Abstract
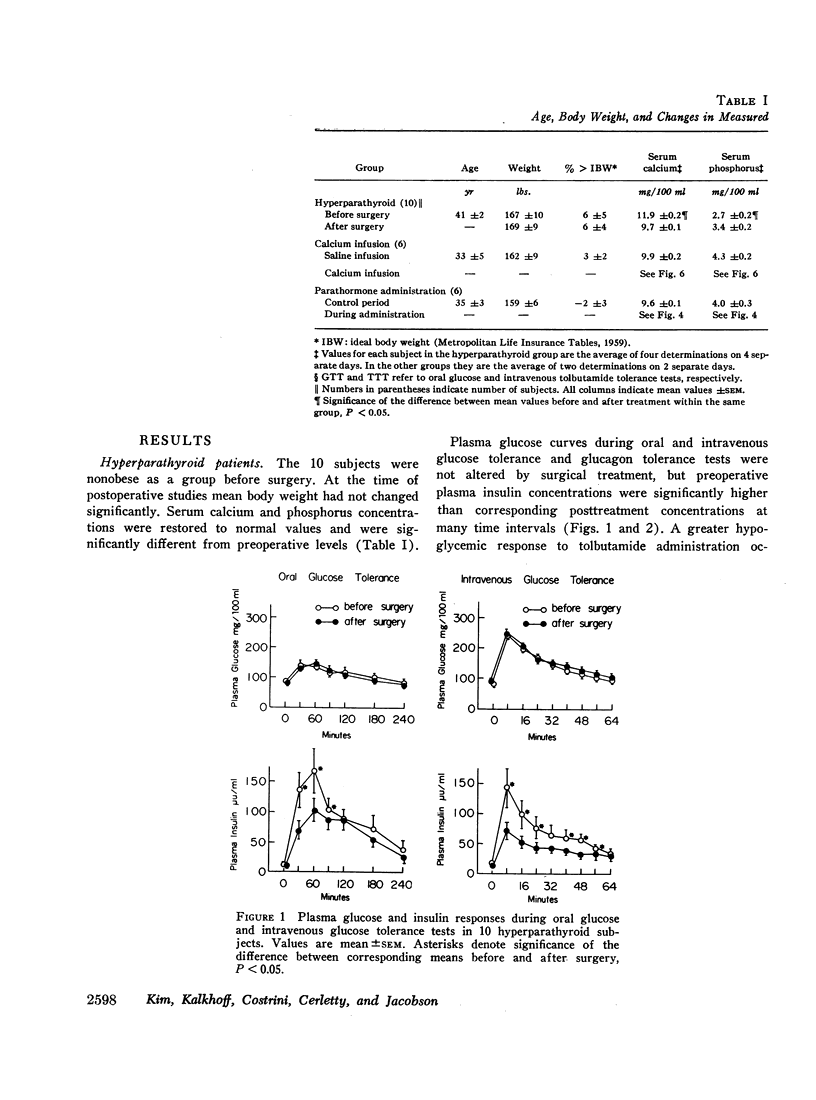
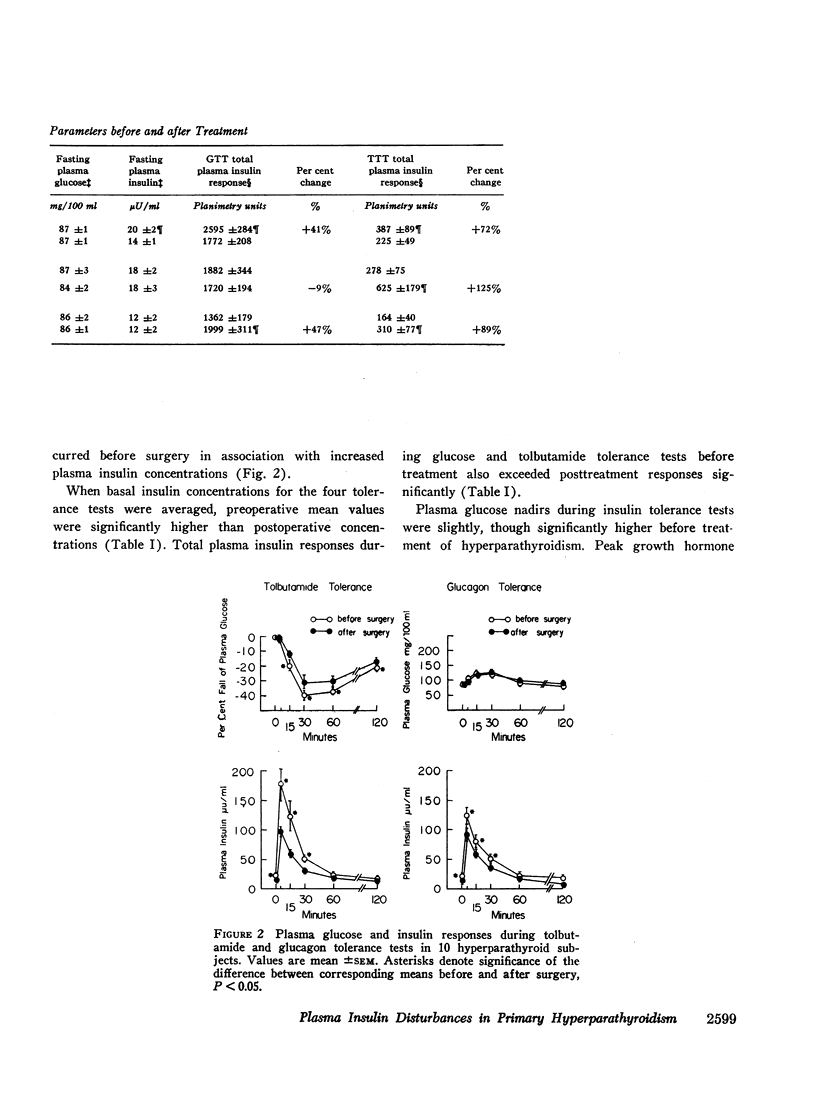
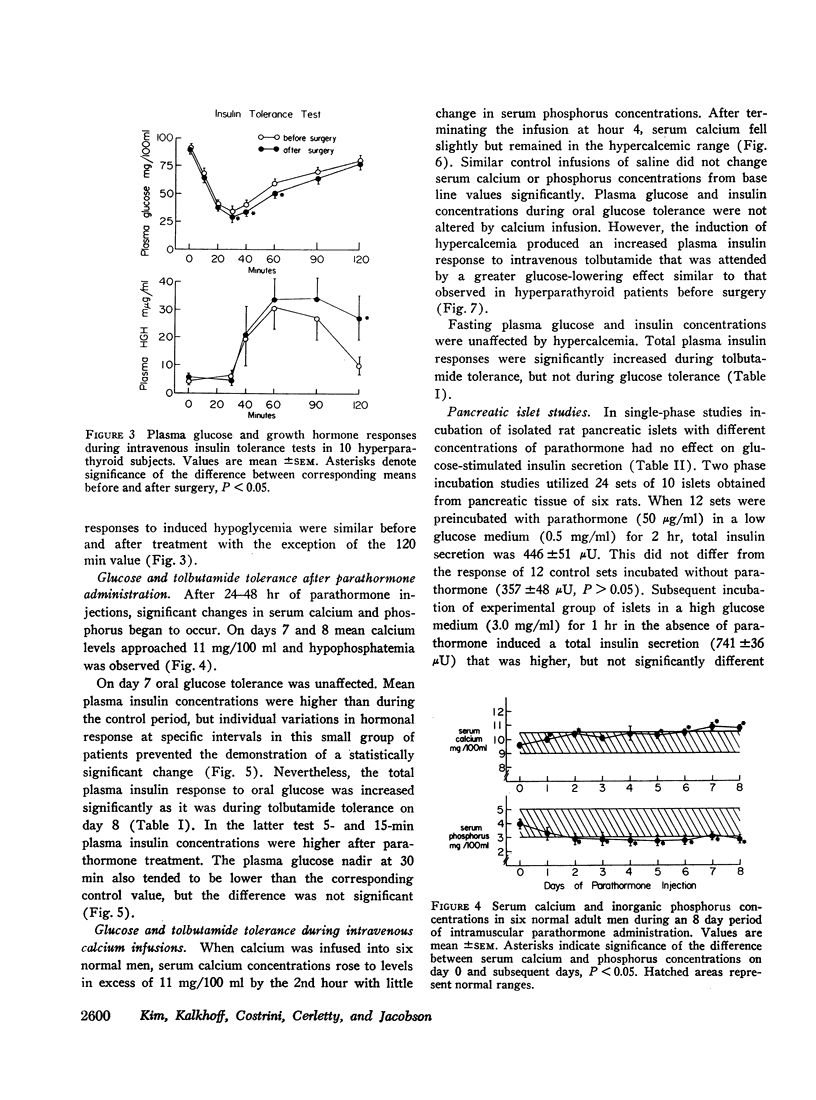
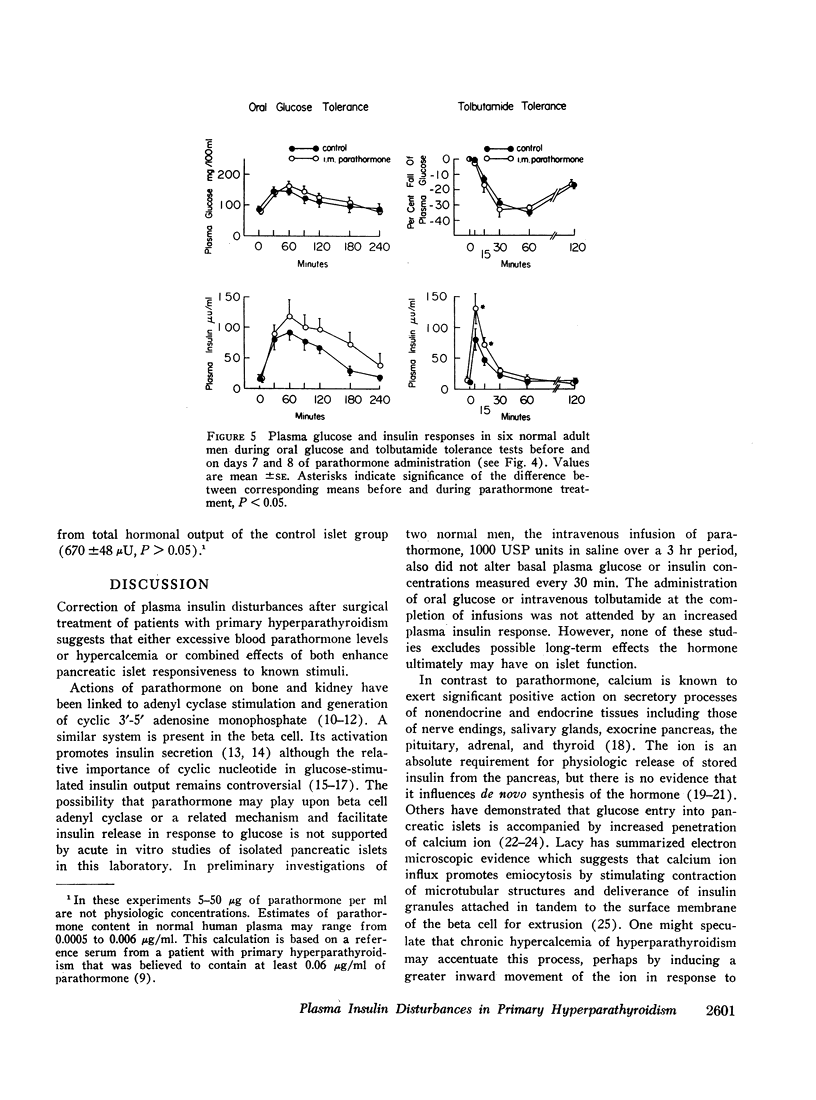
Plasma insulin dynamics were evaluated in 10 patients with primary hyperparathyroidism before and after parathyroidectomy and correction of hypercalcemia. Before surgery fasting plasma insulin concentrations and insulin responses to administered glucose, tolbutamide, and glucagon were significantly greater than postoperative values. Hyperinsulinemia was not associated with altered glucose curves during glucose or glucagon tolerance tests, but a relatively greater insulin response to tolbutamide resulted in an increased hypoglycemic effect following its administration. The glucose-lowering action of intravenous insulin was slightly impaired before treatment. Intramuscular injections of parathormone to six normal men for 8 days induced mild hypercalcemia and hypophosphatemia and reproduced augmented plasma insulin responses to oral glucose and intravenous tolbutamide. 4-hr intravenous infusions of calcium to another group of six normal men raised serum calcium concentrations above 11 mg/100 ml. This did not alter glucose or insulin curves during oral glucose tolerance but markedly accentuated insulin responses to tolbutamide and potentiated its hypoglycemic effect. When highly purified parathormone was incubated with isolated pancreatic islets of male rats, glucose-stimulated insulin secretion was unaffected.
These findings suggest that chronic hypercalcemia of hyperparathyroidism sustains a form of endogenous insulin resistance that necessitates augmented insulin secretion to maintain plasma glucose homeostasis. This state is insufficient to oppose tolbutamide-induced hypoglycemia because of an additional direct, selective enhancement of hypercalcemia on pancreatic beta cell responsiveness to the sulfonylurea. The possible direct role of parathormone in these events has not been established.
Full text
PDF

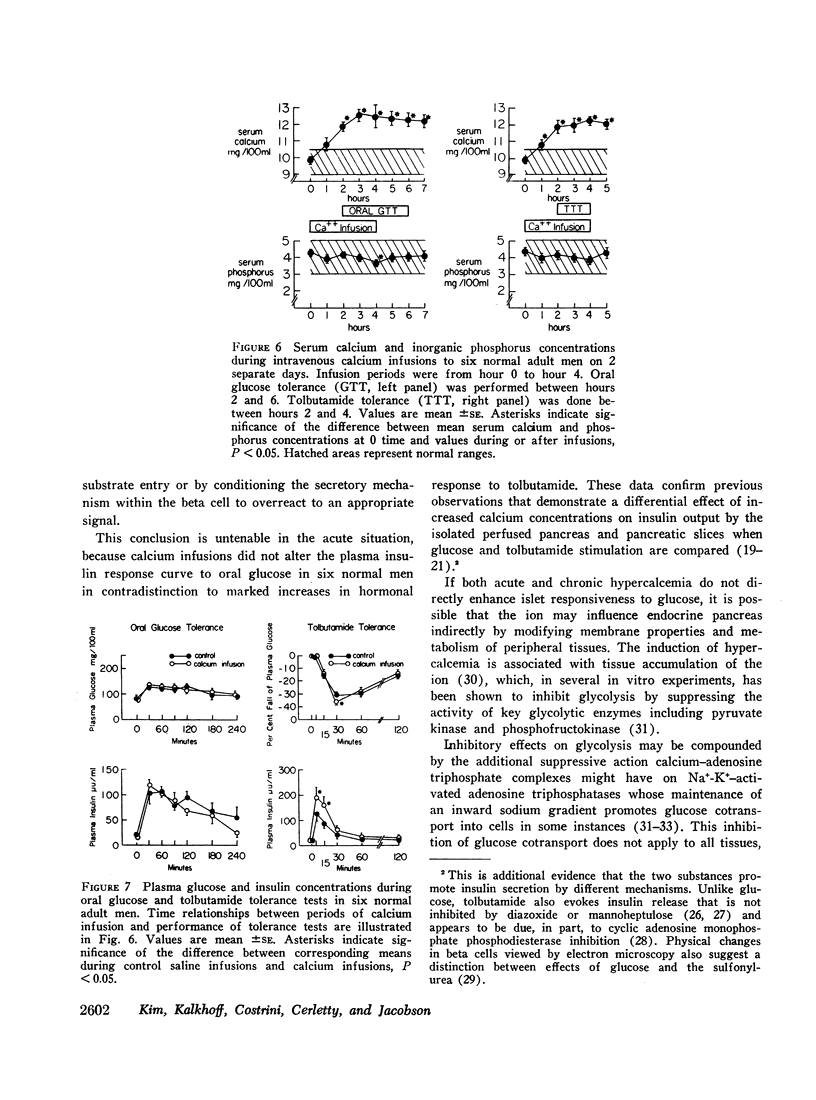
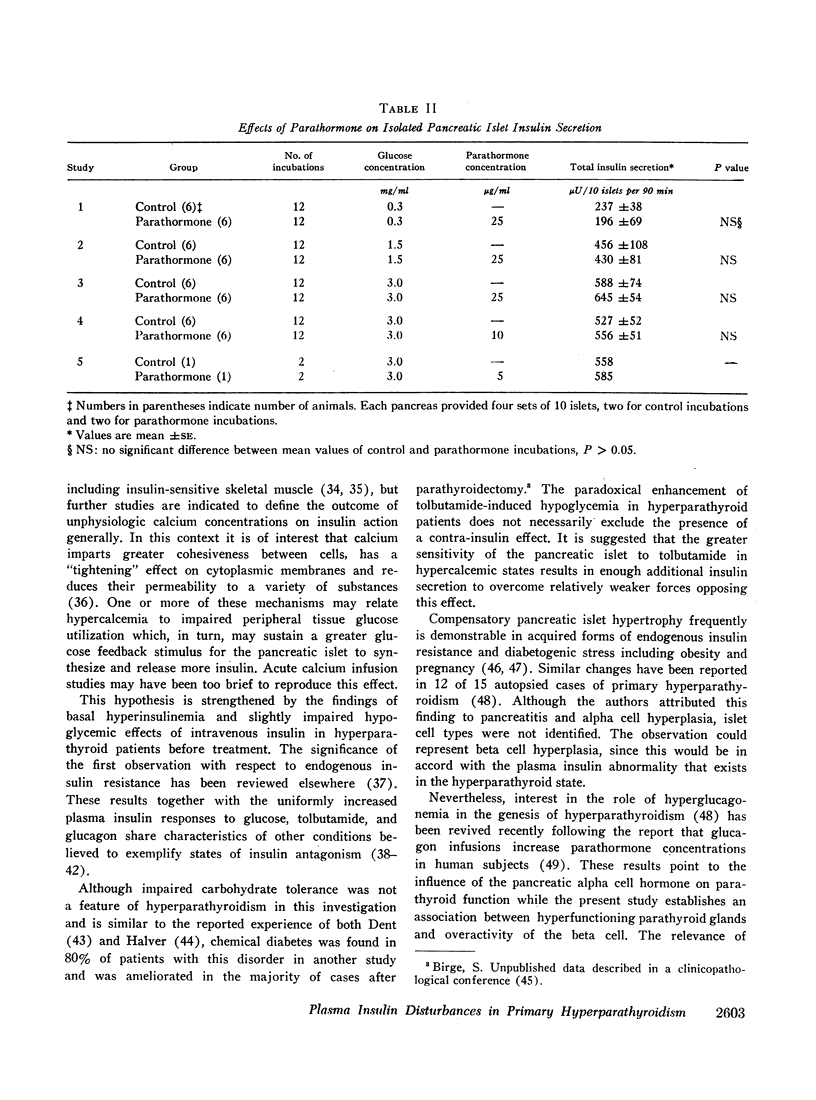


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALLARD H. S., FAME B., HARTSOCK R. J. FAMILIAL MULTIPLE ENDOCRINE ADENOMA-PEPTIC ULCER COMPLEX. Medicine (Baltimore) 1964 Jul;43:481–516. [PubMed] [Google Scholar]
- Berson S. A., Yalow R. S. Parathyroid hormone in plasma in adenomatous hyperparathyroidism, uremia, and bronchogenic carcinoma. Science. 1966 Nov 18;154(3751):907–909. doi: 10.1126/science.154.3751.907. [DOI] [PubMed] [Google Scholar]
- Bihler I., Sawh P. C. The effect of alkali metal ions on sugar transport in muscle: interaction with the sugar carrier or indirect effect. Biochim Biophys Acta. 1971 Jan 5;225(1):56–63. doi: 10.1016/0005-2736(71)90283-5. [DOI] [PubMed] [Google Scholar]
- Bygrave F. L. The ionic environment and metabolic control. Nature. 1967 May 13;214(5089):667–671. doi: 10.1038/214667a0. [DOI] [PubMed] [Google Scholar]
- Chase L. R., Aurbach G. D. Parathyroid function and the renal excretion of 3'5'-adenylic acid. Proc Natl Acad Sci U S A. 1967 Aug;58(2):518–525. doi: 10.1073/pnas.58.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase L. R., Fedak S. A., Aurbach G. D. Activation of skeletal adenyl cyclase by parathyroid hormone in vitro. Endocrinology. 1969 Apr;84(4):761–768. doi: 10.1210/endo-84-4-761. [DOI] [PubMed] [Google Scholar]
- Clinicopathologic conference. Multiple endocrine adenomatosis. Am J Med. 1969 Oct;47(4):608–618. [PubMed] [Google Scholar]
- Coore H. G., Randle P. J. Regulation of insulin secretion studied with pieces of rabbit pancreas incubated in vitro. Biochem J. 1964 Oct;93(1):66–78. doi: 10.1042/bj0930066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costrini N. V., Kalkhoff R. K. Relative effects of pregnancy, estradiol, and progesterone on plasma insulin and pancreatic islet insulin secretion. J Clin Invest. 1971 May;50(5):992–999. doi: 10.1172/JCI106593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane R. K. Na+ -dependent transport in the intestine and other animal tissues. Fed Proc. 1965 Sep-Oct;24(5):1000–1006. [PubMed] [Google Scholar]
- Curry D. L., Bennett L. L., Grodsky G. M. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology. 1968 Sep;83(3):572–584. doi: 10.1210/endo-83-3-572. [DOI] [PubMed] [Google Scholar]
- Curry D. L., Bennett L. L., Grodsky G. M. Requirement for calcium ion in insulin secretion by the perfused rat pancreas. Am J Physiol. 1968 Jan;214(1):174–178. doi: 10.1152/ajplegacy.1968.214.1.174. [DOI] [PubMed] [Google Scholar]
- DENT C. E. Some problems of hyperparathyroidism. Br Med J. 1962 Dec 1;2(5317):1419–1425. doi: 10.1136/bmj.2.5317.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughaday W. H., Kipnis D. M. The growth-promoting and anti-insulin actions of somatotropin. Recent Prog Horm Res. 1966;22:49–99. doi: 10.1016/b978-1-4831-9825-5.50005-1. [DOI] [PubMed] [Google Scholar]
- Epstein F. H., Whittam R. The mode of inhibition by calcium of cell-membrane adenosine-triphosphatase activity. Biochem J. 1966 Apr;99(1):232–238. doi: 10.1042/bj0990232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL J. B., KESSLER G. An automated determination of glucose utilizing a glucose oxidase-peroxidase system. J Lab Clin Med. 1961 Jun;57:970–980. [PubMed] [Google Scholar]
- Hales C. N., Milner R. D. Cations and the secretion of insulin from rabbit pancreas in vitro. J Physiol. 1968 Nov;199(1):177–187. doi: 10.1113/jphysiol.1968.sp008647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halver B. Glucose metabolism in parathyroid disease. Acta Med Scand. 1967 Dec;182(6):737–740. doi: 10.1111/j.0954-6820.1967.tb10902.x. [DOI] [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. Effects of diazoxide on insulin secretion in vitro. Lancet. 1966 Jan 15;1(7429):128–129. doi: 10.1016/s0140-6736(66)91263-3. [DOI] [PubMed] [Google Scholar]
- KARAM J. H., GRODSKY G. M., FORSHAM P. H. Excessive insulin response to glucose in obese subjects as measured by immunochemical assay. Diabetes. 1963 May-Jun;12:197–204. doi: 10.2337/diab.12.3.197. [DOI] [PubMed] [Google Scholar]
- KREBS H. A. Body size and tissue respiration. Biochim Biophys Acta. 1950 Jan;4(1-3):249–269. doi: 10.1016/0006-3002(50)90032-1. [DOI] [PubMed] [Google Scholar]
- Kipnis D. M., Parrish J. E. Role of Na+ and K+ on sugar (2-deoxyglucose) and amino acid (alpha-aminoisobutyric acid) transport in striated muscle. Fed Proc. 1965 Sep-Oct;24(5):1051–1059. [PubMed] [Google Scholar]
- Lacy P. E. Beta cell secretion--from the standpoint of a pathobiologist. Diabetes. 1970 Dec;19(12):895–905. doi: 10.2337/diab.19.12.895. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Malaisse-Lagae F., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. 3. Uptake of 45 calcium by isolated islets of Langerhans. Endocrinology. 1971 Jan;88(1):72–80. doi: 10.1210/endo-88-1-72. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Brisson G., Malaisse-Lagae F. The stimulus-secretion coupling of glucose-induced insulin release. I. Interaction of epinephrine and alkaline earth cations. J Lab Clin Med. 1970 Dec;76(6):895–902. [PubMed] [Google Scholar]
- Manery J. F. Effects of Ca ions on membranes. Fed Proc. 1966 Nov-Dec;25(6):1804–1810. [PubMed] [Google Scholar]
- Matschinsky F. M., Ellerman J. E., Krzanowski J., Kotler-Brajtburg J., Landgraf R., Fertel R. The dual function of glucose in islets of Langerhans. J Biol Chem. 1971 Feb 25;246(4):1007–1011. [PubMed] [Google Scholar]
- Matschinsky F. M., Ellerman J. E. Metabolism of glucose in the islets of Langerhans. J Biol Chem. 1968 May 25;243(10):2730–2736. [PubMed] [Google Scholar]
- Paloyan E., Lawrence A. M., Straus F. H., 2nd, Paloyan D., Harper P. V., Cummings D. Alpha cell hyperplasia in calcific pancreatitis associated with hyperparathyroidism. JAMA. 1967 May 29;200(9):757–761. [PubMed] [Google Scholar]
- Perley M., Kipnis D. M. Effect of glucocorticoids on plasma insulin. N Engl J Med. 1966 Jun 2;274(22):1237–1241. doi: 10.1056/NEJM196606022742205. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Pechet M., Fast D. Effect of dibutyryl cyclic adenosine 3',5'-monophosphate, theophylline, and other nucleotides upon calcium and phosphate metabolism. J Clin Invest. 1968 Aug;47(8):1843–1850. doi: 10.1172/JCI105874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- SCHALCH D. S., PARKER M. L. A SENSITIVE DOUBLE ANTIBODY IMMUNOASSAY FOR HUMAN GROWTH HORMONE IN PLASMA. Nature. 1964 Sep 12;203:1141–1142. doi: 10.1038/2031141a0. [DOI] [PubMed] [Google Scholar]
- SPELLACY W. N., GOETZ F. C. Plasma insulin in normal late pregnancy. N Engl J Med. 1963 May 2;268:988–991. doi: 10.1056/NEJM196305022681805. [DOI] [PubMed] [Google Scholar]
- Turtle J. R., Littleton G. K., Kipnis D. M. Stimulation of insulin secretion by theophylline. Nature. 1967 Feb 18;213(5077):727–728. doi: 10.1038/213727a0. [DOI] [PubMed] [Google Scholar]
- WALLACH S., BELLAVIA J. V., SCHORR J., REIZENSTEIN D. L. TISSUE DISTRIBUTION OF ELECTROLYTES, CA47, AND MG28 IN ACUTE HYPERCALCEMIA. Am J Physiol. 1964 Sep;207:553–560. doi: 10.1152/ajplegacy.1964.207.3.553. [DOI] [PubMed] [Google Scholar]



