Abstract
Diabetes mellitus is characterized by either the inability to produce insulin (type 1 diabetes) or as insensitivity to insulin secreted by the body (type 2 diabetes). In either case, the body is unable to move blood glucose efficiently across cell membranes to be used. This leads to a variety of local and systemic detrimental effects. Current treatments for diabetes focus on exogenous insulin administration and dietary control. Here, we describe a potential cure for diabetes using a cellular therapy to ameliorate symptoms associated with both reduced insulin secretion and insulin sensitivity. Using induced pluripotent stem (iPS) cells, we were able to derive β-like cells similar to the endogenous insulin-secreting cells in mice. These β-like cells secreted insulin in response to glucose and corrected a hyperglycemic phenotype in two mouse models of type 1 and 2 diabetes via an iPS cell transplant. Long-term correction of hyperglycemia was achieved, as determined by blood glucose and hemoglobin A1c levels. These data provide an initial proof of principle for potential clinical applications of reprogrammed somatic cells in the treatment of diabetes type 1 or 2.
Keywords: cellular therapy, diabetes, stem cells, somatic cell programming
Approximately 3.5% of the US population is diagnosed with diabetes mellitus, which makes this is a major health care problem. Type 1 diabetes mellitus is an autoimmune disease in which insulin-secreting β cells in pancreatic islets are irreversibly destroyed by autoimmune assault, resulting in a lack of insulin production. Type 2 diabetes mellitus occurs when the pancreas produces insufficient amounts of the hormone insulin and/or the body's tissues become resistant to normal or even high levels of insulin. In either case, introduction of exogenous insulin is usually enough to ameliorate the symptoms. Cell-based therapies are intended to reduce the dependence of diabetic patients on insulin injections via the introduction of exogenous insulin-secreting cells, resulting in endogenous insulin production. One extremely promising avenue by which this can occur is through the use of stem cells (1–7).
Current cell therapies for diabetes focus primarily on either whole pancreas transplantation or the introduction of pancreatic islets into the portal vein of a diabetic individual (8). Although these techniques are largely effective, they are hindered by immune rejection and the lack of primary tissues for transplantation. The concept of autologous grafting of insulin-secreting cells derived from the patient's own tissue stem cells, particularly those derived from bone marrow (BM) or liver, is attractive (3). However, transplantation of these cells in animals encounters problems with restricted growth capacity, low levels of insulin expression, and poor or nonexistent insulin secretion. More recent studies suggest that BM stem cells improve experimental diabetes in vivo by enhancing the regeneration and survival of existing β cells rather than by repopulating the islets with newly transdifferentiated β cells (2, 9).
Recently, methods to retrodifferentiate adult somatic cells into induced pluripotent stem (iPS) cells using defined transcription factors have been established (10–13). These iPS cells, to date, are quite similar to ES cells and have the same pluripotent characteristics. Because iPS cells can potentially have the same haplotype as the host, immune rejection may be avoided. Specific cell types derived from iPS cells have been used to treat various diseases in mouse models, including sickle cell anemia (14), Parkinson's disease (15), and hemophilia A (16).
Here, we demonstrate that iPS cells derived from skin fibroblasts can be differentiated into insulin-secreting β-like cells in vitro and that they respond to glucose stimulation under physiological or pathological conditions. Insulin-secreting β-like cells were introduced via intraportal vein injection into the livers of two different mouse systems modeling type 1 and type 2 diabetes. The β-like cells engrafted and corrected the hyperglycemic phenotype in both models.
Results
Reprogramming of Normal Mouse Skin Fibroblasts into iPS Cells.
Normal fibroblasts from GFP transgenic mice were transduced with four retroviral transcription factors (10–12) using methods that we have previously described (16). In the current study, two iPS cell subclone colonies were picked and expanded at 20 d posttransduction (Fig. S1A). mRNA expression profiles of these two iPS cell subclones show up-regulation of the endogenous stem cell markers Oct4, Sox2, Klf4, and c-Myc similar to those of ES cells (Fig. S1B). There was minimal expression of exogenous viral transgenes. Immunofluorescent staining of surface and intracellular antigens in these iPS cells was positive for the well-established pluripotent markers Oct4, Sox2, Nanog, and SSEA-1 (Fig. S2A). To determine their capacity for proliferation and differentiation, roughly 2 × 106 dissociated iPS cells were injected s.c. into the flanks of two nude mice. Thirty days postinjection, a teratoma had formed in the injected mice, suggesting full proliferative capacity of the injected cells. Differentiation was established via H&E staining of tissue from the teratomas, showing the presence of cell types belonging to the three germ layers: ectoderm (skin), endoderm (gut-like), and mesoderm (cartilage) (Fig. S2B). The morphology, mRNA expression profile, immunofluorescent staining, and teratoma formation confirm the in vitro generation of ES cell-like iPS cells.
Selective Differentiation of iPS Cells into Insulin-Producing Pancreatic β-Like Cells.
iPS cells were driven to undergo a three-stage differentiation protocol into β-like cells, which can produce insulin, using modification of a previously published protocol for ES cells (17). The stages of this differentiation protocol are shown in Fig. S3. At stage 1, the ES cell-like iPS cell cultures were dissociated into single cells and placed in suspension culture, where they formed embryoid bodies (EBs; Fig. S3 Top). After 5 d in suspension culture, the EBs were returned to adherent culture to undergo further differentiation for 9 d. During this time, cells migrated from the attached spheroids (Fig. S3 Middle). After 9 d, the medium was replaced with selective medium containing laminin, insulin, nicotinamide, selenic acid, transferrin, progesterone, and KO replacement serum, marking the beginning of stage 3. During the ensuing 20 d, the cells continued to differentiate (Fig. S3 Bottom). During the in vitro differentiation of the iPS cells to β-like cells, we determined that a small refinement of the differentiation protocol significantly improved the efficiency of the differentiation process. The prior protocol (17) had included enzymatic detachment of the differentiated cells at the end of stage 2. By eliminating this trypsinization step between stages 2 and 3, we saw a substantial increase (up to 80%) in the survival of the differentiating cells, resulting in a more robust β-like–cell yield. Using the original protocol (17), our viable cell yield was only 20%.
During stage 3 differentiation, multilineage cells were seen to be present at day 6 of selective media treatment (Fig. S4, column 2). At this time, the cultures were trypsinized and replated onto previously unused dishes in fresh selective medium. On day 13, numerous clusters had begun to form (Fig. S4, column 3). By day 20, large cell clusters were evident (Fig. S4, column 4). GFP was highly expressed throughout the differentiation process.
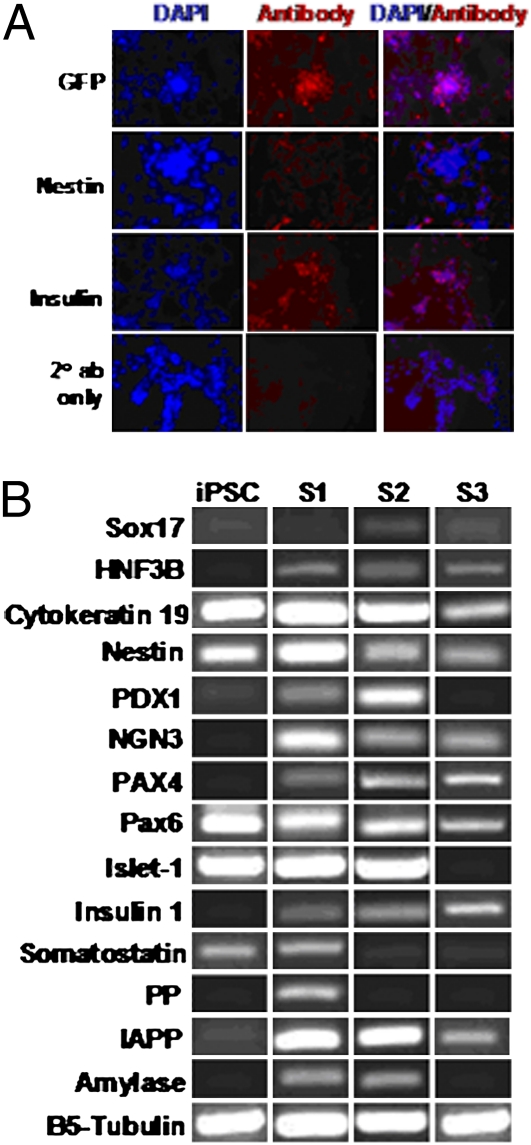
Cellular levels of insulin and nestin were analyzed at stage 3 by immunofluorescent staining. At day 7, both insulin and nestin were expressed (Fig. S5A). Expression of nestin peaked at day 13 (Fig. S5B) and decreased by day 20 (Fig. 1A). Stage 3 cells showed increasing percentages of insulin staining during the course of differentiation. At stage 3 on day 7 (Fig. S5A), ∼10–20% of the differentiated cells were insulin-positive; at stage 3 on day 13 (Fig. S5B), ∼50% of the differentiated cells were insulin-positive; and at stage 3 on day 20 (Fig. 1A), more than 50% of the cells were observed to be insulin-positive.
Fig. 1.
Characterization of iPS cell-derived insulin-producing β-like cells. (A) Immunofluorescent staining: stage 3 on day 20. In vitro-derived β-like cells were stained with antibodies to GFP, nestin, and insulin on day 20 of stage 3. Cells were counterstained with DAPI. Nestin staining was reduced to minimal, although insulin staining persisted. (B) Gene expression profile of in vitro-derived β-like cells. Gene expression analysis by RT-PCR with various pancreatic cell markers during iPS cell-derived β-like cell differentiation. Total RNA was isolated from cells at stage 1 (S1), stage 2 (S2), and stage 3 (S3) of β-like cell development. B5-tubulin was used as an endogenous control. IAPP, islet amyloid polypeptide; iPSC, cells before differentiation.
mRNA analysis revealed expression of several specific markers of in vitro pancreatic β-cell differentiation, as shown in Fig. 1B. The definitive endoderm markers Sox17 and HNF3B (FoxA2) were coexpressed in stage 2 and were detectable up to stage 3. Coexpression of cytokeratin 19 and nestin, suggesting the presence of multilineage progenitors, was seen during stages 1 through 3. Coexpression of PDX1 and HNF3B, indicative of pancreatic endoderm or epithelium generation, was seen in stages 1 and 2. NGN3, which is highly expressed in all endocrine progenitors (18), was coexpressed with PDX1 in stages 1 and 2, reflecting further commitment of the differentiated cells to the endocrine lineage. Pax4, Pax6, and Islet1 are important transcription factors controlling endocrine cell differentiation and were detected in all three stages. Islet-1 was, however, down-regulated early in stage 3 as the cells progressed into a mature differentiated phenotype.
There are five endocrine cell types, α, β, δ, pancreatic polypeptide (PP), and ε cells, which produce the hormones glucagon, insulin, somatostatin, PP, and ghrelin, respectively. Insulin expression was detected as early as stage 1 and remained up-regulated until stage 3. Furthermore, islet amyloid polypeptide, secreted by pancreatic β cells at the same time as insulin (19, 20), was also highly expressed in stages 1–3. Expression of somatostatin and PP was only evident in stage 1. Amylase-positive acinar cells were detected in stages 1 and 2 but were undetectable at the selective differentiation stage 3.
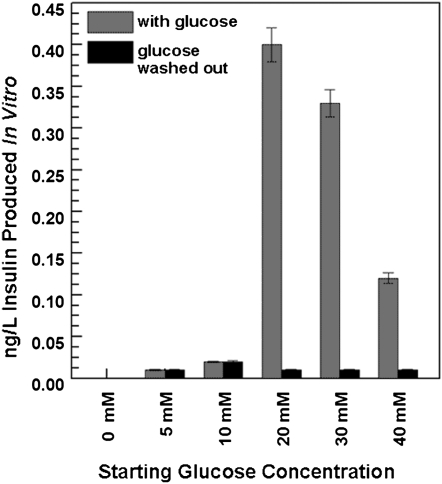
Glucose responsiveness of iPS cell-derived β-like cells was tested in vitro by exposure of glucose-starved stage 3 cells to five glucose concentrations for 90 min. As shown in Fig. 2, day 20 differentiated cells responded to glucose in a dose-dependent manner. At the lower glucose concentrations (5 and 10 mM), the differentiated cells were marginally responsive. At 20 mM glucose, insulin production peaked, at ∼8-fold higher than readings from 5- and 10-mM glucose-treated cells. At 30 and 40 mM, the insulin levels began to decrease again. Following glucose induction, the cells were washed thoroughly to remove residual insulin and were then reincubated with basal Krebs-Ringer bicarbonate/Hepes buffer (KRBH) solution for 90 min. As shown in Fig. 2, no insulin was produced when glucose was removed, suggesting that the in vitro-derived β-like cells only produce insulin in response to glucose treatment. Insulin was also produced by day 7 and 13 differentiated cells when exposed to glucose treatment.
Fig. 2.
In vitro insulin induction. On glucose treatment, stage 3 on day 20, iPS cell-derived β-like cells secrete insulin in vitro. Cells were first exposed to five glucose concentrations (5, 10, 20, 30, and 40 mM), and the insulin levels in their supernatants were determined. Glucose was then removed from the medium, and insulin levels were again measured in supernatants from the same cells.
Type 2 Diabetic Mouse Model Phenotypic Tests Reveal Hyperglycemia, Abnormal Insulin Levels, and Resistance to Exogenous Insulin Therapy.
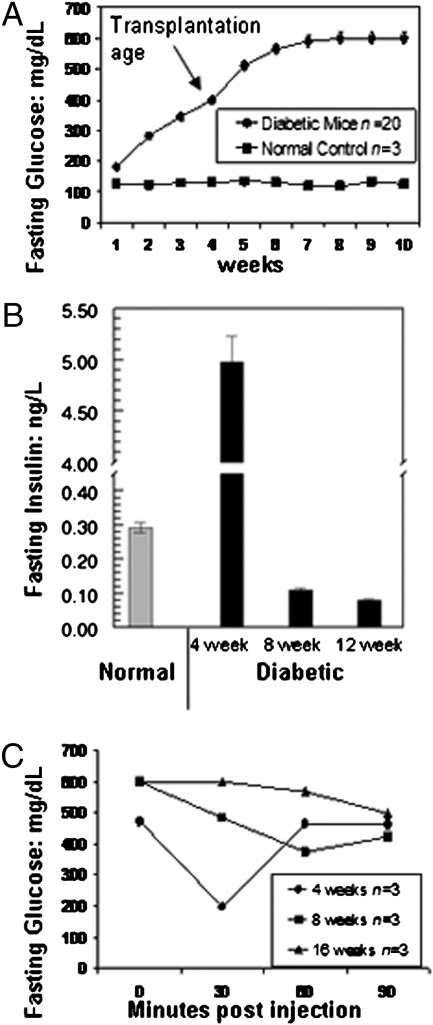
The type 2 diabetes mouse model (Leprdb, C57BLKS; Dock7m, DBA/J) used for this study shows depleted insulin production as the mice age, although maintaining a hyperglycemic phenotype. By the age of 3–4 wk, diabetic mice demonstrated hyperglycemia after 6–8 h of fasting, with glucose concentrations greater than 300 mg/dL as compared with the normal C57BL6 mouse strain used as a control (Fig. 3A). At 3 wk of age, diabetic mouse blood glucose concentrations (n = 20) increased ∼2.6-fold as compared with normal controls. At 4–5 wk of age, glucose concentrations of the diabetic mice increased ∼3.8-fold. After 6 wk of age, the hyperglycemia is very severe and glucose levels were dangerously high at >600 mg/dL.
Fig. 3.
In vivo characterization of diabetes mellitus type 2 model. (A) Glucose concentration of the untransplanted type 2 diabetes mellitus mouse model. Basal glucose concentrations were obtained from untransplanted type 2 diabetes mellitus mouse models (n = 20). As shown, this model began to exhibit diabetic features (glucose >300 mg/dL) at 3 wk of age. Glucose levels continued to increase until week 6 and then remained elevated at 600 mg/dL. Normal control animals retained lower glucose levels throughout the entire period. (B) Insulin levels of the untransplanted type 2 diabetes mellitus mouse model. Basal insulin concentrations were obtained from untransplanted type 2 diabetes mellitus mouse models at 4 wk old (n = 20), 8 wk old (n = 3), and 12 wk old (n = 3). Insulin was initially high at 4 wk but dropped off and remained reduced from 8 wk on. Insulin concentrations from normal mice (n = 3) are shown for comparison. (C) Insulin tolerance test. Untransplanted type 2 diabetes mellitus mouse models at 4 wk old (n = 3), 8 wk old (n = 3), and 16 wk old (n = 3) were i.p. injected with 0.75 U/kg human insulin. Fasting glucose concentration was measured before injection and at 30, 60, and 90 min postinjection via tail vein bleed and a handheld glucometer. At 4 wk, the mice showed sensitivity to insulin therapy, but they were resistant by 8 and 16 wk of age.
Insulin levels in serum samples collected from 4-wk-old diabetic mice (n = 20) (Fig. 3B) were highly variable, ranging from 0.064 to 5.32 ng/L. The mean insulin level was ∼17-fold higher than that of normal control mice (n = 3). As the diabetic mice continued to age, insulin levels decreased. Diabetic mice at 8 wk (n = 3) had ∼2.6-fold lower insulin levels than the normal controls, and at 12 wk (n = 3), they had ∼3.6-fold lower insulin levels than the normal controls.
Insulin resistance also developed as the mice aged (Fig. 3C). Exogenous insulin injected into 4-wk-old (n = 3) diabetic mice led to a drop in fasting glucose, occurring between 0 and 30 min postinjection. With increased age, the mice showed minimal response to the injection at 8 wk (n = 3) and 16 wk (n = 3), indicating resistance to insulin. These findings are consistent with previous reports that fully describe this model (21, 22).
iPS Cell-Derived β-Like Cells Were Able to Engraft in Liver Parenchyma and Ameliorate Hyperglycemia in the Type 2 Diabetes Mouse Model.
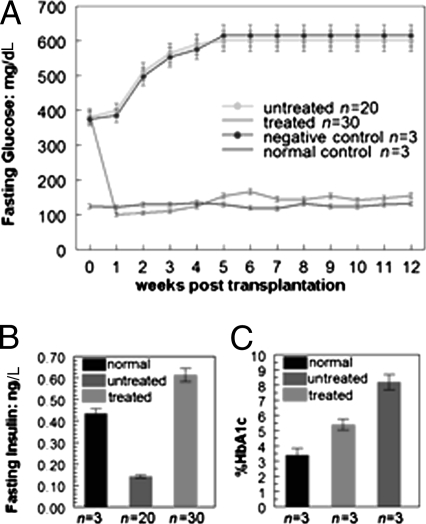
Approximately 200,000 iPS cell-derived insulin-secreting β-like cells were isolated by FACS sorting to yield GFP+/SSEA1− cells, which were transplanted into diabetic mice (n = 30) by intraportal vein injection. Fasting glucose measurements, begun 2 d posttransplantation, are shown in Fig. 4A) All 30 transplanted mice (exhibiting 100% engraftment efficiency) exhibited normal glucose concentrations with levels comparable to those of C57BL6 normal controls (n = 3). Three diabetic mice transplanted with GFP−/SSEA1− control cells, (feeder cells) remained hyperglycemic. Their unengrafted diabetic counterparts (n = 20) remained hyperglycemic as well.
Fig. 4.
Transplanted diabetes mellitus type 2 model. (A) Glucose concentration of engrafted type 2 diabetes mellitus mouse model. Before transplantation, all mice presented a hyperglycemic phenotype. Stage 3 day 7 iPS cell-derived pancreatic β cells engrafted into type 2 diabetes mellitus mice (n = 30) were able to regulate glucose levels and ameliorate hyperglycemia for more than 3 mo. Glucose levels were obtained from the tail vein and measured by a handheld glucometer every 2–3 d. For untreated and control mice, n = as marked. Weekly tracking of the decrease in treated mice attributable to both postsurgical loss and harvest of animals for histology is illustrated in Fig. S6. (B) Insulin production in the engrafted type 2 diabetes mellitus mouse model. Serum insulin levels were measured 3 wk posttransplantation in type 2 diabetes mellitus mice (n = 30) engrafted with stage 3 day 7 iPS cell-derived β-like cells. Data indicate that treated mice had markedly increased insulin levels as compared with untreated mice. (C) Hemoglobin (Hb) A1c levels of the engrafted type 2 diabetes mellitus mouse model. At 4 wk posttransplantation, blood samples from engrafted type 2 diabetes mellitus mice (n = 3) were tested by an independent company (DTI) to measure the level of Hb A1c. The Hb A1c level of unengrafted mice was ∼2.5 times higher than normal. After engraftment, the Hb A1c level in the treated mice (n = 3), although still higher than that in normal control mice, had decreased to a level ∼40% lower than that of the untreated cohort (n = 3).
At 1 wk posttransplantation, 25 of the original 30 β-like cell–transplanted mice survived and maintained normal glycemic control. At that time, we killed 3 mice for histological evaluation, leaving 22 mice. At 8 wk posttransplantation, 15 of these mice were still alive and normoglycemic. Of the seven mice lost by this time, three were killed at week 4 for additional histology. Our most recent data (to week 12) show continued maintenance of normal glucose levels in the treated mice. The mortality rate in the transplanted mice (Fig. S6) appeared to be primarily the result of complications (i.e., thrombosis) resulting from surgical access of the portal vein (Discussion). However, all the mice survived for at least 1 wk posttransplantation and exhibited normal glucose levels during that time. Two mice had relapsed into hyperglycemia after maintaining a regulated glucose level for 8 wk. These two mice appeared to have redeveloped insulin resistance (Fig. S7).
Engrafted iPS Cell-Derived β-Like Cells Were Able to Produce Insulin in Vivo and Normalized Hemoglobin A1c Levels.
The amelioration of hyperglycemia in the type 2 diabetic mouse model transplanted with iPS cell-derived β-like cells occurred concomitantly with an increase in in vivo insulin concentration measured by mouse insulin ELISAs. By 21–56 d posttransplantation, β-like cell–transplanted mice had an ∼4.35-fold increase in insulin levels as compared with untreated mice (P < 0.05; Fig. 4B). Insulin levels in the treated mice were somewhat higher than those in the untreated controls.
Hemoglobin A1c tests to measure the amount of glucose attached to the hemoglobin of red blood cells were done 4 wk posttransplantation (Fig. 4C).
The hemoglobin A1c levels of untreated diabetic mice (n = 3) were ∼2.4-fold higher than those of normal C57BL6 controls (n = 3). Transplanted mice with normal glucose levels 4 wk posttransplantation (n = 3) had significantly improved hemoglobin A1c levels.
Cell Distribution of Engrafted Cells in the Liver Parenchyma.
Intraportal vein injection of in vitro-derived β-like cells is an efficient way to engraft the cells directly into the murine hepatic sinusoids. At 7 d and 4 wk posttransplantation, three mice were killed to obtain tissues for immunohistochemical and immunofluorescence analysis to assess the distribution of engrafted cells in the liver. The engrafted cells stably expressed GFP, enabling us to recognize and distinguish the transplanted cells from other hepatic cell types.
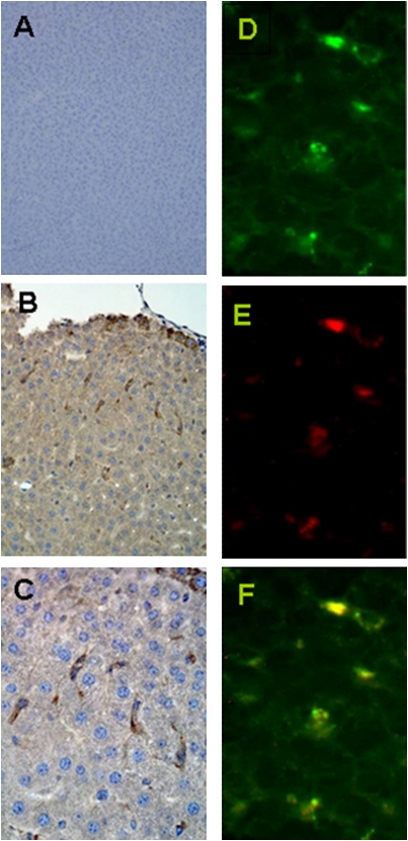
As shown in Fig. 5, cells were able to engraft stably into the liver parenchyma of the diabetic mouse model. Spindle-shaped GFP-positive cells detected by anti-GFP antibodies (brown) were evenly distributed throughout the microscopic liver sections of 7-d treated mice (Fig. 5 B and C). Minimal GFP-positive cells were detected in the lungs and spleen. Liver sections from the C57BL6 normal mouse strain were used as negative controls (Fig. 5A). Engrafted β-like cells coexpress insulin and GFP 7 d posttransplantation, as detected by immunofluorescence (Fig. 5 D–F).
Fig. 5.
Immunohistochemistry and immunofluorescent staining of liver tissue from the engrafted type 2 diabetes mellitus mouse model. Mice were killed at 7 d (n = 3 for each) for immunostaining of liver tissues to analyze the distribution of engrafted cells. (A) Liver of C57BL6 control as a negative control for GFP staining. (B) At low magnification, the engraftment of spindle-shaped GFP-positive cells (brown) scattered throughout the liver parenchyma is shown. (C) Higher magnification of the GFP-positive cells. (D) At high magnification, GFP-positive cells (green) are scattered throughout the tissue. (E) Same section with insulin-positive cells (red). (F) Merged images demonstrate colocalization of GFP and insulin.
Normoglycemia in Streptozotocin-Treated Mice After Transplantation with iPS Cell-Derived β-Like Cells.
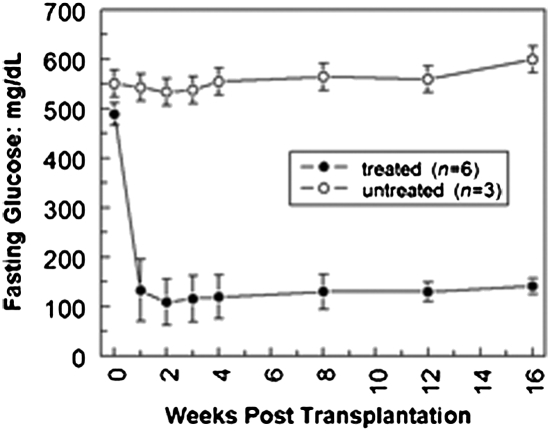
To test whether our in vitro-derived β-like cells are functional in an environment in which islet cells are severely depleted, a model of type 1 diabetes, in vitro-derived insulin-secreting β-like cells were transplanted via intraportal vein injection into streptozotocin (STZ)-treated mice with glucose concentrations of >400 mg/dL (Fig. 6). At 2 d posttransplantation with β-like cells, the glucose levels of the STZ-treated mice (n = 6) became normal, with glucose concentrations of 160 ± 5 mg/dL. Untransplanted STZ-treated mice (n = 3) maintained hyperglycemia with glucose concentrations >400 mg/dL. Glucose levels of treated mice from day 2 up to 16 wk posttransplantation continued to be normal, whereas the untransplanted mice remained hyperglycemic.
Fig. 6.
Type 1 diabetes mellitus mouse model. The type 1 diabetes mellitus mouse model was derived from a single i.p. injection of 180 mg/kg STZ and stabilized for 10 d before transplantation. The mice used in this study (n = 9) all demonstrated hyperglycemia of greater than 400 mg/dL glucose in at least three levels before transplantation. Six mice were then injected with iPS cell-derived β-like cells via hepatic portal vein injection. Fasting glucose levels obtained every 2–3 d, starting at 2 d posttransplantation and continuing for ∼4 mo, show amelioration of hyperglycemia in this type 1 diabetic mouse model. The data shown here are for weeks 0 through 16.
Discussion
In this study, we explore the ability of iPS cells to differentiate in vitro into insulin-secreting cells and test whether the cells are sufficiently functional in vivo to correct a hyperglycemic phenotype in diabetic mouse models. We used iPS cells in this study because (i) the iPS cells were derived from fibroblasts of C57BL6 background mice, which are close relatives of the diabetic mouse models used here, and thus could reduce the possibility of graft rejection on transplantation; (ii) insulin produced from the transplanted iPS cell-derived β-like cells can be considered endogenous insulin, thereby allowing maintenance of normal glucose homeostasis as the mice age; and (iii) a virtually unlimited supply of cells for differentiation can be produced from the iPS cells.
This study was primarily focused on the reconstitution of β cell-mediated secretion of insulin via iPS cell transplantation. However, the question of what other cell types might be found among our differentiated cells is intriguing. We have some preliminary immunostaining data from our in vitro cells, which demonstrated the presence of glucagon. This suggests to us that we actually have a heterogeneous population, including the presence of at least both α and β cells in our stage 3 population. As a result of these limited data, we hypothesize that the transplanted cells included both types and that they might work synergistically in engraftment. We will be actively pursuing this question in our ongoing studies.
We tested three potential approaches for implantation of the iPS cell-derived β-like cells before choosing the method described here. Each was evaluated to determine engraftment efficiency. The i.v. injection of the cells produced no effect on circulating blood glucose levels. It is likely that the engraftment of circulating β-like cells was much too low for any efficacy. Subrenal capsule injection was also tried, resulting in little effect. Portal vein injection, the method described in this study, was by far the most effective in reducing the hyperglycemic phenotype within the mouse models.
The use of two different mouse models allowed us to explore the potential for treating either type 1 or 2 diabetes via the cellular transplant route. Establishing our system in both of these models also allows us to pursue future comparisons of the effects of this treatment on diabetes with and without insulin resistance or an immune component. Our most extensive studies were done in the type 2 model with mice that maintained a hyperglycemic phenotype and had insulin production that was rapidly depleted as they aged. Three key features are hallmarks of type 2 diabetes: hyperglycemia, progressive failure of insulin secretion, and the development of insulin resistance. Impairment in insulin secretion is likely caused by β-cell exhaustion attributable to a constant unsuccessful attempt to compensate for the existing insulin resistance. In addition, β-cell function is adversely affected by glucotoxicity, generating a downward cycle of hyperglycemia leading to decreased insulin secretion, which further worsens hyperglycemia.
Transplantation of these animals with one dose containing as few as 200,000 iPS cell-derived β-like cells showed that the cells engrafted well and persistently in the liver, resulting in restored insulin secretion and normalization of glucose levels within 2 d postimplantation. The injection of 1 × 106 cells provided no detectable improvement in serum insulin levels above the response obtained with the lower cell dose. The control of glycemia was maintained for ∼20–30% of the life expectancy of these mice. However, no significant weight reduction was observed in the iPS cell-derived cell-transplanted mice as compared with the control mice, Leprdb.
Our studies indicate that transplanted cells are able to prevent insulin resistance and progressive endogenous β-cell failure, which are key components of the pathogenesis of type 2 diabetes. In the future, it will be important to investigate the putative mechanisms underlying these results. The important issues to address are as follows: (i) Does early transplantation of iPS cell-derived islet-like cells reduce insulin resistance and progression to overt diabetes? (ii) Are islet-like cell transplantations able to reverse the “glucose toxicity” component of insulin resistance in the diabetes?
The preliminary data from a small cohort of type 1 diabetic mice (n = 9: 6 treated and 3 controls) showed similarly promising results. These animals have now been followed for 4 mo, and the glucose levels of the treated mice dropped rapidly and have remained stable for the entire 4-mo period.
Although there were some losses in the treated mice of our type 2 study, there are a number of possible causes for the mortality seen in this group. Of the eight mice lost during the first week, three were killed for tissue analysis. The other deaths were determined to be attributable to side effects of the surgery. The death rate was higher in mice from the earlier cohorts; however, as we refined the transplantation protocol, the mortality rate dropped. The needles used for injection played a crucial factor in mouse survival postsurgery. We initially used a 27-gauge needle, but the vein puncture was too big and pressure applied to the vein after injection was insufficient to suppress blood loss. However, using a smaller 32-gauge needle to reduce bleeding and postsurgical warming via a heating pad to reduce shock greatly improved the survival of later study cohorts.
Of the 12 mice lost between weeks 3 and 12, the first 3 mice (lost between weeks 3 and 4) were actually killed for tissue analysis, and thus cannot be counted as deaths attributable to experimental manipulations. Some of these losses also included initial survivors of our earliest transplant attempts and, as such, had been subjected to much more stressful conditions. This particular genetic type 2 model also has a greatly reduced life span (10 mo) to begin with and is especially susceptible to a variety of complications attributable to obesity. Our future studies will look more closely at survival times and long-term complications in general as well as in this model in particular.
On a more positive note, the six transplanted type 1 mice (STZ-induced) were all still alive at 4 mo posttransplantation and maintaining normal glucose levels. The difference in survival between the two groups may be attributable to the fact that the type 1 animals were transplanted later when we had more experience, but this variation in survival is an issue that we are actively pursuing.
Unfortunately, in an animal as small as a mouse, surgery is necessary to expose the portal vein for the introduction of the cells. The intraportal vein injection would be expected to be less taxing to larger animals and humans. Even the mice that died posttransplantation showed a complete return to normal glucose levels before death. The drop in glucose was observed in all 30 treated type 2 animals and all 6 treated type 1 animals within 2 d of the transplantation procedure.
Seventy-three days posttransplantation, one transplanted type 2 mouse was determined to have a tumor. Fluorescent analysis of a frozen section showed that the tumor was GFP-negative, which indicated that the tumor was probably not derived from transplanted iPS cells. No evidence of tumor formation in transplanted type 1 mice was evident by 3 mo, although additional long-term follow-up is needed.
Two of the treated type 2 mice appeared to have redeveloped hyperglycemia after maintaining normal glucose levels for 8 wk posttransplantation. One of the mechanisms in type 2 diabetes is a loss of sensitivity to insulin. It is unclear whether the improvements seen in this study in the type 2 phenotype were attributable to changes in the level of glucose sensitivity or merely to increased amounts of insulin being secreted by the transplanted cells. The hyperglycemia in the two relapsed mice could be attributable to either a return of insulin insensitivity or a loss of insulin secretion because the engrafted cells may have begun to die in vivo. It is also possible that hyperglycemia resulted from immunorejection of the transplanted cells because they were derived from BL6 mice and were not a perfect match to the Leprdb, C57BLKS; Dock7m, DBA/J strain. We will be examining these problems in greater detail as we go forward.
Collectively, our data show that an iPS cell-derived β-like cell–based therapy is able to correct a hyperglycemic phenotype by restoring in vivo insulin secretion and normal glucose levels. These β-like cells, derived in vitro from iPS cells, secreted insulin in response to glucose both in vitro and after transplantation via the hepatic portal vein in vivo. This was extensively demonstrated in a mouse model of type 2 diabetes mellitus, and additional preliminary data indicate that a similar strategy may work for type 1 diabetes.
Materials and Methods
In Vitro Cell Differentiation and Analysis.
The three-stage differentiation protocol of iPS cells into β-like cells was adapted from the protocol for differentiation of ES cells into insulin-producing cells developed by Wobus and co-workers (17), with modifications. In vitro insulin induction was carried out by the addition of 2.5 mM glucose to the media of stage 3 on days 7, 13, and 20 when iPS cell-derived β-like cells and insulin secretion were measured by ELISA. Day 7 iPS cell-derived β-like cells were analyzed for expression of the stem cell marker as described (17). Details of the methods are provided in SI Text.
Mouse Models.
Two mouse models were used: STZ-treated C57BL6 mice (type 1) and Leprdb, C57BLKS; Dock7m, DBA/J mice (type 2). An insulin tolerance test was performed as previously described (21). Cellular transplantation was done by portal vein injection using previously established protocols (22). Details of the methods are provided in SI Text. Information on primer sequences for PCR analysis and antibodies for immunohistochemical studies are listed in Tables S1 and S2.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant R01HL087948.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007884107/-/DCSupplemental.
References
- 1.Jones PM, Courtney ML, Burns CJ, Persaud SJ. Cell-based treatments for diabetes. Drug Discov Today. 2008;13:888–893. doi: 10.1016/j.drudis.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Urbán VS, et al. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. 2008;26:244–253. doi: 10.1634/stemcells.2007-0267. [DOI] [PubMed] [Google Scholar]
- 3.Karnieli O, Izhar-Prato Y, Bulvik S, Efrat S. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells. 2007;25:2837–2844. doi: 10.1634/stemcells.2007-0164. [DOI] [PubMed] [Google Scholar]
- 4.Sun B, Roh KH, Lee SR, Lee YS, Kang KS. Induction of human umbilical cord blood-derived stem cells with embryonic stem cell phenotypes into insulin producing islet-like structure. Biochem Biophys Res Commun. 2007;354:919–923. doi: 10.1016/j.bbrc.2007.01.069. [DOI] [PubMed] [Google Scholar]
- 5.Lumelsky N, et al. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- 6.Docherty K, Bernardo AS, Vallier L. Embryonic stem cell therapy for diabetes mellitus. Semin Cell Dev Biol. 2007;18:827–838. doi: 10.1016/j.semcdb.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Kroon E, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 8.Lakey JR, Mirbolooki M, Shapiro AM. Current status of clinical islet cell transplantation. Methods Mol Biol. 2006;333:47–104. doi: 10.1385/1-59745-049-9:47. [DOI] [PubMed] [Google Scholar]
- 9.Sordi V, et al. Mesenchymal cells appearing in pancreatic tissue culture are bone marrow-derived stem cells with the capacity to improve transplanted islet function. Stem Cells. 2010;28:140–151. doi: 10.1002/stem.259. correction (2010) 28:386. [DOI] [PubMed] [Google Scholar]
- 10.Park IH, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 11.Lewitzky M, Yamanaka S. Reprogramming somatic cells towards pluripotency by defined factors. Curr Opin Biotechnol. 2007;18:467–473. doi: 10.1016/j.copbio.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Hanna J, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 15.Soldner F, et al. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu D, et al. Phenotypic correction of murine hemophilia A using an iPS cell-based therapy. Proc Natl Acad Sci USA. 2009;106:808–813. doi: 10.1073/pnas.0812090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroeder IS, Rolletschek A, Blyszczuk P, Kania G, Wobus AM. Differentiation of mouse embryonic stem cells to insulin-producing cells. Nat Protoc. 2006;1:495–507. doi: 10.1038/nprot.2006.71. [DOI] [PubMed] [Google Scholar]
- 18.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 19.Höppener JW, et al. Molecular physiology of the islet amyloid polypeptide (IAPP)/amylin gene in man, rat, and transgenic mice. J Cell Biochem. 1994;55(Suppl):39–53. doi: 10.1002/jcb.240550006. [DOI] [PubMed] [Google Scholar]
- 20.Pittner RA, et al. Molecular physiology of amylin. J Cell Biochem. 1994;55(Suppl):19–28. doi: 10.1002/jcb.240550004. [DOI] [PubMed] [Google Scholar]
- 21.Mathews ST, et al. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes. 2002;51:2450–2458. doi: 10.2337/diabetes.51.8.2450. [DOI] [PubMed] [Google Scholar]
- 22.Kemp CB, Knight MJ, Scharp DW, Lacy PE, Ballinger WF. Transplantation of isolated pancreatic islets into the portal vein of diabetic rats. Nature. 1973;244:447. doi: 10.1038/244447a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.