Summary
The CCN proteins contain six members, namely CCN1 to CCN6, which are small secreted cysteine-rich proteins. The CCN proteins are modular proteins, containing up to four functional domains. Many of the CCN members are induced by growth factors, cytokines, or cellular stress. The CCNs show a wide and highly variable expression pattern in adult and in embryonic tissues. The CCN proteins can integrate and modulate the signals of integrins, BMPs, VEGF, Wnts, and Notch. The involvement of integrins in mediating CCN signaling may provide diverse context-dependent responses in distinct cell types. CCN1 and CCN2 play an important role in development, angiogenesis and cell adhesion, whereas CCN3 is critical to skeletal and cardiac development. CCN4, CCN5 and CCN6 usually inhibit cell growth. Mutations of Ccn6 are associated with the progressive pseudorheumatoid dysplasia and spondyloepiphyseal dysplasia tarda. In stem cell differentiation, CCN1, CCN2, and CCN3 play a principal role in osteogenesis, chondrogenesis, and angiogenesis. Elevated expression of CCN1 is associated with more aggressive phenotypes of human cancer, while the roles of CCN2 and CCN3 in tumorigenesis are tumor type-dependent. CCN4, CCN5 and CCN6 function as tumor suppressors. Although CCN proteins may play important roles in fine-tuning other major signaling pathways, the precise function and mechanism of action of these proteins remain undefined. Understanding of the biological functions of the CCN proteins would not only provide insight into their roles in numerous cellular processes but also offer opportunities for developing therapeutics by targeting CCN functions.
Keywords: CCN family, Chondrogenesis, Osteogenesis, Stem Cell Differentiation, Tumorigenesis
Introduction
The CCN (CYR61/CTGF/NOV) family is a small group of structurally similar matrix proteins composed of six members (CCN1 to CCN6). The extracellular protein products of this CCN gene family are approximately 40 kDa and regulate numerous biological processes, such as differentiation, migration, proliferation, and cell adhesion (Katsube et al., 2009). The roles of CCN proteins in cancer, injury repair, and embryonic development have led to a surge in research surrounding CCN proteins since their discoveries. The first reports of CCN proteins began in the early 1990s (Erwin, 2008). The first three CCN members that were discovered include cysteine rich 61 (CYR 61/CCN1), connective tissue growth factor (CTGF/CCN2), and nephroblastoma overexpressed (NOV/CCN3). The names of these first three proteins were used to derive the acronym CCN to establish a name for this family of proteins sharing a similar structure.
In 2003, a common nomenclature for the CCN family was proposed to simplify the numerous names that had been assigned to each multifunctional protein (Brigstock et al., 2003) (Table 1). The proteins are numbered based on the order of their discovery. For example, cysteine rich 61 is now called CCN1, which was first reported in 1991 as an early gene activated by platelet-derived growth factor in mouse fibroblasts (O’Brien et al., 1990). As the 61st gene identified it was given the name cysteine rich 61 (cyr61). CCN2 was discovered in the cDNA library of an human umbilical vein endothelial cell and was termed connective tissue growth factor (Bradham et al., 1991). In 1992, CCN3 was discovered in myeloblastosis-associated virus type 1 (MAV1)-induced nephroblastomas where it regulated cell growth (Joliot et al., 1992). Since their initial discoveries, the CCN family has added three additional members that exhibit the basic structure of CCN founding members. The three additional members are involved in the Wnt-1 inducible signaling pathway and include Wnt-1 induced secreted protein-1 (WISP-1/CCN4), Wnt-1 induced secreted protein-2 (WISP-2/CCN5), and Wnt-1 induced secreted protein-3 (WISP-3/CCN6) (Pennica et al., 1998). In this review, we use the CCN nomenclature for each of the CCN family members.
Table 1.
CCN family members and their known functions.
| CCN nomenclature | First reported | Synonym (also known as) | NM number (human/mouse) | cDNA Length (human/mouse) | Location (human/mouse) | Main functions | Knock-out Phenotypes |
|---|---|---|---|---|---|---|---|
| CCN1 | 1990 | Cyr61, CEF10, IGFBP10 | NM_001554.4/NM_010516.2 | 1146/1140 | 1p31-p22/3 H2; 3 72.9 cM | Cell migration, angiogenesis, cell adhesion, apoptosis, gastrulation, tumorigenesis | Embryonic death, duo to failure in chorioallantoic fusion, placental vascular insufficiency and compromised vessel integrity (severe atrioventricular septal defects). |
| CCN2 | 1991 | CTGF, Fisp-12, HCS24, IGFBP8 | NM_001901.2/NM_010217.2 | 1050/1047 | 6q23.1/10 A3-B1; 10 17.0 cM | Fibrogenesis, osteogenesis, chondrogenesis, angiogenesis, diabetic nephropathy, tumorigenesis | Lethal; major skeletal defects; dramatic decrease in beta-cell proliferation (at late gestation). |
| CCN3 | 1992, 1994 | Nov, IGFBP9 | NM_002514.3/NM_010930.4 | 1074/1065 | 8q24.1/15 D1; 15 22.5 cM | Angiogenesis, tumorigenesis | Enhanced chondrogenesis and osteogenesis, enlargement and abnormal modelling of the endocardial cushions; hypertrophy and calcification of the septum and left ventricle dilation; muscle atrophy; premature tissue degeneration in the lens. |
| CCN4 | 1998 | WISP1, ELM-1 | NM_003882.2, NM_080838.1/NA | 1104, 1035/NA | 8q24.1-q24.3/NA | Tumorigenesis | NA |
| CCN5 | 1998 | WISP2; CTGF-L, rCop-1 | NM_003881.2/NA | 753/NA | 20q12-q13.1/NA | Tumorigenesis | NA |
| CCN6 | 1998 | WISP3 | NM_003880.3, NM_198239.1/NA | 1065, 1119/NA | 6q21/NA | Tumorigenesis | Progressive pseudorheumatoid dysplasia were seen in CCN6 loss-of-function mutant mice, while CCN6 knockout mouse has no phenotype |
Functional domains of the CCN proteins
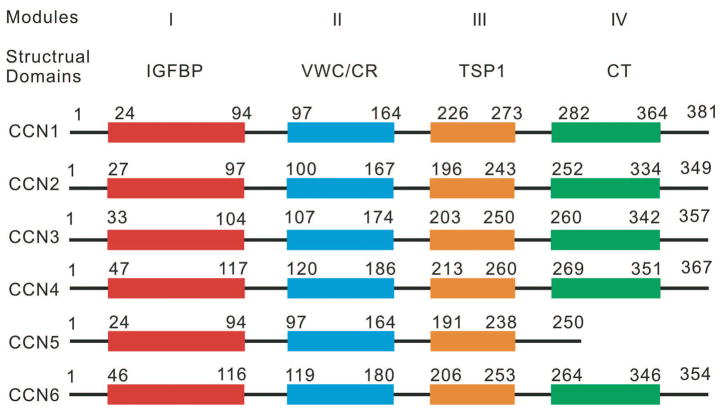
The members of the CCN family exhibit similar structural properties at both the gene and protein levels. The Ccn genes share approximately 30 to 50% overall nucleotide sequences and CCN proteins share about 40 to 60% similar amino acid sequences (Rachfal and Brigstock, 2005) (Fig. 1). The basic gene structure for the CCN family contains five exons and four introns. The origin of the CCN family gene goes back over 40 million years in the evolutionary history of vertebrate. The CCN proteins have been found in a diverse collection of vertebrate including humans, zebra fish, mice, rats, and chickens (Desnoyers, 2004). The gene is conveniently structured such that each exon codes a modular domain in the resulting translational product. This translational organization suggests that the CCN family evolved through exon shuffling (Bork, 1993). The resulting CCN proteins have numbers of amino acids ranging from 348 to 381 with the exception of CCN5 (Brigstock, 1999).
Fig. 1.
Structural comparison of the six CCN proteins. The amino acid sequences of the six human CCN proteins were compared. The locations of the four structural domains are shown. It is noteworthy that slicing variants of some CCN transcripts are not shown.
The CCN proteins are mosaic proteins characterized by four unique globular modules that share homology with various extracellular mosaic protein domains (Fig. 1). Module I contains high homology to insulin-like growth factor (IGF) binding domain. Despite conformational similarities at module I, it has been shown that CCN2 exhibits a much lower affinity for insulin-like growth factor than expected (Vorwerk et al., 2002). CCN2 IGF binding module may interact with other factors (Desnoyers, 2004). Module II is a von Williebrand factor type C (VWC) repeat module and plays a role in oligomerization. Module III is a thrombospondin type 1 repeat domain (TSP-1) and plays a role in cell attachment in most CCN proteins. It has been found that an amino acid residue in Module III is involved in the binding of CCN1 to integrins. Module IV is a C-terminal domain that contains a cystine knot (CT). CCN5 lacks the CT domain (Desnoyers, 2004). The CT domain may play a role in the initial dimerization followed by the von Williebrand factor type C domain carrying out the following oligomerization (Bork, 1993). The two N-terminal modules (Modules I & II) of the CCN proteins are separated from the two C-modules (Modules III & IV) by a linker with variable sequence of amino acids (Desnoyers, 2004). Despite the structural similarities to other protein’s domains, the CCN proteins have unique interactions through modulation with extracellular factors.
Interactions with signaling molecules by the CNN proteins
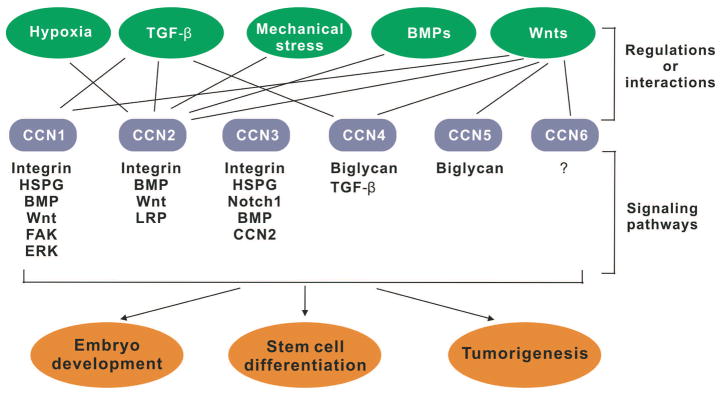
The CCN proteins are involved in numerous biological processes (Fig. 2). The modular property of CCN proteins gives them the ability to bind and interact with a broad range of factors. It is known that these modules can bind to molecules such as heparan sulfate proteoglycans (HSPGs), integrins, and lipoprotein receptor-related proteins (LRPs) (Rachfal and Brigstock, 2005). It is mainly through the direct binding of CCN proteins to cell adhesion receptors that CCN proteins bring about their regulation of numerous cell functions (Fig. 2). CCN1 was the first CCN protein to demonstrate a direct ligand binding by its divalent cation association with integrin αvβ3 in the adhesion of human umbilical vein endothelial cells (Kireeva et al., 1998; Chen and Lau, 2009). The ability of CCN proteins to bind low density lipoprotein receptor-related proteins has been specifically important to CCN2. Module III has been credited for the binding between CCN2 and low density lipoprotein receptor-associated protein, suggesting that module III may be responsible for other low density lipoprotein receptor interactions in CCN proteins (Gao and Brigstock, 2003). The diverse but specific interactions of CCN proteins with cell surface receptors give them the ability to participate in a broad spectrum of cellular processes.
Fig. 2.
Schematic representation of the interplays between the CCN proteins and other signaling networks. A partial list of potential upstream regulators or factors that may directly interact with the CCN proteins is shown on the top row. Downstream signaling pathways or interacting factors are also shown.
The role of the CCN1 protein in development, angiogenesis and cell adhesion
CCN1 has been found to play a regulatory role in several physiological processes within vertebrates, most importantly cell migration, angiogenesis, cell adhesion, and apoptosis (Fig. 2). CCN1 interacts with an assortment of cell surface integrins including α6β1, αvβ5, αvβ3, αMβ2, and αIIbβ3 to regulate these processes (Desnoyers, 2004). During gastrulation, correct levels of CCN1 are imperative in regulating the rearrangement and migration of cells. Overexpression or presence of antisense CCN1 causes a disruption in gastrulation and results in defects in morphogenesis in zebrafish. It is suspected that CCN1 exhibits this disruption in gastrulation through the stimulation or repression of the Wnt signaling pathway and the inhibition of the BMP pathway at various levels (Latinkic et al., 2003). The Ccn1-null mice most commonly died due to placental vascular inefficiency and compromised blood vessels (Mo et al., 2002), suggesting that CCN1 may play a prominent role in angiogenesis. In fact, the Ccn knockout mice showed the worst vascular development (Mo et al., 2002). Both CCN1 and CCN2 have been found to regulate angiogenesis through integrins αvβ3 and α6β1 (Lau and Lam, 1999). Their function as angiogenesis promoters or inhibitors may be dependent on the induction of TGFβ or fibroblast growth factor (Holbourn et al., 2008). CCN1 is also up-regulated at injury sites.
CCN1 also plays a role in cell adhesion along with several other CCN proteins. The CCN proteins interact with integrins or HSPGs to regulate the adhesion and migration of cells in endothelial cells, smooth muscle cells, and fibroblasts (Babic et al., 1999; Leu et al., 2004; Kubota and Takigawa, 2007). In endothelial cells, CCN1 regulates adhesion and migration through integrins (Babic et al., 1999; Leu et al., 2004) and HSPGs while CCN2 regulates through integrin αv3β (Chen et al., 2001; Leu et al., 2004). In smooth muscle cells, CCN1 also interacts with integrin α6β1 and HSPGs to regulate adhesion and migration (Leu et al., 2004). At cutaneous injury sites, CCN1 along with CCN2 is up-regulated in response to growth factor to stimulate fibroblast adhesion. CCN1 and CCN2 have been found to mediate fibroblast adhesion signaling through integrin α6β1 and HSPGs (Chen et al., 2001). CCN1 may act on the integrin receptor and HSPGs of the cells to cause an increase in cellular reactive oxygen species (ROS) resulting in cell death (Juric et al., 2009).
Regulatory roles of CCN2 in development and cell adhesion and migration
CCN2 is mainly expressed in endothelial, smooth muscle, and cartilaginous cells (Ihn, 2002). Mice lacking Ccn2 showed a deficit of pro-adhesive, pro-inflammatory, and pro-angiogenic gene expression in fibroblasts (Kennedy et al., 2007). The Ccn2 knockout mice were found to die at birth, because of respiratory failure that was resulted from hypoplastic lungs and poor thoracic development (Baguma-Nibasheka and Kablar, 2008). Phenotypically, these mice expressed skeletal dysmorphisms, decreased extracellular matrix components, and decreased chondrocyte proliferation (Katsube et al., 2009). Ccn2 knockdown zebrafish expressed similar phenotypes with bone defects and disruption in notochord development (Chiou et al., 2006). The upregulation of CCN2 is responsible for diabetic nephropathy in humans. CCN2 brings about diabetic nephropathy phenotype symptoms by inhibiting BMP-7 signaling and contributing to abnormal gene expression in kidney cells (Nguyen et al., 2008).
The CCN3 protein in skeletal and cardiac development and cell adhesion
CCN3 shares a similar role as CCN1 and CCN2 in vertebrates. CCN3 is expressed in a diverse collection of tissues, including muscle, cartilage, bone, and nervous tissue, and provides a regulatory function in their development (Katsuki et al., 2008). Ccn3 mutant mice exhibit skeletal and cardiac abnormalities, such as cardiomyopathy, muscle atrophy, and cataract formation (Heath et al., 2008). In the endothelial cells of human umbilical veins, CCN3 was found to promote adhesion through integrins αvβ3, α5β1, α6β1, and HSPGs while regulating cell migration only through integrins αvβ3 and α5β1 (Lin et al., 2003). CCN3 can be secreted by hematopoietic progenitor cells into the serum of humans where it can form a complex with fibulin-1 in blood (Thibout et al., 2003).
Inhibition of wnt signaling pathway by CCN4
CCN4 is expressed in developing mesenchymal, pre-osteoblastic, and cartilage cells (French et al., 2004). It is believed to serve an important regulatory function in skeletal growth and bone repair. The truncated form of CCN4, WISP1v, has been found to regulate the differentiation of chondrocytes towards endochondral ossification (Yanagita et al., 2007). CCN4 prevents apoptosis in cells by inhibiting c-Myc in the Wnt/β-catenin signaling pathway and p53 induced apoptosis (Su et al., 2002; You et al., 2002).
Inhibition of cell growth and motility by CCN5
CCN5 is expressed in nearly all tissues of developing mice and human embryos until protein levels eventually differentiate with growth (Jones et al., 2007). This diverse expression in unique tissue types at varying levels suggests that CCN5 may play a multifunctional and tissue specific regulatory role. In adult mice, CCN5 expression is varied with the highest levels being found in the heart, brain, spleen, lung and uterus (Gray et al., 2007). CCN5 is a heparin induced gene in vascular smooth muscle cells with anti-proliferative properties (Lake et al., 2003). The binding of heparin to vascular smooth muscle surface receptors increases the expression of CCN5 and results in the inhibition of cell growth and motility, without a noticeable effect on cell adhesion and apoptosis (Lake et al., 2003). CCN5 overexpression decreases the motility of vascular smooth muscle by reducing matrix metalloproteinase-2 (MMP- 2) level. In contrast, cell motility and MMP-2 level increases as CCN5 expression is reduced (Lake and Castellot, 2003). In vivo studies on the uterus smooth muscle cells of rats found that CCN5 gene expression is positively regulated by estrogen, as CCN5 expression increases in ovariectomized rats up to eight fold in response to estrogen administration (Mason et al., 2004), suggesting that CCN5 may play a regulatory role in uterine maintenance in vertebrate.
Role of CCN6 in Musculoskeletal Development and Disorders
Mutations in the Ccn6 gene are associated with human diseases, such as the progressive pseudorheumatoid dysplasia (PD), an autosomal recessive skeletal disorder. The PD disease is marked by cartilage loss and destructive bone changes as young patients age through childhood (Hurvitz et al., 1999). Ccn6 gene mutation is also responsible for spondyloepiphyseal dysplasia tarda with progressive arthropathy, which is marked by symptoms of cartilage loss (Yang and Liao, 2007). However, in contrast to humans, CCN6 is not an essential participant during skeletal growth or homeostasis in mice (Kutz et al., 2005). Ccn6-null mice and Ccn6 overexpression mice exhibit no gross abnormal phenotypes; and the developing Ccn6-null mice show mature endplates throughout their skeletal system (Kutz et al., 2005). Overexpression of CCN6 in human chondrocyte lines C-28/I2 and T/C-28a2 is associated with an increased production of type II collagen and aggrecan matrix molecules (Sen et al., 2004), which may be caused by CCN6-mediated inhibition of IGF-IR, IRS-1, and ERK kinase signaling pathways (Cui et al., 2007). CCN6 also regulates the cellular level of reactive oxygen species (ROS) in cells, as abnormal CCN6 levels or mutations in the Ccn6 gene cause the accumulation of ROS and disturb balances in cellular homeostasis (Miller and Sen, 2007).
CCN proteins and stem cell differentiation
CCN proteins as important mediators of major signal pathways in stem cells
Mesenchymal stem cells are pluripotent progenitors that can give rise to osteogenic, chondrogenic, adipogenic, myogenic, and fibroblastic lineages upon the stimulation with appropriate differentiation cues (Deng et al., 2008; Tang et al., 2008). A complex interaction of signaling molecules is required to orchestrate the dynamic processes of stem cell differentiation, proliferation, and migration. CCN proteins participate and interact with several important signaling pathways to regulate differentiation of progenitor cells and the development, maintenance, and repair of the skeletal system (Ivkovic et al., 2003; Safadi et al., 2003; Katsuki et al., 2008) (Fig. 2). The expression of CCN proteins has been shown to be coordinated with many components of Wnt, BMP, and TGF‚ pathways (Abreu et al., 2002; Si et al., 2006; Maeda et al., 2009). The Wnt pathway is activated by the binding of secreted Wnt ligands to Wnt receptors, followed by a cascade of intracellular signaling (Luo et al., 2007). The best known Wnt pathway is canonical Wnt/β-catenin pathway that begins with the binding of Wnt ligands to Frizzled receptors and co-receptor LRP5 or 6. The Wnt/β-catenin pathway has been shown to regulate many cellular processes, such as bone formation (Krishnan et al., 2006).
CCN1, CCN2, and CCN5 are up-regulated in the early stages of mesenchymal stem cell differentiation by Wnt3A stimulation. CCN1 has been shown to be an important target of canonical Wnt/β-catenin signaling and plays a regulatory role in the migration and differentiation of the mesenchymal stem cells into osteoblasts (Si et al., 2006; Schutze et al., 2007). CCN1 protein is believed to regulate cell migration through binding to cell surface integrins in Xenopus (Latinkic et al., 2003). In human primary mesenchymal progenitor cells, CCN1 promotes cell proliferation (Schutze et al., 2005). CCN1 also plays a regulatory role in the formation of blood vessels where it acts as an important regulatory signal for endothelial progenitor cells (Yu et al., 2008). The similarities between Ccn1 knockout mice and Notch knockout mice in exhibiting poor vascular development and defects suggest that CCN1 may regulate the Notch/VEGF pathway (Katsube et al., 2009).
CCN2 in chondrogenenic and osteogenic differentiation of MSCs
CCN2 plays an important role in embryogenesis and bone formation. The skeletal system of the developing vertebrae forms through the process of endochondral ossification, which originates with mesenchymal cells that differentiate into chondrocytes. As the chondrocytes embed in the extracellular matrix they differentiate into pre-hypertrophic and hypertrophic cells, and eventually ossified to form bone (Maeda et al., 2009). CCN2 is found at its highest levels in the vascular tissue and the maturing chondrocytes of the embryo (Ivkovic et al., 2003). CCN2 promotes the steps of proliferation, maturation, and hypertrophy in these chondrocytes. It has been shown that CCN2 is a mutual target of both Wnt and BMP signaling pathways (Luo et al., 2004). CCN2 has shown to interact with BMP-2 to form a complex that regulates the proliferation and differentiation of pre-hypertrophic and hypertrophic chondrocytes (Maeda et al., 2009). CCN2’s binding to BMP-4 prevents BMP-4 from binding to BMP receptors and hence modulates the activity of BMP-9 (Abreu et al., 2002; Luo et al., 2004). CCN2 also modulates the Wnt pathway in Xenopus embryos by binding to LRP6 co-receptor (Mercurio et al., 2004). The expression of CCN2 in chondrocytes is controlled by both Rac1 and actin pathways mediated by TGFβ/Smad signaling (Woods et al., 2009). In adult skeletal systems, CCN2 is most highly expressed in the osteoblasts lining metaphyseal trabeculae and in osteogenic surfaces lining fracture calluses, suggesting that increased CCN2 may play an important role in bone growth and fracture repair (Safadi et al., 2003). Additionally, CCN2 and CCN3 may function in chondrocytes with concerted but opposite roles in regulating gene expression. Studies of null mice suggest that CCN2-stimulated proliferation and differentiation is balanced by CCN3-mediated inhibition of growth and differentiation (Kawaki et al., 2008).
Interaction between CCN3 and Notch1 in regulating osteogenesis and hematopoiesis
CCN3 is an important secreted extracellular protein in regards to mesenchymal stem cell differentiation, and regulates differentiation, growth, and maturation of osteogenic, chondrogenic, and hematopoietic progenitor cells. CCN3 functions as a positive mediator while acting inhibitory in both muscle and osteoblast differentiation (Katsuki et al., 2008). CCN3 may regulate differentiation of mesenchymal stem cells through binding with Notch1 in a co-activator manner. CCN3 has been shown to upregulate the downstream members of the Notch signaling pathway, namely Hes/Hey and p21, by binding with Notch1 by the fourth module and inhibiting osteogenic activity (Katsuki et al., 2008). A study on CCN3 overexpression in ST-2 cells found that CCN3 prevents BMP-2 from phosphorylating Smad1/5/8 in BMP signaling (Rydziel et al., 2007), resulting in the inhibition of osteoblastogenesis. CCN3 also plays an important role in regulating primitive hematopoietic stem cells, as studies involved in CCN3 knockdown or forced expression in CD34+ cells has demonstrated that the presence of CCN3 is necessary for the functional self-renewal of hematopoietic stem cells (Gupta et al., 2007).
CCN4 as an Important Mediator of Wnt Signaling in MSCs
CCN4 is a target of the Wnt1 pathway and plays a major role in controlling the expression of key regulators in the chondrogenic and osteoblastic differentiation (Pennica et al., 1998). CCN4 is expressed at the highest levels in osteoblasts and its progenitor cells during embryonic development. CCN4 may promote mesenchymal proliferation and osteoblastic differentiation while inhibiting chondrogenic differentiation (French et al., 2004). In human bone marrow, CCN4 has been found in both full-length and WISP-1va truncated forms. CCN4 may regulate osteoblast proliferation by limiting the activation of the TGF-β1 signaling member, Smad-2 (Inkson et al., 2008). The truncated version of CCN4, WISP1v, is also expressed in human chondrosarcomas, and is believed to play a role in endochondral ossification (Yanagita et al., 2007). Upon injury, CCN4 expression is up-regulated by the signaling activity of nitric oxide at the site of inflammation to partake in tissue repair (Wang et al., 2009). To date, CCN5 and CCN6 have no known roles in stem cell differentiation. Experiments to study the possible role of CCN5 in pre-adipocyte 3T3-L1 cells have found that CCN5 plays little to no functional role in adipogenic differentiation (Inadera et al., 2009).
Roles of CCN proteins in tumorigenesis
Increased CCN1 expression associated with cancer aggressiveness
While abnormal levels or altered forms of CCN proteins have been widely reported in human cancers, it has been shown that CCN proteins can play either as a positive growth regulator or growth inhibitor of cancer cells; and their functional outcomes are often dependent on cell and/or tissue types (Fig. 2).
CCN1 is overexpressed in prostate cancer, gliomas, pancreatic cancer, and breast cancer. Overexpression of CCN1 levels in prostate cancer cells and samples is closely associated with the status of p53 gene. CCN1 levels in prostate cancer cells with mutant or null p53 genes are much higher than that of wild-type (Lv et al., 2009). This overexpression of CCN1 may be responsible for the uncontrolled growth of some prostate cancer (Lv et al., 2009). Overexpression of CCN1 in U343 glioma cells rapidly increases cell growth and the possibilities to form large tumors. It has been subsequently found that CCN1 activates both β-catenin-TCF/Lef and Akt signaling pathways in these cells (Xie et al., 2004). CCN1 levels have been found to be over two times higher in metastatic lesions than in primary pancreatic tumors, suggesting that CCN1 may be responsible for the development of the pancreatic metastasis (Holloway et al., 2005).
CCN1 has also been associated with the increased aggressiveness, vascularization, and estrogen independence in breast cancer, suggesting a major role of CCN1 in breast cancer progression (Babic et al., 1998; Tsai et al., 2002). A recent study suggests that hypoxia may induce alternative splicing in CCN1 in which intron 3 is retained in the breast cancer, and this alternative splicing could subsequently lead to tumorigenesis (Hirschfeld et al., 2009). It has been reported that overexpression of CCN1 causes the development of breast cancer through up-regulating the expression of the enzyme MMP-1 in adjacent stromal fibroblasts, which subsequently activates on protease-activated receptor 1 to promote cancer cell migration and invasion (Nguyen et al., 2006). Nonetheless, below normal levels of CCN1 have been detected in lung cancer samples and in rhabdomyosarcomas (Chen et al., 2007).
Tumor- and Tissue-Specific Roles of CCN2 and CCN3
In the majority of ovarian tumors it has been found that CCN2 expression is reduced (Kikuchi et al., 2007). The restoration of normal CCN2 levels in ovarian cancer cells has been shown to inhibit cancer cell growth while the deletion of CCN2 expression has accelerated cancer cell growth, suggesting that the inactivation of the CCN2 gene though hypermethylation of CCN2 promoter may play a prominent role in ovarian tumorigenesis (Kikuchi et al., 2007). Unlike in ovarian cancer, CCN2 is over-expressed in pancreatic cancer and breast cancer (Jiang et al., 2004; Bennewith et al., 2009). In pancreatic cancer, increased CCN2 expression was found in hypoxic cells in vitro, and cancer cells with higher levels of CCN2 are more resistant to hypoxia-mediated apoptosis in vivo (Bennewith et al., 2009). Studies suggest that CCN2 is also associated with breast cancer metastasis. In breast cancer cells, CCN2 can increase in levels in response to prometastatic cytokine TGFβ. The combinational increase of CCN2 and interleukin-11 expression in response to TGFβ positively correlates with osteolytic metastasis of breast cancer (Kang et al., 2003). In chondrosarcomas and enchondromas, the level of CCN2 also correlates with the grade of malignancy (Shakunaga et al., 2000). In astrocytomas, CCN2 levels correlate strongly with mitogenic activity of tumor cells (Rubenstein et al., 2003).
CCN3 has been found to promote the growth of prostate cancer, Wilm’s tumors, and osteosarcomas (Glukhova et al., 2001; Maillard et al., 2001; McCallum and Irvine, 2009). While in rhabdomyosarcoma and cartilage tumors the level of CCN3 expression correlates with level of tumor differentiation (Manara et al., 2002). In contrast, CCN3 has been found to suppress the proliferation and growth of glioma cells, Ewing’s sarcoma, melanoma, brain, and adrenocortical tumors (McCallum and Irvine, 2009). In glioma cells, in vivo and in vitro studies have shown that with the increase in the gap junction protein, Connexin-43, the expression of CCN3 is increased in the glioma cells, and tumor growth is halted (Gupta et al., 2001). CCN3 also inhibits tumor growth of Ewing’s sarcoma in nude mice (Benini et al., 2005). Interestingly, despite the decreased growth the Ewing’s sarcoma cells still exhibit increased migration and invasion characteristics (Manara et al., 2002; Benini et al., 2005). It is noteworthy that the expression of truncated forms of CCN3 may also be associated with tumorigenesis, given the fact that an amino-truncated form has been found in MAV-induced nephroblastoma (Perbal, 2001).
Tumor suppression role of CCN4, CCN5 and CCN6
CCN4 was initially identified by using differential display method in comparing high and low metastatic K-1735 mouse melanoma cells. CCN4 inhibits growth and metastasis of the K-1735 melanoma cells; and CCN4 was up-regulated in the low metastatic cells in comparison to high metastatic cells (Hashimoto et al., 1996). When the high metastatic K-1735 cells were transfected with the CCN4 expression vector, the growth rate and metastatic potential of these cells decreased in vivo (Hashimoto et al., 1998). CCN4 may also be a tumor suppressor of breast cancer (Davies et al., 2007). In lung cancer, overexpression of CCN4 inhibits cancer metastasis and cell motility, probably through inhibiting the activation of Rac (Soon et al., 2003). While CCN4 functions as a tumor suppressor in its full form, truncated forms of CCN4 may act as oncogenes and promote tumor growth. For example, CCN4 lacking the second domain (WISP1v) is associated with invasive cholangiocarcinomas in humans and aggressive progression of scirrhous gastric carcinoma (Tanaka et al., 2001, 2003). WISP1v may activate p38 and p42/44 mitogen-activated protein kinases (MAPKS) in cholangiocarcinoma cells (Tanaka et al., 2001, 2003).
CCN5 seems to play a preventive role in the progression of breast cancer. CCN5 is not expressed in normal cells while it is expressed at increased levels in noninvasive breast lesion samples (Banerjee et al., 2008). Thus, CCN5 acts as a negative inhibitor of migration and invasion and inhibits the progression from noninvasive to invasive cancer type (Banerjee et al., 2008). The activation of p53 mutants may be responsible for the silencing of CCN5 expression in both breast cancer and pancreatic adenocarcinoma (Dhar et al., 2007a, 2008). CCN5 may preserve estrogen-dependent growth in many breast cancer cells, as the estrogen independent growth has been observed in CCN5 knocked down MCF-7 breast cancer cells (Fritah et al., 2008). CCN5 controls cell growth by modulating signaling activity of insulin-like growth factor-1 (IGF-1) in estrogen receptor positive breast tumor cells (Dhar, 2007b). Down-regulation of CCN5 is correlated with the development of salivary gland tumors and the formation of leiomyomas in women, suggesting that the loss of CCN5 may be the cause for uncontrolled cell growth in these tumor cells (Mason et al., 2004; Kouzu et al., 2006).
Mutations in the Ccn6 gene are closely correlated with the development of colorectal carcinomas, inflammatory breast cancer, and hepatocellular carcinomas (van Golen et al., 1999; Thorstesen et al., 2001; Cervello et al., 2004). In inflammatory breast cancer, CCN6 levels are reduced in over 80% of the samples (van Golen et al., 1999). Restoration of CCN6 inhibits tumor growth and invasiveness in inflammatory breast cancer cells (Kleer et al., 2004). Mechanistically, CCN6 is believed to inhibit tumor motility and invasion by preserving cell-cell to junctions in mammary epithelial cells, as CCN6 can increase E-cadherin protein levels through possible transcriptional regulators Snail and ZEB1 (Huang et al., 2008).
Concluding remarks and future directions
The CCN proteins are multifunctional proteins that play important roles in skeletal development, angiogenesis, tumorigenesis, cell proliferation, adhesion, migration, and survival. Many of the CCN family members are induced by growth factors, cytokines, and cellular stress, such as hypoxia. CCN proteins are modular proteins, containing up to four distinct functional domains, at least two of which are involved in binding to cell surfaces molecules, including integrins, HSPGs, and LRP. The involvement of integrins in mediating CCN signaling allows for considerable plasticity in context-dependent responses in distinct cell types, as certain integrin subtypes and integrin signaling are coordinated with other signaling pathways in the cell. CCNs show a wide and highly variable expression pattern in adult and embryonic tissues. Most studies have focused on their principal role in osteogenesis, chondrogenesis, and angiogenesis from the aspect of mesenchymal cell differentiation. Furthermore, CCN proteins can integrate and modulate the signals of integrins, BMPs, vascular endothelial growth factor, Wnts, and Notch by direct binding.
Although most CCN family members were discovered over a decade ago, the precise physiological function and mechanism of action of these proteins remain elusive. Future directions should focus on the basic studies and translational aspects of CCN proteins. As matrix proteins, CCN proteins may play important roles in fine-tuning other major signaling pathways, such as TGFβ/BMP and Wnt signaling. It is important to investigate how CCN proteins modulate these pathways. Similarly, little is known about what factors determines the tissue and/or cell-type specific response to CCN proteins. On the clinical application fronts, it remains to be determined if some of CCN proteins can serve as anti-cancer targets. It is conceivable that some of the CCN proteins may be used as wound healing agents, while some (e.g., CCN2) can be targeted with inhibitors or antibodies to treat fibrotic disorders in clinical setting. Therefore, a thorough understanding of the biological functions of the CCN proteins would not only provide insight into their roles in numerous cellular processes but also offer opportunities for developing potential therapeutics by targeting CCN functions.
Acknowledgments
We apologize to the authors whose original work was not cited due to space constraints. The reported work was supported in part by research grants from The Brinson Foundation (TCH), Natural Science Foundation of China (QL, #30500602; QK #30600625), Musculoskeletal Transplant Foundation (RCH), National Institutes of Health (RCH, TCH and HHL), and Orthopaedic Research and Education Foundation (RCH and HHL).
References
- Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic AM, Chen CC, Lau LF. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphavbeta3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol. 1999;19:2958–2966. doi: 10.1128/mcb.19.4.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic AM, Kireeva ML, Kolesnikova TV, Lau LF. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci USA. 1998;95:6355–6360. doi: 10.1073/pnas.95.11.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguma-Nibasheka M, Kablar B. Pulmonary hypoplasia in the connective tissue growth factor (Ctgf) null mouse. Dev Dyn. 2008;237:485–493. doi: 10.1002/dvdy.21433. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Dhar G, Haque I, Kambhampati S, Mehta S, Sengupta K, Tawfik O, Phillips TA, Banerjee SK. CCN5/WISP-2 expression in breast adenocarcinoma is associated with less frequent progression of the disease and suppresses the invasive phenotypes of tumor cells. Cancer Res. 2008;68:7606–7612. doi: 10.1158/0008-5472.CAN-08-1461. [DOI] [PubMed] [Google Scholar]
- Benini S, Perbal B, Zambelli D, Colombo MP, Manara MC, Serra M, Parenza M, Martinez V, Picci P, Scotlandi K. In Ewing’s sarcoma CCN3(NOV) inhibits proliferation while promoting migration and invasion of the same cell type. Oncogene. 2005;24:4349–4361. doi: 10.1038/sj.onc.1208620. [DOI] [PubMed] [Google Scholar]
- Bennewith KL, Huang X, Ham CM, Graves EE, Erler JT, Kambham N, Feazell J, Yang GP, Koong A, Giaccia AJ. The role of tumor cell-derived connective tissue growth factor (CTGF/CCN2) in pancreatic tumor growth. Cancer Res. 2009;69:775–784. doi: 10.1158/0008-5472.CAN-08-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett. 1993;327:125–130. doi: 10.1016/0014-5793(93)80155-n. [DOI] [PubMed] [Google Scholar]
- Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991;114:1285–1294. doi: 10.1083/jcb.114.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigstock DR. The connective tissue growth factor/cysteine-Rich 61/Nephroblastoma Overexpressed (CCN) Family. Endocrine Rev. 1999;20:189–206. doi: 10.1210/edrv.20.2.0360. [DOI] [PubMed] [Google Scholar]
- Brigstock DR, Goldschmeding R, Katsube KI, Lam SC, Lau LF, Lyons K, Naus C, Perbal B, Riser B, Takigawa M, Yeger H. Proposal for a unified CCN nomenclature. Mol Pathol. 2003;56:127–128. doi: 10.1136/mp.56.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervello M, Giannitrapani L, Labbozzetta M, Notarbartolo M, D’Alessandro N, Lampiasi N, Azzolina A, Montalto G. Expression of WISPs and of their novel alternative variants in human hepatocellular carcinoma cells. Ann NY Acad Sci. 2004;1028:432–439. doi: 10.1196/annals.1322.051. [DOI] [PubMed] [Google Scholar]
- Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41:771–783. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Chen N, Lau LF. The angiogenic factors Cyr61 and connective tissue growth factor induce adhesive signaling in primary human skin fibroblasts. J Biol Chem. 2001;276:10443–10452. doi: 10.1074/jbc.M008087200. [DOI] [PubMed] [Google Scholar]
- Chen PP, Li WJ, Wang Y, Zhao S, Li DY, Feng LY, Shi XL, Koeffler HP, Tong XJ, Xie D. Expression of Cyr61, CTGF, and WISP-1 correlates with clinical features of lung cancer. PLoS One. 2007;2:e534. doi: 10.1371/journal.pone.0000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou MJ, Chao TT, Wu JL, Kuo CM, Chen JY. The physiological role of CTGF/CCN2 in zebrafish notochond development and biological analysis of the proximal promoter region. Biochem Biophys Res Commun. 2006;349:750–758. doi: 10.1016/j.bbrc.2006.08.095. [DOI] [PubMed] [Google Scholar]
- Cui RR, Huang J, Yi L, Xie H, Zhou HD, Yuan LQ, Wang M, Peng YQ, Luo XH, Liao EY. WISP3 suppresses insulin-like growth factor signaling in human chondrocytes. Mol Cell Endocrinol. 2007;279:1–8. doi: 10.1016/j.mce.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Davies SR, Watkins G, Mansel RE, Jiang WG. Differential expression and prognostic implications of the CCN family members WISP-1, WISP-2, and WISP-3 in human breast cancer. Ann Surg Oncol. 2007;14:1909–1918. doi: 10.1245/s10434-007-9376-x. [DOI] [PubMed] [Google Scholar]
- Deng ZL, Sharff KA, Tang N, Song WX, Luo J, Luo X, Chen J, Bennett E, Reid R, Manning D, Xue A, Montag AG, Luu HH, Haydon RC, He TC. Regulation of osteogenic differentiation during skeletal development. Front Biosci. 2008;13:2001–2021. doi: 10.2741/2819. [DOI] [PubMed] [Google Scholar]
- Desnoyers L. Structural basis and therapeutic implication of the interaction of CCN proteins with glycoconjugates. Curr Pharm Des. 2004;10:3913–3928. doi: 10.2174/1381612043382567. [DOI] [PubMed] [Google Scholar]
- Dhar G, Mehta S, Banerjee S, Gardner A, McCarty BM, Mathur SC, Campbell DR, Kambhampati S, Banerjee SK. Loss of WISP-2/CCN5 signaling in human pancreatic cancer: a potential mechanism for epithelial-mesenchymal-transition. Cancer Lett. 2007a;254:63–70. doi: 10.1016/j.canlet.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Dhar K, Banerjee S, Dhar G, Sengupta K, Banerjee SK. Insulin-like growth factor-1 (IGF-1) induces WISP-2/CCN5 via multiple molecular cross-talks and is essential for mitogenic switch by IGF-1 axis in estrogen receptor-positive breast tumor cells. Cancer Res. 2007b;67:1520–1526. doi: 10.1158/0008-5472.CAN-06-3753. [DOI] [PubMed] [Google Scholar]
- Dhar G, Banerjee S, Dhar K, Tawfik O, Mayo MS, Vanveldhuizen PJ, Banerjee SK. Gain of oncogenic function of p53 mutants induces invasive phenotypes in human breast cancer cells by silencing CCN5/WISP-2. Cancer Res. 2008;68:4580–4587. doi: 10.1158/0008-5472.CAN-08-0316. [DOI] [PubMed] [Google Scholar]
- Erwin WM. The Notochord, Notochordal cell and CTGF/CCN-2: ongoing activity from development through maturation. J Cell Commun Signal. 2008;2:59–65. doi: 10.1007/s12079-008-0031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French DM, Kaul RJ, D’Souza AL, Crowley CW, Bao M, Frantz GD, Filvaroff EH, Desnoyers L. WISP-1 is an osteoblastic regulator expressed during skeletal development and fracture repair. Am J Pathol. 2004;165:855–867. doi: 10.1016/S0002-9440(10)63348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritah A, Saucier C, De Wever O, Bracke M, Bieche I, Lidereau R, Gespach C, Drouot S, Redeuilh G, Sabbah M. Role of WISP-2/CCN5 in the maintenance of a differentiated and noninvasive phenotype in human breast cancer cells. Mol Cell Biol. 2008;28:1114–1123. doi: 10.1128/MCB.01335-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Brigstock DR. Low density lipoprotein receptor-related protein (LRP) is a heparin-dependent adhesion receptor for connective tissue growth factor (CTGF) in rat activated hepatic stellate cells. Hepatol Res. 2003;27:214–220. doi: 10.1016/s1386-6346(03)00241-9. [DOI] [PubMed] [Google Scholar]
- Glukhova L, Angevin E, Lavialle C, Cadot B, Terrier-Lacombe MJ, Perbal B, Bernheim A, Goguel AF. Patterns of specific genomic alterations associated with poor prognosis in high-grade renal cell carcinomas. Cancer Genet Cytogenet. 2001;130:105–110. doi: 10.1016/s0165-4608(01)00477-0. [DOI] [PubMed] [Google Scholar]
- Gray MR, Malmquist JA, Sullivan M, Blea M, Castellot JJ., Jr CCN5 Expression in mammals. II. Adult rodent tissues. J Cell Commun Signal. 2007;1:145–158. doi: 10.1007/s12079-007-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Wang H, McLeod TL, Naus CC, Kyurkchiev S, Advani S, Yu J, Perbal B, Weichselbaum RR. Inhibition of glioma cell growth and tumorigenic potential by CCN3 (NOV) Mol Pathol. 2001;54:293–299. doi: 10.1136/mp.54.5.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Hong D, Iborra F, Sarno S, Enver T. NOV (CCN3) functions as a regulator of human hematopoietic stem or progenitor cells. Science. 2007;316:590–593. doi: 10.1126/science.1136031. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Shindo-Okada N, Tani M, Nagamachi Y, Takeuchi K, Shiroishi T, Toma H, Yokota J. Expression of the Elm1 gene, a novel gene of the CCN (connective tissue growth factor, Cyr61/Cef10, and neuroblastoma overexpressed gene) family, suppresses In vivo tumor growth and metastasis of K-1735 murine melanoma cells. J Exp Med. 1998;187:289–296. doi: 10.1084/jem.187.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Shindo-Okada N, Tani M, Takeuchi K, Toma H, Yokota J. Identification of genes differentially expressed in association with metastatic potential of K-1735 murine melanoma by messenger RNA differential display. Cancer Res. 1996;56:5266–5271. [PubMed] [Google Scholar]
- Heath E, Tahri D, Andermarcher E, Schofield P, Fleming S, Boulter CA. Abnormal skeletal and cardiac development, cardiomyopathy, muscle atrophy and cataracts in mice with a targeted disruption of the Nov (Ccn3) gene. BMC Dev Biol. 2008;8:18. doi: 10.1186/1471-213X-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld M, zur Hausen A, Bettendorf H, Jager M, Stickeler E. Alternative splicing of Cyr61 is regulated by hypoxia and significantly changed in breast cancer. Cancer Res. 2009;69:2082–2090. doi: 10.1158/0008-5472.CAN-08-1997. [DOI] [PubMed] [Google Scholar]
- Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: structure-function relationships. Trends Biochem Sci. 2008;33:461–473. doi: 10.1016/j.tibs.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway SE, Beck AW, Girard L, Jaber MR, Barnett CC, Jr, Brekken RA, Fleming JB. Increased expression of Cyr61 (CCN1) identified in peritoneal metastases from human pancreatic cancer. J Am Coll Surg. 2005;200:371–377. doi: 10.1016/j.jamcollsurg.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Huang W, Zhang Y, Varambally S, Chinnaiyan AM, Banerjee M, Merajver SD, Kleer CG. Inhibition of CCN6 (Wnt-1- induced signaling protein 3) down-regulates E-cadherin in the breast epithelium through induction of snail and ZEB1. Am J Pathol. 2008;172:893–904. doi: 10.2353/ajpath.2008.070899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurvitz JR, Suwairi WM, Van Hul W, El-Shanti H, Superti-Furga A, Roudier J, Holderbaum D, Pauli RM, Herd JK, Van Hul EV, Rezai-Delui H, Legius E, Le Merrer M, Al-Alami J, Bahabri SA, Warman ML. Mutations in the CCN gene family member WISP3 cause progressive pseudorheumatoid dysplasia. Nat Genet. 1999;23:94–98. doi: 10.1038/12699. [DOI] [PubMed] [Google Scholar]
- Ihn H. The role of TGF-beta signaling in the pathogenesis of fibrosis in scleroderma. Arch Immunol Ther Exp (Warsz) 2002;50:325–331. [PubMed] [Google Scholar]
- Inadera H, Shimomura A, Tachibana S. Effect of Wnt-1 inducible signaling pathway protein-2 (WISP-2/CCN5), a downstream protein of Wnt signaling, on adipocyte differentiation. Biochem Biophys Res Commun. 2009;379:969–974. doi: 10.1016/j.bbrc.2008.12.185. [DOI] [PubMed] [Google Scholar]
- Inkson CA, Ono M, Kuznetsov SA, Fisher LW, Robey PG, Young MF. TGF-beta1 and WISP-1/CCN-4 can regulate each other’s activity to cooperatively control osteoblast function. J Cell Biochem. 2008;104:1865–1878. doi: 10.1002/jcb.21754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang WG, Watkins G, Fodstad O, Douglas-Jones A, Mokbel K, Mansel RE. Differential expression of the CCN family members Cyr61, CTGF and Nov in human breast cancer. Endocr Relat Cancer. 2004;11:781–791. doi: 10.1677/erc.1.00825. [DOI] [PubMed] [Google Scholar]
- Joliot V, Martinerie C, Dambrine G, Plassiart G, Brisac M, Crochet J, Perbal B. Proviral rearrangements and overexpression of a new cellular gene (nov) in myeloblastosis-associated virus type 1- induced nephroblastomas. Mol Cell Biol. 1992;12:10–21. doi: 10.1128/mcb.12.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JA, Gray MR, Oliveira BE, Koch M, Castellot JJ., Jr CCN5 expression in mammals: I. Embryonic and fetal tissues of mouse and human. J Cell Commun Signal. 2007;1:127–143. doi: 10.1007/s12079-007-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juric V, Chen CC, Lau LF. Fas-mediated apoptosis is regulated by the extracellular matrix protein CCN1 (CYR61) in vitro and in vivo. Mol Cell Biol. 2009;29:3266–3279. doi: 10.1128/MCB.00064-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Katsube K, Sakamoto K, Tamamura Y, Yamaguchi A. Role of CCN, a vertebrate specific gene family, in development. Dev Growth Differ. 2009;51:55–67. doi: 10.1111/j.1440-169X.2009.01077.x. [DOI] [PubMed] [Google Scholar]
- Katsuki Y, Sakamoto K, Minamizato T, Makino H, Umezawa A, Ikeda MA, Perbal B, Amagasa T, Yamaguchi A, Katsube K. Inhibitory effect of CT domain of CCN3/NOV on proliferation and differentiation of osteogenic mesenchymal stem cells, Kusa-A1. Biochem Biophys Res Commun. 2008;368:808–814. doi: 10.1016/j.bbrc.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Kawaki H, Kubota S, Suzuki A, Lazar N, Yamada T, Matsumura T, Ohgawara T, Maeda T, Perbal B, Lyons KM, Takigawa M. Cooperative regulation of chondrocyte differentiation by CCN2 and CCN3 shown by a comprehensive analysis of the CCN family proteins in cartilage. J Bone Miner Res. 2008;23:1751–1764. doi: 10.1359/jbmr.080615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy L, Liu S, Shi-Wen X, Chen Y, Eastwood M, Sabetkar M, Carter DE, Lyons KM, Black CM, Abraham DJ, Leask A. CCN2 is necessary for the function of mouse embryonic fibroblasts. Exp Cell Res. 2007;313:952–964. doi: 10.1016/j.yexcr.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Kikuchi R, Tsuda H, Kanai Y, Kasamatsu T, Sengoku K, Hirohashi S, Inazawa J, Imoto I. Promoter hypermethylation contributes to frequent inactivation of a putative conditional tumor suppressor gene connective tissue growth factor in ovarian cancer. Cancer Res. 2007;67:7095–7105. doi: 10.1158/0008-5472.CAN-06-4567. [DOI] [PubMed] [Google Scholar]
- Kireeva ML, Lam SC, Lau LF. Adhesion of human umbilical vein endothelial cells to the immediate-early gene product Cyr61 is mediated through integrin alphavbeta3. J Biol Chem. 1998;273:3090–3096. doi: 10.1074/jbc.273.5.3090. [DOI] [PubMed] [Google Scholar]
- Kleer CG, Zhang Y, Pan Q, Merajver SD. WISP3 (CCN6) is a secreted tumor-suppressor protein that modulates IGF signaling in inflammatory breast cancer. Neoplasia. 2004;6:179–185. doi: 10.1593/neo.03316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzu Y, Uzawa K, Kato M, Higo M, Nimura Y, Harada K, Numata T, Seki N, Sato M, Tanzawa H. WISP-2 expression in human salivary gland tumors. Int J Mol Med. 2006;17:567–573. [PubMed] [Google Scholar]
- Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–1209. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S, Takigawa M. CCN family proteins and angiogenesis: from embryo to adulthood. Angiogenesis. 2007;10:1–11. doi: 10.1007/s10456-006-9058-5. [DOI] [PubMed] [Google Scholar]
- Kutz WE, Gong Y, Warman ML. WISP3, the gene responsible for the human skeletal disease progressive pseudorheumatoid dysplasia, is not essential for skeletal function in mice. Mol Cell Biol. 2005;25:414–421. doi: 10.1128/MCB.25.1.414-421.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake AC, Bialik A, Walsh K, Castellot JJ., Jr CCN5 is a growth arrest-specific gene that regulates smooth muscle cell proliferation and motility. Am J Pathol. 2003;162:219–231. doi: 10.1016/S0002-9440(10)63813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake AC, Castellot JJ., Jr CCN5 modulates the antiproliferative effect of heparin and regulates cell motility in vascular smooth muscle cells. Cell Commun Signal. 2003;1:5. doi: 10.1186/1478-811X-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latinkic BV, Mercurio S, Bennett B, Hirst EM, Xu Q, Lau LF, Mohun TJ, Smith JC. Xenopus Cyr61 regulates gastrulation movements and modulates Wnt signalling. Development. 2003;130:2429–2441. doi: 10.1242/dev.00449. [DOI] [PubMed] [Google Scholar]
- Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res. 1999;248:44–57. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- Leu SJ, Chen N, Chen CC, Todorovic V, Bai T, Juric V, Liu Y, Yan G, Lam SC, Lau LF. Targeted mutagenesis of the angiogenic protein CCN1 (CYR61). Selective inactivation of integrin alpha6beta1-heparan sulfate proteoglycan coreceptor-mediated cellular functions. J Biol Chem. 2004;279:44177–44187. doi: 10.1074/jbc.M407850200. [DOI] [PubMed] [Google Scholar]
- Lin CG, Leu SJ, Chen N, Tebeau CM, Lin SX, Yeung CY, Lau LF. CCN3 (NOV) is a novel angiogenic regulator of the CCN protein family. J Biol Chem. 2003;278:24200–24208. doi: 10.1074/jbc.M302028200. [DOI] [PubMed] [Google Scholar]
- Luo J, Chen J, Deng ZL, Luo X, Song WX, Sharff KA, Tang N, Haydon RC, Luu HH, He TC. Wnt signaling and human diseases: what are the therapeutic implications? Lab Invest. 2007;87:97–103. doi: 10.1038/labinvest.3700509. [DOI] [PubMed] [Google Scholar]
- Luo Q, Kang Q, Si W, Jiang W, Park JK, Peng Y, Li X, Luu HH, Luo J, Montag AG, Haydon RC, He TC. Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279:55958–55968. doi: 10.1074/jbc.M407810200. [DOI] [PubMed] [Google Scholar]
- Lv H, Fan E, Sun S, Ma X, Zhang X, Han DM, Cong YS. Cyr61 is up-regulated in prostate cancer and associated with the p53 gene status. J Cell Biochem. 2009;106:738–744. doi: 10.1002/jcb.22075. [DOI] [PubMed] [Google Scholar]
- Maeda A, Nishida T, Aoyama E, Kubota S, Lyons KM, Kuboki T, Takigawa M. CCN family 2/connective tissue growth factor modulates BMP signalling as a signal conductor, which action regulates the proliferation and differentiation of chondrocytes. J Biochem. 2009;145:207–216. doi: 10.1093/jb/mvn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard M, Cadot B, Ball RY, Sethia K, Edwards DR, Perbal B, Tatoud R. Differential expression of the ccn3 (nov) protooncogene in human prostate cell lines and tissues. Mol Pathol. 2001;54:275–280. doi: 10.1136/mp.54.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manara MC, Perbal B, Benini S, Strammiello R, Cerisano V, Perdichizzi S, Serra M, Astolfi A, Bertoni F, Alami J, Yeger H, Picci P, Scotlandi K. The expression of ccn3(nov) gene in musculoskeletal tumors. Am J Pathol. 2002;160:849–859. doi: 10.1016/S0002-9440(10)64908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason HR, Grove-Strawser D, Rubin BS, Nowak RA, Castellot JJ., Jr Estrogen induces CCN5 expression in the rat uterus in vivo. Endocrinology. 2004;145:976–982. doi: 10.1210/en.2003-0823. [DOI] [PubMed] [Google Scholar]
- Mason HR, Lake AC, Wubben JE, Nowak RA, Castellot JJ., Jr The growth arrest-specific gene CCN5 is deficient in human leiomyomas and inhibits the proliferation and motility of cultured human uterine smooth muscle cells. Mol Hum Reprod. 2004;10:181–187. doi: 10.1093/molehr/gah028. [DOI] [PubMed] [Google Scholar]
- McCallum L, Irvine AE. CCN3--a key regulator of the hematopoietic compartment. Blood Rev. 2009;23:79–85. doi: 10.1016/j.blre.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Mercurio S, Latinkic B, Itasaki N, Krumlauf R, Smith JC. Connective-tissue growth factor modulates WNT signalling and interacts with the WNT receptor complex. Development. 2004;131:2137–2147. doi: 10.1242/dev.01045. [DOI] [PubMed] [Google Scholar]
- Miller DS, Sen M. Potential role of WISP3 (CCN6) in regulating the accumulation of reactive oxygen species. Biochem Biophys Res Commun. 2007;355:156–161. doi: 10.1016/j.bbrc.2007.01.114. [DOI] [PubMed] [Google Scholar]
- Mo FE, Muntean AG, Chen CC, Stolz DB, Watkins SC, Lau LF. CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Biol. 2002;22:8709–8720. doi: 10.1128/MCB.22.24.8709-8720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N, Kuliopulos A, Graham RA, Covic L. Tumorderived Cyr61(CCN1) promotes stromal matrix metalloproteinase-1 production and protease-activated receptor 1-dependent migration of breast cancer cells. Cancer Res. 2006;66:2658–2665. doi: 10.1158/0008-5472.CAN-05-2082. [DOI] [PubMed] [Google Scholar]
- Nguyen TQ, Roestenberg P, van Nieuwenhoven FA, Bovenschen N, Li Z, Xu L, Oliver N, Aten J, Joles JA, Vial C, Brandan E, Lyons KM, Goldschmeding R. CTGF inhibits BMP-7 signaling in diabetic nephropathy. J Am Soc Nephrol. 2008;19:2098–2107. doi: 10.1681/ASN.2007111261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien TP, Yang GP, Sanders L, Lau LF. Expression of cyr61, a growth factor-inducible immediate-early gene. Mol Cell Biol. 1990;10:3569–3577. doi: 10.1128/mcb.10.7.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D, Swanson TA, Welsh JW, Roy MA, Lawrence DA, Lee J, Brush J, Taneyhill LA, Deuel B, Lew M, Watanabe C, Cohen RL, Melhem MF, Finley GG, Quirke P, Goddard AD, Hillan KJ, Gurney AL, Botstein D, Levine AJ. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci USA. 1998;95:14717–14722. doi: 10.1073/pnas.95.25.14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. NOV (nephroblastoma overexpressed) and the CCN family of genes: structural and functional issues. Mol Pathol. 2001;54:57–79. doi: 10.1136/mp.54.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachfal AW, Brigstock DR. Structural and functional properties of CCN proteins. Vitam Horm. 2005;70:69–103. doi: 10.1016/S0083-6729(05)70003-0. [DOI] [PubMed] [Google Scholar]
- Rubenstein J, Shen A, Haqq C, Ginziger D, Hyun W, Brigstock D, Shuman M. Connective tissue growth factor is expressed in malignant astrocytic tumors and is involved in cell-cycle regulation. Mol Pathol. 2003;56:72. [Google Scholar]
- Rydziel S, Stadmeyer L, Zanotti S, Durant D, Smerdel-Ramoya A, Canalis E. Nephroblastoma overexpressed (Nov) inhibits osteoblastogenesis and causes osteopenia. J Biol Chem. 2007;282:19762–19772. doi: 10.1074/jbc.M700212200. [DOI] [PubMed] [Google Scholar]
- Safadi FF, Xu J, Smock SL, Kanaan RA, Selim AH, Odgren PR, Marks SC, Jr, Owen TA, Popoff SN. Expression of connective tissue growth factor in bone: its role in osteoblast proliferation and differentiation in vitro and bone formation in vivo. J Cell Physiol. 2003;196:51–62. doi: 10.1002/jcp.10319. [DOI] [PubMed] [Google Scholar]
- Schutze N, Kunzi-Rapp K, Wagemanns R, Noth U, Jatzke S, Jakob F. Expression, purification, and functional testing of recombinant CYR61/CCN1. Protein Expr Purif. 2005;42:219–225. doi: 10.1016/j.pep.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Schutze N, Schenk R, Fiedler J, Mattes T, Jakob F, Brenner RE. CYR61/CCN1 and WISP3/CCN6 are chemoattractive ligands for human multipotent mesenchymal stroma cells. BMC Cell Biol. 2007;8:45. doi: 10.1186/1471-2121-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M, Cheng YH, Goldring B, Lotz MK, Carson DA. WISP3-dependent regulation of type II collagen and aggrecan production in chondrocytes. Arthritis Rheum. 2004;50:488–497. doi: 10.1002/art.20005. [DOI] [PubMed] [Google Scholar]
- Shakunaga T, Ozaki T, Ohara N, Asaumi K, Doi T, Nishida K, Kawai A, Nakanishi T, Takigawa M, Inoue H. Expression of connective tissue growth factor in cartilaginous tumors. Cancer. 2000;89:1466–1473. [PubMed] [Google Scholar]
- Si W, Kang Q, Luu HH, Park JK, Luo Q, Song WX, Jiang W, Luo X, Li X, Yin H, Montag AG, Haydon RC, He TC. CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A-induced osteoblast differentiation of mesenchymal stem cells. Mol Cell Biol. 2006;26:2955–2964. doi: 10.1128/MCB.26.8.2955-2964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soon LL, Yie TA, Shvarts A, Levine AJ, Su F, Tchou-Wong KM. Overexpression of WISP-1 down-regulated motility and invasion of lung cancer cells through inhibition of Rac activation. J Biol Chem. 2003;278:11465–11470. doi: 10.1074/jbc.M210945200. [DOI] [PubMed] [Google Scholar]
- Su F, Overholtzer M, Besser D, Levine AJ. WISP-1 attenuates p53-mediated apoptosis in response to DNA damage through activation of the Akt kinase. Genes Dev. 2002;16:46–57. doi: 10.1101/gad.942902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Sugimachi K, Kameyama T, Maehara S, Shirabe K, Shimada M, Wands JR, Maehara Y. Human WISP1v, a member of the CCN family, is associated with invasive cholangiocarcinoma. Hepatology. 2003;37:1122–1129. doi: 10.1053/jhep.2003.50187. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Sugimachi K, Saeki H, Kinoshita J, Ohga T, Shimada M, Maehara Y, Sugimachi K. A novel variant of WISP1 lacking a Von Willebrand type C module overexpressed in scirrhous gastric carcinoma. Oncogene. 2001;20:5525–5532. doi: 10.1038/sj.onc.1204723. [DOI] [PubMed] [Google Scholar]
- Tang N, Song WX, Luo J, Haydon RC, He TC. Osteosarcoma development and stem cell differentiation. Clin Orthop Relat Res. 2008;466:2114–2130. doi: 10.1007/s11999-008-0335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibout H, Martinerie C, Creminon C, Godeau F, Boudou P, Le Bouc Y, Laurent M. Characterization of human NOV in biological fluids: an enzyme immunoassay for the quantification of human NOV in sera from patients with diseases of the adrenal gland and of the nervous system. J Clin Endocrinol Metab. 2003;88:327–336. doi: 10.1210/jc.2002-020304. [DOI] [PubMed] [Google Scholar]
- Thorstensen L, Diep CB, Meling GI, Aagesen TH, Ahrens CH, Rognum TO, Lothe RA. WNT1 inducible signaling pathway protein 3, WISP-3, a novel target gene in colorectal carcinomas with microsatellite instability. Gastroenterology. 2001;121:1275–1280. doi: 10.1053/gast.2001.29570. [DOI] [PubMed] [Google Scholar]
- Tsai MS, Bogart DF, Castaneda JM, Li P, Lupu R. Cyr61 promotes breast tumorigenesis and cancer progression. Oncogene. 2002;21:8178–8185. doi: 10.1038/sj.onc.1205682. [DOI] [PubMed] [Google Scholar]
- van Golen KL, Davies S, Wu ZF, Wang Y, Bucana CD, Root H, Chandrasekharappa S, Strawderman M, Ethier SP, Merajver SD. A novel putative low-affinity insulin-like growth factor-binding protein, LIBC (lost in inflammatory breast cancer), and RhoC GTPase correlate with the inflammatory breast cancer phenotype. Clin Cancer Res. 1999;5:2511–2519. [PubMed] [Google Scholar]
- Vorwerk P, Hohmann B, Oh Y, Rosenfeld RG, Shymko RM. Binding properties of insulin-like growth factor binding protein-3 (IGFBP-3), IGFBP-3 N- and C-terminal fragments, and structurally related proteins mac25 and connective tissue growth factor measured using a biosensor. Endocrinology. 2002;143:1677–1685. doi: 10.1210/endo.143.5.8760. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang R, Wen S, McCafferty DM, Beck PL, MacNaughton WK. Nitric oxide increases Wnt-induced secreted protein-1 (WISP-1/CCN4) expression and function in colitis. J Mol Med. 2009;87:435–445. doi: 10.1007/s00109-009-0445-4. [DOI] [PubMed] [Google Scholar]
- Woods A, Pala D, Kennedy L, McLean S, Rockel JS, Wang G, Leask A, Beier F. Rac1 signaling regulates CTGF/CCN2 gene expression via TGFbeta/Smad signaling in chondrocytes. Osteoarthritis Cartilage. 2009;17:406–413. doi: 10.1016/j.joca.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Xie D, Yin D, Tong X, O’Kelly J, Mori A, Miller C, Black K, Gui D, Said JW, Koeffler HP. Cyr61 is overexpressed in gliomas and involved in integrin-linked kinase-mediated Akt and beta-catenin- TCF/Lef signaling pathways. Cancer Res. 2004;64:1987–1996. doi: 10.1158/0008-5472.can-03-0666. [DOI] [PubMed] [Google Scholar]
- Yanagita T, Kubota S, Kawaki H, Kawata K, Kondo S, Takano-Yamamoto T, Tanaka S, Takigawa M. Expression and physiological role of CCN4/Wnt-induced secreted protein 1 mRNA splicing variants in chondrocytes. FEBS J. 2007;274:1655–1665. doi: 10.1111/j.1742-4658.2007.05709.x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liao E. Mutant WISP3 triggers the phenotype shift of articular chondrocytes by promoting sensitivity to IGF-1 hypothesis of spondyloepiphyseal dysplasia tarda with progressive arthropathy (SEDT-PA) Med Hypotheses. 2007;68:1406–1410. doi: 10.1016/j.mehy.2006.06.046. [DOI] [PubMed] [Google Scholar]
- You Z, Saims D, Chen S, Zhang Z, Guttridge DC, Guan KL, MacDougald OA, Brown AM, Evan G, Kitajewski J, Wang CY. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J Cell Biol. 2002;157:429–440. doi: 10.1083/jcb.200201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Gao Y, Wang H, Huang L, Qin J, Guo R, Song M, Yu S, Chen J, Cui B, Gao P. The matrix protein CCN1 (CYR61) promotes proliferation, migration and tube formation of endothelial progenitor cells. Exp Cell Res. 2008;314:3198–3208. doi: 10.1016/j.yexcr.2008.08.001. [DOI] [PubMed] [Google Scholar]