Abstract
The amount of neurotransmitter released from a presynaptic terminal is the product of the quantal content (number of vesicles) and the presynaptic quantal size (QSpre, amount of transmitter per vesicle). QSpre varies with synaptic use, but its regulation is poorly understood. The motor nerve terminals at the neuromuscular junction (NMJ) contain TGF-β receptors. We present evidence that TGF-β2 regulates QSpre at the NMJ. Application of TGF-β2 to the rat diaphragm NMJ increased the postsynaptic response to both spontaneous and evoked release of acetylcholine, whereas antibodies to TGF-β2 or its receptor had the converse effect. L-vesamicol and bafilomycin blocked the actions of TGF-β2, indicating that TGF-β2 acts by altering the extent of vesicular filling. Recordings of the postsynaptic currents from the diaphragm were consistent with TGF-β2 having this presynaptic action and a lesser postsynaptic effect on input resistance. TGF-β2 also decreased quantal content by an atropine-sensitive pathway, indicating that this change is secondary to cholinergic feedback on vesicular release. Consequently, the net actions of TGF-β2 at the NMJ were to amplify the postsynaptic effects of spontaneous transmission and to diminish the number of vesicles used per evoked stimulus, without diminishing the amount of acetylcholine released.
Keywords: motoneuron, quantal size, synaptic plasticity, synaptic vesicle, motor nerve terminal
Synapses need to be efficient and malleable to account for the dynamic features of neuronal networks. The number of synaptic vesicles released with each stimulus (quantal content) and the postsynaptic response to each vesicle (quantal size) are actively regulated to achieve this malleability and are major determinants of whether synaptic transmission occurs (1). The amount of neurotransmitter released from each vesicle (presynaptic quantal size, QSpre) appears to vary inversely with synaptic activity and is a component of quantal size (2–4). However, it is unclear whether QSpre is actively regulated as an important component of the characteristics of a synapse.
QSpre cannot be measured directly at most synapses; variation in QSpre is inferred from the analysis of either evoked (EPP) or spontaneous postsynaptic potentials. This inference tacitly assumes that the function of QSpre is to influence postsynaptic quantal size. Although this influence may occur in many circumstances, the existence of negative feedback on vesicle release (5) challenges the universality of this assumption. Because any QSpre-dependent change in the amount of neurotransmitter released should be attenuated rapidly by an opposite change in quantal content, up-regulation of QSpre would restrict the number of vesicles released per evoked stimulus but would not acutely affect evoked postsynaptic currents unless the negative feedback loop was ineffectual. In that case, the regulation of QSpre would not be readily apparent from studies of EPPs.
The major features of synapses are profoundly shaped by extracellular signals from the pre- and postsynaptic cells (6). Consequently, if QSpre is a determinant of synaptic function, then it also may be regulated by locally acting signals. The identification of these putative signals is critical for understanding the physiological function of QSpre.
TGF-βs are extracellular regulators that are widely expressed in the nervous system (7, 8). At the rat and human neuromuscular junctions (NMJ), TGF-β receptors are located in the synaptic membrane of presynaptic motor nerve terminals (8, 9), where they are potentially activated by two sources of TGF-β2: the muscle fiber, which selectively produces TGF-β2 in its synaptic region (9), and the nerve terminal itself after anterograde transport from the perikaryon (10). This molecular arrangement raises the possibility that TGF-β2 is a local regulator of motor nerve terminal function. Consistent with this possibility, we report here that TGF-β2 and its antagonists have multiple effects on rat diaphragm NMJs, the most direct and significant of which is regulation of QSpre.
Results
Miniature End-Plate Potential Amplitude Is Regulated by TGF-β2.
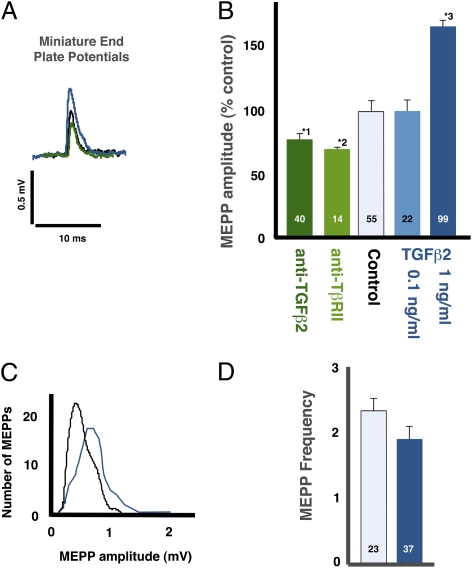
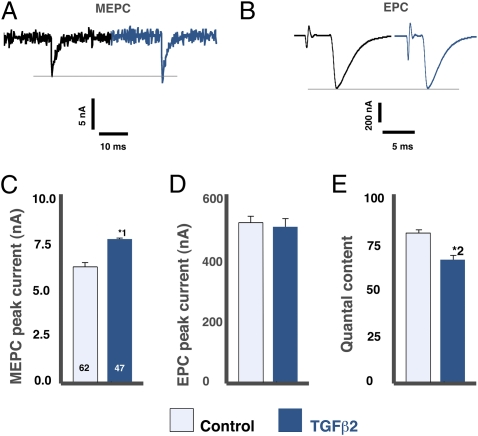
The putative interaction between QSpre and quantal content can be by-passed by examining miniature end-plate potentials (MEPPs), which are the postsynaptic response to the release of a single synaptic vesicle. Administration of 80 ng/mL of neutralizing antibodies to either TGF-β2 or the type II TGF-β receptor (TβRII) to isolated diaphragms decreased the amplitude of the MEPP at their NMJs by 28–29% (Fig. 1 A and B; P < 0.002). Conversely, the application of 1 ng/mL of TGF-β2 increased MEPP amplitude by 67% (Fig. 1 A–C; P < 0.0001) without altering MEPP frequency (Fig. 1D). TGF-β2 required 1 h to change the MEPPs, with longer incubations having no additional effect (not shown). As expected, there was a corresponding change in the magnitude of the postsynaptic current elicited by the spontaneous release of a synaptic vesicle (Fig. 2 A and C; P < 0.001).
Fig. 1.
TGF-β2 regulates MEPP amplitude. (A) TGF-β2 (1 ng/mL, blue) increased the amplitude of MEPPs (spontaneous, single vesicle releases) relative to controls (black), whereas anti-TGFβ2 (green) had the converse effect. (B) The mean amplitude of the MEPPs was significantly reduced by anti-TGF-β2 (dark green; *1P < 0.0001) and anti-TβRII (type II TGF-β receptor, light green; *2P < 0.002) antibodies and was increased significantly by TGF-β2 (blue; *3P < 0.0001). The bars in this and subsequent figures are the mean ± SE. The number of NMJs examined is indicated on each bar. The mean value for the control data was 0.39 ± 0.02 mV. (C) TGF-β2 affected MEPP amplitude across the whole MEPP population (TGF-β2, 1 ng/mL, dark blue; control, black). (D) TGF-β2 did not affect the frequency of MEPPs (P = 0.20).
Fig. 2.
TGF-β2 acts presynaptically to increase MEPC and decrease quantal content. Two-electrode voltage clamp showed that 1 ng/mL TGF-β2 significantly increased MEPC (*1P < 0.001) (A and C) but had no effect on EPCs (evoked multivesicular release) (B and D). (E) The calculated quantal content was significantly decreased in the TGF-β2 group (*2P < 0.0004). Data are from 21 BSA-treated and 20 TGF-β2–treated muscles, 1–4 NMJs per muscle.
TGF-β2 Has a Lesser Effect on the Amplitude of EPPs.
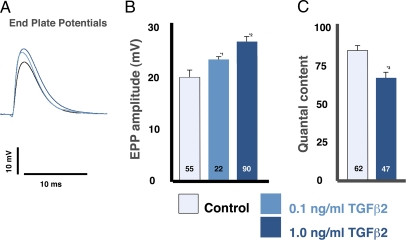
The application of TGF-β2 to the diaphragm increased EPPs by 30% (Fig. 3 A and B; P < 0.001). This change in EPP could arise if TGF-β2 had a postsynaptic effect and/or if the putative increase in QSpre were incompletely offset by a decreased quantal content. However, any effect of TGF-β2 purely on the postsynaptic fiber should affect MEPPs and EPPs equally. Consequently, the observation that the TGF-β2–induced change in MEPP amplitude is twice the change in EPP amplitude (Figs. 1 and 3 and Fig. S1) suggests that TGF-β2 has a significant presynaptic effect.
Fig. 3.
TGF-β2 increased EPP amplitude and decreases quantal content. (A) Single EPPs (evoked multivesicular releases) showed a TGF-β2–dependent increase in amplitude (TGF-β2, 1 ng/mL, dark blue; TGF-β2, 0.1 ng/mL light blue; control, black). (B) TGF-β2 at 0.1 and 1 ng/mL caused significant increases in EPP amplitude (*1P < 0.003; *2P < 0.001). (C) TGF-β2–induced decreases in quantal content were confirmed from potentials (*3P < 0.04).
TGF-β2 Increases Muscle Fiber Input Resistance.
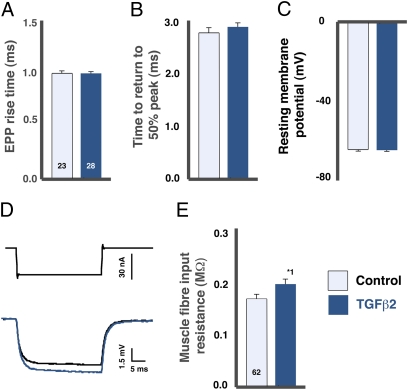
The putative postsynaptic actions of TGF-β2 were analyzed further using a combination of single- and dual-electrode recordings. TGF-β2 had no effect on evoked postsynaptic current amplitudes or time courses at voltage-clamped NMJs (Fig. 2 B and D; P = 0.8). By the same reasoning used above for potentials, this lack of effect indicates that the TGF-β2–induced changes in MEPP and EPP amplitude are not caused simply by an increase in either AChR single-channel currents or channel open time. TGF-β2 also had no effect on the resting potential of muscle fibers (Fig. 4C) or on the time course of the EPPs (Fig. 4 A and B); together with the unchanged EPC kinetics, this lack of effect indicates that the inactivation of released ACh by cholinesterase was unaffected.
Fig. 4.
TGF-β2 had no effect on resting membrane potential or EPP time course but slightly increased muscle fiber input resistance. (A–C) TGF-β2 (1 ng/mL) had no significant effect on EPP rise time (A), time to return to 50% of peak (B), or resting membrane potential (C). (D and E) Two-electrode recording revealed a small increase in muscle fiber input resistance with bath application of TGF-β2 (dark blue trace) relative to control (black trace). *1P < 0.02.
TGF-β2 did, however, increase the muscle fiber input resistance by 18% (Fig. 4 D and E), a significant proportion of the 30% increase in EPPs. Input resistance influences MEPPs and EPPs equally. Consequently, less than one third of the 67% increase in MEPPs caused by TGFβ2 is attributable to this mechanism.
TGFβ2 Increases Vesicle Loading.
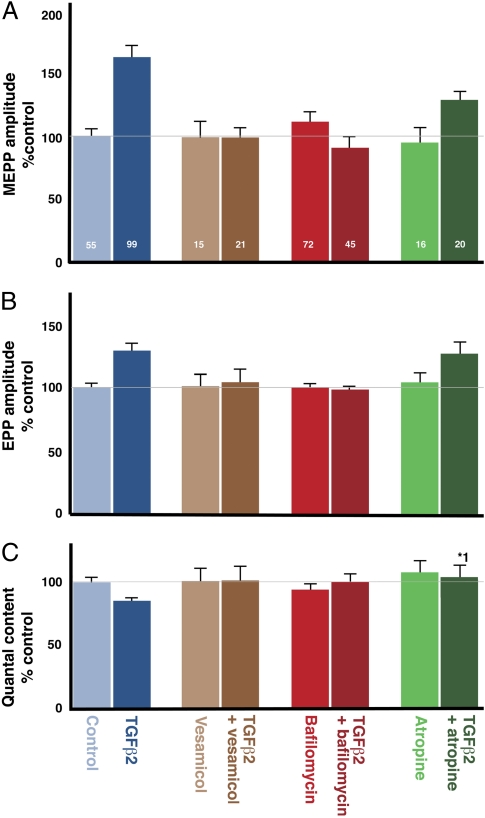
The size of QSpre cannot be measured directly, but QSpre-dependent mechanisms should be sensitive to drugs that affect the filling of synaptic vesicles, whereas such drugs would not be expected to block postsynaptic mechanisms. We therefore examined whether inhibitors of vesicular ACh transport antagonized the effect of TGF-β2 on MEPPs. Consistent with this hypothesis, both direct (L-vesamicol) (11) and indirect (bafilomycin) (12, 13) inhibition of ACh transport completely inhibited the TGF-β2–induced increases in MEPP and EPP amplitude (Fig. 5 A and B). Neither vesamicol nor bafilomycin had any effect when applied in the absence of TGF-β2 (Fig. 5 A and B).
Fig. 5.
Inhibitors of vesicle filling block the TGF-β2–induced changes in MEPP (A) and EPP (B) amplitudes, and quantal content (C). The vesicular ACh transporter inhibitor L-vesamicol (5 μM) (dark brown) and the vesicular proton pump inhibitor bafilomycin (0.1 μM) (deep red) abolished the TGF-β2–nduced change in MEPP amplitude (P = 0.9 and P = 0.2) (A), EPP amplitude (P = 0.7 and P = 0.6) (B), and quantal content (P = 0.9 and P = 0.63) (C). Application of 2 μM of atropine, a presynaptic muscarinic AChR inhibitor (dark green), did not prevent TGF-β2 from increasing MEPP (P = 0.1) (A) or EPP (P = 0.6) (B) amplitude. (C) However, atropine blocked the TGF-β2–induced decrease in quantal content (*1P = 0.03). Thus, increased vesicle loading may increase presynaptic inhibition, which reduces quantal content. None of the drugs alone (vesamicol, light brown; bafilomycin, light red; atropine, light green) had any effect on these parameters. The effect of TGF-β2 alone (dark blue) is significantly different from control (light blue), as documented in the previous figures.
TGF-β2 Indirectly Decreases Quantal Content.
We then tested whether TGF-β2 had a lesser effect on EPP amplitude than on MEPP amplitude because of increased negative feedback on the release of vesicles, as postulated. Consistent with this assumption, 1 ng/mL of TGF-β2 produced a depression in quantal content (Fig. 3C; P < 0.04), an effect that was confirmed by calculations from the previous two-electrode voltage-clamp recordings of the miniature end-plate currents and the evoked potential currents (EPCs) (Fig. 2E; P < 0.03). The negative feedback mechanism would have a greater influence when the diaphragm is stimulated with a physiological train than when it is stimulated with single stimuli because of the greater accumulation of transmitter in the synaptic cleft. Consequently, if TGF-β2 acts as postulated, its effect should be more evident after a train. As predicted, 1 ng/mL of TGF-β2 halved the rate of vesicle use in murine diaphragm stimulated at 20 Hz for 10 min (FM1-43 technique; Fig. S2).
The TGF-β2–induced decrease in quantal content did not occur in the presence of vesamicol and bafilomycin (Fig. 5C), indicating that the effect of TGF-β2 on quantal content is secondary to an increase in vesicle filling. Furthermore, as expected, blockade of the presynaptic cholinergic muscarinic receptors with 2 μM of atropine prevented the TGF-β2–induced decrease in quantal content (Fig. 5C) but not the increase in MEPP and EPP amplitude. Thus, the presynaptic action of TGF-β2 appears to be limited to increased filling of synaptic vesicles, with the observed decrease in quantal content being a down-stream consequence.
Discussion
The current study implicates TGF-β2 as a local modulator of the NMJ, through the control of QSpre and a lesser postsynaptic effect on input resistance. It also provides some validation of the hypothesis that QSpre and negative feedback combine to produce a physiology that is not apparent when either mechanism is considered in isolation.
TGF-β2 Regulates QSpre.
The evidence that TGF-β2 increases the amount of ACh released from a synaptic vesicle comes from multiple experiments. First, the mean postsynaptic response to the release of a single vesicle (MEPP) was increased by TGF-β2 and decreased by anti-TGF-β2 (Fig. 1), indicating that TGF-β2 is a physiological regulator of either QSpre and/or postsynaptic sensitivity. Second, nerve terminals contain TGF-β2 receptors (9), and drugs that act presynaptically blocked the affect of TGF-β2 (Fig. 5). Third, TGF-β2 reduced synaptic vesicle use by half during an extended stimulus of the diaphragm (Fig. S2). Fourth, as discussed later, TGF-β2’s effects could not be explained solely by an increase in receptor sensitivity/response to neurotransmitter. Most noticeably, the EPCs were unaffected, and the predominant postsynaptic effect was a small change in input resistance (Fig. 4) that was insufficient to explain the magnitude of TGF-β2’s affect on MEPPs. Hence, there is direct evidence for a presynaptic action and clear evidence against the major effects of TGF-β2 being postsynaptic.
Decreases in postsynaptic sensitivity at the NMJ increase quantal content (14, 15) via an unknown mechanism. TGF-β2 is located postsynaptically (8, 9), creating the possibility that postsynaptic sensitivity influences quantal content by regulating the release of TGF-β2. This possibility is broadly consistent with studies of Drosophila, where presynaptic motor nerve terminals are regulated by postsynaptic release of glass bottom boat, a member of the TGF-β superfamily (16, 17). If so, the mechanism described here may be an ancient and fundamental aspect of synaptic biology.
Alternatively, the observed effect of TGF-β2 on quantal content (Figs. 2 and 3 and Fig. S2) could be indirect, with TGF-β2 acting to increase postsynaptic sensitivity which, in turn, controls quantal content via an unidentified retrograde signal. We do not favor this alternative, because the location of TGF-β2 and its receptor support its being a retrograde signal rather than the trigger of a retrograde signal.
QSpre May Influence the Rate of Vesicular Use at NMJs.
Postsynaptic receptors usually are not saturated during synaptic transmission (4). Consequently, any variation in QSpre should lead to an equivalent variation in EPPs if QSpre is able to vary in isolation from other synaptic parameters. However, this variation in EPP size was not observed in this study. TGF-β2 induced a comparatively large increase in QSpre during the spontaneous release of vesicles, but its effect on EPPs was smaller and was largely the result of a postsynaptic effect, apparently because the increase in QSpre led to diminution in the release of vesicles through increased negative feedback by ACh. This effect was not an artifact of receptor saturation during evoked release, because larger EPPs were recorded when the decrease in quantal content was blocked by atropine. Hence, the net effect of increasing QSpre was to reduce the number of vesicles used per stimulus, with little change in the quantity of total ACh release during evoked activity.
The conservation of vesicles may increase the sustainability of neurotransmission, as indicated by the reduced rate of vesicular depletion during evoked stimuli (Fig. S2). In this context, it is noteworthy that TGF-β2 is not detectable at immature NMJs (9); its initial concentration in the postsynaptic domain is temporally correlated with the NMJ becoming able to sustain large-amplitude, high-frequency neurotransmission (18, 19). Furthermore, it may explain the unique ability of TGF-β2 to restore muscle function acutely in the early stage of motor neuron disease (20), when nerve terminals are beginning to fail (21).
Relationship Between QSpre and Evoked Potential Could Be Synapse-Type Dependent.
TGF-β2 is widely expressed in the brain (7), with TGF-β2 deficiency in mice leading to age-related aberration in dopamine turnover in the striatum (22). This observation raises the possibility that TGF-β2 is a modifier of many synapses, through alterations in QSpre. However, the physiological consequence of such regulation would be expected to vary with synaptic type. In particular, QSpre should have immediate effects on evoked synaptic potentials when the number of vesicles released per stimulus is small and/or negative feedback is absent. Consistent with this proposal, inhibitory single-vesicle responses are significantly reduced in pre-Botzinger complex synapses of TGF-β2−/− neonates (23), whereas excitatory single-vesicle responses are enhanced by long-term cultures of TGF-β2–treated hippocampal neurons (24). This observation is of intellectual interest but also may be of practical importance, given that TGF-β2 is up-regulated in Alzheimer's disease (25) and that TGF-β2 polymorphisms have been linked with Parkinson's disease (26). TGF-β1 deficiency in mice also has been linked to an Alzheimer's-like condition (27), but the relevance of this observation to the current observations is unclear, because TGF-β1 and TGF-β2 are activated through different mechanisms (28).
QSpre May Affect MEPP-Dependent Phenomena.
The suggestion that TGF-β2 regulation of QSpre has minimal effect on the evoked ACh does not exclude the possibility that altering QSpre leads to subsequent postsynaptic changes, for at least two reasons. First, a change in QSpre alters the amount of ACh derived from spontaneously released vesicles. The physiological significance of spontaneous release is poorly understood, although there is evidence from central synapses that spontaneous and evoked release activate different postsynaptic receptors, leading to activation of different intracellular pathways (29). If this difference in activation also occurs at the NMJ, then TGF-β2 would be expected to modulate selectively the pathways controlled by spontaneous release; such modulation might subtly regulate the postsynaptic cell. Second, cholinergic vesicles also release compounds such as ATP that have both pre- and postsynaptic targets (30, 31). Hence, the relative strengths of the cholinergic and noncholinergic pathways should change when QSpre is altered unless all the contents of synaptic vesicles are coordinately regulated by TGF-β2.
Postsynaptic Actions of TGF-β2 May Be Indirect.
The mechanism by which TGF-β2 increases the input resistance of muscle fibers is unclear. Muscle fibers express TGF-β receptors, but TGF-β receptor protein is not detectable in the postsynaptic domain of the fiber under conditions where the receptors are readily apparent in the presynaptic terminal (9), thus suggesting that the postsynaptic actions of TGF-β2 noted here may be caused by an extrasynaptic action and/or secondary to the TGF-β2 regulation of QSpre (see above). The fact that TGF-β2 did not increase EPPs when coadministered with inhibitors of vesicular filling (Fig. 5) is consistent with a presynaptic action.
TGF-β2’s Effect May Be Limited to Recycling Vesicles.
The effect of TGF-β2 was slow, with a 1-h incubation needed to produce a significant effect. The rate of entry of exogenous TGF-β2 into the synaptic cleft is unknown, and the slow onset of TGF-β2’s action may simply reflect barriers to its diffusion. However, TGF-β2 is similar in size to α-bungarotoxin, which acts rapidly at the NMJ (32). Alternatively, TGF-β2 may affect vesicles only after their use, an effect that would lead to the slow onset observed, because a large proportion of the pool of readily releasable recycling vesicles (33) must turn over in the 1-h period of drug incubation of the current experiment. If so, endogenous TGF-β2 would regulate QSpre at the NMJ with a time course that varies with the pattern of activation but in all instances would be too slow to produce impulse-by-impulse regulation.
In conclusion, the extracellular regulation of synapses historically has focused on the control of quantal content and postsynaptic sensitivity. The data presented here suggest that the QSpre is subject to extracellular regulation, in this case via TGF-β2–dependent control of the neurotransmitter-carrying capacity of synaptic vesicles. The characterization of this mechanism opens up new possibilities for how the characteristics of synapses are defined and/or linked to their pattern of use.
Materials and Methods
Animals and Materials.
Adult male rats (>230–530 g) were stunned and then killed by cervical dislocation in accordance with Schedule 1 of the UK Animals (Scientific Procedures) Act, 1986. All drugs were purchased from Sigma unless otherwise stated.
Electrophysiological Recordings.
Electrical recording procedures were similar to those used previously (19, 33, 34). Briefly, nerve-evoked muscle contraction was first blocked using 2 μM μ-conotoxin GIIIB (Peptide Institute), which selectively inhibits muscle voltage-gated sodium channels and excitation–contraction coupling (35) but leaves synaptic transmission unaffected (15). The nerve then was stimulated with 0.2-ms pulses, with double a previously determined maximal stimulus (2–3 V). All experiments were performed in a paired-control paradigm. For example, TGF-β2 (gift of Genzyme Corp), anti-TGF-β2 (LS-C48870; Bioscience), or anti-TβRII (SC-1700; Santa Cruz) was added to one hemidiaphragm preparation, and the other hemidiaphragm was bathed in vehicle/blocker, including BSA, and used as a control. TGF-β2 and other drugs were applied at least 1 h before electrophysiological recording to facilitate penetration into the synaptic cleft.
Single-electrode recording.
MEPPs and EPPs were recorded via a sharp glass microelectrode, as previously described (33, 34). Impalements within 100 μm of an NMJ were determined by the presence of MEPPs as well as an EPP with a rise time <1.5 ms (0–100%).
Two-electrode recording.
The voltage-recording electrode was positioned at an NMJ, and the same fiber then was impaled within 200 μm with a current-passing electrode (36). The initial resting membrane potential was noted, and fibers then were held at −70 mV for subsequent current- and voltage-clamp recordings. Muscle fiber input resistance was estimated by injecting hyperpolarizing pulses of 30 nA and 100 ms into the muscle fiber through the current electrode, and the potential at steady-state hyperpolarization was recorded with the voltage electrode. Input resistance was calculated using Ohm's law.
MEPPs at each NMJ were recorded for 60 s before single EPPs were provoked by nerve stimulation. For two-electrode recording, input resistance was recorded first in current clamp, at a holding potential of −70 mV. Miniature excitatory postsynaptic currents (MEPCs) (60 s) and single excitatory postsynaptic currents (EPCs) (five at 0.1 Hz) then were recorded with the voltage clamped at −70 mV.
Data Analysis and Statistics.
Quantal content, the number of vesicles released per action potential, was determined by the direct method for potentials (EPP/MEPP amplitude with nonlinear correction) and currents (EPC/MEPC) using standard criteria (15, 33, 36). The data are expressed as mean ± SE of NMJs taken from at least three (single-electrode recording) or 20–21 (two-electrode recording) rat hemidiaphragm preparations, with the number of NMJs (n ≤ 10 per muscle) indicated in each figure. Differences between means were compared using a two-sample Student's t test for equal or unequal variance according to a prior F test. P <0.05 was taken as significant.
Supplementary Material
Acknowledgments
We thank Prof. Arild Njå (University of Oslo, Oslo, Norway) for helpful discussions during the preparation of the manuscript and Prof. Clarke Slater (University of Newcastle upon Tyne, UK) for discussions, as well as technical expertise and equipment in the two-electrode voltage clamp recording experiments. This work was supported by the Neurobiology Programme (S.W.F., PhD Studentship) and the Institute of Medical Sciences (A.M., MSc Studentship; J.R., Tait Neuroscience Studentship) of the University of Aberdeen, the Marsden Fund of the Royal Society, New Zealand (I.S.M.), by a travel grant from the Physiological Society, by the Biotechnology and Biological Sciences Research Council Institutional Research and Career Development Award Scholars in Science Programme, and by the Royal Society of Edinburgh Open Exchange Programme (G.S.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1001695107/-/DCSupplemental.
References
- 1.Van der Kloot W, Molgó J. Quantal acetylcholine release at the vertebrate neuromuscular junction. Physiol Rev. 1994;74:899–991. doi: 10.1152/physrev.1994.74.4.899. [DOI] [PubMed] [Google Scholar]
- 2.Naves LA, Van der Kloot W. Repetitive nerve stimulation decreases the acetylcholine content of quanta at the frog neuromuscular junction. J Physiol. 2001;532:637–647. doi: 10.1111/j.1469-7793.2001.0637e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, et al. Activity-dependent presynaptic regulation of quantal size at the mammalian neuromuscular junction in vivo. J Neurosci. 2005;25:343–351. doi: 10.1523/JNEUROSCI.3252-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards RH. The neurotransmitter cycle and quantal size. Neuron. 2007;55:835–858. doi: 10.1016/j.neuron.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira L, Timóteo MA, Correia-de-Sá P. Negative crosstalk between M1 and M2 muscarinic autoreceptors involves endogenous adenosine activating A1 receptors at the rat motor endplate. Neurosci Lett. 2009;459:127–131. doi: 10.1016/j.neulet.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Regehr WG, Carey MR, Best AR. Activity-dependent regulation of synapses by retrograde messengers. Neuron. 2009;63:154–170. doi: 10.1016/j.neuron.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flanders KC, Ren RF, Lippa CF. Transforming growth factor-betas in neurodegenerative disease. Prog Neurobiol. 1998;54:71–85. doi: 10.1016/s0301-0082(97)00066-x. [DOI] [PubMed] [Google Scholar]
- 8.McLennan IS, Koishi K. The transforming growth factor-betas: Multifaceted regulators of the development and maintenance of skeletal muscles, motoneurons and Schwann cells. Int J Dev Biol. 2002;46:559–567. [PubMed] [Google Scholar]
- 9.McLennan IS, Koishi K. Transforming growth factor-β-2 (TGF-β 2) is associated with mature rat neuromuscular junctions. Neurosci Lett. 1994;177:151–154. doi: 10.1016/0304-3940(94)90889-3. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Y, McLennan IS, Koishi K, Hendry IA. Transforming growth factor-beta 2 is anterogradely and retrogradely transported in motoneurons and up-regulated after nerve injury. Neuroscience. 2000;97:735–742. doi: 10.1016/s0306-4522(00)00084-1. [DOI] [PubMed] [Google Scholar]
- 11.Prior C, Marshall IG, Parsons SM. The pharmacology of vesamicol: An inhibitor of the vesicular acetylcholine transporter. Gen Pharmacol. 1992;23:1017–1022. doi: 10.1016/0306-3623(92)90280-w. [DOI] [PubMed] [Google Scholar]
- 12.Cousin MA, Robinson PJ. Ba2+ does not support synaptic vesicle retrieval in rat cerebrocortical synaptosomes. Neurosci Lett. 1998;253:1–4. doi: 10.1016/s0304-3940(98)00610-7. [DOI] [PubMed] [Google Scholar]
- 13.Cavelier P, Attwell D. Neurotransmitter depletion by bafilomycin is promoted by vesicle turnover. Neurosci Lett. 2007;412:95–100. doi: 10.1016/j.neulet.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cull-Candy SG, Miledi R, Trautmann A, Uchitel OD. On the release of transmitter at normal, myasthenia gravis and myasthenic syndrome affected human end-plates. J Physiol. 1980;299:621–638. doi: 10.1113/jphysiol.1980.sp013145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plomp JJ, van Kempen GT, Molenaar PC. Adaptation of quantal content to decreased postsynaptic sensitivity at single endplates in alpha-bungarotoxin-treated rats. J Physiol. 1992;458:487–499. doi: 10.1113/jphysiol.1992.sp019429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goold CP, Davis GW. The BMP ligand Gbb gates the expression of synaptic homeostasis independent of synaptic growth control. Neuron. 2007;56:109–123. doi: 10.1016/j.neuron.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frank CA, Kennedy MJ, Goold CP, Marek KW, Davis GW. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron. 2006;52:663–677. doi: 10.1016/j.neuron.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly SS. The effect of age on neuromuscular transmission. J Physiol. 1978;274:51–62. doi: 10.1113/jphysiol.1978.sp012133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bewick GS, Reid B, Jawaid S, Hatcher T, Shanley L. Postnatal emergence of mature release properties in terminals of rat fast- and slow-twitch muscles. Eur J Neurosci. 2004;19:2967–2976. doi: 10.1111/j.0953-816X.2004.03418.x. [DOI] [PubMed] [Google Scholar]
- 20.Day WA, Koishi K, Nukuda H, McLennan IS. Transforming growth factor-beta 2 causes an acute improvement in the motor performance of transgenic ALS mice. Neurobiol Dis. 2005;19:323–330. doi: 10.1016/j.nbd.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Gould TW, et al. Complete dissociation of motor neuron death from motor dysfunction by Bax deletion in a mouse model of ALS. J Neurosci. 2006;26:8774–8786. doi: 10.1523/JNEUROSCI.2315-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrews ZB, et al. Transforming growth factor β2 haploinsufficient mice develop age-related nigrostriatal dopamine deficits. Neurobiol Dis. 2006;21:568–575. doi: 10.1016/j.nbd.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Heupel K, et al. Loss of transforming growth factor-beta 2 leads to impairment of central synapse function. Neural Develop. 2008;3:25. doi: 10.1186/1749-8104-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukushima T, Liu RY, Byrne JH. Transforming growth factor-beta2 modulates synaptic efficacy and plasticity and induces phosphorylation of CREB in hippocampal neurons. Hippocampus. 2007;17:5–9. doi: 10.1002/hipo.20243. [DOI] [PubMed] [Google Scholar]
- 25.Flanders KC, Lippa CF, Smith TW, Pollen DA, Sporn MB. Altered expression of transforming growth factor-beta in Alzheimer's disease. Neurology. 1995;45:1561–1569. doi: 10.1212/wnl.45.8.1561. [DOI] [PubMed] [Google Scholar]
- 26.Goris A, et al. Investigation of TGFB2 as a candidate gene in multiple sclerosis and Parkinson's disease. J Neurol. 2007;254:846–848. doi: 10.1007/s00415-006-0414-6. [DOI] [PubMed] [Google Scholar]
- 27.Brionne TC, Tesseur I, Masliah E, Wyss-Coray T. Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron. 2003;40:1133–1145. doi: 10.1016/s0896-6273(03)00766-9. [DOI] [PubMed] [Google Scholar]
- 28.Kusakabe M, Cheong PL, Nikfar R, McLennan IS, Koishi K. The structure of the TGF-beta latency associated peptide region determines the ability of the proprotein convertase furin to cleave TGF-betas. J Cell Biochem. 2008;103:311–320. doi: 10.1002/jcb.21407. [DOI] [PubMed] [Google Scholar]
- 29.Sutton MA, Schuman EM. Partitioning the synaptic landscape: Distinct microdomains for spontaneous and spike-triggered neurotransmission. Sci Signal. 2009;2:pe19. doi: 10.1126/scisignal.265pe19. [DOI] [PubMed] [Google Scholar]
- 30.Silinsky EM. On the association between transmitter secretion and the release of adenine nucleotides from mammalian motor nerve terminals. J Physiol. 1975;247:145–162. doi: 10.1113/jphysiol.1975.sp010925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santos DA, Salgado AI, Cunha RA. ATP is released from nerve terminals and from activated muscle fibres on stimulation of the rat phrenic nerve. Neurosci Lett. 2003;338:225–228. doi: 10.1016/s0304-3940(02)01419-2. [DOI] [PubMed] [Google Scholar]
- 32.Akaaboune M, Grady RM, Turney S, Sanes JR, Lichtman JW. Neurotransmitter receptor dynamics studied in vivo by reversible photo-unbinding of fluorescent ligands. Neuron. 2002;34:865–876. doi: 10.1016/s0896-6273(02)00739-0. [DOI] [PubMed] [Google Scholar]
- 33.Reid B, Slater CR, Bewick GS. Synaptic vesicle dynamics in rat fast and slow motor nerve terminals. J Neurosci. 1999;19:2511–2521. doi: 10.1523/JNEUROSCI.19-07-02511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reid B, Martinov VN, Njå A, Lømo T, Bewick GS. Activity-dependent plasticity of transmitter release from nerve terminals in rat fast and slow muscles. J Neurosci. 2003;23:9340–9348. doi: 10.1523/JNEUROSCI.23-28-09340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gray WR, Olivera BM, Cruz LJ. Peptide toxins from venomous Conus snails. Annu Rev Biochem. 1988;57:665–700. doi: 10.1146/annurev.bi.57.070188.003313. [DOI] [PubMed] [Google Scholar]
- 36.Wood SJ, Slater CR. The contribution of postsynaptic folds to the safety factor for neuromuscular transmission in rat fast- and slow-twitch muscles. J Physiol. 1997;500:165–176. doi: 10.1113/jphysiol.1997.sp022007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.