Abstract
Although the apoptotic role of caspases has been largely understood, accumulating evidence in Drosophila suggests that caspases also control other processes than apoptotic cell death. However, how caspases contribute to the development of the mammalian nervous system remains obscure. Here, we provide unique evidence that Apaf-1/caspase-9–mediated caspase signaling regulates the development of olfactory sensory neurons (OSNs), which includes axonal projection, synapse formation, and maturation of these neurons. This caspase signaling leads to a cleavage of Semaphorin 7A, a membrane-anchored semaphorin that is required for the proper axonal projection. Mutant mice deficient for apaf-1 or caspase-9 exhibit misrouted axons, impaired synaptic formation, and defects in the maturation of OSNs without affecting the number of these cells. Our findings suggest that Apaf-1/caspase-9–mediated nonapoptotic caspase signaling is required for the proper neural network formation during olfactory development.
Keywords: neural development, nonapoptotic function, axonal projection, synapse formation, Sema7A
The caspase family of proteins comprises intracellular cysteine proteases that cleave a variety of specific target proteins to facilitate apoptosis. A deficiency of caspase-3 in the 129S1/SvImJ mouse causes neurodevelopmental abnormalities such as an expanded ventricular zone, ectopic neural structures, and gross brain malformations (1). Severe neural phenotypes are also found in mice deficient for apaf-1 or caspase-9, the apoptosome components required for the mitochondria-activated caspase signaling (2–4). Because ≈50–70% of neurons are estimated to die during mammalian neural development, Apaf-1/caspase-9 signaling has been considered to play an apoptotic role in the formation and morphogenesis of the nervous system. However, in the absence of caspases or apaf-1, caspase-independent cell death pathways, including type 2 autophagic cell death, are alternatively used to eliminate unwanted cells (5–7). This implies that the neural abnormalities observed in apaf-1 or caspase-9–deficient mice could also be caused by impairment of nonapoptotic functions of these proteins. Indeed, caspases have been shown to function as regulatory molecules not only in cell death process but also in other various biological processes such as sperm differentiation, dendritic pruning, and border-cell migration in Drosophila (8). However, nonapoptotic roles of caspases in mammalian neural development have been largely elusive.
We have recently found that a considerable number of OSNs in the developing olfactory epithelium (OE) exhibited caspase-3 activity without showing apoptotic changes of histone H1 (9). This prompted us to assess whether caspases exert nonapoptotic functions in OSNs during olfactory development. Here, we show that Apaf-1/caspase-9–mediated signaling causes the cleavage of a membrane-anchored member of the semaphorin family of guidance proteins, Sema7A, in the axons of OSNs during development. Analysis of mutant mice deficient for apaf-1 or caspase-9 revealed that this Apaf-1/caspase-9–mediated nonapoptotic casapase signaling is important for the development of olfactory sensory neurons (OSNs) by affecting axonal pathfinding, synapse formation and maturation status in the olfactory bulb (OB).
Results
Caspase-3 Is Activated in OSNs During Late Embryogenesis.
During olfactory development, axons from early-born OSNs begin to penetrate the OB around embryonic day (E)14; these axons contact with projection neurons, mitral/tufted cells, at around E16 (10). We have previously found that a large number of OSNs with activated caspases were dispersed throughout the OE in late embryonic stage (Fig. 1A) (9). We therefore examined the patterns of caspase-3 activation in detail using an anti–active caspase–3 antibody. Caspase-3 was strongly activated not only in the cell body but also in the axons of OSNs during late embryogenesis. Interestingly, caspase-3–activated OSN axons were specifically observed in the anterior–medial and posterior–ventral regions of the olfactory nerve layer (ONL) of the OB (Fig. 1 A–C). This caspase-3 activation was completely abolished in apaf-1 or caspase-9–deficient mice (Fig. 1 D and E and Fig. S1), suggesting that Apaf-1/caspase-9-signaling activates caspase-3 in OSN during late embryogenesis.
Fig. 1.
Mitochondrial Apaf-1/caspase-9 pathway-mediated caspase-3 activation in the developing olfactory system. (A) Coronal sections (80 μm thick) of E16 WT mouse OB were stained with anti–active-caspase–3 (green) and anti-NCAM (magenta; a marker for OSN axons) antibodies. (B) Whole-mount staining of an E16.5 WT mouse embryo with the anti–active-caspase–3 antibody. Caspase-3 was strongly activated in the OSN axons projecting to the anterior–medial (arrow) and posterior–ventral (arrowhead) regions of the OB indicated by arrows in A and B. A, anterior; M, medial; P, posterior; V, ventral. (C) A coronal section (80-μm thick) of the E16.5 WT mouse OB was stained with the anti–active-caspase–3 (green) and anti-OCAM (magenta) antibodies. OCAM is specifically expressed in a subpopulation of the OSNs (30, 31). (D) Coronal sections (5 μm thick) through the E16.5 WT and caspase-9–deficient OB were stained with anti–active-caspase–3 (green) and anti-OMP (magenta; a marker for mature OSNs) antibodies. Arrowhead shows the ONL, containing active-caspase-3–positive OSN axons, of the WT mouse. (E) Serial coronal sections (5-μm thick) were prepared from the entire OE of E16.5 WT and caspase-9 homozygous mutant mice, and stained with the active-caspase-3 antibody. Active-caspase-3–positive cells in every 10th section were counted. Values are mean ± SEM (t test, P < 0.01). (F and G) Serial coronal sections (5-μm thick) were prepared from the entire OE of E16.5 WT mice. The apoptotic cells in every 10th section were labeled by TUNEL (magenta), following immunofluorescence labeling with the anti–active-caspase–3 antibody (green). Arrow in G shows a TUNEL-negative/active-caspase-3–positive cell. Arrowhead shows a TUNEL-positive/active-caspase-3–positive cell. The percentage of TUNEL-negative (−) and -positive (+) cells among the active-caspase-3–positive cells is summarized in F. Data shown are representative of three independent experiments with one mouse each. Values are mean ± SEM (n = 3; t test, P < 0.01). (Scale bars, A and C, 100 μm; D, 50 μm; and G, 20 μm.)
To examine whether these caspase-3–activated cells in the late embryonic OE were dying, sections of E16.5 OE were double-labeled for activated caspase-3 and DNA fragmentation (TUNEL) Fig. 1 F and G). A considerable number of caspase-activated cells were TUNEL-negative/nonpyknotic cells. Furthermore, we have previously shown in late-stage embryos that significant numbers of OSNs show caspase-3 activation without changing the immunoreactivity for histone H1, another marker for apoptotic cell death (9). These data suggest that some OSNs activate caspase-3 through Apaf-1/caspase-9 signaling without undergoing apoptotic morphology during late embryogenesis.
Impaired Olfactory Development in apaf-1−/− and caspase-9−/− Embryos.
To investigate the putative nonapoptotic functions of Apaf-1/caspase-9 signaling in the olfactory development, we first examined the gross organization of the olfactory system in apaf-1−/− and caspase-9−/− mice. For this analysis, we used a gene-targeted mouse line P2-IRES-taulacZ, which enabled us to label defined P2 odorant receptor–expressing OSNs (P2 OSNs), as described below (Fig. 2). Although a previous study has suggested that caspase-3−/− and caspase-9−/− embryos have a thicker OE neuronal layer (11), apaf-1−/− and caspase-9−/− embryos in our colony showed the grossly normal anatomic structure of the OE, probably because of a different genetic background (12) (Fig. S2A). In addition, the number of anti–phospho-H3 antibody-positive mitotic cells in the OE of our apaf-1−/− mice was comparable to that of age-matched heterozygous control embryos (Fig. S2B). Intriguingly, however, we found abnormalities in the development of OSN axons in apaf-1−/− or caspase-9−/− mice, including aberrant projection to the deep layer of the OB (Fig. 2B, arrowheads) and impaired maturation (Fig. 2B, arrows). On the other hand, the distribution of the olfactory ensheathing cells surrounding OSN axons fascicles was not affected in apaf-1−/− mice (Fig. S3). These data indicate that the Apaf-1/caspase-9 signaling contributes to the development of OSN axons.
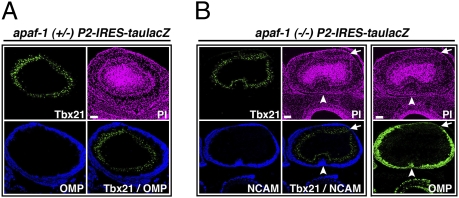
Fig. 2.
Disturbed olfactory development in apaf-1−/− and caspase-9−/− mice. (A) Coronal sections (5 μm thick) of E18.5 apaf-1+/− P2-IRES-taulacZ OB were stained with anti-Tbx21 [green; a mitral/tufted cell marker (29)] and anti-OMP (blue) antibodies. Cell nuclei were visualized by propidium iodide (PI; magenta) staining. (B) Serial coronal sections (5 μm thick) of E18.5 apaf-1−/− P2-IRES-taulacZ OB were stained with anti-OMP (green) antibody and double stained with anti-Tbx21 (green) and anti-NCAM (blue) antibodies, respectively. Arrow shows an OMP-negative region in the ONL of the OB. Arrowheads show the aberrant extension of NCAM-positive OSN axons to the deep layer of the OB. Cell nuclei were visualized by PI staining (magenta). (Scale bars, 100 μm.)
Apaf-1/caspase-9–Mediated Cleavage of Sema7A in the Developing Olfactory System.
Several families of axon guidance cues, including semaphorin 3a and Ephrin-As, have critical roles in the axonal wiring and the organization of the olfactory system. We have recently found that Sema7A, a membrane-anchored member of the semaphorin family of guidance proteins required for the proper formation of the lateral olfactory tract (13), is a substrate for caspase-9 (14). A rabbit polyclonal antibody against amino acid 1–100 of mouse Sema7A (Abcam) immunolabeled Sema7A specifically in caspase-9–activated cells (14). To examine whether Apaf-1/caspase-9 signaling in OSN axons cleaved Sema7A during development, we performed immunofluorescent analysis of OB sections from WT, apaf-1–deficient, or caspase-9–deficient mice. We found that the anti-Sema7A antibody strongly immunolabeled WT OSN axons in anterior–medial and posterior–ventral regions of the ONL (Fig. 3A, arrow), the regions where caspase-3 was strongly activated. In contrast, the apaf-1 or caspase-9 deficiency abolished the immunoreactivity of the OSN axons (Fig. 3A and Fig. S4). In addition, immunoblot analysis showed that Sema7A is cleaved in the E17.5 OB of WT but not that of apaf-1−/−mice (Fig. 3B). In the apaf-1+/− OB, smaller amount of cleaved Sema7A was observed in comparison with that of WT mice. Together, these data indicate that Sema7A was cleaved in the OSN axons in a caspase-dependent manner during late embryogenesis.
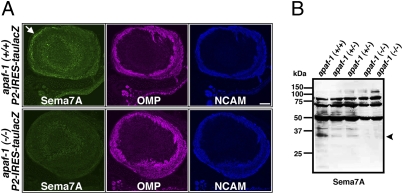
Fig. 3.
Caspase-dependent cleavage of Sema7A in the developing olfactory system. (A) Coronal sections (5-μm thick) of E18.5 WT and apaf-1–deficient OB were stained with anti-Sema7A, anti-OMP, and anti-NCAM antibodies. Arrow shows olfactory nerve layer containing OSN axons labeled with anti-Sema7A antibody in WT mouse. (B) Whole-cell extracts (30 μg) of OB of WT, apaf-1+/−, and apaf-1−/− mice were analyzed by immunoblotting using anti-Sema7A antibody. Arrowhead shows caspase-dependent cleaved fragment of Sema7A. (Scale bar, 100 μm.)
Abnormal Projection of P2 Neurons in apaf-1−/− and caspase-9−/− Mice.
Semaphorins are a large class of secreted and membrane-anchored proteins that are important in neuronal pathfinding and axon guidance in selected areas of the developing nervous system (14–16), raising the possibility that the Apaf-1/caspase-9 signaling contribute to axonal wiring of OSNs. To clarify the role of the Apaf-1/capase-9 signaling in OSN development, especially in their projection patterns, we used the P2-IRES-taulacZ transgenic mouse lines that label P2 OSNs (17). During normal embryogenesis, P2 axons are detected in the ONL of the OB by E14.5 (18, 19). These axons begin to form a protoglomerulus by E17.5. No significant difference was observed in the projection patterns of P2 OSNs between E14 apaf-1+/− and apaf-1−/− mice. However, at E18.5/P0, the P2 OSNs in apaf-1−/− and caspase-9−/− mice extended their axons more posteriorly than those in WT mice and heterozygotes (Fig. 4 A and B and Fig. S5). We quantified the patterns of the P2 axonal trajectories to the OB by measuring the angle of the most anterior axons to the cribriform plate (Fig. 4C). In all apaf-1−/− and caspase-9−/− mice, the angle was smaller than that in WT mice or heterozygotes. As caspase signaling has been shown to control the number of neural cells by executing cell death during neural development, this nonapoptotic phenotype in the projection patterns of OSN axons could be a result from increased number of P2 OSNs in apaf-1−/− or caspase-9−/− mice. To evaluate this possibility, we compared the number of P2 OSNs in apaf-1+/− and apaf-1−/− mice (Fig. 4D). We found no difference in the overall number of P2 OSNs between these mice at E18.5. In addition, caspase-9 deficient mice also showed no difference in the total number of P2 OSNs (Fig. 4E). Together, these data indicate that Apaf-1/caspase-9 signaling contributes to P2 axon pathfinding from the OE to the OB without affecting the number of OSNs.
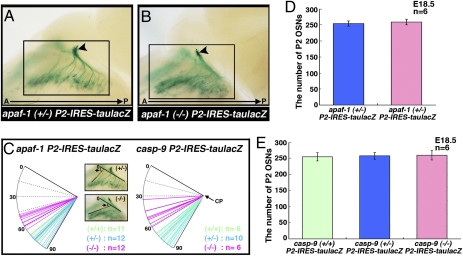
Fig. 4.
Abnormal axon trajectories of P2 neurons in apaf-1– or caspase-9–deficient P2-IRES-taulacZ mice. (A and B) View of nasal septum and medial aspect of OB of E18.5 apaf-1+/− P2-IRES-taulacZ (A) and apaf-1−/− P2-IRES-taulacZ (B) mice. Arrowheads show P2 glomeruli in mice. (C) Projection patterns of P2 neurons were quantified by the angle of the most anterior axons to the cribriform plate. The representative pictures are low-magnification images of the areas boxed in (A) and (B), respectively. Wild type, green; heterozygote, blue; homozygote, magenta. CP, cribriform plate. (D) Total number of X-gal-positive P2 neurons in every two coronal sections (50 μm thick) of E18.5 apaf-1+/− and apaf-1−/− P2-IRES-taulacZ OE. Values are mean ± SEM (n = 6; t test, P < 0.01). (E) Total number of X-gal–positive P2 neurons in every two coronal sections (50 μm thick) of E18.5 WT, caspase-9+/−, and caspase-9−/− P2-IRES-taulacZ OE. Values are mean ± SEM (n = 6; t test, P < 0.01).
Impaired Glomerular Development in apaf-1−/− and caspase-9−/− Mice.
Whole-mount X-gal staining revealed that all of the protoglomeruli in the half-bulbs of E18.5 apaf-1−/− and caspase-9−/− mice developed poorly compared with those of WT or heterozygous mice (Fig. 4 A and B and Fig. S5). To inquire whether Apaf-1/caspase-9 signaling is involved in the synaptic formation of OSNs, we examined the expression level of a presynaptic marker synaptophysin (20) in the P2 protoglomeruli on each side of the OB in three apaf-1+/− or apaf-1−/− mice (Fig. 5 A and B and E–H). In apaf-1+/− or caspse-9+/− mice, synaptophysin was strongly expressed in P2 glomeruli, especially on the medial side of the OB, suggesting that P2 axons underwent synapse formation. In apaf-1−/− or caspase-9−/− mice; however, few P2 axons reached to their glomeruli with a low level expression of synaptophysin, which suggests that they largely fail to contact with the olfactory neurons. We further examined synaptic marker expression using the antibody against bassoon, a cytoskeletal matrix protein that is enriched in the presynaptic active zone (21). The deficiency of caspase-9 dramatically reduced the expression level of bassoon in P2 protoglomeruli (Fig. 5 C and D). These data suggest that impaired caspase activation largely abolishes the expression of synaptic marker in P2 axons, resulting in a defect in their synapse maturation with the proper neurons in the OB.
Fig. 5.
Impaired development of P2 glomeruli in apaf-1- or caspase-9–deficient P2-IRES-taulacZ mice. (A and B) OB sections (5-μm thick) from E18.5 caspase-9−/−, apaf-1−/−, and their heterozygous P2-IRES-taulacZ embryos were stained with anti–β-gal (green), antisynaptophysin (blue), and anti-OMP (magenta) antibodies. (C and D) OB sections (5-μm thick) of caspase-9+/− P2-IRES-taulacZ and caspase-9−/− P2-IRES-taulacZ mice were stained with anti–β-gal (green), anti-OMP (blue), and antibassoon (magenta) antibodies. (E–H) Percentages of synaptophysin-positive or -negative (including slightly expressing cells) P2 protoglomeruli in medial (left) and lateral (right) sides of six half bulbs from three individuals are shown. (Scale bar, 20 μm.)
Impaired P2 Maturation in apaf-1−/− and caspase-9−/− Mice.
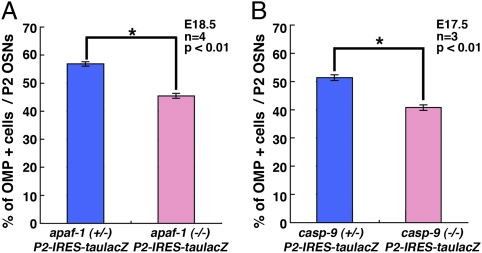
To examine whether the Apaf-1/caspase-9 signaling is involved in the differentiation or maturation of OSNs, we examined the proportion of mature versus immature P2 OSNs in apaf-1+/− and apaf-1−/− mice at E18.5 (Fig. 6). In both genotypes, all of the β-gal–positive P2 OSNs were categorized as either immature (TUJ-1–positive/OMP-negative) or mature (TUJ-1–positive/OMP-positive) OSNs. Interestingly, the proportion of mature P2 OSNs was lower in the apaf-1−/− mice than that in the heterozygotes (Fig. 6A). In addition, E17.5 caspase-9−/− mice exhibited a similar reduction in mature OSNs (Fig. 6B). These observations show that the Apaf-1/caspase-9 signaling specifically influences the maturation status of P2 OSNs but not the proliferation of OSNs.
Fig. 6.
Impairment of P2 maturation in apaf-1−/− and caspase-9−/− P2-IRES-taulacZ mice. (A) Coronal sections (5-μm thick) of E18.5 apaf-1+/− and −/− OEs were stained with anti–β-gal, anti-TUJ1, and anti-OMP antibodies. Percentage of OMP-positive cells among β-gal–positive P2 neurons was determined for every fifth section. (B) Coronal sections (5-μm thick) through E17.5 caspase-9+/− and −/− OEs were stained with anti–β-gal, anti-TUJ1, and anti-OMP antibodies. Percentage of OMP-positive cells among β-gal–positive P2 neurons was determined for every fifth section. Values are mean ± SEM (t test, *P < 0.01).
Impaired Maturation of M71 Neurons in apaf-1−/− and caspase-9−/− Embryos.
In addition to the P2-expressing OSNs, we also examined the M71 OR-expressing OSNs at E18.5 using a gene-targeted mouse line M71-IRES-taulacZ that labels defined M71-expressing OSNs (22). Because M71 axons begin to coalesce between postnatal days 2 and 3, the time point after almost all apaf-1−/− mice die, we examined the effects of the Apaf-1/caspase-9 signaling on the number and maturation of M71 OSNs at E18.5 (Fig. 7). We found that the deficiency of apaf-1 did not influence the number of M71 neurons (Fig. 7A), but resulted in a reduced population of OMP-positive mature OSNs (Fig. 7B). Thus, the Apaf-1/caspase-9 signaling influences the maturation status of both P2 and M71 OSNs during olfactory development.
Fig. 7.
Deficiency of apaf-1 in mice affects maturation, but not cell number, in M71 neurons. (A) Total number of X-gal–positive M71 neurons in every two coronal sections (10-μm thick) through OE of E18.5 apaf-1+/− and −/− mice. (B) Coronal sections (5-μm thick) through OE of E18.5 apaf-1+/− and −/− mice were stained with anti–β-gal, anti-TUJ-1, and anti-OMP antibodies. Percentage of OMP-positive cells among β-gal–positive cells was determined for every fifth section. Values are mean ± SEM (t test, *P < 0.01).
Discussion
The present study shows a nonapoptotic role of the Apaf-1/caspase-9 signaling during olfactory development. Although the number of OSNs is unchanged, their maturation is impaired in both apaf-1−/− and caspase-9−/− mice. Our data suggest that the impairment of OSN maturation in the mutant mice is due to a defect in their axon pathfindings. Indeed, there is growing evidence for the implication of the role of caspase signaling in the molecules related to axon guidance and differentiation. For example, the axon guidance molecule Eph/Ephrin controls the size of the mouse cerebral cortex by regulating caspase signaling in cortical progenitor cells (23). In addition, DCC, a receptor for axon guidance molecule netrin-1, is cleaved by caspase-3 (24). In this study, we provide unique evidence showing the role of the Apaf-1/caspase-9 signaling in the pathfinding, maturation, and synaptic formation of OSNs in vivo. The Apaf-1/caspase-9 signaling caused the cleavage of Sema7A in OSN axons that project to the anterior–medial and posterior–ventral regions of the OB, the region where the active form of caspase-3 was observed (Figs. 1 and 7). To address the consequence of Sema7A cleavage by caspase-9, we examined whether caspase-cleaved fragment of Sema7A was functional. Previous studies have suggested that Sema7A promote axon outgrowth or initiate T-cell–mediated inflammatory responses through integrins and MAPK signaling (i.e., phosphorylation of Erk1/2) (13, 25). We thus examined the effect of full-length or caspase-cleaved fragment of Sema7A on Erk1/2 phosphorylation in Neuro2a cells. In contrast to Fc-tagged full-length Sema7A, overexpression of Fc-tagged caspase-cleaved N-terminal fragment of Sema7A did not enhance the phosphorylation of Erk1/2 compared with control (Fig. S6). These data suggest that Sema7A loses its activity through the cleavage by caspase. Our results suggest that the Apaf-1/caspase-9 signaling regulates OSN development and participates in the establishment of axonal wiring in the olfactory system by modulating the amount of functional Sema7A. Consistent with this idea, a previous study reported that the thalamocortical axon growth in vitro requires optimal concentration of Sema7A (26). Further studies will elucidate the precise mechanism of axon wiring and neuronal maturation through proteolytic cleavage of axon guidance molecules by nonapoptotic caspase activity.
Materials and Methods
Mutant Mice.
We used apaf-1– or caspase-9–deficient mice in which the P2 OR gene was replaced with the P2-IRES-taulacZ or M71-IRES-taulacZ mutation. The apaf-1+/− mice and caspase-9+/− mice were described previously (3, 4). The P2-IRES-taulacZ and M71-IRES-taulacZ mouse strains were a kind gift from P. Mombaerts (Max Planck Institute of Biophysics, Frankfurt, Germany) (17, 22).
Immunohistochemistry.
X-gal staining was performed as previously described (27). The immunostaining procedure used, the peroxidase-labeled avidin-biotin method, has been described (28). The following antibodies were used: rabbit anti–active caspase–3 C92-605 (BD), goat anti–olfactory marker protein (OMP; Wako Chemicals), rat anti-NCAM (Chemicon), mouse anti-TUJ1/class III β-tubulin (Covance), mouse anti-OCAM (BD), mouse antisynaptophysin (DAKO), rabbit anti-Sema7A (Abcam), rabbit anti-Tbx21 (kindly provided by Y. Yoshihara, RIKEN Brain Science Institute, Wako, Saitama, Japan) (29), rabbit anti-S100 (DAKO), and rabbit anti–β-gal (Cappel). The TUNEL assay (In Situ Cell Death Detection kit; Roche) was performed according to the manufacturer's instructions.
OB Extracts.
Mouse OBs were dissected, immediately frozen in liquid nitrogen, and stored at –80 °C. Cell extracts were prepared adding 1 mL of 2× SDS sample buffer (28) to the tissue, followed by sonication. The following antibodies were used for immunoblotting: rabbit anti-Sema7A antibody (Abcam) and rabbit anti–active caspase–3 antibody (Cell Signaling).
Supplementary Material
Acknowledgments
We thank T. Yagi, (Osaka University), Y. Yoshihara (Riken BSI), T. Chihara (University of Tokyo), M. Furuse (Kobe University), N. Yamamoto (Osaka University), and A. Kumanogoh (Osaka University) for helpful discussions and all members of the M.M. laboratory for encouragement. We thank Y. Yamaguchi (University of Tokyo) for experiments and helpful comments, and M. Sasaki (University of Tokyo) for experiments on our study. We also thank P. Mombaerts (Max Planck Institute of Biophysics) for the P2-IRES-taulacZ and M71-IRES-taulacZ mouse strains, Y. Yoshihara (RIKEN Brain Science Institute) for the rabbit polyclonal anti-Tbx21 antibody, and A. L. Kolodkin (The Johns Hopkins University School of Medicine, Baltimore) for pEX-Sema7A-Fc plasmid. This work was supported in part by grants from the Japanese Ministry of Education, Science, Sports, Culture and Technology (to M.M.), a RIKEN Bioarchitect Research Grant (to M.M.), and Research Fellowships of the Japan Society for the promotion of Science for Young Scientists (to S.O).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0910488107/-/DCSupplemental.
References
- 1.Kuida K, et al. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- 2.Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 1998;94:727–737. doi: 10.1016/s0092-8674(00)81732-8. [DOI] [PubMed] [Google Scholar]
- 3.Kuida K, et al. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida H, et al. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell. 1998;94:739–750. doi: 10.1016/s0092-8674(00)81733-x. [DOI] [PubMed] [Google Scholar]
- 5.Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 6.Oppenheim RW, et al. Developing postmitotic mammalian neurons in vivo lacking Apaf-1 undergo programmed cell death by a caspase-independent, nonapoptotic pathway involving autophagy. J Neurosci. 2008;28:1490–1497. doi: 10.1523/JNEUROSCI.4575-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaginuma H, et al. Caspase activity is involved in, but is dispensable for, early motoneuron death in the chick embryo cervical spinal cord. Mol Cell Neurosci. 2001;18:168–182. doi: 10.1006/mcne.2001.1009. [DOI] [PubMed] [Google Scholar]
- 8.Kuranaga E, Miura M. Nonapoptotic functions of caspases: Caspases as regulatory molecules for immunity and cell-fate determination. Trends Cell Biol. 2007;17:135–144. doi: 10.1016/j.tcb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Ohsawa S, Hamada S, Yoshida H, Miura M. Caspase-mediated changes in histone H1 in early apoptosis: Prolonged caspase activation in developing olfactory sensory neurons. Cell Death Differ. 2008;15:1429–1439. doi: 10.1038/cdd.2008.71. [DOI] [PubMed] [Google Scholar]
- 10.Blanchart A, De Carlos JA, López-Mascaraque L. Time frame of mitral cell development in the mice olfactory bulb. J Comp Neurol. 2006;496:529–543. doi: 10.1002/cne.20941. [DOI] [PubMed] [Google Scholar]
- 11.Cowan CM, Roskams AJ. Caspase-3 and caspase-9 mediate developmental apoptosis in the mouse olfactory system. J Comp Neurol. 2004;474:136–148. doi: 10.1002/cne.20120. [DOI] [PubMed] [Google Scholar]
- 12.Leonard JR, Klocke BJ, D'Sa C, Flavell RA, Roth KA. Strain-dependent neurodevelopmental abnormalities in caspase-3-deficient mice. J Neuropathol Exp Neurol. 2002;61:673–677. doi: 10.1093/jnen/61.8.673. [DOI] [PubMed] [Google Scholar]
- 13.Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424:398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- 14.Ohsawa S, et al. Caspase-9 activation revealed by semaphorin 7A cleavage is independent of apoptosis in the aged olfactory bulb. J Neurosci. 2009;29:11385–11392. doi: 10.1523/JNEUROSCI.4780-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He Z, Wang KC, Koprivica V, Ming G, Song HJ. Knowing how to navigate: Mechanisms of semaphorin signaling in the nervous system. Sci STKE. 2002;2002:re1. doi: 10.1126/stke.2002.119.re1. [DOI] [PubMed] [Google Scholar]
- 16.Pasterkamp RJ, Kolodkin AL. Semaphorin junction: Making tracks toward neural connectivity. Curr Opin Neurobiol. 2003;13:79–89. doi: 10.1016/s0959-4388(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 17.Mombaerts P, et al. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- 18.Royal SJ, Key B. Development of P2 olfactory glomeruli in P2-internal ribosome entry site-tau-LacZ transgenic mice. J Neurosci. 1999;19:9856–9864. doi: 10.1523/JNEUROSCI.19-22-09856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang F, Nemes A, Mendelsohn M, Axel R. Odorant receptors govern the formation of a precise topographic map. Cell. 1998;93:47–60. doi: 10.1016/s0092-8674(00)81145-9. [DOI] [PubMed] [Google Scholar]
- 20.Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985;41:1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- 21.tom Dieck S, et al. Bassoon, a novel zinc-finger CAG/glutamine-repeat protein selectively localized at the active zone of presynaptic nerve terminals. J Cell Biol. 1998;142:499–509. doi: 10.1083/jcb.142.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vassalli A, Rothman A, Feinstein P, Zapotocky M, Mombaerts P. Minigenes impart odorant receptor-specific axon guidance in the olfactory bulb. Neuron. 2002;35:681–696. doi: 10.1016/s0896-6273(02)00793-6. [DOI] [PubMed] [Google Scholar]
- 23.Depaepe V, et al. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature. 2005;435:1244–1250. doi: 10.1038/nature03651. [DOI] [PubMed] [Google Scholar]
- 24.Forcet C, et al. The dependence receptor DCC (deleted in colorectal cancer) defines an alternative mechanism for caspase activation. Proc Natl Acad Sci USA. 2001;98:3416–3421. doi: 10.1073/pnas.051378298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki K, et al. Semaphorin 7A initiates T-cell-mediated inflammatory responses through alpha1beta1 integrin. Nature. 2007;446:680–684. doi: 10.1038/nature05652. [DOI] [PubMed] [Google Scholar]
- 26.Maruyama T, Matsuura M, Suzuki K, Yamamoto N. Cooperative activity of multiple upper layer proteins for thalamocortical axon growth. Dev Neurobiol. 2008;68:317–331. doi: 10.1002/dneu.20592. [DOI] [PubMed] [Google Scholar]
- 27.Zou DJ, et al. Postnatal refinement of peripheral olfactory projections. Science. 2004;304:1976–1979. doi: 10.1126/science.1093468. [DOI] [PubMed] [Google Scholar]
- 28.Ohsawa S, Hamada S, Kakinuma Y, Yagi T, Miura M. Novel function of neuronal PAS domain protein 1 in erythropoietin expression in neuronal cells. J Neurosci Res. 2005;79:451–458. doi: 10.1002/jnr.20365. [DOI] [PubMed] [Google Scholar]
- 29.Yoshihara S, Omichi K, Yanazawa M, Kitamura K, Yoshihara Y. Arx homeobox gene is essential for development of mouse olfactory system. Development. 2005;132:751–762. doi: 10.1242/dev.01619. [DOI] [PubMed] [Google Scholar]
- 30.Alenius M, Bohm S. Identification of a novel neural cell adhesion molecule-related gene with a potential role in selective axonal projection. J Biol Chem. 1997;272:26083–26086. doi: 10.1074/jbc.272.42.26083. [DOI] [PubMed] [Google Scholar]
- 31.Yoshihara Y, et al. OCAM: A new member of the neural cell adhesion molecule family related to zone-to-zone projection of olfactory and vomeronasal axons. J Neurosci. 1997;17:5830–5842. doi: 10.1523/JNEUROSCI.17-15-05830.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.