Abstract
This study compared a new transient sham TENS that delivers current for 45 seconds to an inactive sham and active TENS to determine the degree of blinding and influence on pain reduction. Pressure pain thresholds (PPT), heat pain thresholds (HPT), and pain intensities to tonic heat and pressure were measured in 69 healthy adults before and after randomization. Allocation investigators and subjects were asked to identify the treatment administered. The transient sham blinded investigators 100% of the time and 40% of subjects compared to the inactive sham that blinded investigators 0% of the time and 21% of subjects. Investigators and subjects were only blinded 7% and 13% of the time, respectively, with active TENS. Neither placebo treatment resulted in significant changes in PPT, HPT, or pain intensities. Subjects using higher active TENS amplitudes (≥17mAs) had significantly higher PPTs and lower pain intensities to tonic pressure than subjects using lower amplitudes (<17mAs). HPTs and pain intensities to tonic heat were not significantly changed. The transient TENS completely blinds investigators to treatment and does not reduce pain, thereby providing a true placebo treatment.
Introduction
Transcutaneous electrical nerve stimulation (TENS) is commonly used for the relief of acute and chronic pain. TENS delivers electrical current through the skin to produce pain relief. To examine efficacy of any treatment for pain, including TENS, it should be compared to an adequate placebo that allows evaluation of its physiological effect 43. Placebo-induced expectancies decrease pain and modulate specific neural mechanisms 34, 39, 44. In some studies, placebo TENS has shown similar effects to active TENS 2, 29, 32. Thus, the evaluation of placebo treatment to discerning the true efficacy of active TENS treatment is essential. Historically, placebo methods have involved devices that use a battery and display an active indicator light but do not deliver current12, 29, 31. This method attempts to blind the subject but has the limitation of not blinding the investigator who is applying the treatment. Lack of investigator blinding can result in bias when recording outcome data and influence the findings of studies in favor of active TENS therapy or require a separate investigator to allocate treatment so the investigator assessing outcomes remains blinded. A new sham TENS device has been developed that delivers current for 30 seconds and then gradually ramps down to no current over the next 15 seconds. This approach has the potential of blinding the investigator as well as the subject to TENS treatment but whether this occurs is currently unknown. Further, it is unclear if the short stimulus provided by this placebo device elicits a physiologic response that may result in a treatment effect.
The primary purpose of this study was to determine the degree of blinding that occurs with the new transient sham TENS device that delivers current for approximately 45 seconds. We hypothesized that this device would result in significantly higher subject and investigator blinding than the inactive sham TENS therapy. A secondary aim was to determine if the brief 45 seconds of stimulation provided by the new, transient TENS placebo influenced pressure and heat pain thresholds and pain intensities to tonic heat and pressure compared to active TENS therapy. We hypothesized that this short term stimulation would not significantly increase pain thresholds or decrease pain intensities as expected for the active TENS therapy.
Materials and Methods
Subjects
Spixty-nine healthy adults (mean age 27.19 ± 1.75 years) were tested. After approval from the Institutional Review Board, subjects were recruited through posters, campus e-mail, and advertisements in campus publications. Individuals were excluded if they had: 1) any current acute or chronic pain condition; 2) prior use of TENS; 3) myocardial infarction or stroke within the last 12 months; 4) pacemaker or other contraindication to TENS; 5) pregnancy; and 6) any known neuromuscular disorders or loss of sensation (defined by lack of dull or sharp sensations when a cotton swab or the end of a paper clip was brushed over any of 3 dermatomes on the extremity being tested prior to data collection). After providing written informed consent, the subjects were stratified by gender and randomized using a computer generated randomization list and SNOSE (sequentially numbered, opaque sealed envelopes) allocation concealment method described by Doig and Simpson 16. The envelopes were stored in a secure area that only the allocation investigators had access to and were opened after consent was obtained. After data collection on the first 42 subjects, the effect of intensity seemed to be important so the remaining subjects were randomized to either transient placebo or active TENS to further distinguish the effect of TENS amplitude.
Demographic information including age, gender, height, and weight were recorded. There were no significant differences between groups based on age, gender, or body mass index (BMI) (Table 1).
Table 1.
Subject Demographics. No significant difference between groups on gender, age, or BMI (Body Mass Index).
| Treatment Group – random assignment | Inactive Placebo (n = 14) | Transient Placebo (n = 25) | Active TENS (n = 30) |
|---|---|---|---|
| Gender | |||
| Male | 7 (50%) | 14 (56%) | 15 (50%) |
| Female | 7 (50%) | 11 (44%) | 15 (50%) |
| Age (Mean ± SD) | 29.92 ± 3.08 | 28.16 ± 1.48 | 25.10 ± 1.35 |
| BMI (Mean ± SD) | 25.62 ± 1.20 | 27.54 ± 1.24 | 25.14 ± 1.12 |
TENS Treatment
Subjects were randomized to receive one of three TENS treatments: 1) active TENS therapy (n=30); 2) a new, transient placebo TENS therapy using a custom made unit that is active for the first 30 seconds then ramps down to zero stimulus over 15 seconds (n=25); or 3) inactive placebo TENS therapy using a non-functional unit that appears to work but provides no stimulus (n=14) (Figure 1). All devices were Rehabilicare Maxima TENS units (Empi Inc., St. Paul, MN).
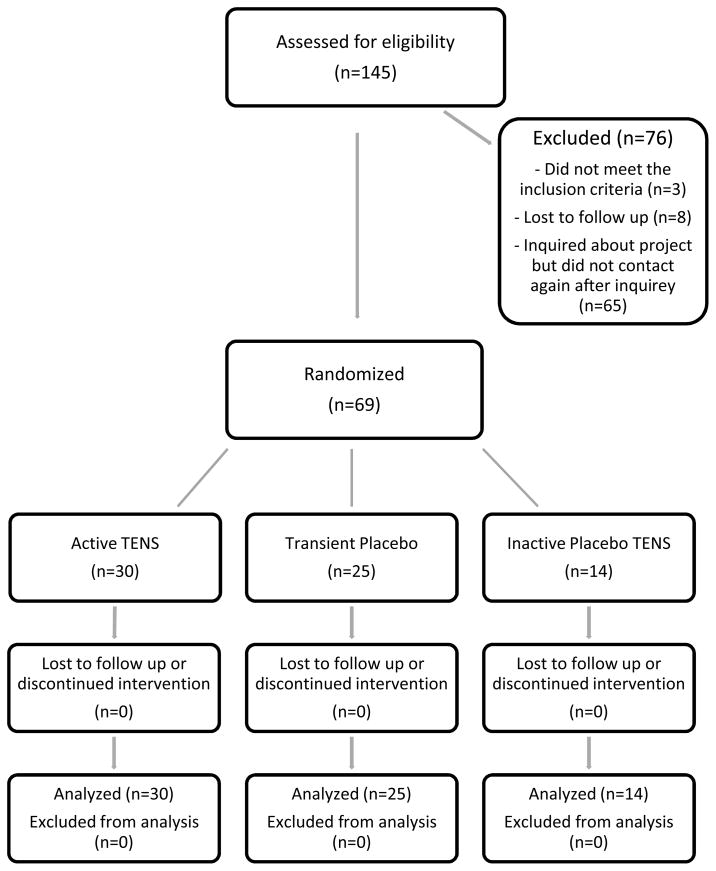
Figure 1.
CONSORT trail flow-chart.
Stimulation parameters were constant mode, 100 Hz pulse rate, and 100 μsec pulse duration. Pulse amplitude was determined for the active and transient placebo TENS in all cases as a strong, but comfortable intensity. A planned interim analysis for effects of intensity on PPT was done for the following reasons: 1) Previous work in animals had utilized the initial intensity of 10% below motor threshold37 but this had never been tested in humans to know if it was an adequate intensity. 2) Prior clinical literature5, 31 had shown an intensity dependent effect of TENS. It was therefore important to ensure we had adequate intensity to get analgesia to test the hypothesis and validate the new placebo. In the first series of data collected, intensity was increased until a motor contraction was produced, and then the pulse amplitude was decreased to 10% below motor contraction (active n=15 or transient placebo n=11) as previously used in animal studies36. An interim analysis, using Pearson’s correlations, showed that those able to tolerate a greater intensity gave a greater degree of analgesia. We subsequently allocated the remaining subjects into a different protocol to maximize analgesia with a greater intensity. This protocol was achieved by increasing pulse amplitude until reaching the maximum intensity that the participant could tolerate, regardless of muscle contraction (active n=15; transient placebo n=14). At all times, there was equal randomization between placebo (old and transient) and active TENS groups, and the randomization schedule did not change. We did not randomize on intensity. Before the interim analysis the high intensity group had 6 subjects in the active (n=9, low intensity) and 3 in the transient placebo (n=8, low intensity), and after interim analysis the high intensity group had 11 subjects in the active (n=4 low intensity) and 11 in the transient placebo group (n=3 low intensity). The TENS units were calibrated using an oscilloscope prior to starting the study. For each pulse amplitude setting on the devices, peak to peak voltage was measured across a 1 kΩ resistor to calculate the corresponding current in mAs.
Parameters were set within the first 30 seconds of stimulation. Pulse amplitude was recorded for each subject. The inactive placebo units were set at an amplitude of 25 mA. TENS was applied with two, 2-inch square, self-adhesive electrodes placed at two points on the posterior aspect of the non-dominant forearm: one distal to the elbow and the other above the wrist crease (Figure 2). Treatment was applied by two investigators who did not participate in outcome assessments.
Figure 2.
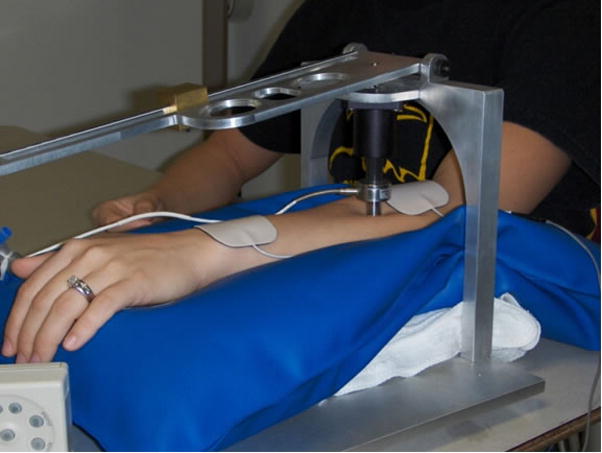
Pressure temporal summation measurement during TENS application. Pressure device includes a pressure transducer and a lever with a movable weight to grade the force delivered. The pressure stimulus is delivered through a 1 cm2 probe over the extensor muscle mass.
Pressure Pain Threshold
PPT was assessed using a digital pressure algometer (Somedic AB, Farsta, Sweden) with a 1 cm2 tip and a controlled rate of stimulus delivery (40 kPA/s). Subjects were instructed to activate a button when the sensation of pressure clearly became one of painful pressure and were familiarized with the assessment by completing two practice trials on their dominant forearm. PPTs were assessed at three marked sites on the subject’s non-dominant forearm, 2 cm apart over the wrist extensor muscle mass and distal to the elbow. With this method, mean pressure pain thresholds (PPT) of the knee average approximately 250 kPa 28. Previous studies demonstrate that anesthetic blockade of the skin under the algometer has no effect on the PPT, thus this is a measure of deep tissue hyperalgesia 4, 25.
Pain Intensities to Tonic Pressure
Pain intensities to tonic pressure were used to determine pressure temporal summation. Tonic pressure was applied using a custom built device incorporating a pressure transducer and a lever with a movable weight to grade the force delivered (see Figure 2). The forearm was secured in place with a vacuum pillow (VersForm, SammonsPreston, Bolingbrook, IL). The pressure stimulus was delivered through a 1 cm2 probe over the extensor muscle mass proximal to the location of the PPT readings. Pressure threshold was reassessed with this device and 130% of this threshold value was used as the tonic pressure stimulus for each subject. This same pressure was applied continuously for two minutes while subjects marked the intensity of their pain on a 100 mm visual analog scale (VAS) at 10 s intervals starting at initial application of the stimulus. The VAS has high reliability, validity, and sensitivity, particularly when attempting to determine the therapeutic effect of specific treatments 11, 35.
Heat Pain Threshold
Heat pain threshold (HPT) was assessed using a TSAII NeuroSensory Analyzer (Medoc Ltd, Ramat Yishai, Israel) as previously described 17, 42 with a 16×16 mm stimulator. Use of a 16×16mm stimulator was based on our preliminary work showing more consistent development of temporal summation with this smaller probe size compared to the larger (30×30mm) probe (unpublished). Temperature started at 37 °C and increased by 1 °C/s to a maximum of 52 °C. The thermal stimulus was terminated when the subject first perceived pain. If pain was not perceived by 52 °C, the test was stopped for subject safety and this temperature was recorded as the pain threshold. Subjects were familiarized with the assessment by completing two practice trials on their dominant forearm. HPTs were assessed at the same three marked sites as the PPTs on the subject’s non-dominant forearm over the extensor muscle mass. Threshold occurs at temperatures of 44.5 to 45.0°C on normal skin.
Pain Intensities to Tonic Heat
Pain intensities to tonic heat stimuli were used to determine heat temporal summation. Tonic heat was applied using the same TSAII NeuroSensory Analyzer and 16×16 mm stimulator used for HPTs. A continuous stimulus of 46 °C was applied for 100 seconds on a marked site over the extensor muscle mass, adjacent to the testing sites for the heat and pressure pain thresholds on the non-dominant forearm. Subjects rated their pain response every 10 s on the 100 mm VAS described above, beginning with the application of the thermal stimulus. Subjects were familiarized with this assessment by completing one trial on their dominant forearm. The room temperature was maintained between 20 and 23°C. This method has been used by other investigators to successfully measure temporal summation 19, 21, 33.
Protocol
Pain measures were performed by an outcome assessor who was blind to group allocation in the following order: HPT, PPT, tonic heat, tonic pressure. Prior to each test, participants were familiarized with the assessment by completing practice trials as described above. The outcome assessor then left the room and another investigator randomized the subject to treatment. The assigned TENS treatment was applied to the subject’s forearm by a third investigator. After a 20 minute treatment interval, the outcome assessor returned and repeated the four pain measures. The TENS device was then discontinued and the subject’s height and weight were measured.
Blinding Assessment
At the conclusion of testing, the outcome assessor asked the subject “Do you think you received an active or placebo treatment?” The investigator applying the TENS treatment was asked “Do you think the subject received an active or placebo treatment?” Their responses to these questions were recorded and used to gauge the adequacy of subject and investigator blinding.
Statistical Analysis
Blinding was coded as 0 for correct group assignment and 1 for incorrect (blinded) group assignment. PPTs and HPTs were calculated as the mean of the three sites tested at each time point. Changes in PPT and HPT were calculated as % of baseline, where no change is equivalent to 0%. Changes in pain intensities for both tonic heat and pressure were calculated as a change in the area under the curve for the duration of testing (i.e. 100 seconds for heat and 120 seconds for pressure) post-TENS to pre-TENS. Descriptive statistics were calculated for all variables. χ tests were used to compare blinding between groups, and to compare against an expected result of 50:50 blinding (i.e. chance). One-way ANOVA compared differences between groups and repeated measures ANOVA was used to compare differences across time for treatment data (i.e. PPT, HPT, area under the curve for tonic heat and pressure). Post hoc testing was performed with a Tukey’s test for differences between groups and with a paired t-test for differences across time. Pearson product-moment correlation coefficients were used to assess levels of associations between TENS pulse amplitudes and the pressure pain sensitivity measures: PPT and pain intensities to tonic pressure. All data are presented as mean +/− S.E.M; significance was set at p<0.05.
Results
Adequacy of Blinding
The new transient placebo TENS completely blinded the investigators to treatment. Investigators correctly identified the transient placebo TENS as a placebo treatment 0% of the time (0/25). In other words, they thought they were applying active TENS therapy 100% of the time when using this sham device. Investigators correctly identified the inactive placebo TENS as a placebo treatment 100% of the time (14/14) and correctly identified the active treatment 93% of the time (28/30). Investigator responses were significantly different when comparing the new transient placebo to both the inactive placebo and the active treatment (p<0.0001).
Subjects were blinded to the new transient placebo 40% of the time (10/25), the inactive placebo 21% of the time (3/14), and the active treatment 13% of the time (4/30). The new transient placebo was no different than chance (random 50:50 probability) suggesting adequate blinding. In contrast, the inactive placebo and the active TENS were more frequently identified as correct than chance alone (p<0.05) suggesting that subjects were not blinded to treatment group. Correct responses in the inactive placebo group were not significantly different from either the new transient placebo or the active TENS groups (p>0.05). Correct responses were identified less frequently in the new transient placebo compared to the active treatment (p<0.05).
Treatment Effects
There were no significant changes in PPT, HPT, or pain intensities to tonic heat or pressure after the new transient placebo TENS or inactive placebo TENS treatments were applied (Figures 3a & b and 4 a, b, c, & d). There were also no significant differences in effect between the two placebo groups.
Figure 3.
Percent change from baseline to after treatment for: A) heat pain thresholds; and B) pressure pain thresholds for each group (Active TENS, Inactive Placebo TENS, and Transient Placebo TENS.
Figure 4.
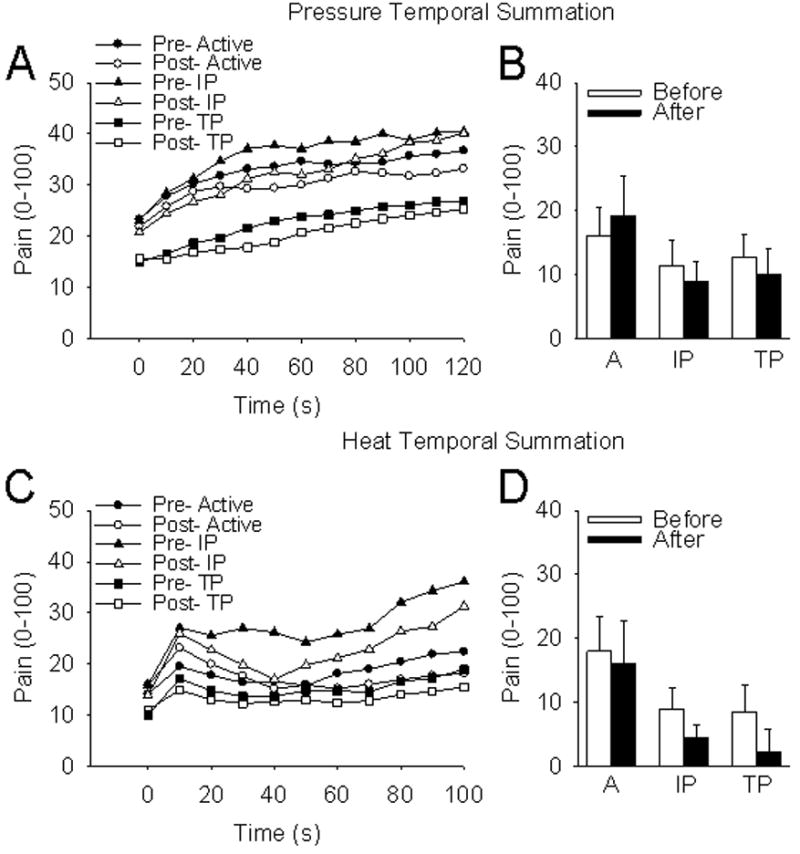
Temporal summation to pressure and heat stimuli. A) Pressure pain intensity scores pre and post treatment for each group from 0–120 seconds; B) Average pressure pain intensity scores pre and post treatment for each group; C) Heat pain intensity scores pre and post treatment for each group from 0–120 seconds; D) Average heat pain intensity scores pre and post treatment for each group. A=Active TENS; IP=Inactive Placebo TENS; TP=Transient Placebo TENS.
In the group receiving active TENS, changes in PPT were not significant overall (Figure 3b). However, when considering those receiving higher pulse amplitudes (defined as peak amplitudes between 17–25mA; N = 17 subjects with or without muscle contraction), there was a significant increase in PPT (17.5% ± 5.0%) compared to those receiving lower pulse amplitudes (i.e. peak amplitudes < 17mA; N = 13)(F1,29 = 9.8, p = 0.004) and increases in PPT were significantly correlated with increases in pulse amplitude (Pearson’s correlation, r = 0.3, p = 0.05). In the new transient placebo group, PPT increased only 3.5% ± 4.4% (Figure 5a & b) and increases in PPT were not significantly correlated with increases in pulse amplitudes (r = −0.03, P = 0.44).
Figure 5.

A) Scatter plot and correlation between percent change in Pressure Pain Threshold (PPT) and TENS pulse amplitudes; B) percent change in PPT for low (0–16mA) and high (17–25mA) TENS pulse amplitudes; C) scatter plot and correlation between change in average pain intensity during temporal summation and TENS pulse amplitudes; D) change in average pain intensity scores during pressure temporal summation and low (0–16mA) and high (17–25mA) TENS pulse amplitudes. Those receiving higher TENS pulse amplitudes in the active TENS group (17–25mA; N = 17), had a significant increase in PPT compared to those receiving lower pulse amplitudes (< 17mA; N = 13)(F1,29 = 9.8, p = 0.004).
Similarly, changes in pain intensities to tonic pressure were not significantly different overall in the group receiving active TENS. However, those receiving higher pulse amplitudes (17–25mA; N = 17) had significantly lower pain intensities to tonic pressure compared to those receiving lower pulse amplitudes (<17mA; N= 13)(F1,29 = 5.3, p = 0.028). A reduction in pressure temporal summation was observed for the group receiving active TENS with mean baseline pain rating increases of 20.5 ± 7.3 mm and post-treatment pain rating increases of only 2.4 ± 3.9 mm. Whereas, in the transient placebo TENS group, no change in temporal summation occurred: baseline increase of 14 ± 0.6 mm and post-treatment increase of 21 ± 0.6 mm. A significant negative correlation was observed between change in pressure temporal summation and pulse amplitude for the active TENS group (r = −0.42, p = 0.02) but not for the transient placebo group (r = 0.03, p = 0.88) (Figure 5c & d).
Changes in HPTs and pain intensities to tonic heat were not significantly different after active TENS treatment. Comparing high and low TENS amplitudes in this group did not result in significant differences.
Discussion
Adequacy of Blinding
The new transient placebo completely blinded the allocation investigators to treatment while these investigators were always aware when they were applying the inactive placebo. This finding supports our hypothesis that the transient placebo results in significantly higher investigator blinding than the inactive placebo TENS. The high level of investigator blinding with the new transient sham device was due to the presence of muscle contraction each time this device was applied, similar to what they saw when applying the active treatment. This resulted in the investigator thinking they were applying an active unit. There was no muscle contraction when the inactive placebo was applied making it obvious that a placebo unit was being used.
The new transient placebo TENS could also be applied using the same script and parameters as the active TENS treatment, whereas a separate script and parameters were needed for application of the inactive placebo (see Table 2). This approach has obvious cost and protocol advantages for prospective randomized clinical trials by allowing the same investigator to both apply the treatment and assess outcomes. It also eliminates investigator expectation bias. Investigators, having been exposed to research goals and hypotheses, can inadvertently influence respondents to produce outcomes consistent with those expectations10, 43. During application of an inactive placebo TENS, the investigator, knowing a placebo treatment is being applied, may inadvertently approach this application differently and in a manner subtly suggesting lack of effect which may influence the subject’s expectations and responses. This influence on outcome results is particularly possible when measuring subjective outcomes like pain.
Table 2.
TENS protocol scripts. Same script used for active and transient placebo TENS application. Separate script used for inactive placebo TENS application.
| Active and Transient Placebo TENS Script: |
| “The goal of this study is to test the effect of a high intensity TENS treatment. I am going to increase the intensity level until you feel a strong but comfortable sensation. If the stimulus becomes uncomfortable, I will turn the intensity down from that point.” Press channel up arrows, in alternating fashion, to increase the intensity to the point the subject indicates it is a strong but comfortable sensation. If muscle contraction occurs, reduce amplitude 10% from point of muscle contraction (protocol 1) or if muscle contraction causes movement of the wrist or fingers turn amplitude down (protocol 2). . |
| Placebo TENS Script: |
| “The goal of this study is to test the effect of a low intensity TENS treatment. I am going to set the intensity at a level that you may or may not feel.” Set intensity level to “2” for both channels. “Do you feel anything?” If subject responds “no” state “That is alright. The stimulus will still get to your nerve fibers even if you don’t feel it.” If the subject answers “yes” state, “Good. If the feeling goes away, that is alright because the stimulus will still get to your nerve fibers even if you don’t feel it.” |
Subject blinding for the new transient placebo TENS was not significantly different than subject blinding for the inactive placebo TENS (which occurred in frequencies consistent with prior studies 15). Thus, our hypothesis that subject blinding would significantly improve with the new transient sham device was not supported. However, it is possible that the 40% of correct responses occurred by chance and subjects were totally blinded to treatment when using the new transient sham device. If subjects did not know what treatment they received and had to guess, they would have a 50% chance of guessing the correct treatment. In this case, 40% of subjects guessed correctly.
It is also possible that these subjects felt the stimulation ramp off suggesting to them that they are receiving a placebo treatment. Since use of a high amplitude is associated with improved outcomes5, 9, the intensity goal was set to a strong but comfortable level. It is necessary to approach a transient placebo in this same manner to maintain investigator blinding and eliminate the effects of expectation bias. Another approach may be to use the transient placebo in a sub-sensory manner so subjects feel the stimulation initially but not once it is set to the sub-sensory level or when ramping off. The disadvantage of this approach is eliminating investigator blinding and requiring a separate investigator to measure outcomes. This approach is costly and presents challenges of providing multiple individuals to apply treatment and assess outcomes in a randomized clinical investigation.
An important but not surprising finding is that both subjects and investigators correctly identified active treatment with the majority of applications (87% and 93%, respectively) and correctly identified active treatment significantly more often than placebo treatment when the transient sham device was used. These results are consistent with prior research15 and demonstrate a lack of blinding for both subjects and investigators to active treatment when using physical modalities such as TENS. This lack of blinding can lead to an expectation bias that may overestimate treatment effect in groups receiving active TENS. The constant stimulation may influence subject responses to outcome measures because it confirms to them that active treatment is being delivered. Investigators may inadvertently influence respondents due to their expectation that active treatment should produce better outcomes. These findings highlight the importance of assessing the degree of blinding when evaluating the efficacy of physical modalities such as TENS. It is important to keep this effect in mind when interpreting results to avoid overestimating the effects of treatment.
Treatment effects
Subjects treated with the transient placebo had no significant physiologic effects from the short duration of treatment they received. PPT, HPT, and pain intensities to tonic heat and pressure were unchanged after treatment with the new transient placebo TENS. Therefore, this new sham device performed as an adequate placebo, mimicking the treatment without producing treatment effects.
Subjects treated with active TENS had the greatest increases in PPT and tonic pressure pain intensities compared to inactive and transient placebos, though these changes were only significant with higher pulse amplitudes. Further, there was a reduction in pain intensities to tonic pressure with active TENS at higher pulse amplitudes when compared to placebo TENS. It should be noted, however, that subjects were not randomized on intensity, but rather randomized between active and placebo. Thus, as the first group had more low intensity and the second had more high intensity, there could have been a difference between protocols. However, these data are in agreement with previous studies displaying an effect of TENS on PPT in humans and animals12, 13, 23, 27, 36 and an intensity effect of TENS treatment1, 5, 8, 9, 40. These data showing effects on reduction of temporal summation suggest a central mechanism, as wind-up of dorsal horn neurons is thought to be the underlying mechanism for temporal summation. This central effect of TENS further validates animal studies showing reduction in sensitization and nociceptive activity of dorsal horn neurons and activation of central inhibitory pathways by TENS20, 24, 26, 37.
Surprisingly, there was no significant difference in HPTs during active TENS, contrary to our initial hypothesis. This is in agreement with prior studies who similarly showed no differences in HPT with high-frequency TENS18, 38 but in contrast with other studies showing an effect in healthy subjects6, 7, 14, 41. Differences in experimental design may account for these findings. For example, positive effects for high-frequency TENS were observed if electrodes were placed over the radial or median nerves or over acupoints with the heat pain stimuli applied over the skin area of the stimulated nerve6, 14, 38. Electrode placement in the current study was over the extensor muscle of the posterior forearm with heat pain stimuli applied over the muscle body. Other possible differences include: use of high frequency TENS versus a modulated frequency which has resulted in higher HPT elevations 38, use of a 16×16mm stimulator instead of a larger stimulator which has shown positive effects on HPT 6, 30, 38, and conducting measurements only during TENS stimulation rather than continuing to measure HPT after TENS application which has demonstrated a positive effect on HPT 6, 14.
These results provide evidence for the use of a new transient sham TENS device, although several study limitations should be addressed in future studies. We used a young healthy cohort, thus these findings may vary with older individuals or patients with specific diseases/illnesses. The subject blinding effects to the active treatment may even improve in clinical cohorts, although the subject blinding to the transient placebo was similar to chance and thus may remain similar. With two placebo devices and an increased number of placebo groups there could have been an increased chance that the subject chose the placebo treatment. However this is unlikely since the subject guessed the active TENS device more accurately than chance. It is likely that the strong intensity of stimulation used in this study, which is necessary to produce an analgesic effect, made it difficult to blind the subject to the active unit. Investigator blinding is not likely to be strongly influenced by study population, thus the improved investigator blinding with the transient device would be expected to be a robust finding. Our study involved a relatively small, although adequately powered, sample size. Larger studies may be able to detect even smaller differences between the sham devices than reported here. While we assessed for blinding, we did not assess for expectation of treatment effect. Expectation of treatment effect can clearly influence outcome3, 22. Lastly, variations in pain sensitivity observed here may not translate to other quantitative pain assessments (e.g. cold presser task) or pain ratings resulting from clinical or experimental pain conditions. Future studies may help to clarify these issues.
Conclusions
These data support the use of a new transient placebo TENS in future single-blind, randomized, clinical trials of TENS. It provides cost and protocol advantages by allowing the same script for active and placebo allocation and use of the same investigator for treatment allocation and outcome assessment.
Acknowledgments
Supported by NIH R03 NR010405, Congdon Faculty Development fund, and the Carver College of Medicine at the University of Iowa. We wish to thank Shannon Lehman for assistance with recruitment and scheduling, and Matthew J. Granquist with is assistance with data collection.
Footnotes
Perspective
This article presents the benefits of a new transient sham TENS device for use in prospective, randomized, clinical trials. This device facilitates blinding of subjects and investigators to eliminate expectation bias and determine the true efficacy of TENS for use in clinical populations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aarskog R, Johnson MI, Demmink JH, Lofthus A, Iversen V, Lopes-Martins R, Joensen J, Bjordal JM. Is mechanical pain threshold after transcutaneous electrical nerve stimulation (TENS) increased locally and unilaterally? A randomized placebo-controlled trial in healthy subjects. Physiother Res Int . 2007;12(Suppl 4):251–263. doi: 10.1002/pri.384. [DOI] [PubMed] [Google Scholar]
- 2.Agency for Health Care Policy and Research: Agency for Health Care Policy and Research. Acute pain management: Guideline technical report. 1995;1:95–0034. [Google Scholar]
- 3.Bialosky JE, Bishop MD, Robinson ME, Barabas JA, George SZ. The influence of expectation on spinal manipulation induced hypoalgesia: an experimental study in normal subjects. BMC Musculoskelet Disord. 2008;9:19. doi: 10.1186/1471-2474-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjerring P, Arendt-Nielsen L. Depth and duration of skin analgesia to needle insertion after topical application of EMLA cream. Br J Anaesth . 1990;64(Suppl 2):173–177. doi: 10.1093/bja/64.2.173. [DOI] [PubMed] [Google Scholar]
- 5.Bjordal JM, Johnson MI, Ljunggreen AE. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta-analysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain . 2003;7(Suppl 2):181–188. doi: 10.1016/S1090-3801(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 6.Buonocore M, Camuzzini N. Increase of the heat pain threshold during and after high-frequency transcutaneous peripheral nerve stimulation in a group of normal subjects. Eura Medicophys . 2007;43(Suppl 2):155–160. [PubMed] [Google Scholar]
- 7.Cheing GL, Hui-Chan CW. Analgesic effects of transcutaneous electrical nerve stimulation and interferential currents on heat pain in healthy subjects. J Rehabil Med . 2003;35(Suppl 1):15–19. doi: 10.1080/16501970306101. [DOI] [PubMed] [Google Scholar]
- 8.Chesterton LS, Barlas P, Foster NE, Lundeberg T, Wright CC, Baxter GD. Sensory stimulation (TENS): effects of parameter manipulation on mechanical pain thresholds in healthy human subjects. Pain . 2002;99(Suppl 1–2):253–262. doi: 10.1016/s0304-3959(02)00118-5. [DOI] [PubMed] [Google Scholar]
- 9.Chesterton LS, Foster NE, Wright CC, Baxter GD, Barlas P. Effects of TENS frequency, intensity and stimulation site parameter manipulation on pressure pain thresholds in healthy human subjects. Pain . 2003;106(Suppl 1–2):73–80. doi: 10.1016/s0304-3959(03)00292-6. [DOI] [PubMed] [Google Scholar]
- 10.Clarke P, Sproston K, Thomas R. An investigation into expectation-led interviewer effects in health surveys. Soc Sci Med . 2003;56(Suppl 10):2221–2228. doi: 10.1016/s0277-9536(02)00238-1. [DOI] [PubMed] [Google Scholar]
- 11.Clavel-Chapelon F, Paoletti C, Benhamou S. Smoking cessation rates 4 years after treatment by nicotine gum and acupuncture. Prev Med . 1997;26(Suppl 1):25–28. doi: 10.1006/pmed.1996.9997. [DOI] [PubMed] [Google Scholar]
- 12.Claydon LS, Chesterton LS, Barlas P, Sim J. Effects of simultaneous dual-site TENS stimulation on experimental pain. Eur J Pain . 2008;12(Suppl 6):696–704. doi: 10.1016/j.ejpain.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Cowan S, McKenna J, McCrum-Gardner E, Johnson MI, Sluka KA, Walsh DM. An Investigation of the Hypoalgesic Effects of TENS Delivered by a Glove Electrode. J Pain. 2009 doi: 10.1016/j.jpain.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean J, Bowsher D, Johnson MI. The effects of unilateral transcutaneous electrical nerve stimulation of the median nerve on bilateral somatosensory thresholds. Clin Physiol Funct Imaging . 2006;26(Suppl 5):314–318. doi: 10.1111/j.1475-097X.2006.00689.x. [DOI] [PubMed] [Google Scholar]
- 15.Deyo RA, Walsh NE, Schoenfeld LS, Ramamurthy S. Can trials of physical treatments be blinded? The example of transcutaneous electrical nerve stimulation for chronic pain. Am J Phys Med Rehabil . 1990;69(Suppl 1):6–10. doi: 10.1097/00002060-199002000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Doig GS, Simpson F. Randomization and allocation concealment: a practical guide for researchers. J Crit Care. 20(Suppl 2):187–91. doi: 10.1016/j.jcrc.2005.04.005. discussion 191–3, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Drummond PD, de Silva-Rossdeutscher E. Transcutaneous iontophoresis of methadone provokes local flushing and thermal hyperalgesia. Inflamm Res . 2003;52(Suppl 9):366–371. doi: 10.1007/s00011-003-1188-2. [DOI] [PubMed] [Google Scholar]
- 18.Ekblom A, Hansson P. Thermal sensitivity is not changed by acute pain or afferent stimulation. J Neurol Neurosurg Psychiatry . 1987;50(Suppl 9):1216–1220. doi: 10.1136/jnnp.50.9.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fillingim RB, Ness TJ, Glover TL, Campbell CM, Price DD, Staud R. Experimental pain models reveal no sex differences in pentazocine analgesia in humans. Anesthesiology . 2004;100(Suppl 5):1263–1270. doi: 10.1097/00000542-200405000-00031. [DOI] [PubMed] [Google Scholar]
- 20.Garrison DW, Foreman RD. Classification of dorsal horn neurons based on somatic receptive fields in cats with intact and transected spinal cords: neural plasticity. Brain Res . 1997;762(Suppl 1–2):228–230. doi: 10.1016/s0006-8993(97)00486-1. [DOI] [PubMed] [Google Scholar]
- 21.Gibson W, Arendt-Nielsen L, Graven-Nielsen T. Referred pain and hyperalgesia in human tendon and muscle belly tissue. Pain . 2006;120(Suppl 1–2):113–123. doi: 10.1016/j.pain.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 22.Goffaux P, Redmond WJ, Rainville P, Marchand S. Descending analgesia--when the spine echoes what the brain expects. Pain . 2007;130(Suppl 1–2):137–143. doi: 10.1016/j.pain.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Gopalkrishnan P, Sluka KA. Effect of varying frequency, intensity, and pulse duration of transcutaneous electrical nerve stimulation on primary hyperalgesia in inflamed rats. Archives of Physical Medicine and Rehabilitation. 2000;81(Suppl 7):984–990. doi: 10.1053/apmr.2000.5576. [DOI] [PubMed] [Google Scholar]
- 24.Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS) J Pharmacol Exp Ther . 2001;298(Suppl 1):257–263. [PubMed] [Google Scholar]
- 25.Kosek E, Ekholm J, Hansson P. Pressure pain thresholds in different tissues in one body region. The influence of skin sensitivity in pressure algometry. Scand J Rehabil Med . 1999;31(Suppl 2):89–93. doi: 10.1080/003655099444597. [DOI] [PubMed] [Google Scholar]
- 26.Ma YT, Sluka KA. Reduction in inflammation-induced sensitization of dorsal horn neurons by transcutaneous electrical nerve stimulation in anesthetized rats. Exp Brain Res . 2001;137(Suppl 1):94–102. doi: 10.1007/s002210000629. [DOI] [PubMed] [Google Scholar]
- 27.Marchand S, Bushnell MC, Duncan GH. Modulation of heat pain perception by high frequency transcutaneous electrical nerve stimulation (TENS) Clin J Pain . 1991;7(Suppl 2):122–129. doi: 10.1097/00002508-199106000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Moss P, Sluka K, Wright A. The initial effects of knee joint mobilization on osteoarthritic hyperalgesia. Manual Therapy. 2007;12(Suppl 2):109–118. doi: 10.1016/j.math.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Oosterhof J, Samwel HJ, de Boo TM, Wilder-Smith OH, Oostendorp RA, Crul BJ. Predicting outcome of TENS in chronic pain: a prospective, randomized, placebo controlled trial. Pain . 2008;136(Suppl 1–2):11–20. doi: 10.1016/j.pain.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Palmer ST, Martin DJ, Steedman WM, Ravey J. Effects of electric stimulation on C and A delta fiber-mediated thermal perception thresholds. Arch Phys Med Rehabil . 2004;85(Suppl 1):119–128. doi: 10.1016/s0003-9993(03)00432-5. [DOI] [PubMed] [Google Scholar]
- 31.Rakel B, Frantz R. Effectiveness of transcutaneous electrical nerve stimulation on postoperative pain with movement. J Pain . 2003;4(Suppl 8):455–464. doi: 10.1067/s1526-5900(03)00780-6. [DOI] [PubMed] [Google Scholar]
- 32.Razavi M, Jansen GB. Effects of acupuncture and placebo TENS in addition to exercise in treatment of rotator cuff tendinitis. Clin Rehabil . 2004;18(Suppl 8):872–878. doi: 10.1191/0269215504cr849oa. [DOI] [PubMed] [Google Scholar]
- 33.Robinson ME, Wise EA, Gagnon C, Fillingim RB, Price DD. Influences of gender role and anxiety on sex differences in temporal summation of pain. J Pain . 2004;5(Suppl 2):77–82. doi: 10.1016/j.jpain.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry . 2008;65(Suppl 2):220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- 35.Seymour RA. The use of pain scales in assessing the efficacy of analgesics in postoperative dental pain. Eur J Clin Pharmacol. 1982;23(Suppl 5):441–444. doi: 10.1007/BF00605995. [DOI] [PubMed] [Google Scholar]
- 36.Sluka KA, Bailey K, Bogush J, Olson R, Ricketts A. Treatment with either high or low frequency TENS reduces the secondary hyperalgesia observed after injection of kaolin and carrageenan into the knee joint. Pain . 1998;77(Suppl 1):97–102. doi: 10.1016/S0304-3959(98)00090-6. [DOI] [PubMed] [Google Scholar]
- 37.Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J Pharmacol Exp Ther . 1999;289(Suppl 2):840–846. [PubMed] [Google Scholar]
- 38.Tong KC, Lo SK, Cheing GL. Alternating frequencies of transcutaneous electric nerve stimulation: does it produce greater analgesic effects on mechanical and thermal pain thresholds? Arch Phys Med Rehabil . 2007;88(Suppl 10):1344–1349. doi: 10.1016/j.apmr.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 39.Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci U S A . 2007;104(Suppl 26):11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang B, Tang J, White PF, Naruse R, Sloninsky A, Kariger R, Gold J, Wender RH. Effect of the intensity of transcutaneous acupoint electrical stimulation on the postoperative analgesic requirement. Anesth Analg . 1997;85(Suppl 2):406–413. doi: 10.1097/00000539-199708000-00029. [DOI] [PubMed] [Google Scholar]
- 41.Wang N, Hui-Chan C. Effects of acupoints TENS on heat pain threshold in normal subjects. Chin Med J (Engl) . 2003;116(Suppl 12):1864–1868. [PubMed] [Google Scholar]
- 42.Werner MU, Duun P, Kraemer O, Lassen B, Kehlet H. Arthroscopic knee surgery does not modify hyperalgesic responses to heat injury. Anesthesiology . 2003;99(Suppl 5):1152–1157. doi: 10.1097/00000542-200311000-00023. [DOI] [PubMed] [Google Scholar]
- 43.Wigley R. When is a placebo effect not an effect? Clin Med. 2007;7(Suppl 5):450–452. doi: 10.7861/clinmedicine.7-5-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci . 2005;25(Suppl 34):7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]