphatase substrate (Moss, Pasadena, MD) was added to each well. The plates were incubated at room temperature for 10 min. After washing with water, the plates were air dried. Spots were counted with an automated ELISPOT plate reader (Immunospot counting system; CTL, Cleveland, OH) and expressed as spot-forming cells (SFCs) per 106 splenocytes.
Results
In vivo activity of PEGylated adenoviral vectors
Previous data have shown that PEGylation of Ad5 ablates the ability of the virus to infect cells via the coxsackievirus–adenovirus receptor (CAR) (Ogawara et al., 2004). However, after intravenous injection, 5-kDa PEG still allowed the vector to transduce the liver whereas 20- and 35-kDa PEG blocked this transduction (Hofherr et al., 2008), perhaps by increasing viral particle size. The effects of PEG on the tropism of Ad administered by the intravenous and intraperitoneal routes were tested in this previous work. Intramuscular and intranasal routes, relevant to systemic and mucosal immunization, were not tested. In addition, the effects of PEG on immune responses against the virus and its transgene products were also not tested.
To test the effects of PEGylation on vaccination, Ad-LIG was modified with amine-reactive 5-, 20-, or 35-kDa PEG as described in Hofherr and coworkers (2007, 2008) to produce Ad-LIG-5K, Ad-LIG-20K, and Ad-LIG-35K. Reactive PEG molecules are also available that display galactose or glucose on their end. These PEG molecules were also tested, because there are carbohydrate-binding receptors on immune cells and mucosa. For example, galactose moieties can target gene delivery to monocytes and dendritic cells via their mannose receptor (Erbacher et al., 1996; Parrott et al., 2003). Similarly, transfection of airway epithelial cells can be increased by targeting with glucose or galactose (Fajac et al., 1999). 35K PEG-galactose and 35K PEG-glucose were therefore also tested on Ad-LIG (Ad-LIG-35K-Gal and Ad-LIG-35K-Glu) to determine whether appending either ligand on the PEG shield would mediate increased immune responses.
Each PEGylated virus was characterized in vitro to determine the extent of PEGylation and the inhibition of in vitro activity. PEGylation of all the reagents resulted in conjugation of 65 to 75% of the free amines on the viral surface as determined by the CBCQA assay. Consistent with previous work, PEGylation reduced in vitro transduction by 99% (Table 1). Pretreatment of the viruses with mouse serum for 1 hr did not increase this transduction, suggesting that the PEG coating was stable in biological fluids. Viral diameter increased from 111 to 158 nm with the various PEG molecules. The predicted molecular weight of the modified adenoviruses increased up to two times that of unmodified Ad (Table 1).
Table 1.
In Vitro Characterization of Polyethylene Glycol-Modified Virus
| Ad-LIG | Ad-LIG-5K | Ad-LIG-20K | Ad-LIG-35K | Ad-LIG-35K-Gal | Ad-LIG-35K-Glu | |
|---|---|---|---|---|---|---|
| Percent of PEG-conjugated free aminesa | 0.0 | 64.96 | 70.54 | 74.41 | 72.07 | 69.70 |
| Percent transduction of A549 cells | 100.00 | 0.18 | 0.01 | 0.02 | 0.02 | 0.01 |
| Effective diameter (nm) | 111.7 ± 0.5 | 132.7 ± 0.7 | 145.9 ± 1.2 | 158.5 ± 1.5 | 150.5 ± 2.7 | 153.9 ± 2.5 |
| Polydispersity | 0.031 ± 0.012 | 0.019 ± 0.013 | 0.049 ± 0.021 | 0.93 ± 0.023 | 0.044 ± 0.020 | 0.077 ± 0.001 |
| Calculated MWb (× 107 g/ml) | 3.7 | 5.49 | 6.88 | 8.35 | 7.40 | 7.79 |
| Percent activity after serum treatmentc | 100.0 | 0.14 | 0.00 | 0.06 | 0.06 | 0.17 |
Abbreviations: PEG, polyethylene glycol; MW, molecular weight.
As measured by the CBCQA assay.
As determined using the Mark–Houwink–Sakurada equation.
Virus was incubated with normal mouse serum for 1 hr at 37°C before infection.
In vivo luciferase activity
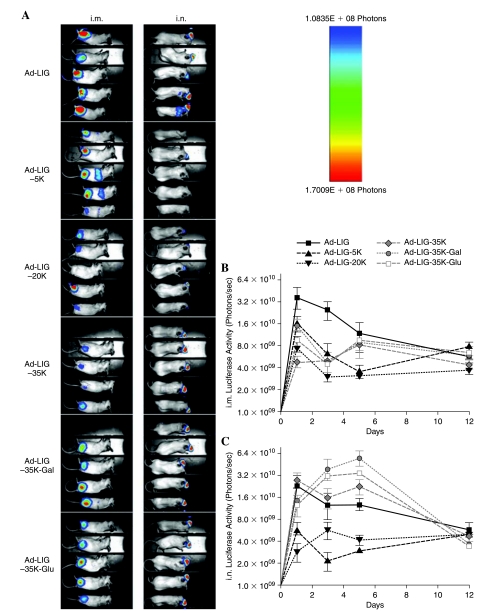
Groups of five BALB/c mice were immunized on day 0 by the intramuscular and intranasal routes with 1 × 1010 viral particles (VP) of PEGylated and unmodified Ad. The mice were then imaged for luciferase activity on days 1, 3, 5, and 12 after administration (Fig. 1). Introduction of unmodified Ad generated robust luciferase activity by both the intramuscular and intranasal routes. Intramuscular injection of Ad-LIG-5K bearing 5-kDa PEG produced signal primarily in the injection site, but with some signal from the abdomen (Fig. 1A). On the basis of previous imaging, this signal is consistent with expression in the liver (Hofherr et al., 2007, 2008). This suggests that some 5K PEG-modified Ad virus translocated from the muscle injection site into the blood and mediated some level of liver transduction. Intranasal injection generated luciferase activity primarily in the nasal region, but with some activity observed in the lungs, particularly for the unmodified virus (Fig. 1A).
FIG. 1.
In vivo luciferase activity induced by unmodified and PEGylated Ad-LIG after intramuscular and intranasal immunization. Groups of five mice were administered 1010 VP of the indicated vectors by the indicated routes. Twenty-four hours later the animals were anesthetized, injected with luciferin, and imaged for luciferase activity (A). Mice were imaged on days 1, 3, 5, and 12. The luciferase activity for intramuscularly and intranasally immunized mice is shown in panel A. Graphs reflect luciferase activity from the entire mouse. Error bars represent the standard error.
Quantitation of luciferase activity by measurement of photons per second showed differing kinetics of bulk transgene expression (Fig. 1B and C). Modification of Ad with all of the PEGs reduced transduction by the intramuscular route from 50 to 90% on day 1 and beyond. Expression was highest on day 1 and this expression decayed for all the vectors over 12 days (Fig. 1B). The kinetics of expression by the intranasal route was markedly different from that by the intramuscular route (Fig. 1C). In this case, expression by most vectors peaked on day 1, but remained relatively high for 5 days. In contrast, expression by Ad-LIG-35K-Gal and -Glu (bearing galactose and glucose ligands) peaked on day 5 and was higher than with the other vectors. Area under the curve (AUC) calculations for intranasal luciferase expression showed that viruses modified with 5- or 20-kDa PEG produced approximately 50% expression compared with unmodified Ad. Ad modified with 35-kDa PEG generated nearly equal AUC as compared with unmodified Ad. Viruses modified with 35-kDa PEG-Gal and 35-kDa PEG-Glu had AUCs that were approximately 3- and 2-fold higher than that of unmodified Ad, respectively (data not shown).
Effect of PEGylation on primary antibody responses against transgene product
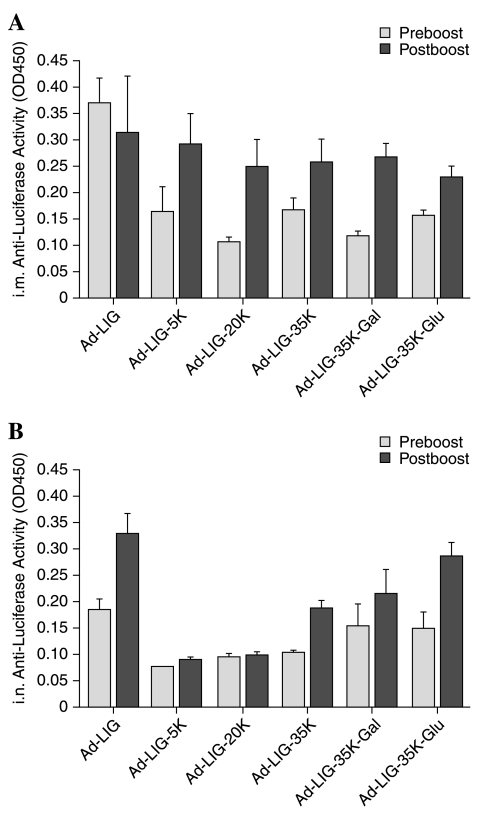
Serum was collected from mice 3 weeks after immunization and antibodies against luciferase were measured by enzyme-linked immunosorbent assay (ELISA) (hrGFP protein was unavailable for this test). By the intramuscular route antibody responses were highest with unmodified Ad (Fig. 2A). By the intranasal route antibody responses were highest with unmodified Ad and lowest for viruses modified with 5- or 20-kDa PEG (Fig. 2B). Ad-LIG modified with any of the 35-kDa PEGs generated antibody responses that were nearly as strong as that with unmodified Ad. These antibody data generally correlated with cumulative luciferase expression (Fig. 1) and the AUC calculations.
FIG. 2.
Anti-luciferase antibody responses after priming and boosting. Sera from the mice in Fig. 1 were collected before and after boosting with the same indicated vector and were tested by ELISA against luciferase protein. Data are shown for immunization by the intramuscular (A) and intranasal (B) routes. Data represent the means of five animals and error bars represent the standard error.
Effect of PEGylation on induction of primary antibody responses against adenoviral vector
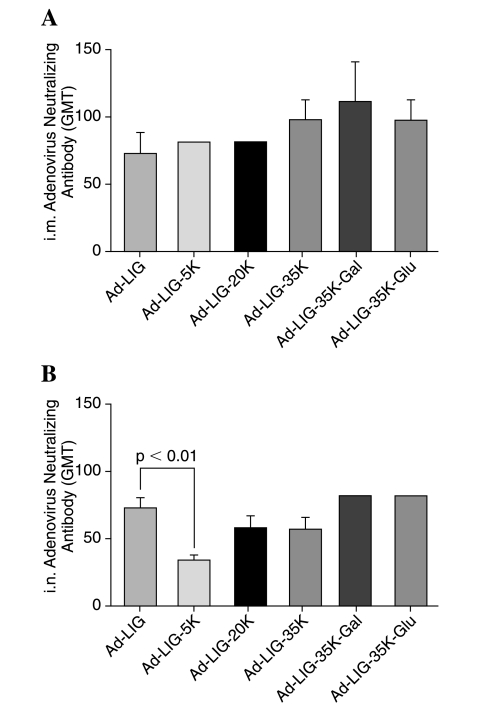
Previous work has suggested PEG may shield Ad from neutralizing antibodies and reduce the production of new immune responses against the virus (O'Riordan et al., 1999; Croyle et al., 2001, 2002; Wortmann et al., 2008). To test this, serum Ad-neutralizing antibodies were assayed 3 weeks after priming (Fig. 3). Intramuscular or intranasal injection of unmodified or PEGylated Ad produced geometric mean titers (GMTs) of Ad-neutralizing antibodies of less than 100. PEGylated Ad did not reduce the induction of neutralizing antibodies (Fig. 3A). Interestingly, neutralizing antibodies by the 5-kDa PEGylated virus in the intranasal group were 2-fold lower than those generated by unmodified Ad (p < 0.01 by ANOVA with Bonferroni post-hoc test) (Fig. 3B).
FIG. 3.
Anti-Ad-neutralizing antibodies before and after boosting. Serum was tested for neutralizing antibody levels against unmodified Ad5. Geometric mean titers (GMTs) were determined as the reciprocal dilution of sera to inhibit 90% luciferase activity as compared with the control. Data are shown as the mean GMT from five mice. Error bars represent the standard error. Statistical analysis was performed by one-way ANOVA.
Effect of PEG on production of anti-luciferase antibodies in the face of preexisting immunity
Five weeks after priming, the groups of mice from Figs. 1 to 3 were boosted with the same vector by the same route as was used for priming (e.g., intranasal Ad-LIG-5K and then intranasal Ad-LIG-5K, etc.). Two weeks after boost, anti-luciferase antibodies were again measured. When the intramuscularly injected mice were boosted, unmodified Ad did not further boost luciferase antibody levels. In contrast, all of the PEGylated vectors boosted, with most luciferase antibody levels approaching the same level as those generated by unmodified Ad (Fig. 2A). When the intranasally injected mice were boosted with the same vectors, anti-luciferase antibodies increased in all groups except the 5- and 20-kDa PEG groups (Fig. 2B). These data suggest that the PEGylated vectors mediated transduction in the face of neutralizing antibodies to boost anti-transgene immune responses. The lack of boosting by unmodified Ad by the intramuscular route may be due to vector neutralization.
Effect of PEGylation on induction of cellular immune responses in the absence of previous exposure to adenovirus
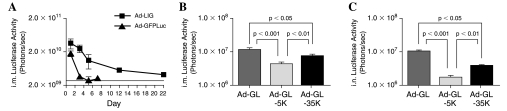
These data show that PEGylated Ad5 vectors can generate antibody responses against transgene products. Because there are no known H-2Kd T cell epitopes in either the luciferase or Renilla GFP transgenes, an alternative Ad5 vector, Ad-GL, was used to assess cellular immune responses. Ad-GL expresses the GFPLuc fusion protein of the codonoptimized Aequorea victoria green fluorescent protein and firefly luciferase. Unlike Renilla GFP, this jellyfish GFP protein bears a known H-2Kd-restricted peptide, HYLSTQSAL, that can be used to test CD8+ T cell responses in BALB/c mice (Andersson and Barry, 2004; Andersson et al., 2005). Luciferase imaging of Ad-GL demonstrated that it can also be used for luciferase imaging, but has lower activity and a shorter time course of detection than Ad-LIG (Fig. 4A).
FIG. 4.
In vivo luciferase activity induced by unmodified and PEGylated Ad. Mice were immunized with Ad-LIG and Ad-GL administered intramuscularly at 1010 VP. The mice were imaged over time and luciferase activity was plotted (A). (B and C) Mice were administered 1010 VP of the indicated vectors by the indicated routes. Twenty-four hours later the animals were anesthetized, injected with luciferin, and imaged for luciferase activity. Quantitation of luciferase expression induced by unmodified and PEGylated Ad-LIG after intramuscular (B) and intranasal (C) immunization is shown. Data represent the mean luciferase activity from 20 mice. Error bars show the standard error associated with this mean. Statistical analysis was performed by one-way ANOVA.
Ad-GL was PEGylated with either 5- or 35-kDa PEG (Table 2) and groups of 20 BALB/c mice were immunized with either buffer, Ad-GL, Ad-GL-5K, or Ad-GL-35K. Luciferase imaging 1 day after injection revealed that transduction by Ad-GL-5K was 60 to 80% lower than by unmodified Ad via the intramuscular and intranasal routes (p < 0.001 by ANOVA; Fig. 4B and C). Transduction by Ad-GL-35K was 25 to 60% lower than by Ad via both the intramuscular and intranasal routes (p < 0.05). These data are similar to the day 1 data from Ad-LIG in Fig. 1B and C, with the exception that Ad-LIG expression was not significantly different from that of Ad-LIG-5K and Ad-LIG-35K. The difference between the two vectors may be due to the lower and shorter persistence of luciferase expression by Ad-GL versus Ad-LIG (Fig. 4A).
Table 2.
In Vitro Characterization of Polyethylene Glycol-Modified Ad-GL and Ad-gag
| Ad-GL | Ad-GL-5K | Ad-GL-35K | Ad-gag | Ad-gag-5K | Ad-gag-35K | |
|---|---|---|---|---|---|---|
| Luciferase activitya | 100.0% | 1.8% | 3.7% | ND | ND | ND |
| Effective diameter (nm) | 116.0 ± 0.8 | 129.5 ± 0.9 | 149.8 ± 1.0 | 109.4 ± 0.7 | 122.3 ± 1.9 | 165.6 ± 1.8 |
| Gag expressionb | ND | ND | ND | 100.0% | 0.0% | 0.0% |
| β-Actin expressionb | ND | ND | ND | 100.0% | 103.2% | 95.4% |
| Polydispersity | 0.004 ± 0.002 | 0.007 ± 0.002 | 0.061 ± 0.017 | 0.012 ± 0.007 | 0.036 ± 0.027 | 0.043 ± 0.021 |
| MWc (× 107 g/ml) | 4.05 | 5.21 | 7.23 | 3.53 | 4.54 | 9.31 |
Abbreviation: ND, not determined.
Detected by luminescence emission.
Determined by densitometry using Kodak In Vivo imaging system F software analysis.
As determined using the Mark–Houwink–Sakurada equation.
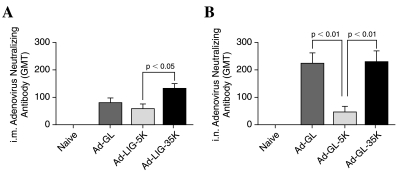
Serum samples were collected 3 weeks after priming with Ad-GL. All vectors generated Ad-neutralizing titers greater than 50 by the intramuscular route (Fig. 5A). The highest levels were produced by the 35-kDa PEG-modified vector and the lowest by the 5-kDa PEGylated vector. By the intranasal route, both unmodified and 35-kDa PEG-modified Ad generated high levels of anti-Ad antibodies (Fig. 5B). In contrast, the 5-kDa PEGylated vector produced 4-fold lower neutralizing antibodies (p < 0.01 for both by ANOVA). These data are consistent with neutralizing antibodies generated by Ad-LIG (Fig. 3).
FIG. 5.
Anti-Ad neutralizing antibodies before boosting. Geometric mean titers (GMTs) were determined as the reciprocal dilution of sera to inhibit 90% luciferase activity as compared with the control. Data are shown as the mean GMT from 20 mice. Error bars show the standard error associated with this mean. Statistical analysis was performed by one-way ANOVA.
Five weeks after priming with buffer or Ad-GL, each group of 20 mice was divided into groups of 5 and these were boosted with either buffer, Ad-gag, Ad-gag-5K, or Ad-gag-35K by the same route as was used at priming (i.e., intranasal and then intranasal or intramuscular and then intramuscular). Two weeks after boost, the resulting T cell immune responses against both transgenes were detected by ELISPOT (Figs. 6 and 7). GFP T cell responses generated by priming were measured 7 weeks after Ad-GL immunization (Fig. 6A and B). Gag T cell responses generated at boost were measured 2 weeks after Ad-gag immunization.
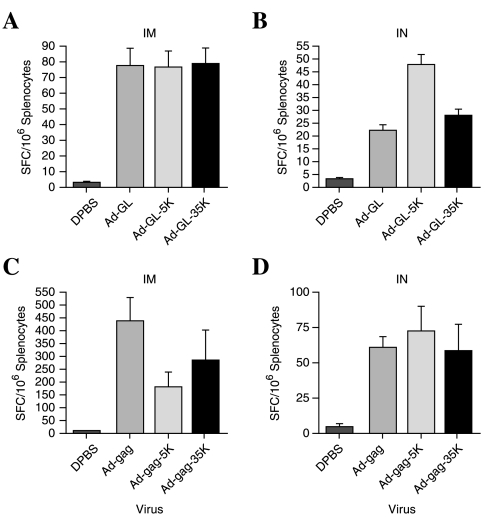
FIG. 6.
Cellular immune responses mediated by unmodified and PEGylated Ad immunization of naive mice. Anti-GFP T cell responses 7 weeks after intramuscular and intranasal immunization were measured by ELISPOT and are shown in (A) and (B) (n = 20). Anti-Gag ELISPOT responses 2 weeks after intramuscular and intranasal immunization are shown in (C) and (D) (n = 5). Bars indicate the standard error.
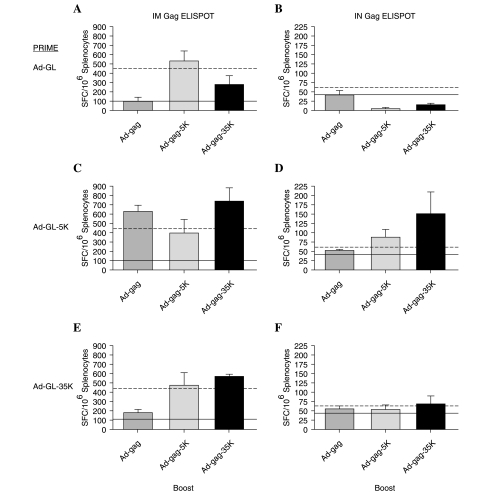
FIG. 7.
Cellular immune responses induced in mice with preexisting anti-Ad immunity. Anti-Gag T cell responses were measured 2 weeks after immunization with Ad-gag in mice that were previously primed with unmodified or PEG-modified Ad-GL. Anti-Gag immune responses induced by intramuscular and intranasal boosting in mice previously immunized with unmodified Ad-GL are shown in (A) and (B), respectively. The anti-Gag immune response in mice that were primed with Ad-GL-5K is shown in (C) and (D). The anti-Gag immune response in mice that were primed with Ad-GL-35K is shown in (E) and (F). The dotted line indicates the immune responses induced with unmodified Ad-gag in naive mice from Fig. 6C and D. The solid line represents the anti-Gag immune responses induced with unmodified Ad-gag in the presence of preexisting anti-Ad immunity. Data are shown as the mean ELISPOT numbers from five mice. Error bars show the standard error.
Figure 6 represents T cell responses against GFP and Gag in mice that had no prior exposure to Ad. For GFP, this corresponds to all the mice that were primed originally. For Gag, this corresponds to the mice that received only buffer at priming (i.e., no virus) and then were immunized with Ad-gag vectors. Figure 7 represents T cell responses generated against Gag in mice that were already immunized with Ad-GL, Ad-GL-5K, or Ad-GL-35K. Figure 7 therefore represents how well each of the vectors mediates immunization in the presence of preexisting anti-Ad immunity that was established during the original prime with each of the Ad vectors.
There were no statistically significant differences in T cell responses against GFP between the vectors when they were delivered intramuscularly into mice with no prior immunity to Ad (n = 20; Fig. 6A). By the intranasal route, Ad-GL-5K generated significantly higher anti-GFP responses than both unmodified Ad and the Ad-GL-35K (n = 20; p ≤ 0.001) (Fig. 6B). This was interesting given that this vector also generated the lowest levels of neutralizing antibodies against Ad by the intranasal route. Anti-Gag T cell responses in naive mice were highest with the unmodified vector after intramuscular injection, but these responses were not significantly higher than those generated with the PEGylated virus (n = 5; Fig. 6C). By the intranasal route, Gag responses in naive animals were all substantially lower than by the intramuscular route (n = 5; Fig. 6D). The pattern of responses after intranasal immunization against Gag was similar to those against GFP. These were not significantly different, perhaps because of the lower n value of the group, the low-level responses, or the increased resting time for GFP responses as compared with the Gag T cell responses (7 vs. 2 weeks, respectively).
Effect of PEG modification on T cell responses against Gag in the presence of preexisting anti-adenoviral immunity
To test whether PEG could shield Ad vaccines from antibodies after systemic and mucosal immunization, animals primed with unmodified and PEGylated Ad-GL were immunized with unmodified and PEGylated Ad-gag by the same routes. T cell responses against Gag were assessed 2 weeks later (Fig. 7). Listed to the left of each panel is the virus that was used for priming. The dotted line in each panel shows the benchmark level of anti-Gag T cell responses generated in Fig. 6 with Ad-gag in naive mice. Each solid line represents the anti-Gag T cell response induced by unmodified Ad-gag in mice with preexisting immunity, a real-world example of virus-vectored vaccines.
Intramuscular priming and boosting
In mice preimmunized with unmodified Ad-GL, both the Ad-gag-5K and Ad-gag-35K viruses induced higher T cell responses against Gag than unmodified Ad-gag. Reciprocally, Gag T cell responses generated by Ad-gag were higher in mice primed with Ad-GL-5K or Ad-GL-35K than in mice primed with unmodified Ad-GL (Fig. 7C and E). In mice primed with Ad-GL-5K, all Ad-gag vectors induced anti-Gag T cell responses that were at least equal to those produced in naive mice (Fig. 7C). Priming with Ad-GL-35K did not allow unmodified Ad-gag to generate as robust T cell responses. However, priming with Ad-GL-35K followed by either 5-kDa or 35-kDa PEG-modified Ad-gag produced anti-Gag T cell responses equal to or greater than those induced in naive mice (Fig. 7E).
Intranasal priming and boosting
T cell immune responses induced by intranasal immunization with Ad-gag, Ad-gag-5K, and Ad-gag-35K in the presence of neutralizing antibodies are shown in Fig. 7B, D, and F, respectively. Prior intranasal immunization with unmodified Ad generally resulted in lower T cell responses than were generated by Ad-gag in naive mice (Fig. 7B). In this case, PEGylation of Ad-gag afforded little protection against neutralizing antibodies. In contrast, mice primed intranasally with Ad-GL-5K or Ad-GL-35K generally produced anti-Gag T cell responses equivalent to or higher than responses generated with Ad-gag in naive mice (Fig. 7D and F). The highest level of Gag T cell responses after intranasal immunization was induced with the Ad-GL-5K/Ad-gag-35K combination, possibly because of the use of heterologous PEG (Fig. 7D).
Discussion
One of the most common criticisms of using Ad as a viral vector for vaccine development is the fact that, depending on the region, 25–50% of the population has preexisting Ad antibodies due to natural infection. These antibodies have long been anticipated to blunt the ability of the vector to mediate efficient transduction and, by extension, efficient immunization against the transgene product. An additional negative role for preexisting immune responses against Ad has been speculated in the failure of the anti-HIV STEP trial, in which higher levels of anti-Ad immunity appeared to increase the likelihood of infection with HIV, rather than prevent it (Sekaly, 2008). Given these issues, efforts to evade preexisting and vector-induced immunity to Ad5 and other adenoviral vectors may be crucial in applying these as vaccines in humans.
We have investigated the use of PEG to modify or shield Ad from preexisting or vector-induced immune responses and to reduce the induction of new anti-vector immune responses. We show that the PEGylated viruses have significantly larger effective diameters and their predicted molecular weight has been increased up to 3-fold. PEGylated Ad appears “dead” in vitro; however, the modified viruses are capable of transduction and transgene expression as shown by in vivo imaging. This dichotomy between in vitro and in vivo transduction by PEGylated Ad is consistent with prior observations of PEGylated Ad applied by intravenous and intraperitoneal injection (Mok et al., 2005; Hofherr et al., 2007, 2008).
The effects of PEGylation were explored in the context of performing systemic immunization by the intramuscular route and by performing mucosal immunization by the intranasal route. Interestingly, the various PEG modifications had different effects on Ad-mediated immune responses. By the intramuscular route, all PEGs reduced transduction. This reduced transduction correlated with reduced antibody responses against the luciferase protein product after priming. Priming with all the vectors generated similar levels of anti-Ad neutralizing antibodies by the intramuscular route. After the second administration of unmodified Ad in the face of these neutralizing responses, antibodies against luciferase failed to boost. In contrast, boosting with the PEGylated vectors did boost luciferase antibody to levels similar to those produced with unmodified Ad. These data suggest that PEGylated vectors may evade these neutralizing responses better than unmodified Ad, to retain a better ability to boost immune responses. Alternatively, the lack of boost by unmodified Ad may be due to its having already achieved the maximal level of anti-luciferase antibodies after priming. Or, the high level of anti-luciferase antibodies in that group might have quenched the boost by absorbing all released antigen. Although these alternatives are possible, the relatively low antibody levels against luciferase suggest that these explanations may not apply.
When T cell responses were monitored against GFP and Gag after intramuscular immunization, the PEGylated vectors produced equal to slightly lower responses after priming compared with unmodified Ad. GFP T cell responses measured from 20 animals were essentially identical by unmodified and PEGylated vectors by the intramuscular route. These data suggest that lower transduction by the PEGylated vectors may affect antibody responses, because these generally require large amounts of antigen delivered, but not T cell responses, which may require a lower threshold of protein production for priming. When animals were boosted intramuscularly in the presence of anti-Ad immunity, the PEGylated vectors generated superior T cell responses than the unmodified vector. The simplest comparison is observed in mice primed with unmodified Ad-GL. In this case of simple preexisting immunity to Ad5, 5-kDa PEG generated higher anti-Gag T cell responses than either unmodified or 35-kDa PEG-modified Ad-gag viruses. Similarly, the 5-kDa PEGylated vector appeared to be a better T cell-priming agent by the intramuscular route and retained or generated higher anti-Gag T cell responses regardless of which virus was used for boosting. In summary for the intramuscular route, PEGylation modestly reduced priming responses, but 5-kDa PEGylation appeared to be generally the most optimal modification for priming or boosting when more than one immunization was performed.
Ad PEGylation had different effects when the viruses were used for mucosal intranasal immunization. Priming with 5- and 20-kDa PEGs generated weak transduction and weak anti-luciferase antibody responses. This is consistent with their effects on in vitro transduction and may be due to inhibition of CAR binding. This effect may be more pronounced by the intranasal route than by the intramuscular route, because intranasal administration bathes the surface of the mucosa with virus in a manner similar to that in a cell culture dish. In contrast, intramuscular injection delivers the virus under pressure and traps the virus in the enclosed muscle bed, where perhaps integrin interactions are enhanced by the virus.
Modification of Ad with the “naked” 35-kDa PEG lacking sugar moieties gave somewhat intermediate effects. It gave similar to lower transduction after intranasal administration than unmodified Ad 1 day after injection and its expression did not increase over time, like the sugar-modified 35-kDa PEGs. This intermediate expression profile reduced antibody priming, but was sufficient to give a better boost than the 5- or 20-kDa PEG-modified vectors. The sugar-modified 35-kDa PEGs, surprisingly, increased intranasal gene expression. These differences in expression correlated well with anti-luciferase antibody responses. Priming antibody levels generated by both 35K-Gal and 35K-Glu were similar to that of unmodified Ad and boosting with Ad-35K-Glu generated similar anti-luciferase antibodies as compared with unmodified Ad. The effects of the sugar moieties by the intranasal, but not the intramuscular, route suggest they may affect vector targeting to antigen-presenting cells or mucosa (Erbacher et al., 1996; Fajac et al., 1999; Parrott et al., 2003). Alternatively, they may affect vector stability or pharmacology. Given that these sugar modifications overcome the deficiencies of the unmodified PEGs after intranasal immunization, they merit further testing.
When T cell responses were explored after intranasal immunization, priming responses were equal to or higher for PEGylated vectors versus unmodified Ad despite the fact that this modification reduced transduction and antibody responses. This could again be due a lower threshold of antigen expression being needed to prime T cell versus antibody responses. After priming with unmodified Ad, the PEGylated vectors were, surprisingly, less able to generate anti-Gag responses by the intranasal route. It is possible that the combination of reduced CAR binding and neutralization may simply push transduction below the needed threshold of T cell priming. In contrast, when the 5-kDa PEGylated vector was used for priming by the intranasal route, boosting T cell responses with unmodified Ad-gag were as good as those produced in naive mice. Indeed, priming with the 5-kDa PEGylated Ad enabled all of the boosting vectors to generate anti-Gag T cell responses that were equal to or even higher than those generated in naive animals. This effect may be due to the significantly lower production of Ad-neutralizing antibody responses with the 5-kDa PEG. Whether 5-kDa PEG effects extend to reducing anti-Ad T cell responses remains to be demonstrated in a better model. Priming with 35K-modified Ad allowed T cell responses equal to those in naive animals with all the vectors. These data suggest that an optimal approach for intranasal immunization for T cell responses may involve priming and boosting with the same or different PEGylated vectors. It is possible that combined PEGylation with 5 kDa PEG for shielding and sugar-targeted PEGs on one vector may enhance both prime and boost immunization by the intranasal route.
Our attempts to test ELISPOT responses against Ad failed with the peptides that were available. Therefore, it is unclear whether prior immunization with Ad attenuates boosting because of effects on neutralizing antibodies or on Ad-directed T cell responses. It is therefore possible that PEG may mediate its beneficial effects by protection against either antibody or T cell responses or both. Given the potential problem of vector-directed immune responses in the STEP trial, teasing out the effects of PEG on these responses will be an important area of exploration.
In summary, we have shown that PEGylation of Ad5 mediates significant boosting of antibody and T cell responses in the face of prior immunity to Ad5. We also show that priming with PEGylated vectors can enable unmodified and PEGylated vectors to produce stronger immune responses than are produced after priming with unmodified Ad. On the basis of this, PEGylation of Ad5 and other Ad serotypes may have utility for immunoevasion, is applicable for use in vaccine development, and shows promise for readministration in naive animals, immune animals, and, perhaps, in humans.
Acknowledgments
The authors thank Mary E. Barry and Jenica Schwegel for excellent technical assistance. This study was supported by grant R01 AI067095 to M.A.B. from the National Institutes of Health. HIV and SIV reagents were obtained from the NIH AIDS Research and Reagent Program.
Author Disclosure Statement
E.W. and M.B. have no competing financial interests.
References
- Andersson H.A. Barry M.A. Maximizing antigen targeting to the proteasome for gene-based vaccines. Mol. Ther. 2004;10:432–446. doi: 10.1016/j.ymthe.2004.05.035. [DOI] [PubMed] [Google Scholar]
- Andersson H.A. Passeri M.F. Barry M.A. Rad23 as a reciprocal agent for stimulating or repressing immune responses. Hum. Gene Ther. 2005;16:634–641. doi: 10.1089/hum.2005.16.634. [DOI] [PubMed] [Google Scholar]
- Barouch D.H. Pau M.G. Custers J.H. Koudstaal W. Kostense S. Havenga M.J. Truitt D.M. Sumida S.M. Kishko M.G. Arthur J.C. Korioth-Schmitz B. Newberg M.H. Gorgone D.A. Lifton M.A. Panicali D.L. Nabel G.J. Letvin N.L. Goudsmit J. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 2004;172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- Crawford-Miksza L. Schnurr D.P. Analysis of 15 adenovirus hexon proteins reveals the location and structure of seven hypervariable regions containing serotype-specific residues. J. Virol. 1996;70:1836–1844. doi: 10.1128/jvi.70.3.1836-1844.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croyle M.A. Chirmule N. Zhang Y. Wilson J.M. “Stealth” adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lung. J. Virol. 2001;75:4792–4801. doi: 10.1128/JVI.75.10.4792-4801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croyle M.A. Chirmule N. Zhang Y. Wilson J.M. PEGylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver. Hum. Gene Ther. 2002;13:1887–1900. doi: 10.1089/104303402760372972. [DOI] [PubMed] [Google Scholar]
- Croyle M.A. Le H.T. Linse K.D. Cerullo V. Toietta G. Beaudet A. Pastore L. PEGylated helper-dependent adenoviral vectors: Highly efficient vectors with an enhanced safety profile. Gene Ther. 2005;12:579–587. doi: 10.1038/sj.gt.3302441. [DOI] [PubMed] [Google Scholar]
- Erbacher P. Bousser M.T. Raimond J. Monsigny M. Midoux P. Roche A.C. Gene transfer by DNA/glycosylated polylysine complexes into human blood monocyte-derived macrophages. Hum. Gene Ther. 1996;7:721–729. doi: 10.1089/hum.1996.7.6-721. [DOI] [PubMed] [Google Scholar]
- Fajac I. Briand P. Monsigny M. Midoux P. Sugar-mediated uptake of glycosylated polylysines and gene transfer into normal and cystic fibrosis airway epithelial cells. Hum. Gene Ther. 1999;10:395–406. doi: 10.1089/10430349950018841. [DOI] [PubMed] [Google Scholar]
- Fields P.A. Kowalczyk D.W. Arruda V.R. Armstrong E. McCleland M.L. Hagstrom J.N. Pasi K.J. Ertl H.C.J. Herzog R.W. High K.A. Role of vector in activation of T cell subsets in immune responses against the secreted transgene product factor IX. Mol. Ther. 2000;1:225–235. doi: 10.1006/mthe.2000.0032. [DOI] [PubMed] [Google Scholar]
- Gomez-Roman V.R. Grimes G.J., Jr. Potti G.K. Peng B. Demberg T. Gravlin L. Treece J. Pal R. Lee E.M. Alvord W.G. Markham P.D. Robert-Guroff M. Oral delivery of replication-competent adenovirus vectors is well tolerated by SIV- and SHIV-infected rhesus macaques. Vaccine. 2006;24:5064–5072. doi: 10.1016/j.vaccine.2006.03.048. [DOI] [PubMed] [Google Scholar]
- Hofherr S.E. Mok S. Gushiken F.C. Lopez J.A. Barry M.A. Polyethylene glycol modification of adenovirus reduces platelet activation, endothelial cell activation, and thrombocytopenia. Hum. Gene Ther. 2007;18:837–848. doi: 10.1089/hum.2007.0051. [DOI] [PubMed] [Google Scholar]
- Hofherr S.E. Shashkova E.V. Weaver E.A. Khare R. Barry M.A. Modification of adenoviral vectors with polyethylene glycol modulates in vivo tissue tropism and gene expression. Mol. Ther. 2008;16:1276–1282. doi: 10.1038/mt.2008.86. [DOI] [PubMed] [Google Scholar]
- Lubeck M.D. Natuk R. Myagkikh M. Kalyan N. Aldrich K. Sinangil F. Alipanah S. Murthy S.C. Chanda P.K. Nigida S.M., Jr. Markham P.D. Zolla-Pazner S. Steimer K. Wade M. Reitz M.S., JR. Arthur L.O. Mizutani S. Davis A. Hung P.P. Gallo R.C. Eichberg J. Robert-Guroff M. Long-term protection of chimpanzees against high-dose HIV-1 challenge induced by immunization. Nat. Med. 1997;3:651–658. doi: 10.1038/nm0697-651. [DOI] [PubMed] [Google Scholar]
- Mok H. Palmer D.J. Ng P. Barry M.A. Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol. Ther. 2005;11:66–79. doi: 10.1016/j.ymthe.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Ogawara K. Rots M.G. Kok R.J. Moorlag H.E. van Loenen A.M. Meijer D.K. Haisma H.J. Molema G. A novel strategy to modify adenovirus tropism and enhance transgene delivery to activated vascular endothelial cells in vitro and in vivo. Hum. Gene Ther. 2004;15:433–443. doi: 10.1089/10430340460745766. [DOI] [PubMed] [Google Scholar]
- O'Riordan C.R. Lachapelle A. Delgado C. Parkes V. Wadsworth S.C. Smith A.E. Francis G.E. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum. Gene Ther. 1999;10:1349–1358. doi: 10.1089/10430349950018021. [DOI] [PubMed] [Google Scholar]
- Parrott M.B. Adams K.E. Mercier G.T. Mok H. Campos S.K. Barry M.A. Metabolically biotinylated adenovirus for cell-targeting, ligand screening, and vector purification. Mol. Ther. 2003;8:689–702. doi: 10.1016/s1525-0016(03)00213-2. [DOI] [PubMed] [Google Scholar]
- Roberts D.M. Nanda A. Havenga M.J. Abbink P. Lynch D.M. Ewald B.A. Liu J. Thorner A.R. Swanson P.E. Gorgone D.A. Lifton M.A. Lemckert A.A. Holterman L. Chen B. Dilraj A. Carville A. Mansfield K.G. Goudsmit J. Barouch D.H. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–243. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- Schnell M.A. Zhang Y. Tazelaar J. Gao G.P. Yu Q.C. Qian R. Chen S.J. Varnavski A.N. Leclair C. Raper S.E. Wilson J.M. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol. Ther. 2001;3:708–722. doi: 10.1006/mthe.2001.0330. [DOI] [PubMed] [Google Scholar]
- Sekaly R.P. The failed HIV Merck vaccine study: A step back or a launching point for future vaccine development? J. Exp. Med. 2008;205:7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprangers M.C. Lakhai W. Koudstaal W. Verhoeven M. Koel B.F. Vogels R. Goudsmit J. Havenga M.J. Kostense S. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: Addressing preexisting immunity to vaccine and gene therapy vectors. J. Clin. Microbiol. 2003;41:5046–5052. doi: 10.1128/JCM.41.11.5046-5052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortmann A. Vohringer S. Engler T. Corjon S. Schirmbeck R. Reimann J. Kochanek S. Kreppel F. Fully detargeted polyethylene glycol-coated adenovirus vectors are potent genetic vaccines and escape from pre-existing anti-adenovirus antibodies. Mol. Ther. 2008;16:154–162. doi: 10.1038/sj.mt.6300306. [DOI] [PubMed] [Google Scholar]
- Xiang Z.Q. Pasquini S. Ertl H.C. Induction of genital immunity by DNA priming and intranasal booster immunization with a replication-defective adenoviral recombinant. J. Immunol. 1999;162:6716–6723. [PubMed] [Google Scholar]
- Yang Y. Ertl H.C.J. Wilson J.M. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice injected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]