Abstract
Nitrospira are barely studied and mostly uncultured nitrite-oxidizing bacteria, which are, according to molecular data, among the most diverse and widespread nitrifiers in natural ecosystems and biological wastewater treatment. Here, environmental genomics was used to reconstruct the complete genome of “Candidatus Nitrospira defluvii” from an activated sludge enrichment culture. On the basis of this first-deciphered Nitrospira genome and of experimental data, we show that Ca. N. defluvii differs dramatically from other known nitrite oxidizers in the key enzyme nitrite oxidoreductase (NXR), in the composition of the respiratory chain, and in the pathway used for autotrophic carbon fixation, suggesting multiple independent evolution of chemolithoautotrophic nitrite oxidation. Adaptations of Ca. N. defluvii to substrate-limited conditions include an unusual periplasmic NXR, which is constitutively expressed, and pathways for the transport, oxidation, and assimilation of simple organic compounds that allow a mixotrophic lifestyle. The reverse tricarboxylic acid cycle as the pathway for CO2 fixation and the lack of most classical defense mechanisms against oxidative stress suggest that Nitrospira evolved from microaerophilic or even anaerobic ancestors. Unexpectedly, comparative genomic analyses indicate functionally significant lateral gene-transfer events between the genus Nitrospira and anaerobic ammonium-oxidizing planctomycetes, which share highly similar forms of NXR and other proteins reflecting that two key processes of the nitrogen cycle are evolutionarily connected.
Keywords: environmental genomics, nitrification, Nitrospirae
Nitrification, the microbially catalyzed sequential oxidation of ammonia via nitrite to nitrate, is a key process of the biogeochemical nitrogen cycle and of biological wastewater treatment. The second step of nitrification is carried out by chemolithoautotrophic nitrite-oxidizing bacteria (NOB), which are phylogenetically heterogeneous (1) and occur in a wide range of aquatic and terrestrial ecosystems. Most studies on the physiology of NOB used pure cultures of Nitrobacter, which belong to the Alphaproteobacteria (1), and complete genome sequences from NOB are available for three Nitrobacter strains (2, 3) and the marine gammaproteobacterium Nitrococcus mobilis (GenBank accession no. NZ_AAOF00000000). However, cultivation-independent molecular methods revealed that Nitrospira, forming a deeply branching lineage in the bacterial phylum Nitrospirae (4), are by far the most diverse and abundant NOB (5). In addition to their wide distribution in natural habitats such as soils (6), sediments (7), the oceans (8), and hot springs (9), members of the genus Nitrospira are the predominant NOB in wastewater treatment plants (5) and thus belong to the microorganisms most relevant for biotechnology.
The immense ecological and technical significance of Nitrospira contrasts with our scarce knowledge about these bacteria. As the majority of Nitrospira are uncultured, and the available cultures are difficult to maintain, only a few studies have addressed their ecology and physiology (e.g., 5, 10, 11). Furthermore, except for one 137-kbp contig (12), genomic sequences from Nitrospira have not been obtained yet. This situation has been highly unsatisfactory because deeper insight into the biology of these elusive NOB is crucial for a better understanding of nitrogen cycling in natural and engineered systems.
Recently, a Nitrospira strain was enriched from activated sludge and partly characterized (13). This organism, tentatively named “Candidatus Nitrospira defluvii,” belongs to Nitrospira sublineage I, which is most important for sewage treatment (5) but has no representative in pure culture. Here, the complete genome of Ca. N. defluvii was reconstructed from a metagenomic library of the enrichment. More than two decades after Nitrospira were discovered (8), we provide an analysis of a Nitrospira genome with previously unmatched insight into the biology of Nitrospira, show striking differences in key metabolic pathways between Nitrospira and other NOB, and change the current perception on the evolution of NO2− oxidation.
Results and Discussion
Genome Reconstruction.
Quantitative FISH has shown that the NO2−-oxidizing enrichment consisted of 86% of Ca. N. defluvii and did not contain other known NOB (13). The complete genome of Ca. N. defluvii was reconstructed from this enrichment by an environmental genomics approach similar to that used for inferring the genome sequence of the anaerobic ammonium-oxidizing bacterium (“anammox” organism) “Candidatus Kuenenia stuttgartiensis” (14). The completeness and correct assembly of the Nitrospira genome was indicated by the retrieval of all 63 clusters of orthologous groups (COGs) of proteins, which are present in all genomes in the current COG database (Fig. S1), by lack of suspicious redundancy in gene content, and by the presence of all essential genes in key biosynthetic pathways. The low frequency of single nucleotide polymorphisms (about one per 500 kbp) strongly suggests that the enrichment culture contained only one Nitrospira strain. Key features of the genome are summarized in Table S1 and Fig. S1. About 30% of the predicted coding sequences (CDS) have no homologs in other organisms, reflecting the distant relationship of Nitrospira to other bacteria and the lack of genome sequences from the genus Nitrospira in public databases. Furthermore, only two lineages within the phylum Nitrospirae have been explored on a genomic level. The closest genome-sequenced relatives of Ca. N. defluvii belong to the genus Leptospirillum and are aerobic acidophilic iron oxidizers (15–17). In addition, the genome sequence of the anaerobic sulfate reducer Thermodesulfovibrio yellowstonii (GenBank accession no. NC_011296), also belonging to the Nitrospirae, is publicly available.
Nitrite Oxidation and Energy Metabolism.
The key enzyme for NO2− oxidation by NOB is nitrite oxidoreductase (NXR), which shuttles two electrons per oxidized NO2− into the electron transport chain. In Nitrobacter, NXR is an iron-sulfur molybdoprotein (18) located at the inner cell membrane and at the intracytoplasmic membranes (ICM). The reaction catalyzed by this NXR is reversible, so that the enzyme also reduces NO3− with electrons derived from organic compounds. Depending on the applied purification method, this NXR was found to consist of two (18) or three subunits with a supposed α2β2γ1 stoichiometry (19). The α-subunit (NxrA) is thought to contain the substrate-binding site with the molybdopterin cofactor (Mo-co) (18, 19), whereas the β-subunit (NxrB) with [Fe-S] clusters probably channels electrons from the α- to the γ-subunit or directly to the membrane-integral electron transport chain (20).
Nitrospira are Gram-negative bacteria lacking ICM (8). Although no NO3−-reducing activity has been demonstrated yet for their nitrite-oxidizing system, the term NXR is used here to be consistent with established terminology (2). The first insight into the nature of the Nitrospira NXR was obtained by studying a pure culture of Nitrospira moscoviensis (21). Four major proteins were detected in membrane fractions showing a high NO2−-oxidizing activity in vitro. Antibodies originally raised against NxrB of Nitrobacter bound to one of these proteins, which was designated the NxrB of N. moscoviensis (21). Another protein with an apparent molecular mass of 130 kDa resembled the NxrA of Nitrobacter (115–130 kDa). The other two proteins were not further characterized. The NXR of N. moscoviensis was also shown to contain molybdenum and to be located at the inner cell membrane, where it faces the periplasmic space (21).
The genome of Ca. N. defluvii was screened for CDS with a predicted molecular mass resembling the NxrA and NxrB of N. moscoviensis and similarity to known NO2−/NO3−-binding molybdoenzymes, such as the NXR of Nitrobacter or bacterial nitrate reductases (NARs). Two candidates were identified for each NxrA and NxrB (Table S2). The genes are colocalized in two clusters (nxrA1B1 and nxrA2B2), which are separated by 17 other CDS from each other. The amino acid identities are 86.6% for the two NxrA and 100% for the two NxrB copies (the nxrB genes are identical except for a synonymous single-base substitution). NxrA1 and NxrA2 contain binding motifs for one [Fe-S] cluster and for molybdenum, which are indicative of the type II group in the dimethyl sulfoxide (DMSO) reductase family of Mo-co–binding enzymes (SI Results and Fig. S2 A and B). Five residues, which are conserved in the α-subunits of NARs and in the NxrA of Nitrobacter and Nitrococcus, have been proposed to interact with NO2−/NO3− or to affect the conformation of the substrate entry channel (22). Except for one threonine, which is replaced by asparagine (Fig. S2B), these residues are conserved in both NxrA copies of Ca. N. defluvii, suggesting that the α-subunit contains the substrate-binding site. Consistent with the periplasmic orientation of NXR in N. moscoviensis (21), NxrA1 and NxrA2 of Ca. N. defluvii contain an N-terminal twin-arginine motif for export via the twin-arginine protein translocation (Tat) pathway.
Both NxrB copies of Ca. N. defluvii lack a predicted signal peptide, but may be cotranslocated with NxrA into the periplasm by a “hitchhiker” mechanism as proposed for the β-subunits of other periplasmic Mo-co–binding enzymes (e.g., ref. 23). Four cysteine-rich binding motifs for [Fe-S] clusters, which occur also in NxrB of Nitrobacter and Nitrococcus, were identified (Fig. S2 C and D). Homologous [Fe-S] clusters mediate intramolecular electron transfer in nitrate reductase A of Escherichia coli (24).
All NxrA and NxrB copies of Ca. N. defluvii lack transmembrane helices, although NXR is membrane-associated in Nitrospira (21). Theoretically, the α/β-complex might cluster with a membrane-bound terminal oxidase that receives electrons from NXR. However, other enzymes in the DMSO reductase family contain an additional membrane-integral γ-subunit, which is the membrane anchor of the holoenzyme and channels electrons between the β-subunit and the electron transport chain via one or two hemes (25). Four proteins encoded by Ca. N. defluvii could be heme-containing subunits of NXR (Table S2). Each has one transmembrane domain and an N-terminal signal peptide for translocation via the Sec pathway. The largest candidate (66.7 kDa) is a c-type cytochrome with two predicted heme-binding sites. The other three proteins are smaller (29.7–34.3 kDa) and remotely similar to the γ-subunit of chlorate reductase, which contains one b-type heme (26). These genes are not in direct proximity of the nxrAB clusters, but the predicted molecular masses of their products resemble the two uncharacterized major proteins from N. moscoviensis membrane extracts (62 and 29 kDa) (21). Their biological functions and the exact composition of NXR await experimental clarification.
The sequenced Nitrobacter genomes encode a peptidyl-prolyl cis-trans isomerase (NxrX) proposed to assist in the folding of NXR (2, 3). Ca. N. defluvii lacks a homolog of NxrX, but one CDS is similar to chaperones involved in the assembly of other DMSO reductase-family enzymes (26). It is located directly upstream of one putative membrane-integral NXR subunit (Table S2) and could play a role in NXR maturation.
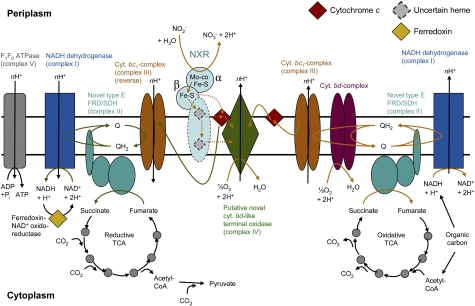
On the basis of biochemical (21) and genomic data for Nitrospira, a membrane-bound periplasmic NXR that consists of at least two subunits is proposed (Fig. 1). High-potential electrons from NO2− are probably transferred to cytochrome (cyt.) c as in Nitrobacter (19) and then to a terminal cyt. c oxidase (Fig. 1). In Nitrobacter, the terminal oxidase is of the aa3 type (3). The lack of detectable cyt. a in Nitrospira cultures (4, 8) and of genes coding for a-type cytochromes in Ca. N. defluvii implies that Nitrospira possess a different type of terminal oxidase. Intriguingly, the genome does not encode any known heme-copper oxidase, which could transfer electrons from cyt. c to O2. However, Ca. N. defluvii has a heterodimeric cyt. bd quinol oxidase (genes cydA and cydB; Table S2) that could receive electrons derived from low-potential donors, such as organic carbon, via the quinol pool (Fig. 1). The genome contains four additional CDS that resemble the CydA subunit of cyt. bd oxidases, but can be distinguished from the canonical proteins by phylogenetic analysis (Fig. S3A). We refer to these uncharacterized proteins as putative “cyt. bd-like oxidases.” They contain 14 predicted transmembrane helices and several histidines that may serve as heme ligands (Fig. S3B). Interestingly, one of these CDS (Nide0901) also contains a putative copper (CuB)-binding site (Fig. S3B). This motif is characteristic for the binuclear center of heme-copper cyt. c oxidases, and it is thus tempting to speculate that Nide0901 could replace the lacking canonical heme-copper oxidases in Nitrospira (Fig. 1). The proposed function of Nide0901 as terminal oxidase gains further support from transcriptional analysis. High levels of nide0901 mRNA were detected in the presence of the electron donor NO2− and the terminal electron acceptor O2, whereas the transcription of this gene decreased markedly in the absence of these substrates (Fig. S3D). An alternative to a membrane-bound terminal oxidase would be a soluble cytoplasmic O2 reductase, but this is not supported by the genomic data.
Fig. 1.
Genome-based model of energy metabolism in Ca. N. defluvii. Orange arrows indicate electron flow in the oxidative branches of the electron transport chain; green arrows indicate reverse electron transport from NO2− to NAD+. Dashed black lines point out that the membrane-integral subunit of NXR is uncertain. Dashed orange arrows show hypothetical possibilities for electron flow from NXR to the putative cyt. c-oxidase. nH+ indicates that the number of translocated protons is unknown because the H+/e− ratio of the respective complexes has not been determined for Nitrospira. FRD, fumarate reductase; SDH, succinate dehydrogenase. See Table S2 for a list of the involved proteins.
The genome-based model of energy metabolism in Ca. N. defluvii composes a branched respiratory chain for NO2− oxidation for the use of low-potential electron donors such as organic substrates and for reverse electron transport (Fig. 1). In addition, two copper-containing nitrite reductases (NirK; Table S2) were identified. NirK forms NO from NO2− in denitrifying organisms, including other nitrifiers (e.g., 27). Although denitrification by Ca. N. defluvii has not been experimentally demonstrated, the nirK genes indicate that this organism may denitrify NO2−, for example, by using organic substrates as the electron donor. If NXR works reversibly in Nitrospira, denitrification could also start from NO3−. Other denitrification genes were not found. In Nitrobacter, NO may function in reverse electron transport (28) and in electron flux regulation (27). It remains unclear whether NO plays similar physiological roles in Nitrospira.
Expression of NXR.
To test whether NO2− induces the expression of NXR, RNA was extracted from enrichment biomass during starvation in NO2−-free medium and after addition of NO2−, and nxrB mRNA was analyzed by reverse transcription (RT)–PCR. Interestingly, a low level of nxrB mRNA was detected after starvation for 11 d in NO2−-free medium (Fig. S2E). Addition of NO2− led to an increased transcription of nxrB, whereas the level of 16S rRNA from Ca. N. defluvii did not change markedly (Fig. S2E). NxrB protein was detected even after starvation in NO2−-free medium for 110 d, and its level increased markedly upon addition of NO2− (Fig. S2E). These results support the annotation of NXR. The constitutive expression of NXR should enable Ca. N. defluvii to use NO2−, whose concentration usually is low and fluctuates in natural habitats, immediately after this energy source becomes available.
Autotrophy.
NOB of the genus Nitrobacter (2) and, on the basis of genomic data, also Nitrococcus use the Calvin–Benson–Bassham (CBB) cycle for CO2 fixation. The key enzymes of this pathway are ribulose-1,5-bisphosphate carboxylase (RubisCO) and ribulose-5-phosphate kinase. Nitrospira also grow chemolithoautotrophically on NO2− and CO2 (4, 13), but their pathway for CO2 fixation was not identified previously. Ca. N. defluvii encodes a form IV RubisCO-like protein (Fig. S4A) lacking functional key residues of canonical RubisCO (Fig. S4B). In Bacillus subtilis, a form IV RubisCO-like protein has no bona fide carboxylating activity (29). The absence of other genes similar to RubisCO and of ribulose-5-phosphate kinase suggests that the CBB cycle does not operate in Ca. N. defluvii. Instead, all genes of the reductive tricarboxylic acid (rTCA) cycle are present, including the key enzymes ATP-citrate lyase and 2-oxoglutarate:ferredoxin oxidoreductase (OGOR), and also pyruvate:ferredoxin oxidoreductase (POR) (Table S2 and SI Results).
Operation of the rTCA cycle in Ca. N. defluvii was confirmed by the small carbon isotopic fractionation factor (ε) between biomass and CO2 of 2–6‰ (Table S3), typical for the rTCA cycle (30). Furthermore, the abundant (∼80% of all fatty acids) and characteristic straight-chain fatty acid for Ca. N. defluvii, C16:1 ω5 (13), was 3–6‰ enriched relative to the biomass, whereas isoprenoid lipids were ∼4‰ depleted (Table S3). This trend of more enriched straight-chain lipids is unusual for almost all carbon fixation pathways except for the rTCA cycle (31).
As POR and OGOR generally are O2-sensitive enzymes (32), the rTCA cycle is found mainly in anaerobic organisms, and its presence in an aerobic nitrifier seems surprising. However, this pathway is functional in some microaerophilic autotrophs such as Hydrogenobacter thermophilus (33), and it was identified in Leptospirillum genomes (16, 17). H. thermophilus has two isozymes of OGOR, a two-subunit enzyme needed under anoxic conditions and a more O2-tolerant unique five-subunit form, which supports mainly aerobic growth (34), and it also has an unusual five-subunit POR (35). Highly similar five-subunit OGOR and POR in Ca. N. defluvii (SI Results) and Leptospirillum (16) may allow the rTCA cycle to function in these aerobic members of the Nitrospirae phylum. Thus, on the basis of genomic and isotopic data, Nitrospira fix CO2 via the rTCA cycle and represent the only nitrifier for which this pathway has been detected.
Use of Organic Substrates.
Ca. N. defluvii and Nitrospira marina benefit from simple organic compounds in nitrite media (8, 13), and uncultured Nitrospira in sewage plants take up pyruvate (5). However, it is unknown whether Nitrospira use organic substrates only as carbon sources or also for energy generation. Interestingly, the Ca. N. defluvii genome encodes pathways for the catabolic degradation and for the assimilation of acetate, pyruvate, and formate (Fig. S5 and SI Results), and candidate genes were found for the degradation of branched amino acids. As the Embden–Meyerhof–Parnas pathway is complete, Ca. N. defluvii should be able to metabolize hexose sugars. This is consistent with carbon being stored as glycogen (SI Results). Two of the three sequenced Nitrobacter genomes also contain the complete glycolysis pathway (3), but growth of Nitrobacter on sugars has not been reported. Whether Ca. N. defluvii can take up and use sugars should depend mainly on functional sugar transport systems. The genome indeed contains putative sugar transporters (Table S1), but their function remains to be determined.
The oxidative tricarboxylic acid (oTCA) cycle shares most enzymes with the rTCA cycle except for citrate synthase and the 2-oxoglutarate dehydrogenase complex (ODH). Ca. N. defluvii encodes citrate synthase but apparently lacks ODH, which may, however, be replaced by OGOR (Table S2 and SI Results). A complete oTCA cycle was reported for Nitrobacter (28), indicating that this pathway is not unusual in NOB.
Purely heterotrophic growth of Nitrospira has not been observed yet. However, if all potentially involved genes are functional, Ca. N. defluvii benefits from a mixotrophic lifestyle using organic compounds from sewage in addition to NO2− and CO2.
Stress Response and Defense.
Ca. N. defluvii is exposed to a plethora of potentially toxic substances in sewage. Accordingly, the genome encodes multidrug efflux systems and transporters for heavy metals, organic solvents, and antimicrobials (Table S1), and it contains genes for cyanate and arsenic resistance (Table S2 and SI Results). As shown previously (12), Ca. N. defluvii has a functional chlorite dismutase that could degrade ClO2− in polluted environments, in chlorinated activated sludge, or in the proximity of chlorate-reducing microbes. Most intriguingly, Ca. N. defluvii lacks key genes for protection from reactive oxygen species (ROS) present in most aerobic organisms. No catalase, superoxide dismutase, or superoxide reductase was found. Two cyt. c peroxidases and several thioredoxin-dependent peroxiredoxins could function as H2O2 scavengers (Table S2 and SI Results). Protection from O2− and H2O2 might be conferred by manganese [Mn(II)] (36). Indeed, the required permease for manganese import was identified in the genome. Bacterioferritin and carotenoids (Table S2) could also contribute to protection from radicals and ROS. Moreover, the intracellular O2 level could be kept low by the canonical cyt. bd oxidase. Homologs in other organisms have a high affinity to O2 and contribute to oxidative stress protection (37). Growth of Nitrospira in biofilms and flocs (e.g., ref. 13) could offer additional protection from ambient O2.
Ca. N. defluvii carries one region of clustered, regularly interspaced short palindromic repeats (CRISPRs) and CRISPR-associated (cas) genes for phage defense (38). The CRISPR repeats of Ca. N. defluvii show no sequence similarity to those of Leptospirillum groups II and III, which also differ in their Cas proteins (17), suggesting that this defense mechanism was independently acquired by different members of the Nitrospirae phylum.
Ecophysiology and Evolutionary History of Nitrospira.
The NXRs of Nitrobacter, Nitrococcus, and Nitrospira differ in their subcellular localization and phylogenetic position within the DMSO reductase family. The NXRs of Nitrobacter and Nitrococcus are closely related to NARs. They are associated with the cytoplasmic membrane and ICM with the active site facing the cytoplasm (39). The unique NXR of Nitrospira does not cluster with the NARs (Fig. 2). It is also attached to the cytoplasmic membrane, but is oriented toward the periplasmic space (21; this study). The periplasmic orientation should be energetically advantageous because proton release by NO2− oxidation in the periplasm and concomitant proton consumption by O2 reduction in the cytoplasm contribute to the membrane potential (Fig. 1). Furthermore, only a cytoplasmic NXR requires the transport of NO2− and NO3− in opposite directions across the inner membrane. Accordingly, putative NO2−/NO3− transporters are found in all sequenced Nitrobacter genomes (3) and in Nitrococcus. Their substrate affinities and turnover rates could be limiting factors for NO2− oxidation by these NOB. This and the catalytic properties of NXR could explain the relatively high apparent Km(NO2−) value of Nitrobacter (11). In contrast, the predicted NO2− and NO3− transporters of Ca. N. defluvii (Table S1) most likely play no role in nitrite oxidation but are required only for nitrogen assimilation and resistance against excess nitrite (SI Results).
Fig. 2.
Maximum-likelihood tree showing the phylogenetic positioning of selected type II enzymes of the DMSO reductase family. For phylogenetic analysis of the catalytic (α) subunits, 1,308 amino acid positions were considered. Names of validated enzymes are indicated: Nxr, nitrite oxidoreductase; Nar, membrane-bound respiratory nitrate reductase; Pcr, perchlorate reductase; Ebd, ethylbenzene dehydrogenase; Ddh, dimethylsulfide dehydrogenase; Clr, chlorate reductase; Ser, selenate reductase. Parentheses contain the number of sequences within a group or the accession number, respectively.
Consistent with the predicted advantages of their periplasmic NXR, Nitrospira are better adapted to low NO2− concentrations (10, 11), which also were key to the selection against coexisting Nitrobacter during enrichment (13). As NO2− rarely accumulates in natural environments, the highly efficient use of this substrate most likely is a main reason for the competitive success and wide natural distribution of Nitrospira.
The use of different key enzymes and pathways (e.g., CO2 fixation) by Nitrospira in contrast to the proteobacterial NOB Nitrobacter and Nitrococcus suggests that chemolithoautotrophic NO2− oxidation evolved independently in these lineages. On the basis of the close phylogenetic affiliation of Nitrobacter and Nitrococcus to phototrophic Proteobacteria, which also possess ICM, Teske et al. (1) hypothesized that these NOB were derived from phototrophic ancestors. Indeed, a recently isolated anaerobic phototroph, which uses NO2− as the electron donor, is closely related to Nitrococcus (40). A cytoplasmically oriented NXR would probably be no disadvantage for phototrophic NOB where the membrane potential is sustained mainly by light-driven cyclic electron flow. The orientation of NXR may not easily be reversed, because it intimately affects the interaction with downstream components of the electron transport chain. Hence, the conservation of a cytoplasmic NXR during the transition from phototrophy to chemolithotrophy could explain the orientation of NXR in Nitrobacter and Nitrococcus. In contrast and consistent with the absence of ICM in Nitrospira, no phototrophic relative of Nitrospira is known and we hypothesize that the capability to gain energy from NO2− oxidation has evolved in this lineage from an anaerobic nonphototrophic ancestor. An anaerobic or microaerophilic origin of Nitrospira would be consistent with the rTCA cycle, the presence of the anaerobic cobalamin biosynthesis pathway (Table S2), and the lack of classic defense mechanisms against ROS. Additional support for this hypothesis stems from estimating genus divergence times within the Nitrospirae phylum by using 16S rRNA as the molecular clock (SI Results). Extant Nitrospira are active at low dissolved O2 levels in bioreactors and might still prefer hypoxic conditions (41).
Intriguingly, comparative genomics revealed an unexpected evolutionary link between Nitrospira and anammox organisms. For example, the closest homolog of the NXR of Ca. N. defluvii was found in Ca. K. stuttgartiensis (Fig. 2). NO2− oxidation is an integral step of the anammox metabolism where it replenishes the electron transport system (14), and this NXR-like protein is the only candidate for a NO2−-oxidizing enzyme in the Kuenenia genome. Its α-subunit contains the signature residues of NO2−/NO3−-binding molybdoenzymes (22) (Fig. S2 A and B). The NXRs of Nitrospira and Kuenenia are highly similar (amino acid identities are 57.4–57.7% for the α-subunit and 62.5% for the β-subunit) and form a monophyletic lineage in the tree of type II enzymes of the DMSO reductase family (Fig. 2). In addition, both Ca. N. defluvii and Ca. K. stuttgartiensis have the putative chaperone for NXR assembly in analogy to NxrX of Nitrobacter. Ca. K. stuttgartiensis also has a putative cyt. bd-like oxidase, which is the closest relative of the four cyt. bd-like oxidases of Ca. N. defluvii (Fig. S3A). Interestingly, its gene is located in close proximity to nxrA, nxrB, two putative membrane subunits of NXR, and the chaperone in the Kuenenia genome (Fig. 3). The same region contains a monoheme cyt. c-like protein and three proteins of unknown function, which also have highly similar homologs in Ca. N. defluvii (Fig. 3). Thus, both organisms share a set of highly similar proteins that function in NO2− oxidation and probably in electron transport and respiration, and these genes are clustered as a small metabolic island in the anammox genome. As anammox organisms are planctomycetes and consequently not closely related to the Nitrospirae (14), these observations are strongly indicative of a horizontal gene transfer (HGT) that established NXR and the other proteins in both lineages. Consistent with the fundamental importance of the transferred genes for the basic metabolism of Nitrospira and anammox, this HGT apparently occurred early during the evolution of these lineages as no remarkable deviation in GC content or codon use of the respective genes was observed in either organism.
Fig. 3.
Schematic of the genomic regions in Ca. K. stuttgartiensis and Ca. N. defluvii, which contain shared genes coding for NXR, putative cyt. bd-like oxidases and electron carriers, and proteins of unknown function. Solid lines connect genes that are the closest homologs on the basis of protein phylogeny. Their predicted functions are in boldface. Dashed lines connect similar genes that are not the closest relatives in the respective phylogenetic protein trees. Predicted CDS and connecting lines are colored according to functional classes. CDS and intergenic regions are drawn to scale.
To explore further the influence of vertical gene transfer and HGT on the evolutionary history of Ca. N. defluvii, we calculated phylogenies for each protein of Ca. N. defluvii and identified the organism encoding the respective most closely related homolog (Fig. S6). Most remarkably, in this analysis Ca. K. stuttgartiensis was the single organism that shared the highest number of closest homologs (71 hits) with Ca. N. defluvii and thus exchanged, compared with all other organisms for which genome sequences are available, the most genes with Nitrospira via HGT. Surprisingly, the 71 hits even exceed the number of best hits with members of the Nitrospirae phylum, namely Thermodesulfovibrio (67 hits) and different Leptospirillum strains (39–66 hits). These findings illustrate a surprisingly small set of the most closely related homologs in the Nitrospirae, most likely reflecting the dramatically different ecological niches inhabited by the genera affiliated with this phylum.
Taken together, the metagenome sequence of Ca. N. defluvii revealed that this globally important nitrite oxidizer differs fundamentally in its enzymatic repertoire (unusual NXR and putative terminal oxidase) and metabolic pathways (rTCA for autotrophy) from all other known nitrifiers, but strikingly exploits almost the same gene repertoire for NO2− oxidation as the anammox organism Ca. K. stuttgartiensis. The unique genomic features of Nitrospira have already provided some well-supported hypotheses for its competitive success in most nitrifying ecosystems and suggest that Nitrospira are well adapted to hypoxic environmental niches where nitrite oxidation has rarely been studied until now. From an applied perspective, the lack of common protection mechanisms against oxidative stress in Nitrospira implies that a good aeration control is crucial for maintaining stable and active populations of these organisms in engineered systems.
Materials and Methods
Genomic Sequencing and Annotation.
Metagenome sequencing and the reconstruction of the whole Ca. N. defluvii genome were carried out by Genoscope (SI Materials and Methods). The MaGe software system (42) was used for the prediction, automatic annotation, and manual annotation refinement of all CDS as described in SI Materials and Methods.
Phylogenetic Analyses.
Amino acid sequences of type II DMSO reductase-family enzymes, of RubisCO and RubisCO-like proteins, and of cyt. bd and cyt. bd-like oxidases were aligned, and phylogenetic trees were computed by using ARB (43). For the calculation of phylogenetic trees for each protein in the proteome, PhyloGenie (44) was used. For details, see SI Materials and Methods.
Expression Analysis of NxrB and the Putative Terminal Cyt. c Oxidase (Nide0901).
Ca. N. defluvii enrichment biomass was incubated in mineral media with or without NO2− and, for Nide0901, also under oxic or anoxic conditions as described in SI Materials and Methods. Following total RNA extraction, 16S rRNA of Nitrospira and nxrB or nide0901 transcripts were detected by RT–PCR (SI Materials and Methods). Translation of NxrB was shown by Western blotting with a monoclonal antibody that binds to the NxrB of Nitrospira (21) (SI Materials and Methods).
Stable Carbon Isotopic Fractionation.
The isotopic fraction of Ca. N. defluvii was measured following methods published earlier (45) (SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank Lisa Stein for analyses of nirK genes and Peter Bottomley, Jim Hemp, Jim Prosser and Andreas Schramm for helpful discussions. Christiane Dorninger, Alexander Galushko, Christian Baranyi, Jan Dolinšek, Patrick Tischler, Irene Rijpstra, and Michiel Kienhuis are acknowledged for technical support. This work was supported by the Vienna Science and Technology Fund (Wiener Wissenschafts-, Forschungs-, und Technologiefonds, Grant LS 216 to H.D., S.L., and F.M. and by Grant LS09-40 to H.D., H.K., and S.L.), the Austrian Research Fund (Fonds zur Förderung der Wissenschaftlichen Forschung, Grant S10002-B17 to H.D., M.W., S.L., and F.M.), and the German Research Foundation (Deutsche Forschungsgemeinschaft, Grant SP 667/3-1 to E.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been depositied in the GenBank database (accession no. FP929003).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003860107/-/DCSupplemental.
References
- 1.Teske A, et al. Evolutionary relationships among ammonia- and nitrite-oxidizing bacteria. J Bacteriol. 1994;176:6623–6630. doi: 10.1128/jb.176.21.6623-6630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starkenburg SR, et al. Genome sequence of the chemolithoautotrophic nitrite-oxidizing bacterium Nitrobacter winogradskyi Nb-255. Appl Environ Microbiol. 2006;72:2050–2063. doi: 10.1128/AEM.72.3.2050-2063.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starkenburg SR, et al. Complete genome sequence of Nitrobacter hamburgensis X14 and comparative genomic analysis of species within the genus Nitrobacter. Appl Environ Microbiol. 2008;74:2852–2863. doi: 10.1128/AEM.02311-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehrich S, Behrens D, Lebedeva E, Ludwig W, Bock E. A new obligately chemolithoautotrophic, nitrite-oxidizing bacterium, Nitrospira moscoviensis sp. nov. and its phylogenetic relationship. Arch Microbiol. 1995;164:16–23. doi: 10.1007/BF02568729. [DOI] [PubMed] [Google Scholar]
- 5.Daims H, Nielsen JL, Nielsen PH, Schleifer KH, Wagner M. In situ characterization of Nitrospira-like nitrite-oxidizing bacteria active in wastewater treatment plants. Appl Environ Microbiol. 2001;67:5273–5284. doi: 10.1128/AEM.67.11.5273-5284.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freitag TE, Chang L, Clegg CD, Prosser JI. Influence of inorganic nitrogen management regime on the diversity of nitrite-oxidizing bacteria in agricultural grassland soils. Appl Environ Microbiol. 2005;71:8323–8334. doi: 10.1128/AEM.71.12.8323-8334.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altmann D, Stief P, Amann R, De Beer D, Schramm A. In situ distribution and activity of nitrifying bacteria in freshwater sediment. Environ Microbiol. 2003;5:798–803. doi: 10.1046/j.1469-2920.2003.00469.x. [DOI] [PubMed] [Google Scholar]
- 8.Watson SW, Bock E, Valois FW, Waterbury JB, Schlosser U. Nitrospira marina gen. nov. sp. nov.: A chemolithotrophic nitrite-oxidizing bacterium. Arch Microbiol. 1986;144:1–7. [Google Scholar]
- 9.Lebedeva EV, et al. Moderately thermophilic nitrifying bacteria from a hot spring of the Baikal rift zone. FEMS Microbiol Ecol. 2005;54:297–306. doi: 10.1016/j.femsec.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Maixner F, et al. Nitrite concentration influences the population structure of Nitrospira-like bacteria. Environ Microbiol. 2006;8:1487–1495. doi: 10.1111/j.1462-2920.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- 11.Schramm A, de Beer D, van den Heuvel JC, Ottengraf S, Amann R. Microscale distribution of populations and activities of Nitrosospira and Nitrospira spp. along a macroscale gradient in a nitrifying bioreactor: Quantification by in situ hybridization and the use of microsensors. Appl Environ Microbiol. 1999;65:3690–3696. doi: 10.1128/aem.65.8.3690-3696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maixner F, et al. Environmental genomics reveals a functional chlorite dismutase in the nitrite-oxidizing bacterium ‘Candidatus Nitrospira defluvii’. Environ Microbiol. 2008;10:3043–3056. doi: 10.1111/j.1462-2920.2008.01646.x. [DOI] [PubMed] [Google Scholar]
- 13.Spieck E, et al. Selective enrichment and molecular characterization of a previously uncultured Nitrospira-like bacterium from activated sludge. Environ Microbiol. 2006;8:405–415. doi: 10.1111/j.1462-2920.2005.00905.x. [DOI] [PubMed] [Google Scholar]
- 14.Strous M, et al. Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature. 2006;440:790–794. doi: 10.1038/nature04647. [DOI] [PubMed] [Google Scholar]
- 15.Tyson GW, et al. Community structure and metabolism through reconstruction of microbial genomes from the environment. Nature. 2004;428:37–43. doi: 10.1038/nature02340. [DOI] [PubMed] [Google Scholar]
- 16.Levicán G, Ugalde JA, Ehrenfeld N, Maass A, Parada P. Comparative genomic analysis of carbon and nitrogen assimilation mechanisms in three indigenous bioleaching bacteria: Predictions and validations. BMC Genomics. 2008;9:581. doi: 10.1186/1471-2164-9-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goltsman DS, et al. Community genomic and proteomic analyses of chemoautotrophic iron-oxidizing “Leptospirillum rubarum” (group II) and “Leptospirillum ferrodiazotrophum” (group III) bacteria in acid mine drainage biofilms. Appl Environ Microbiol. 2009;75:4599–4615. doi: 10.1128/AEM.02943-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meincke M, Bock E, Kastrau D, Kroneck PMH. Nitrite oxidoreductase from Nitrobacter hamburgensis: Redox centers and their catalytic role. Arch Microbiol. 1992;158:127–131. [Google Scholar]
- 19.Sundermeyer-Klinger H, Meyer W, Warninghoff B, Bock E. Membrane-bound nitrite oxidoreductase of Nitrobacter: Evidence for a nitrate reductase system. Arch Microbiol. 1984;140:153–158. [Google Scholar]
- 20.Kirstein K, Bock E. Close genetic relationship between Nitrobacter hamburgensis nitrite oxidoreductase and Escherichia coli nitrate reductases. Arch Microbiol. 1993;160:447–453. doi: 10.1007/BF00245305. [DOI] [PubMed] [Google Scholar]
- 21.Spieck E, Ehrich S, Aamand J, Bock E. Isolation and immunocytochemical location of the nitrite-oxidizing system in Nitrospira moscoviensis. Arch Microbiol. 1998;169:225–230. doi: 10.1007/s002030050565. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Espinosa RM, et al. Look on the positive side! The orientation, identification and bioenergetics of ‘Archaeal’ membrane-bound nitrate reductases. FEMS Microbiol Lett. 2007;276:129–139. doi: 10.1111/j.1574-6968.2007.00887.x. [DOI] [PubMed] [Google Scholar]
- 23.McDevitt CA, Hugenholtz P, Hanson GR, McEwan AG. Molecular analysis of dimethyl sulphide dehydrogenase from Rhodovulum sulfidophilum: Its place in the dimethyl sulphoxide reductase family of microbial molybdopterin-containing enzymes. Mol Microbiol. 2002;44:1575–1587. doi: 10.1046/j.1365-2958.2002.02978.x. [DOI] [PubMed] [Google Scholar]
- 24.Blasco F, et al. The coordination and function of the redox centres of the membrane-bound nitrate reductases. Cell Mol Life Sci. 2001;58:179–193. doi: 10.1007/PL00000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothery RA, Workun GJ, Weiner JH. The prokaryotic complex iron-sulfur molybdoenzyme family. Biochim Biophys Acta. 2008;1778:1897–1929. doi: 10.1016/j.bbamem.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Thorell HD, Stenklo K, Karlsson J, Nilsson T. A gene cluster for chlorate metabolism in Ideonella dechloratans. Appl Environ Microbiol. 2003;69:5585–5592. doi: 10.1128/AEM.69.9.5585-5592.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starkenburg SR, Arp DJ, Bottomley PJ. Expression of a putative nitrite reductase and the reversible inhibition of nitrite-dependent respiration by nitric oxide in Nitrobacter winogradskyi Nb-255. Environ Microbiol. 2008;10:3036–3042. doi: 10.1111/j.1462-2920.2008.01763.x. [DOI] [PubMed] [Google Scholar]
- 28.Bock E, Koops HP, Harms H, Ahlers B. The biochemistry of nitrifying organisms. In: Shively JM, Barton LL, editors. Variations in Autotrophic Life. London: Academic Press; 1991. pp. 171–200. [Google Scholar]
- 29.Ashida H, et al. A functional link between RuBisCO-like protein of Bacillus and photosynthetic RuBisCO. Science. 2003;302:286–290. doi: 10.1126/science.1086997. [DOI] [PubMed] [Google Scholar]
- 30.Quandt L, Gottschalk G, Ziegler H, Stichler W. Isotope discrimination by photosynthetic bacteria. FEMS Microbiol Lett. 1977;1:125–128. [Google Scholar]
- 31.van der Meer MTJ, Schouten S, Damste JSS. The effect of the reversed tricarboxylic acid cycle on the C-13 contents of bacterial lipids. Org Geochem. 1998;28:527–533. [Google Scholar]
- 32.Campbell BJ, Engel AS, Porter ML, Takai K. The versatile ɛ-proteobacteria: Key players in sulphidic habitats. Nat Rev Microbiol. 2006;4:458–468. doi: 10.1038/nrmicro1414. [DOI] [PubMed] [Google Scholar]
- 33.Shiba H, Kawasumi T, Igarashi Y, Kodama T, Minoda Y. The CO2 assimilation via the reductive tricarboxylic-acid cycle in an obligately autotrophic, aerobic hydrogen-oxidizing bacterium, Hydrogenobacter thermophilus. Arch Microbiol. 1985;141:198–203. [Google Scholar]
- 34.Yamamoto M, Arai H, Ishii M, Igarashi Y. Role of two 2-oxoglutarate:ferredoxin oxidoreductases in Hydrogenobacter thermophilus under aerobic and anaerobic conditions. FEMS Microbiol Lett. 2006;263:189–193. doi: 10.1111/j.1574-6968.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 35.Ikeda T, et al. Anabolic five subunit-type pyruvate:ferredoxin oxidoreductase from Hydrogenobacter thermophilus TK-6. Biochem Biophys Res Commun. 2006;340:76–82. doi: 10.1016/j.bbrc.2005.11.155. [DOI] [PubMed] [Google Scholar]
- 36.Horsburgh MJ, Wharton SJ, Karavolos M, Foster SJ. Manganese: Elemental defence for a life with oxygen. Trends Microbiol. 2002;10:496–501. doi: 10.1016/s0966-842x(02)02462-9. [DOI] [PubMed] [Google Scholar]
- 37.Das A, Silaghi-Dumitrescu R, Ljungdahl LG, Kurtz DM., Jr Cytochrome bd oxidase, oxidative stress, and dioxygen tolerance of the strictly anaerobic bacterium Moorella thermoacetica. J Bacteriol. 2005;187:2020–2029. doi: 10.1128/JB.187.6.2020-2029.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barrangou R, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 39.Spieck E, Bock E. The lithoautotrophic nitrite-oxidizing bacteria. In: Staley JT, et al., editors. Bergey's Manual of Systematic Bacteriology. 2nd Ed. Vol. 2. New York: Springer Science+Business Media; 2005. pp. 149–153. [Google Scholar]
- 40.Griffin BM, Schott J, Schink B. Nitrite, an electron donor for anoxygenic photosynthesis. Science. 2007;316:1870. doi: 10.1126/science.1139478. [DOI] [PubMed] [Google Scholar]
- 41.Park HD, Noguera DR. Nitrospira community composition in nitrifying reactors operated with two different dissolved oxygen levels. J Microbiol Biotechnol. 2008;18:1470–1474. [PubMed] [Google Scholar]
- 42.Vallenet D, et al. MaGe: A microbial genome annotation system supported by synteny results. Nucleic Acids Res. 2006;34:53–65. doi: 10.1093/nar/gkj406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ludwig W, et al. ARB: A software environment for sequence data. Nucleic Acids Res. 2004;32:1363–1371. doi: 10.1093/nar/gkh293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frickey T, Lupas AN. PhyloGenie: Automated phylome generation and analysis. Nucleic Acids Res. 2004;32:5231–5238. doi: 10.1093/nar/gkh867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schouten S, et al. Stable carbon isotopic fractionations associated with inorganic carbon fixation by anaerobic ammonium-oxidizing bacteria. Appl Environ Microbiol. 2004;70:3785–3788. doi: 10.1128/AEM.70.6.3785-3788.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.