Abstract
Tissue kallikrein (TK) is a serine protease synthetized in renal tubular cells located upstream from the collecting duct where renal potassium balance is regulated. Because secretion of TK is promoted by K+ intake, we hypothesized that this enzyme might regulate plasma K+ concentration ([K+]). We showed in wild-type mice that renal K+ and TK excretion increase in parallel after a single meal, representing an acute K+ load, whereas aldosterone secretion is not modified. Using aldosterone synthase-deficient mice, we confirmed that the control of TK secretion is aldosterone-independent. Mice with TK gene disruption (TK−/−) were used to assess the impact of the enzyme on plasma [K+]. A single large feeding did not lead to any significant change in plasma [K+] in TK+/+, whereas TK−/− mice became hyperkalemic. We next examined the impact of TK disruption on K+ transport in isolated cortical collecting ducts (CCDs) microperfused in vitro. We found that CCDs isolated from TK−/− mice exhibit net transepithelial K+ absorption because of abnormal activation of the colonic H+,K+-ATPase in the intercalated cells. Finally, in CCDs isolated from TK−/− mice and microperfused in vitro, the addition of TK to the perfusate but not to the peritubular bath caused a 70% inhibition of H+,K+-ATPase activity. In conclusion, we have identified the serine protease TK as a unique kalliuretic factor that protects against hyperkalemia after a dietary K+ load.
Keywords: collecting duct, serine protease, transport
Extracellular K+ concentration ([K+]) is critical for the control of membrane potential and many cellular functions and, hence, has to be maintained within narrow limits in mammals (1). This regulation is primarily achieved by the kidney, which modulates urinary K+ excretion so that it matches exactly the daily K+ intake, thereby maintaining K+ balance. Potassium is freely filtered at the glomerulus and almost totally reabsorbed by the proximal tubule and the loop of Henle; the amount of K+ excreted by the kidney is determined by events beyond the early distal tubule, where either reabsorption or secretion of K+ can occur (1).
Generally, a large amount of K+ has to be eliminated to maintain balance, and potassium secretion occurs mainly through the principal cells (PCs) of the collecting duct where it involves the following: (i) cellular K+ entry across the basolateral membrane via the Na+,K+-ATPase pump; and (ii) K+ exit across the apical membrane via K+ channels allowing its secretion into the lumen. However, under conditions in which potassium has to be retained by the kidney, such as when individuals are fed a K+-depleted diet, the collecting duct epithelium reverses the transepithelial flux of K+ to yield net potassium reabsorption (2). This absorption occurs through the intercalated cells (ICs) and depends on an apical electroneutral H+,K+-ATPase (3). This system and, hence, potassium balance, is under the tight control of the mineralocorticoid hormone aldosterone, which activates and fine-tunes K+ secretion by the collecting duct (4, 5). Despite the fact that aldosterone is the key regulator of renal K+ excretion and K+ balance, this regulation is not sufficient to ensure the stability of plasma [K+]. Indeed, K+ intake during the day is not stable but fluctuates between fasting and feeding periods. Because the extracellular content of K+ is very low and K+ is rapidly absorbed by the digestive tract, the K+ content of a single meal represents a massive and rapid influx of K+ into the extracellular fluid, which would have a major impact on plasma [K+] in the absence of regulation (2). The main protection against postprandial hyperkalemia is extrarenal and achieved by a rapid redistribution of K+ from the extracellular to the intracellular space under the influence of insulin (6). However, a rapid increase in kaliuresis also contributes to the protection against hyperkalemia (7). Even though aldosterone is the main determinant of steady-state plasma [K+], the rapid renal K+ excretion after acute K+ intake is aldosterone-independent (8, 9). Indeed, this rapid regulation acts to limit increases not only in plasma [K+], but also in aldosterone, which could otherwise have a prolonged effect on Na+ and water balance in addition to its kaliuretic action.
The mechanisms that mediate the transient aldosterone-independent increase in renal K+ excretion in response to an acute K+ load are not well understood (7). Tissue kallikrein (TK)† is a serine protease synthesized in large amounts by the renal connecting tubule cells (10). An increase in renal TK secretion consecutive to K+ loading has been reported in both humans (11, 12) and rodents (13, 14). Because the distal nephron segments that are responsible for regulation of K+ excretion (i.e., late distal tubules, connecting tubules (CNTs), and cortical collecting ducts (CCDs)] are bathed by large amounts of this enzyme, and because serine proteases have been described to modulate many different biological functions including ion transport (15), we hypothesized that TK might modulate renal K+ excretion. The goal of our study was to determine: (i) the kinetics of renal TK, aldosterone, and K+ secretion in response to acute K+ loading, (ii) the aldosterone independence of renal TK secretion, (iii) the functional impact of TK gene inactivation on plasma [K+], (iv) whether the lack of TK is associated with abnormal transepithelial transport of K+ in isolated microperfused CCDs, and eventually (v) the transport mechanism responsible for such dysfunction. The results of this study demonstrate that the modulation of renal K+ transport by TK is part of the acute response to a dietary K+ load.
Results
Kinetics of Aldosterone and Kallikrein Excretion in Response to K+ Loading.
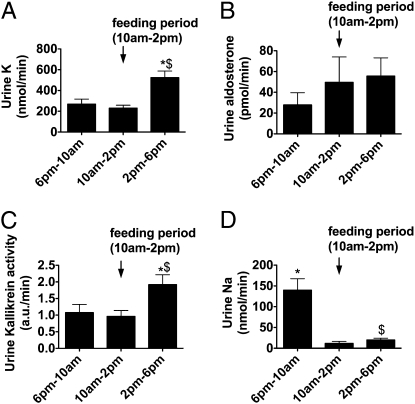
We first looked at the 24-h pattern of urinary aldosterone, kallikrein, Na+, and K+ excretion in wild-type mice, which were allowed to ingest their daily ration over a 4-h period (between 10:00 am and 2:00 pm). Feeding provoked a marked increase in kaliuresis (Fig. 1A), which was accompanied by no significant change in urinary aldosterone level (Fig. 1B). Thus, feeding-induced kaliuresis is not related to changes in aldosterone secretion. In contrast, the release of kallikrein into the urine was significantly increased after the feeding period (Fig. 1C) and could be linked to the increase in K+ excretion.
Fig. 1.
Urinary excretion of K+ (A), aldosterone (B), kallikrein (C), and Na+ (D) in wild-type mice that have been taught to eat their daily ration over a 4-h period. n = 8 mice in each group. Values are means ± SE. Statistical significance is assessed by ANOVA and Newman-Keuls post hoc test. *P < 0.05 vs. the 10:00 am to 2:00 pm period. $P < 0.05 vs. the 6:00 pm to 10:00 am period.
Aldosterone Independence of TK Secretion.
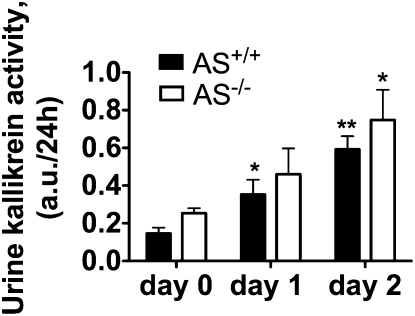
The preceding experiments suggest that dietary K+ loading stimulates directly the secretion of TK independently of aldosterone. To confirm this hypothesis, we determined whether K+-loading could stimulate urinary TK excretion in the absence of aldosterone. We measured urinary TK level in aldosterone synthase-deficient mice (AS−/−) and their wild-type littermates (AS+/+) before and after feeding a high K+ diet. Urinary TK levels in both AS−/− and AS+/+ mice were significantly increased 24 h after K+ loading, and even more after 48 h (Fig. 2). There was no significant difference in urinary kallikrein levels between genotypes at any time. These results confirmed that the increase in urinary TK excretion observed after dietary K+ loading does not require the presence of aldosterone.
Fig. 2.
Effect of aldosterone synthase deletion on urinary kallikrein excretion. Urinary kallikrein activity was measured on a standard (0.8% K+) rodent diet and after 24 or 48 h K+ loading by administration of a high (2% K+) diet. AS+/+, wild-type mice; AS−/−, aldosterone synthase-deficient mice. n = 5–7 mice per group. Values are means ± SE. Statistical significance is assessed by ANOVA followed by Bonferroni multiple comparison post hoc test when appropriate. *P < 0.05; **P < 0.01 vs. day 0, same genotype.
Effect of K+ Loading on Plasma K+ Concentration in TK−/− Mice.
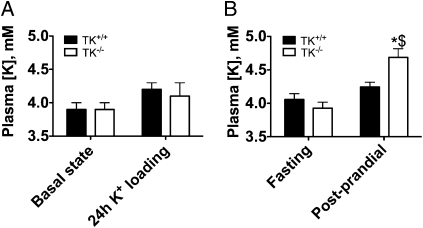
To evaluate the importance of TK in the regulation of plasma K+ concentration, we next measured blood K+ concentration in TK+/+ and TK−/− mice before and 24 h after administration of a high K+ diet (2% K+). As shown in Fig. 3A, wild-type and knockout mice exhibited the same plasma K+ concentrations on standard diet (0.8% K+) and after 24-h feeding with a high (2%) K+ diet. Urinary aldosterone did not differ significantly between genotypes on standard diet (7.0 ± 1.6 vs. 6.8 ± 1.5 pmol/min, in TK+/+ and TK−/− mice; n = 8 and 6, respectively) and was similarly increased in both groups after 24-h feeding with a high K+ diet (63.9 ± 11.1 vs. 79.8 ± 12.9 pmol/min, in TK+/+ and TK−/− mice; n = 8 and 6, respectively). Basal urinary Na+ and K+ excretions were similar in both groups. Urinary K+ excretion increased in both groups to a similar extent after administration of a high K+ diet (Table S1), indicating similar food intake. As food intake represents a physiological K+ load that the body has to handle repeatedly during a day, we next determined the postprandial increment in plasma K+ concentration above fasting value. To maximize the chance of observing a postprandial change in plasma K+ concentration, the effect of a single large feeding (over a 4-h period) was tested on pair-fed TK+/+ and TK−/− mice. Before feeding both genotypes exhibited similar plasma K+ concentration (Fig. 3B), and similar body weight [29.6 ± 1.0 grams versus 28.2 ± 1.0 grams; not significant (NS)]. After feeding, gain of weight was not significantly different between genotypes (change in body weight +2.2 ± 0.3 grams in TK+/+ vs. +2.1 ± 0.1 grams in TK−/−; NS), indicating similar food intake. As shown in Fig. 3B, at the end of the 4-h feeding period, plasma K+ concentration in TK+/+ mice was not different from fasting value. In contrast, TK−/− mice became hyperkalemic (4.7 ± 0.1 mM; n = 7).
Fig. 3.
Effects of TK disruption on blood K+ concentration. (A) Blood K+ concentration on standard diet (0.8% K+) in five TK+/+ and six TK−/− mice, or after 24 h of feeding with a high (2%) K+ diet in eight TK+/+ and six TK−/− mice. (B) Blood K+ concentration in 7 TK+/+ (filled bars) and 7 TK−/− (open bars) littermate mice under fasting and postprandial states. Measurements were performed 1 h before and 5 h after the beginning of the feeding period in which the mice did ingest their daily food intake. Values are means ± SE. Statistical significance is assessed by ANOVA and, when significant, groups were compared by Newman-Keuls post hoc test. *P < 0.01 vs. TK+/+ under postprandial state. $P < 0.001 vs. TK−/− under fasting state.
Taken together, the results from the preceding experiments indicate that TK plays an important role in protecting the organism against an acute K+ load.
CCDs from TK−/− Mice Reabsorb K+.
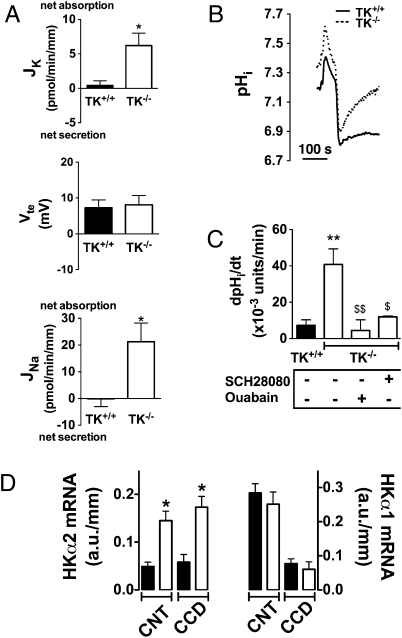
Renal K+ regulation occurs in PCs of connecting tubules and cortical collecting ducts that express ROMK, maxi-K, and ENaC (2, 16). We thus examined the impact of TK disruption on transepithelial K+ (JK) and Na+ (JNa) fluxes and on transepithelial voltage (Vte) by using in vitro microperfusion of isolated CCDs. We and others have shown that CCDs do not exhibit any significant Na+ or K+ transport and do not generate a lumen-negative transepithelial voltage, when mice are fed a Na+-replete diet (17, 18). Accordingly, CCDs isolated from TK+/+ mice neither reabsorbed sodium nor secreted potassium (Fig. 4A). In contrast, CCDs isolated from TK−/− mice exhibited significant Na+ absorption. However, this occurred in the absence of a lumen-negative transepithelial voltage and was accompanied by net K+ absorption rather than K+ secretion (Fig. 4A). Taken together, these results suggest that Na+ absorption in CCD from TK−/− mice is not mediated by ENaC but occurs instead through the electroneutral Na+ absorption pathway that we recently described and characterized in detail (18).
Fig. 4.
K+ transepithelial absorption is due to increased expression and activity of colonic H+,K+-ATPase in CCDs isolated from TK+/+ (filled bars) and TK−/− mice (open bars). (A) CCDs isolated from TK+/+ mice do not absorb Na+ (Bottom), do not generate a lumen-negative transepithelial voltage (Middle) and do not secrete K+ (Top) under basal conditions. CCDs isolated from TK−/− mice absorb Na+ (Bottom) but do not generate a lumen-negative transepithelial voltage (Middle) and exhibited net K+ absorption (Top). The control group consists of littermate mice. Statistical significance is assessed by unpaired Student's t test. n = 7 in each group, *P < 0.05. (B) Intracellular pH recovery after an acute acid load in ICs of CCDs isolated from TK+/+ and TK−/− mice. Each trace represents the mean of pHi changes recorded when NH4Cl was added and removed in 8–9 CCDs (4–5 ICs were analyzed in each CCD). NH4Cl removal leads to rapid intracellular acidification. (C) Average initial rates of pHi recovery after intracellular acidification in ICs in the absence or presence of different inhibitors (30 μM Sch28080 or 1 mM oubain). Values on graph are means ± SE; n = 8 tubules for TK+/+, n = 9 for TK−/−, and n = 4 for TK−/− + Sch28080 and TK−/− + ouabain. Statistical significance is assessed by ANOVA and Newman-Keuls multiple comparison test. **P < 0.01 vs. TK+/+. $P < 0.05. $$P < 0.01 vs. TK−/− in the absence of inhibitor. The nadir pHi achieved after washout of the NH4Cl prepulse was identical in each group, 6.62 ± 0.05 for TK+/+ (n = 8); 6.70 ± 0.11 for TK−/− (n = 9); 6.72 ± 0.05 for TK−/− + Sch28080 (n = 4) and 6.68 ± 0.09 for TK−/− + ouabain (n = 4). Values are means ± SE and were not significantly different (one-way ANOVA). (D) Expression of the α2 subunit of H+,K+-ATPase and the α1 subunit of the H+,K+-ATPase in CNTs and CCDs. Results are expressed as arbitrary units (a.u.)/mm tubules. Data are means ± SE from four to six mice. Statistical significance was assessed by ANOVA followed by Bonferroni post hoc test: *P < 0.01 vs. TK+/+.
Colonic H+, K+-ATPase Activity Was Increased in ICs of CCDs from TK−/− Mice.
K+ transport in the cortical-collecting duct involves two distinct populations of K+ channels (high conductance K+ channels—maxi K channel—and low conductance K+ channels—ROMK) that can contribute to K+ secretion (16, 19) and an electroneutral ATP-dependent H+/K+ exchange that mediates K+ reabsorption (3). Two isoforms of H+,K+-ATPase have been cloned from the rat gastric mucosa and colon, respectively. Molecular studies have demonstrated the presence of both H+,K+-ATPase isoforms in collecting duct cells (20). These isoforms mediate the exchange of intracellular H+ for extracellular K+, mediating absorption of K+ and bicarbonate. It has been shown that the colonic type of the H+,K+-ATPase is responsible for K+ conservation by the kidney (3) and that increased bicarbonate absorption in the collecting duct during potassium depletion is mediated by the colonic type of the H+,K+-ATPase (21).
Therefore, we measured H+,K+-ATPase activity in PCs and ICs of collecting ducts isolated from TK−/− mice or their respective wild-type littermates. To do so, the effect of an acute intracellular acid load on intracellular pH (pHi) recovery was monitored in ICs and PCs. Cells were acidified by the NH4Cl prepulse technique, and the initial rate of intracellular pH recovery in the absence of Na+ was measured. This pH recovery reflects the activity of proton pumps. ICs were distinguished from PCs by their shape, fluorescein-conjugated peanut lectin labeling, and their greater uptake of BCECF, when the fluorophore was applied to the perfusate. Fig. 4i shows that the rate of recovery of intracellular pH after intracellular acidification of ICs from TK−/− mice was larger than that of ICs from WT. No proton pump activity was detected in PCs. Acid extrusion in ICs from TK−/− mice was inhibited either by 30 μM SCH28080, a specific inhibitor of H+,K+-ATPases, or 1 mM ouabain (Fig. 4C). These pharmacological properties correspond to those described for the colonic type of the H+,K+-ATPase (22). Moreover, Fig. 4D shows that mRNA for the α2 subunit of the H+,K+-ATPase (specific to the colonic type of the H+,K+-ATPase) was expressed at low levels all along the aldosterone-sensitive distal nephron of control mice and was markedly increased in connecting tubules and collecting ducts from TK−/− mice (≈3-fold). In contrast, mRNA expression of the α1 subunit of the H+,K+-ATPase (specific to the gastric type of the H+,K+-ATPase) was not different in the CNTs and CCDs of TK−/− mice when compared with those of TK+/+ mice (Fig. 4D). Interestingly, mRNA of the β1 subunit of the Na+,K+-ATPase that assembles with the α2 H+,K+-ATPase in vivo (23) was also increased in the CNT from TK−/− mice (Fig. S1). mRNA expression of ROMK was not different in the CNT and CCD of TK−/− mice when compared with those of TK+/+ mice (Fig. S1).
Our results demonstrate that the absence of TK stimulates the expression and activity of the colonic H+,K+-ATPase of the ICs and that this results in net K+ absorption by the CCD. Conversely, it suggests that under conditions in which K+ has to be excreted, TK exerts a negative tonus on K+ absorption by inhibiting the pump.
Up-Regulation of the Colonic H+,K+-ATPase in TK−/− Mice Leads to an Increase in Net Acid Excretion.
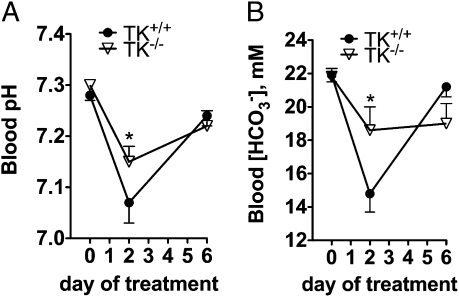
As shown above, despite abnormal transport of K+ in the CCD, TK−/− mice exhibited only mild disturbance of postprandial plasma [K+]. To avoid life-threatening hyperkalemia, K+ homeostasis is tightly controled by different synergistic mechanisms, such as the uptake of K+ by muscle and liver cells, or colon K+ secretion that might have compensated at least in part the excess in collecting duct K+ absorption consecutive to TK disruption. Thus, we next assessed the consequences of the abnormal up-regulation of H+,K+-ATPase in vivo on renal acid-base regulation. Our reasoning was that K+ absorption through H+,K+-ATPase is linked to equimolar H+ secretion; hence, if this regulation is not marginal in vivo, it should represent a net renal loss of acid. TK−/− mice did not exhibit overt alteration in acid base balance (Table 1). However, urinary net acid excretion was higher in TK−/− relative to TK+/+ mice because of an increase in titratable acid (Table 1). Therefore, we tested the ability of TK−/− mice to cope with a chronic acid load. TK+/+ and TK−/− mice were administrated 280 mM NH4Cl in the drinking water. Although this challenge induced a significant reduction in blood pH and [HCO3−] in both genotypes, metabolic acidosis was significantly less severe in TK−/− mice (Fig. 5). These experiments confirm that the up-regulation of the H+,K+-ATPase is not marginal because it has detectable effects on renal acid-base regulation of the animal.
Table 1.
Physiological blood and urine parameters in TK+/+ and TK−/− littermates on a standard diet
| Measurement | TK+/+ | TK−/− | P |
| Blood | |||
| pH | 7.28 ± 0.01 (18) | 7.30 ± 0.01 (18) | NS |
| pCO2, mmHg | 48.3 ± 1.3 (18) | 46.0 ± 2.1 (18) | NS |
| HCO3−, mM | 21.9 ± 0.4 (18) | 21.8 ± 0.5 (18) | NS |
| Urine | |||
| pH | 5.71 ± 0.04 (7) | 5.64 ± 0.06 (6) | NS |
| HCO3−/creatinine, mmol/mmol | 0.06 ± 0.01 (7) | 0.10 ± 0.05 (6) | NS |
| Net acid excretion/creatinine, mmol/mmol | 124.4 ± 6.6 (8) | 148.0 ± 7.0 (7) | 0.028 |
| NH4+/creatinine, mmol/mmol | 86.3 ± 6.3 (8) | 97.5 ± 5.1 (7) | NS |
| Titratable acid/creatinine, mmol/mmol | 38.1 ± 2.9 (8) | 50.6 ± 3.7 (7) | 0.018 |
Values are means ± SE. (n), number of mice studied. NS, not significant. Statistical significance is assessed by unpaired Student t test.
Fig. 5.
Response to an acid load in TK-/ - mice. Values in TK+/+ (•) and TK−/− (△) littermates are compared and represented as means ± SE. Blood pH (A) and HCO3− (B) were measured in mice challenged with an acid load consisting in 280 mM NH4Cl in the drinking water. n = 5–7 mice per genotype. *P < 0.05 vs. TK+/+.
Luminal TK Inhibits H+,K+-ATPase Activity in Intercalated Cells of Cortical Collecting Duct.
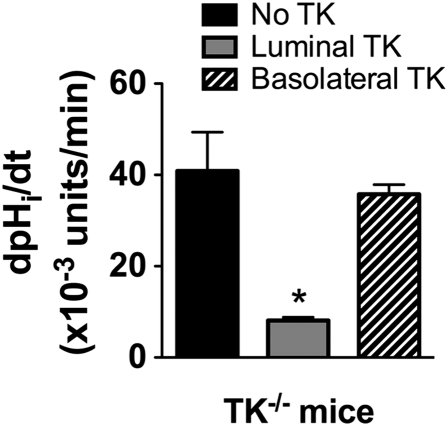
Our preceding experiments indicate that in TK−/− mice, H+,K+-ATPase activity is up-regulated in both CNTs and CCDs. This result, however, does not imply that TK is involved in acute regulation of plasma K+ concentration. To assess directly whether TK is able to inhibit H+,K+-ATPase activity, we next examined the effects of extracellular (luminal or peritubular) TK on H+,K+-ATPase activity in CCDs isolated from TK−/− mice microperfused in vitro. Fig. 6 shows that addition of TK to the perfusate inhibited H+,K+-ATPase activity of ICs by 70%, whereas basolateral addition of TK had no effect. Thus, TK is a factor that acts from the luminal side of CCD to acutely block the H+,K+ ATPase.
Fig. 6.
Intracellular pH recovery after an acute acid load in ICs of CCDs isolated from TK−/− mice. CCDs isolated from TK−/− mice were perfused with either TK (10 μg/mL) or the vehicle alone for 30 min before recording. TK was able to inhibit the rate of recovery of intracellular pH after intracellular acidification of ICs by 70%. Values on graph are means ± SE, n = 9 tubules for TK−/− with no TK, n = 5 for luminal TK, and n = 4 for peritubular TK. Statistical significance is assessed by ANOVA and Newman-Keuls multiple comparison test. *P < 0.05 vs. TK−/− in the absence of TK. The nadir pHi achieved after washout of the NH4Cl prepulse was identical in each group, 6.70 ± 0.11 for no TK (n = 9); 6.61 ± 0.06 for luminal TK (n = 5); 6.60 ± 0.09 for peritubular TK (n = 4). Values are means ± SE and were not significantly different (one-way ANOVA).
Taken together, our results demonstrate that the inhibitory effect by TK on K+ absorption is a significant factor in the acute regulation of plasma K+ concentration.
Discussion
The main factor regulating plasma K+ concentration is the mineralocorticoid aldosterone (5). Aldosterone secretion by adrenals is stimulated in response to chronic hyperkalemia or to an increase in dietary K+. Conversely, it is inhibited by chronic hypokalemia or a K+-depleted diet. Aldosterone controls the relationship between plasma K+ concentration and urinary excretion of K+ by modulating K+ transport across the epithelium of the distal part of the nephron (1, 4). As an illustration of the critical role of aldosterone in setting steady-state plasma K+ concentration, hypoaldosteronemic states are characterized by the presence of a hyperkalemia, whereas hyperaldosteronemic states are frequently associated with hypokalemia.
A typical meal of a western style diet represents a K+ load equivalent to half of the total amount of K+ in the extracellular fluid. As a consequence, every meal represents an acute and massive K+ load in which the body has to cope (2). It is likely that aldosterone is not well adapted to mediate this adaptation because most of the effects of aldosterone are transcriptional and, thus, long-term, and because aldosterone not only modulates K+ transport but also regulates sodium and acid base balance. Hence, the defense against postfeeding hyperkalemia is mediated by factors, such as insulin or β-adrenergic agents, both of which stimulate the transfer of K+ from the extracellular to the intracellular space (7).
In this study, we identified the serine protease TK as a unique aldosterone-independent mechanism that protects the body against postfeeding hyperkalemia by mediating rapid modification of renal tubular K transport (Fig. 7). The main finding of this study is that renal TK secretion increases after an acute potassium load. An early increase in renal kallikrein secretion, which occurs after i.v. infusion of potassium, has been reported in rats (24–26). The mechanism underlying this increase remains unclear. Nevertheless, it has been shown that administration of ATP-sensitive K+ channel blockers also caused an increase in renal kallikrein secretion in superfused slices of kidney cortex (24). It is possible that potassium and KATP channel blockers increase renal kallikrein secretion through the same mechanism. It is known that membrane permeability to potassium through potassium channels mainly determines the membrane potential of excitable cells. Renal K+ channels in distal nephron not only secrete potassium but also maintain the membrane potential of tubule cells (27). A decrease in membrane potential caused by the high concentration of extracellular potassium has been reported in the case of renal tubular cells (28). K+ channel blockers such as barium chloride also produced the same effect (28). A separate study reported that enhanced release of kallikrein by elevated potassium levels or by ATP-sensitive potassium channel blockers was reduced in the absence of calcium ion or in the presence of voltage-dependent calcium channel blockers in the superfused solution (29). Thus, it is possible that the increase in renal kallikrein secretion, induced by high potassium intakes, may result from membrane depolarization of kallikrein-secreting cells in the renal connecting tubules, followed by enhanced calcium influx. The same mechanism has been reported for the release of insulin from the β-cell in the pancreas (30, 31) and for that of aldosterone from the cells of adrenal glands (32).
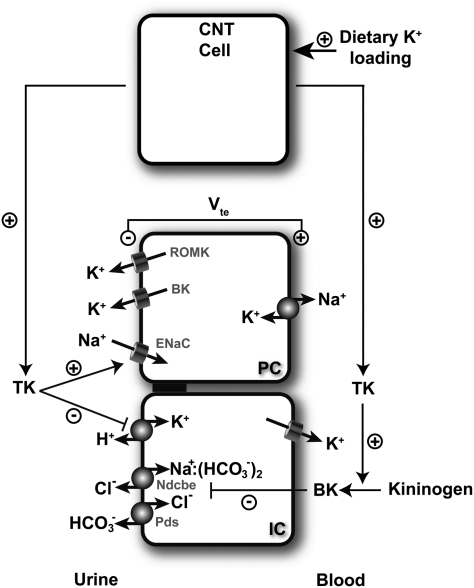
Fig. 7.
Schematic model depicting how TK participate to the response against dietary K+ load. TK production by CNT cells is stimulated by dietary K+ loading. TK is then released into the urinary fluid and reaches the CCD. There, TK might favor K+ secretion by PCs through its stimulating action on ENaC mediated Na+ absorption, an effect that occurs through proteolytic processing of the γ-ENaC subunit (34). TK also inhibits K+ absorption by ICs by decreasing colonic H+/K+-ATPase expression and activity. It also up-regulates ENaC-independent electroneutral NaCl absorption presumably through a decrease in the local production of bradykinin (BK) (35).
In the kidney, TK is synthesized in large amounts by connecting tubule cells and secreted into the urinary fluid and, to a lesser extent, to the peritubular interstitium (10). Therefore, connecting tubules and collecting ducts are exposed to large amounts of the enzyme in the lumen. Because proteolytic regulation of several membrane proteins by serine proteases, including ion channels, has been demonstrated (33), it is possible that urinary TK is involved in autocrine regulation and paracrine regulation of CNT and CCD transport, respectively. Accordingly, we have shown that TK activates the epithelial Na+ channel ENaC by cleaving its gamma subunit (34). In CCDs isolated from rats microperfused in vitro, Tomita et al. (35) showed that basolateral bradykinin inhibits net Na+ absorption by affecting an electroneutral, amiloride-resistant, thiazide-sensitive NaCl transporter that remained unidentified until our recent report (35). In this study, we observed that CCDs from TK−/− mice actually absorb sodium via an electroneutral process. Thus, the absence of TK decreases ENaC proteolysis and activation (34), but it up-regulates ENaC-independent electroneutral NaCl absorption presumably through a decrease in the local production of bradykinin. The combination of these two opposite effects can explain why TK−/− mice do not have a significantly altered sodium balance (34, 36), but it might have important implications for renal potassium handling. Because K+ secretion strictly depends on electrogenic Na+ transport by ENaC, by favoring ENaC-mediated Na+ absorption over electroneutral NaCl absorption, TK is expected to indirectly stimulate K+ secretion by PCs (Fig. 7).
The renal handling of K+ differs notably from that of other solutes. K+ is almost completely absorbed when the tubular fluid exits the loop of Henle; hence, the fraction that needs to be excreted in the urine depends on active secretion, which occurs in the late distal nephron. Thus, except in a situation of K+ depletion, in which net K+ absorption also occurs in the collecting system, the latter is generally a site of net K+ secretion. However, our study reveals that CCDs isolated from TK−/− mice do not secrete K+ but instead absorb K+. This observation suggests that K+ absorption is permanently under the negative control of TK in this nephron segment. K+ reabsorption in isolated CCDs from TK null mice is associated with an increased H+,K+-ATPase activity. The mechanism or signaling pathway by which TK controls H+,K+-ATPase remains unsettled in this study.
In summary, our results suggest that TK is a hypokalemic factor participating in the stability of plasma [K+] during the day (Fig. 7). TK acts by favoring ENaC-mediated Na+ absorption that, in turn, is expected to favor renal K+ secretion and by inhibiting K+ absorption through the colonic type of the H+,K+-ATPase.
Materials and Methods
Animals.
TK−/− mice have been generated in our laboratory (34, 36, 37). Aldosterone synthase-deficient mice (AS−/−) (38) were obtained from O. Smithies (University of North Carolina, Chapel Hill). Mice heterozygous for TK or AS gene disruption were crossed, and wild-type (TK+/+ or AS+/+) and homozygous knock-out mice (TK−/− or AS−/−) were identified in their offspring by genotyping tail DNA as described in ref (36, 38). In all experiments, controls consisted in wild-type littermates. All of the experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication 93-23, revised 1985) and with the French Government animal welfare policy (Agreement RA024647151FR).
Physiological Studies.
Mice were housed in metabolic cages (Techniplast). They were first allowed to adapt for 3–5 d to the cages. At steady state, urine collection was performed daily under mineral oil in the urine collector for electrolyte measurements. For acid-loading experiments, mice were given 0.28 M NH4Cl in their drinking water. To study the ability of mice to adapt to a high K+ diet, TK−/− and TK+/+ mice were equilibrated for a few days on the standard diet (0.8% K+) and then switched to a “high” K+ (2% K+) diet (Institut National de la Recherche Agronomique, Jouy en Josas, France). To maximize feeding-dependent influences on plasma K+ level (39), TK−/− and TK+/+ mice were supplied with their daily ration, which they were allowed to eat over a 4-h period (between 10:00 am and 2:00 pm). For convenience, the 12:12 light/dark cycle was inverted. Mice were allowed to adapt to a once-a-day feeding over a week.
All biochemical and hormonal analyses were performed by using standard methods as detailed in SI Materials and Methods. Urinary kallikrein activity was quantified by an enzymatic assay as described (40).
Microdissection and Reverse Transcription-PCR.
The various segments of nephron were dissected from collagenase-treated kidney slices under RNase-free conditions. Total RNA was immediately extracted from pools of 40–60 nephrons segments by using RNeasy Micro kit (Qiagen). Reverse transcription was performed by using first-strand cDNA synthesis kit for reverse transcription-PCR (Roche Diagnostics). Real-time PCR was performed by using a cDNA quantity corresponding to 0.1 mm of specific nephron segment on a LightCycler (Roche Diagnostics) with LightCycler 480 SYBR Green I Master qPCR kit (Roche Diagnostics). We verified that no amplification was produced when reverse transcriptase was omitted. Sequences of the specific primers are provided as Table S2. Results (arbitrary units per millimeter of tubule length) are expressed as means ± SEM from 5 to 6 mices.
In Vitro Microperfusion and pH Measurements.
Cortical CCDs were isolated and microperfused in vitro as described by Burg (41). Intracellular pH was monitored by using the pH-sensitive dye BCECF (42). [Na+], [K+], and [creatinine] measurements were performed by HPLC (18) to calculate the rate of K+ (JK) and Na+ (JNa) transepithelial transport as described in detail in the SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Carsten Wagner (University of Zurich) for giving us access to the aldosterone synthase-deficient mouse. R.C. and coworkers are funded by the Institut National de La Santé et de la Recherche Médicale. Part of this work is also funded by the Leducq Transatlantic Network in Hypertension, and by a subvention de recherche AMGEN 2008 pour la recherche en Néphrologie to Dominique Eladari. S.E.M. is supported by grants (allocations de recherches doctorales) from the Fondation pour la Recherche Médicale and from the Société de Néphrologie (Bellco-Soludia). B.W. and F.S. are supported by l'Agence Nationale de la Recherche Grants PHYSIO 2007-RPV07087 (to P.H.) and PHYSIO 2007-RPV07084 (to D.E.), respectively.
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0913070107/-/DCSupplemental.
†In this paper, tissue kallikrein (TK) indicates the product of the mouse klk1 gene (accession number: NM_010639); synonyms: KLK1, Kal, mGk-6, renal kallikrein, and Klk1b6.
References
- 1.Giebisch G. Renal potassium transport: Mechanisms and regulation. Am J Physiol. 1998;274:F817–F833. doi: 10.1152/ajprenal.1998.274.5.F817. [DOI] [PubMed] [Google Scholar]
- 2.Wang W. Regulation of renal K transport by dietary K intake. Annu Rev Physiol. 2004;66:547–569. doi: 10.1146/annurev.physiol.66.032102.112025. [DOI] [PubMed] [Google Scholar]
- 3.Meneton P, et al. Increased sensitivity to K+ deprivation in colonic H,K-ATPase-deficient mice. J Clin Invest. 1998;101:536–542. doi: 10.1172/JCI1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young DB, Paulsen AW. Interrelated effects of aldosterone and plasma potassium on potassium excretion. Am J Physiol. 1983;244:F28–F34. doi: 10.1152/ajprenal.1983.244.1.F28. [DOI] [PubMed] [Google Scholar]
- 5.Young DB. Quantitative analysis of aldosterone's role in potassium regulation. Am J Physiol. 1988;255:F811–F822. doi: 10.1152/ajprenal.1988.255.5.F811. [DOI] [PubMed] [Google Scholar]
- 6.DeFronzo RA, Felig P, Ferrannini E, Wahren J. Effect of graded doses of insulin on splanchnic and peripheral potassium metabolism in man. Am J Physiol. 1980;238:E421–E427. doi: 10.1152/ajpendo.1980.238.5.E421. [DOI] [PubMed] [Google Scholar]
- 7.Youn JH, McDonough AA. Recent advances in understanding integrative control of potassium homeostasis. Annu Rev Physiol. 2009;71:381–401. doi: 10.1146/annurev.physiol.010908.163241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabinowitz L, Sarason RL, Yamauchi H. Effects of KCl infusion on potassium excretion in sheep. Am J Physiol. 1985;249:F263–F271. doi: 10.1152/ajprenal.1985.249.2.F263. [DOI] [PubMed] [Google Scholar]
- 9.de Mello-Aires M, Giebisch G, Malnic G. Kinetics of potassium transport across single distal tubules of rat kidney. J Physiol. 1973;232:47–70. doi: 10.1113/jphysiol.1973.sp010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meneton P, Loffing J, Warnock DG. Sodium and potassium handling by the aldosterone-sensitive distal nephron: The pivotal role of the distal and connecting tubule. Am J Physiol Renal Physiol. 2004;287:F593–F601. doi: 10.1152/ajprenal.00454.2003. [DOI] [PubMed] [Google Scholar]
- 11.Murakami E, Hiwada K, Kokubu T, Imamura Y. Effect of oral potassium on urinary kallikrein excretion in essential hypertension. Adv Exp Med Biol. 1989;247B:133–137. doi: 10.1007/978-1-4615-9546-5_22. [DOI] [PubMed] [Google Scholar]
- 12.Valdés G, Vio CP, Montero J, Avendaño R. Potassium supplementation lowers blood pressure and increases urinary kallikrein in essential hypertensives. J Hum Hypertens. 1991;5:91–96. [PubMed] [Google Scholar]
- 13.Vío CP, Figueroa CD. Evidence for a stimulatory effect of high potassium diet on renal kallikrein. Kidney Int. 1987;31:1327–1334. doi: 10.1038/ki.1987.146. [DOI] [PubMed] [Google Scholar]
- 14.Barden A, Vandongen R, Beilin LJ. Increases in urinary kallikrein activity and prostanoid synthesis after dietary potassium supplementation. Clin Exp Pharmacol Physiol. 1987;14:565–572. doi: 10.1111/j.1440-1681.1987.tb01875.x. [DOI] [PubMed] [Google Scholar]
- 15.Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature. 1997;389:607–610. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]
- 16.Bailey MA, et al. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of Type II Bartter's syndrome and in adaptation to a high-K diet. Kidney Int. 2006;70:51–59. doi: 10.1038/sj.ki.5000388. [DOI] [PubMed] [Google Scholar]
- 17.Pech V, Zheng W, Pham TD, Verlander JW, Wall SM. Angiotensin II activates H+-ATPase in type A intercalated cells. J Am Soc Nephrol. 2008;19:84–91. doi: 10.1681/ASN.2007030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leviel F, et al. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest. 2010;120:1627–1635. doi: 10.1172/JCI40145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rieg T, et al. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int. 2007;72:566–573. doi: 10.1038/sj.ki.5002369. [DOI] [PubMed] [Google Scholar]
- 20.Gumz ML, Lynch IJ, Greenlee MM, Cain BD, Wingo CS. The renal H+, K+ ATPases: Physiology, regulation, and structure. Am J Physiol Renal Physiol. 2009;298:F12. doi: 10.1152/ajprenal.90723.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura S, Amlal H, Galla JH, Soleimani M. Colonic H+-K+-ATPase is induced and mediates increased HCO3- reabsorption in inner medullary collecting duct in potassium depletion. Kidney Int. 1998;54:1233–1239. doi: 10.1046/j.1523-1755.1998.00105.x. [DOI] [PubMed] [Google Scholar]
- 22.Dherbecourt O, Cheval L, Bloch-Faure M, Meneton P, Doucet A. Molecular identification of Sch28080-sensitive K-ATPase activities in the mouse kidney. Pflugers Arch. 2006;451:769–775. doi: 10.1007/s00424-005-1508-1. [DOI] [PubMed] [Google Scholar]
- 23.Codina J, Delmas-Mata JT, DuBose TD., Jr The alpha-subunit of the colonic H+,K+-ATPase assembles with beta1-Na+,K+-ATPase in kidney and distal colon. J Biol Chem. 1998;273:7894–7899. doi: 10.1074/jbc.273.14.7894. [DOI] [PubMed] [Google Scholar]
- 24.Fujita T, Hayashi I, Kumagai Y, Inamura N, Majima M. K+ loading, but not Na+ loading, and blockade of ATP-sensitive K+ channels augment renal kallikrein secretion. Immunopharmacology. 1999;44:169–175. doi: 10.1016/s0162-3109(99)00088-0. [DOI] [PubMed] [Google Scholar]
- 25.Fujita T, Hayashi I, Kumagai Y, Inamura N, Majima M. Early increases in renal kallikrein secretion on administration of potassium or ATP-sensitive potassium channel blockers in rats. Br J Pharmacol. 1999;128:1275–1283. doi: 10.1038/sj.bjp.0702899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obika LF. Urinary kallikrein excretion during potassium chloride infusion in potassium-adapted rats: effect of amiloride. Clin Sci (Lond) 1989;77:21–27. doi: 10.1042/cs0770021. [DOI] [PubMed] [Google Scholar]
- 27.Giebisch G. Renal potassium channels: An overview. Kidney Int. 1995;48:1004–1009. doi: 10.1038/ki.1995.382. [DOI] [PubMed] [Google Scholar]
- 28.Stanton BA. Characterization of apical and basolateral membrane conductances of rat inner medullary collecting duct. Am J Physiol. 1989;256:F862–F868. doi: 10.1152/ajprenal.1989.256.5.F862. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi I, Fujita T, Majima M, Katori M. A secretory mechanism of renal kallikrein by a high potassium ion; a possible involvement of ATP-sensitive potassium channel. Immunopharmacology. 1999;44:49–55. doi: 10.1016/s0162-3109(99)00110-1. [DOI] [PubMed] [Google Scholar]
- 30.Henquin JC, Lambert AE. Cationic environment and dynamics of insulin secretion. II. Effect of a high concentration of potassium. Diabetes. 1974;23:933–942. doi: 10.2337/diab.23.12.933. [DOI] [PubMed] [Google Scholar]
- 31.Henquin JC, Meissner HP. Opposite effects of tolbutamide and diazoxide on 86Rb+ fluxes and membrane potential in pancreatic B cells. Biochem Pharmacol. 1982;31:1407–1415. doi: 10.1016/0006-2952(82)90036-3. [DOI] [PubMed] [Google Scholar]
- 32.Arrighi I, et al. Altered potassium balance and aldosterone secretion in a mouse model of human congenital long QT syndrome. Proc Natl Acad Sci USA. 2001;98:8792–8797. doi: 10.1073/pnas.141233398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carattino MD, Hughey RP, Kleyman TR. Proteolytic processing of the epithelial sodium channel gamma subunit has a dominant role in channel activation. J Biol Chem. 2008;283:25290–25295. doi: 10.1074/jbc.M803931200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Picard N, et al. Defective ENaC processing and function in tissue kallikrein-deficient mice. J Biol Chem. 2008;283:4602–4611. doi: 10.1074/jbc.M705664200. [DOI] [PubMed] [Google Scholar]
- 35.Tomita K, Pisano JJ, Knepper MA. Control of sodium and potassium transport in the cortical collecting duct of the rat. Effects of bradykinin, vasopressin, and deoxycorticosterone. J Clin Invest. 1985;76:132–136. doi: 10.1172/JCI111935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meneton P, et al. Cardiovascular abnormalities with normal blood pressure in tissue kallikrein-deficient mice. Proc Natl Acad Sci USA. 2001;98:2634–2639. doi: 10.1073/pnas.051619598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Picard N, et al. Tissue kallikrein-deficient mice display a defect in renal tubular calcium absorption. J Am Soc Nephrol. 2005;16:3602–3610. doi: 10.1681/ASN.2004110923. [DOI] [PubMed] [Google Scholar]
- 38.Lee G, et al. Homeostatic responses in the adrenal cortex to the absence of aldosterone in mice. Endocrinology. 2005;146:2650–2656. doi: 10.1210/en.2004-1102. [DOI] [PubMed] [Google Scholar]
- 39.Clarke LL, Ganjam VK, Fichtenbaum B, Hatfield D, Garner HE. Effect of feeding on renin-angiotensin-aldosterone system of the horse. Am J Physiol. 1988;254:R524–R530. doi: 10.1152/ajpregu.1988.254.3.R524. [DOI] [PubMed] [Google Scholar]
- 40.Bönner G, Marin-Grez M. Measurement of kallikrein activity in urine of rats and man using a chromogenic tripeptide substrate. Validation of the amidolytic assay by means of bradykinin radioimmunoassay. J Clin Chem Clin Biochem. 1981;19:165–168. doi: 10.1515/cclm.1981.19.3.165. [DOI] [PubMed] [Google Scholar]
- 41.Burg M, Grantham J, Abramow M, Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966;210:1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- 42.Chambrey R, et al. Genetic ablation of Rhbg in the mouse does not impair renal ammonium excretion. Am J Physiol Renal Physiol. 2005;289:F1281–F1290. doi: 10.1152/ajprenal.00172.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.