Abstract
Previous studies have demonstrated that considerable amounts of parenterally administered cardiac glycosides are excreted in the bile and reabsorbed across the intestinal mucosa in several species. It is currently believed that the more prolonged action of nonpolar digitalis glycosides is due to their retention and recycling in the enterohepatic circulation. This report describes studies carried out to evaluate the effects of pharmacologic interruption of this enterohepatic cycle with the intraluminal sequestering agent cholestyramine.
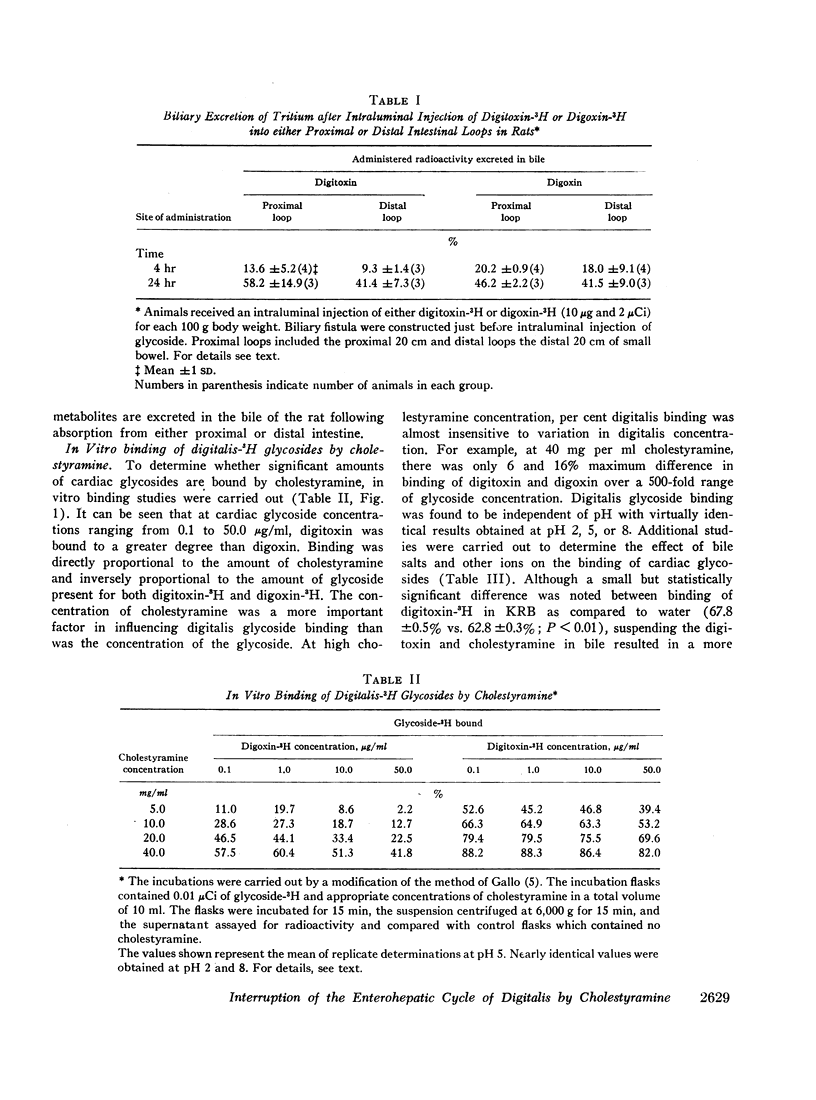
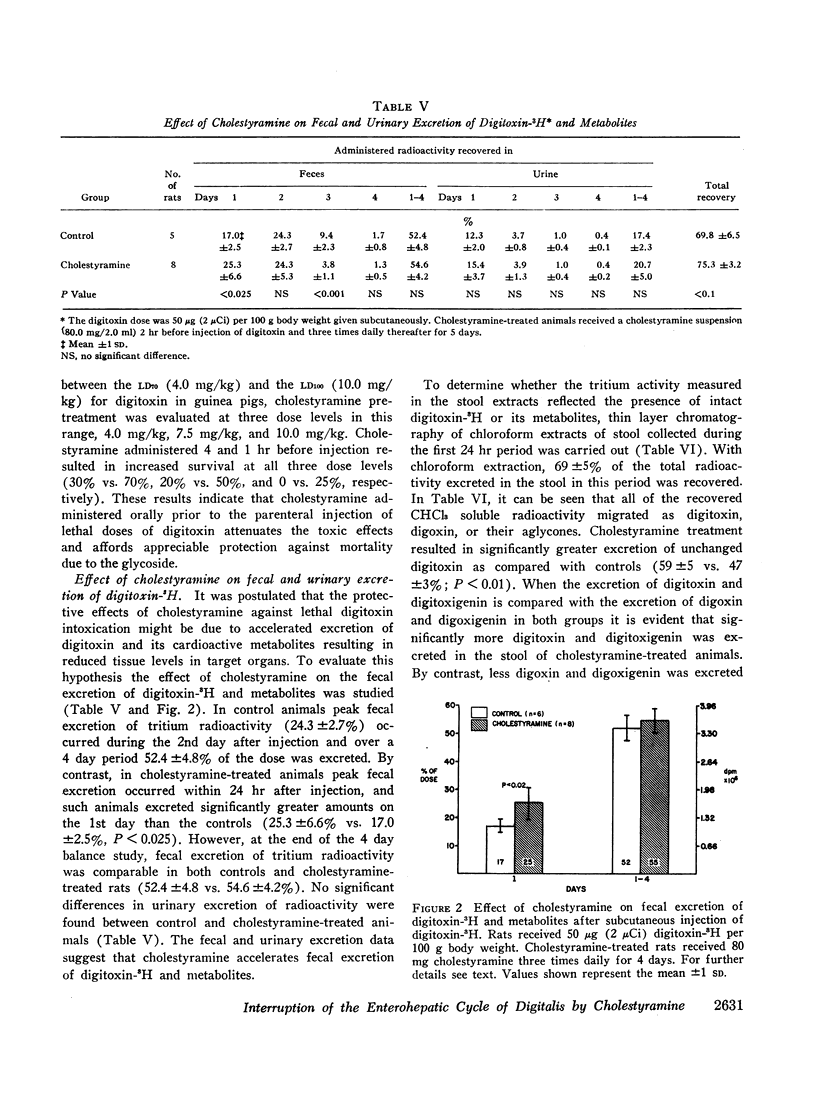
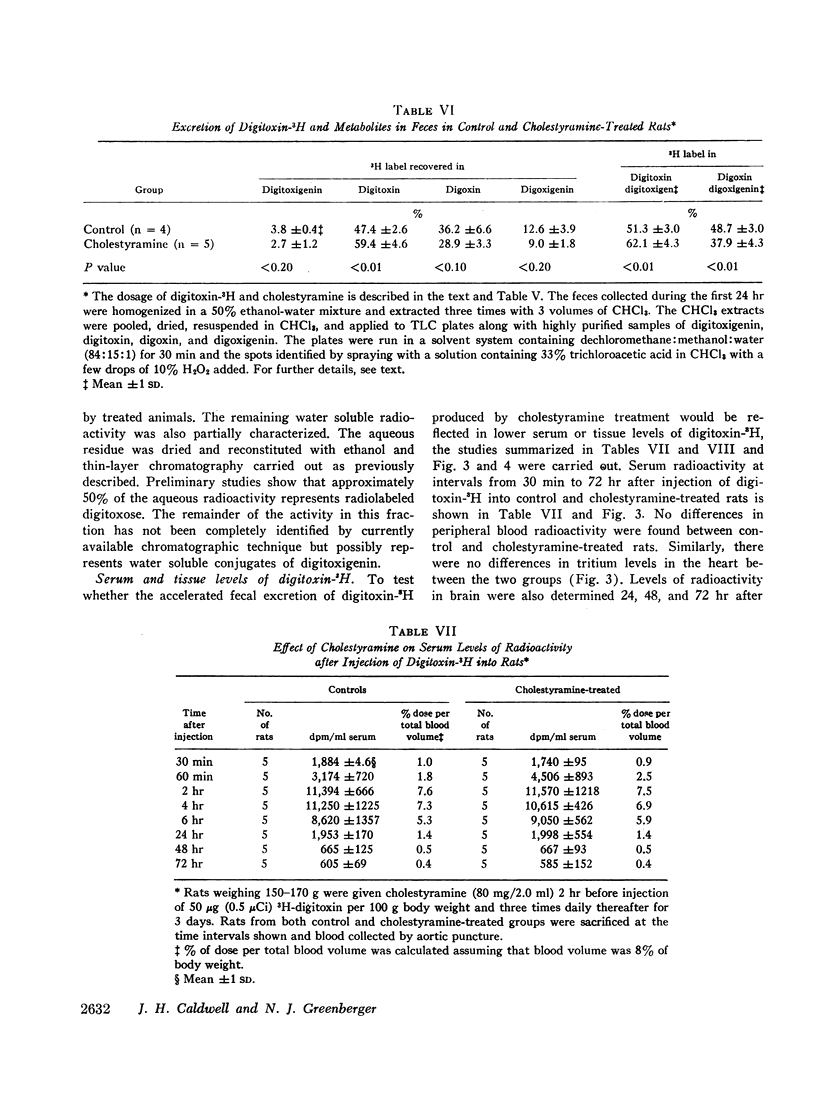
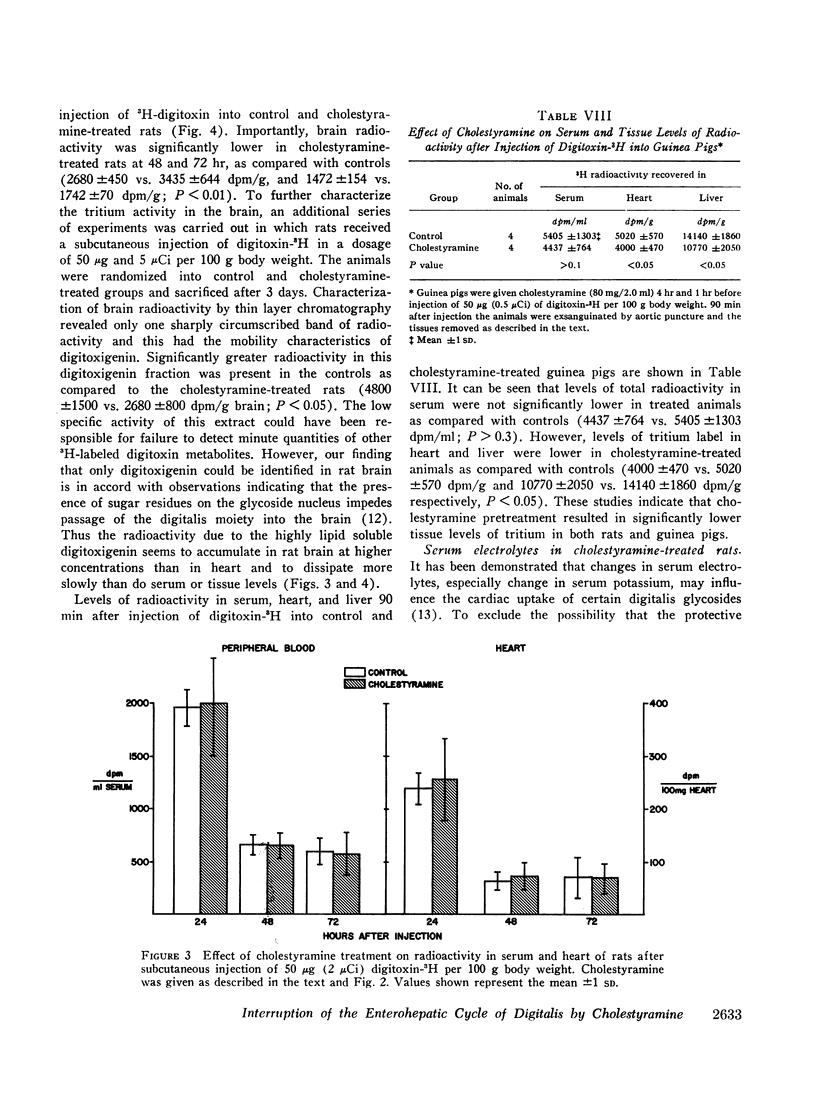
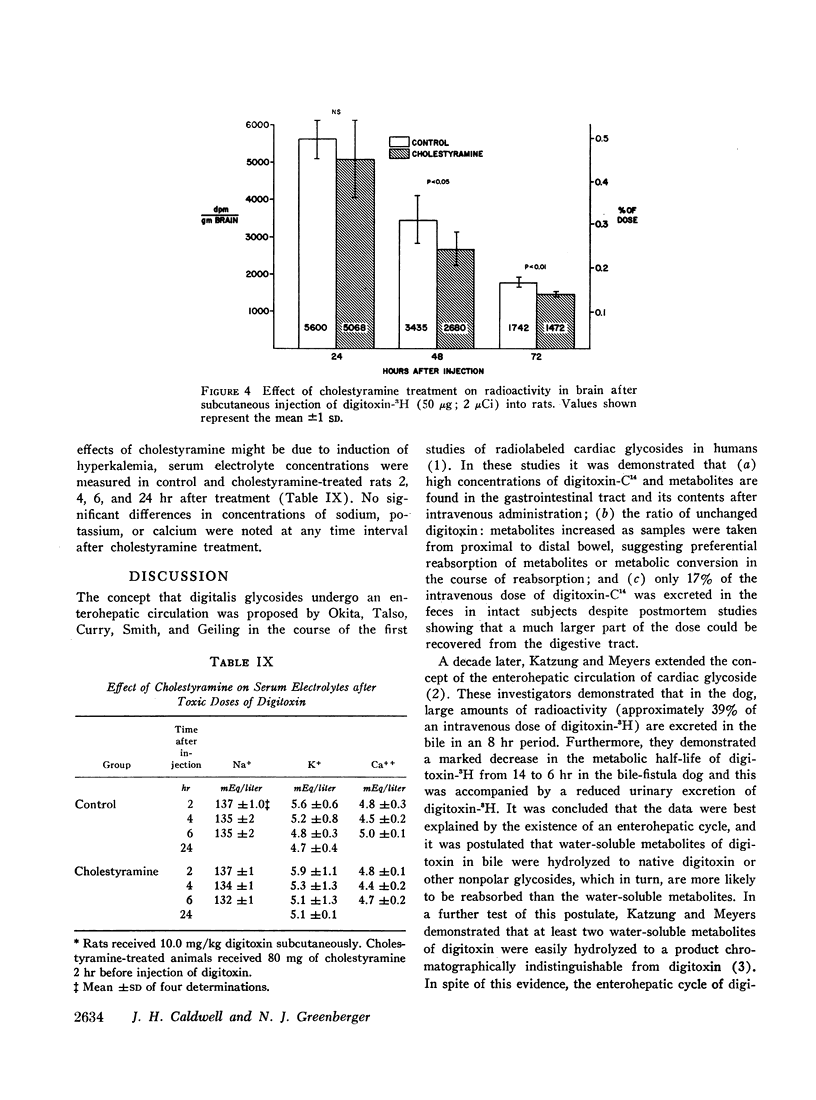
Cholestyramine was found to bind substantial quantities of digitoxin-3H and digoxin-3H in vitro and this binding was only modestly inhibited by the presence of bile. Administration of cholestyramine to rats by intragastric catheter before the subcutaneous injection of the LD100 dose of digitoxin (10 mg/kg) resulted in a 70% survival rate. Further, oral administration of cholestyramine to rats before the subcutaneous injection of digitoxin-3H resulted in accelerated fecal excretion of radioactivity and lower levels of digitoxin-3H and metabolites in brain tissue compared to controls. Similarly, pretreatment of guinea pigs with cholestyramine orally before the injection of digitoxin in dosages of 10.0 and 4.0 mg/kg resulted in a 25 and 70% survival rate respectively as compared to survival rates of 0 and 30% in control animals. Cholestyramine pretreatment of guinea pigs was also accompanied by lower levels of digitoxin-3H and metabolites in heart and liver 90 min after injection of digitoxin-3H. Cholestyramine therapy did not result in significant changes in serum potassium levels excluding the possibility that drug-induced hyperkalemia might have affected the cardiac uptake of digitoxin.
The data obtained in this study indicate that cholestyramine treatment affords a significant degree of protection against lethal digitoxin intoxication in rats and guinea pigs. It is suggested that cholestyramine binds appreciable amounts of digitoxin in the intestinal lumen resulting in reduced reabsorption, increased fecal excretion, and lower tissue levels of glycoside in critical organs. The protective effects of cholestyramine appear to be mediated by interruption of the enterohepatic circulation of digitoxin.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COX E., WRIGHT S. E. The hepatic excretion of digitalis glycosides and their genins in the rat. J Pharmacol Exp Ther. 1959 Jun;126(2):117–122. [PubMed] [Google Scholar]
- DATTA D. V., SHERLOCK S. Treatment of pruritus of obstructive jaundice with cholestyramine. Br Med J. 1963 Jan 26;1(5325):216–219. doi: 10.1136/bmj.1.5325.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty J. E. The clinical pharmacology of digitalis glycosides: a review. Am J Med Sci. 1968 Jun;255:382–414. doi: 10.1097/00000441-196806000-00006. [DOI] [PubMed] [Google Scholar]
- FAUCONNET L., WALDESBUEHL M. ANALYSE DES CARD'ENOLIDES DIGITALIQUES PAR CHROMATOGRAPHIE SUR COUCHE MINCE. Pharm Acta Helv. 1963 Jul-Aug;38:423–429. [PubMed] [Google Scholar]
- Gallo D. G., Bailey K. R., Sheffner A. L. The interaction between cholestyramine and drugs. Proc Soc Exp Biol Med. 1965 Oct;120(1):60–65. doi: 10.3181/00379727-120-30443. [DOI] [PubMed] [Google Scholar]
- Greenberger N. J., MacDermott R. P., Martin J. F., Dutta S. Intestinal absorption of six tritium-labeled digitalis glycosides in rats and guinea pigs. J Pharmacol Exp Ther. 1969 Jun;167(2):265–273. [PubMed] [Google Scholar]
- Johns W. H., Bates T. R. Quantification of the binding tendencies of cholestyramine. II. Mechanism of interaction with bile salt and fatty acid salt anions. J Pharm Sci. 1970 Mar;59(3):329–333. doi: 10.1002/jps.2600590311. [DOI] [PubMed] [Google Scholar]
- Katzung B. G., Meyers F. H. Biotransformation of digitoxin in the dog. J Pharmacol Exp Ther. 1966 Dec;154(3):575–580. [PubMed] [Google Scholar]
- Katzung B. G., Meyers F. H. Excretion of radioactive digitoxin by the dog. J Pharmacol Exp Ther. 1965 Aug;149(2):257–262. [PubMed] [Google Scholar]
- Lage G. L., Spratt J. L. Structure-activity correlation of the lethality and central effects of selected cardiac glycosides. J Pharmacol Exp Ther. 1966 Jun;152(3):501–508. [PubMed] [Google Scholar]
- Marcus F. I., Kapadia G. G., Goldsmith C. Alteration of the body distribution of tritiated digoxin by acute hyperkalemia in the dog. J Pharmacol Exp Ther. 1969 Jan;165(1):136–148. [PubMed] [Google Scholar]
- Marks B. H., Dutta S., Hoffman R. F. Cocaine-H3 binding by the isolated guinea pig vas deferens. Arch Int Pharmacodyn Ther. 1967 Jul;168(1):28–35. [PubMed] [Google Scholar]
- Northcutt R. C., Stiel J. N., Hollifield J. W., Stant E. G., Jr The influence of cholestyramine on thyroxine absorption. JAMA. 1969 Jun 9;208(10):1857–1861. [PubMed] [Google Scholar]
- OKITA G. T., TALSO P. J., CURRY J. H., Jr, SMITH F. D., Jr, GEILING E. M. Metabolic fate of radioactive digitoxin in human subjects. J Pharmacol Exp Ther. 1955 Dec;115(4):371–379. [PubMed] [Google Scholar]
- Okita G. T. Species difference in duration of action of cardiac glycosides. Fed Proc. 1967 Jul-Aug;26(4):1125–1130. [PubMed] [Google Scholar]
- SILBERMAN H., THORP R. H. The estimation of the component cardiac glycosides in digitalis plant samples. II. The estimation of the desgluco-glycosides and some observations on the production of ultra-violet fluorescence with trichloracetic acid. J Pharm Pharmacol. 1954 Aug;6(8):546–551. doi: 10.1111/j.2042-7158.1954.tb10984.x. [DOI] [PubMed] [Google Scholar]
- Saral R., Spratt J. L. Alteration of oral digitoxin toxicity and its in vitro binding by cholestyramine. Arch Int Pharmacodyn Ther. 1967 May;167(1):10–18. [PubMed] [Google Scholar]


