Abstract
The right inferior frontal gyrus (rIFG) and the presupplementary motor area (pre-SMA) have been identified with cognitive control—the top-down influence on other brain areas when nonroutine behavior is required. It has been argued that they “inhibit” habitual motor responses when environmental changes mean a different response should be made. However, whether such “inhibition” can be equated with inhibitory physiological interactions has been unclear, as has the areas’ relationship with each other and the anatomical routes by which they influence movement execution. Paired-pulse transcranial magnetic stimulation (ppTMS) was applied over rIFG and primary motor cortex (M1) or over pre-SMA and M1 to measure their interactions, at a subsecond scale, during either inhibition and reprogramming of actions or during routine action selection. Distinct patterns of functional interaction between pre-SMA and M1 and between rIFG and M1 were found that were specific to action reprogramming trials; at a physiological level, direct influences of pre-SMA and rIFG on M1 were predominantly facilitatory and inhibitory, respectively. In a subsequent experiment, it was shown that the rIFG's inhibitory influence was dependent on pre-SMA. A third experiment showed that pre-SMA and rIFG influenced M1 at two time scales. By regressing white matter fractional anisotropy from diffusion-weighted magnetic resonance images against TMS-measured functional connectivity, it was shown that short-latency (6 ms) and longer latency (12 ms) influences were mediated by cortico-cortical and subcortical pathways, respectively, with the latter passing close to the subthalamic nucleus.
Keywords: connectivity, diffusion-weighted imaging, inhibition, transcranial magnetic stimulation, cognitive control
We humans can engage in a complex repertoire of behaviors geared toward often far-removed goals. We have to override reflexive and habitual reactions to orchestrate behavior in accordance with our intentions. The mechanisms that allow us to act in this way are commonly referred to as “cognitive control processes,” and their functions include controlling, and often preventing, lower level or more automatic sensory, memory, and motor operations (1). In the control of action, a network involving the presupplementary motor area (pre-SMA) and right inferior frontal gyrus (rIFG) has been commonly identified as crucial for the adaptation of actions to changes in the environment (2–10), a process we refer to as “action reprogramming” (6). The precise contributions of these regions, however, remain unknown. For example, at a cognitive level, the rIFG has been suggested to be involved in the inhibition of an incorrect motor program (2, 7). However, whether this cognitive inhibition is also reflected at a physiological level remains subject to investigation (8). Moreover, how each individual node of the cortical network exerts its influence and interacts with other nodes is unknown. Some authors have argued that interactions among cortical regions during action reprogramming occurs via direct cortical routes (11, 12), whereas others have argued for the involvement of subcortical routes, particularly via the subthalamic nucleus (STN) (2, 3, 13). However, the relative contributions of these different routes, particularly at the millisecond time scale during which these control processes take place, remain unclear.
In the present study, we use a combination of techniques to characterize the interaction among different cortical regions during action reprogramming and to study the underlying anatomical networks involved. We use paired-pulse transcranial magnetic stimulation (ppTMS) to characterize the interactions between rIFG and pre-SMA with primary motor cortex (M1) during normal action selection and action reprogramming. We then use a combination of ppTMS and diffusion-weighted (DW) MRI to investigate the anatomical networks that support these interactions. Furthermore, we use repetitive TMS (rTMS) (14) to interfere briefly with pre-SMA functioning, allowing us to make some claims about causality and direction of influence during frontal cortical interactions. We show that during action reprogramming, rIFG and pre-SMA have, respectively, inhibitory and facilitatory influences on M1. Different anatomical networks support these interactions. At short latencies, the influence of rIFG and pre-SMA occurs via relatively direct cortical routes, whereas influence at longer latencies occurs via a route through subcortical structures, including STN. Although only rIFG directly inhibited M1, pre-SMA is identified as a crucial node in the network because interfering with its activity leads to a breakdown of rIFG/M1 interactions.
Results
The ppTMS approach makes it possible to measure causal functional interactions among brain areas during different cognitive states. In the present investigation, it was used to measure the causal influence of rIFG or pre-SMA on M1 corticospinal excitability. A test TMS pulse is delivered over M1, and the amplitude of the motor-evoked potential (MEP) in the electromyographic signal recorded from a hand muscle involved in the task [right first dorsal interosseus (FDI)] is measured to quantify M1 excitability. On some trials, the M1 test pulse is preceded by a conditioning pulse applied over rIFG or pre-SMA (ppTMS). The conditioning pulse can modulate the amplitude of the MEP elicited by the test pulse over M1. Changes in M1 excitability attributable to the conditioning pulse can be quantified by calculating the ratio [ppTMS MEP amplitude/single-pulse (sp) TMS MEP amplitude].
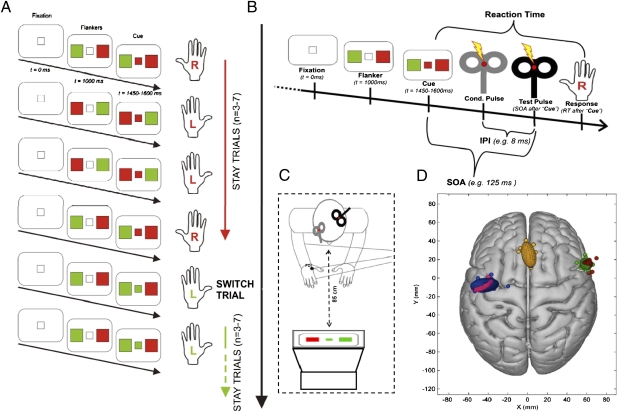
A total of 29 (15 female) right-handed volunteers participated in 1 or more of the 74 TMS experiments using various versions of a task modeled on the paradigm developed by Isoda and Hikosaka (15) (Fig. 1A). This task required participants to either execute a prepared response (“stay trials”) or reprogram the action by inhibiting the prepared response and executing another response (“switch trials”).
Fig. 1.
(A) Behavioral task required participants to respond with the left or right index finger in response to visual stimuli presented on a computer screen. Each trial started with the presentation of two peripheral flankers (red and green, sides random). A center cue taking the color of one of the flankers appeared 450–600 ms later. Participants had to respond with the finger of the hand on the side of the congruent flanker color. The center cue took the same color for trains of three to seven trials. Hence, on each trial, participants could prepare a movement based on their knowledge of the identity of the center cue on the previous trial. However, after taking the same color for a series of trials, the center cue color changed. This manipulation meant that there were two types of trials: stay trials, in which the fixation square turned into the same color as in the previous trial, thus allowing the participants to exert the already prepared response, and switch trials, in which the fixation square turned into a different color from that in the previous trial, thus requiring participants to inhibit an already prepared response and to reprogram their action plans. (B) Time course of the rIFG/M1 interaction experiments. Pulses were delivered through two coils. The conditioning (Cond.) coil (gray) was placed over rIFG, and the test coil was placed over left M1. ppTMS was applied at different SOAs after center cue color onset in the “rIFG/M1 SOA experiment” or at a single SOA but with different IPLs in the rIFG/M1 IPL experiment and pre-SMA/M1 IPL experiment. (C) Schematic representation of the setup, with the gray conditioning coil placed over rIFG and black test coil placed over the left M1. (D) Coil placements on Montreal Neurological Institute (MNI) brain. Circular symbols indicate individual participants’ stimulation locations. Ellipsoids represent 95% confidence limits of the mean group stimulation location. Red and blue ellipsoids show coil location for the “rIFG/M1 interaction experiment.” Green, yellow, and pink ellipsoids show locations for the rIFG/M1 IPL experiment and pre-SMA/M1 IPL experiment.
rIFG/M1 Interaction Experiments.
The first experiments investigated time courses of functional interactions between rIFG and M1 during switch and stay trials in two separate experimental sessions (switch and stay experiments). The exact same behavioral task and experimental setup were used in the switch and stay experiments. However, TMS was delivered mostly on switch trials in the switch experiment and on stay trials in the stay experiment (concern about the total number of TMS pulses received by a participant during a single session and the length of a session precluded combining switch and stay experiments into a single session; SI Text Section 3). In both experimental sessions, TMS was delivered at 75, 125, or 175 ms after center cue color onset to investigate time courses of rIFG/M1 interactions during action execution and action reprogramming.
ANOVAs of median reaction times (RTs) on correct trials and of error rates with “trial type” (switch vs. stay) as a within-subjects factor and “session” (switch vs. stay) as a between-subjects factor showed a main effect of trial type [F(1,18) = 64.493; P < 0.001 for RTs and F(1,18) = 27.135; P < 0.001 for error rates] but no main effect of session and no interaction between session and trial type (P > 0.25). Post hoc paired-samples t tests confirmed that subjects were significantly slower [395.8 vs. 290.6 ms, t(19) = 9.16; P < 0.001] and made significantly more errors [23.24% vs. 2.29%, t(19) = 5.89; P < 0.001] on switch trials in comparison to stay trials. This confirmed the effectiveness of the task manipulation. There was no effect of simply changing response hand from one trial to the next in the absence of reprogramming and change in central cue color (Fig. 1A and SI Text Section 3).
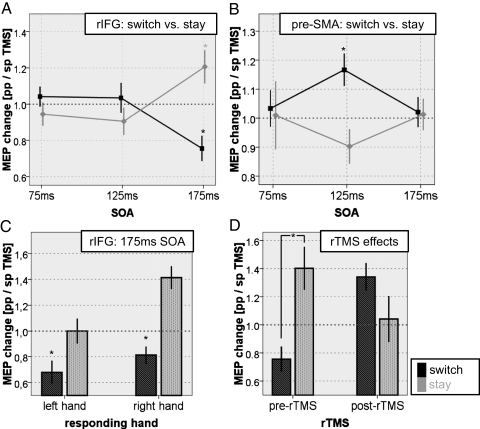
To infer whether rIFG/M1 interactions differed depending on if the participants were just executing the action they had prepared or if they had to reprogram their action plan and inhibit the prepared but incorrect response, an ANOVA on ppTMS MEP/spMS MEP ratios with the within-subjects factors “hand” (left vs. right) and “stimulus-onset asynchrony” (SOA) (75, 125, and 175 ms) and with the between-subjects factor “condition” (switch vs. stay) was conducted and revealed a significant condition × SOA interaction [F(2,36) = 17.015; P < 0.001] and a significant condition × hand × SOA interaction [F(2,36) = 3.386; P = 0.045), indicating that the time course of rIFG/M1 interactions differed between switch and stay trials in a manner that depended on which hand was to respond (note that the M1 TMS coil was placed over the left M1, which has the primary role in the making of right hand movements). Independent sample t tests revealed a significant difference between switch and stay trials for left hand response trials at 175-ms SOA [t(18) = −2.438; P = 0.025; switch < stay] and for right hand response trials at 175-ms SOA [t(18) = −3.472; P = 0.003; switch < stay] (Fig. 2A). One-sample two-tailed t tests of the MEP ratios against baseline (MEP ratio of 1.0) showed that the right hand FDI MEPs were significantly inhibited in left hand response switch trials [trials on which subjects prepared a right hand response but had to switch to making a left hand response, t(9) = −3.563; P = 0.006)] and significantly facilitated in right hand response stay trials [t(9) = 3.189; P = 0.011)] at 175 ms after center cue color onset. Although the effects were clearest for the right hand, contralateral to the stimulated M1, there was evidence of inhibition being exerted even when subjects were switching away from a prepared left hand movement (SI Text Section 3). In summary, rIFG stimulation during action reprogramming unveils an inhibitory influence of rIFG on the M1 that controls the hand that must be stopped. During normal action selection on stay trials, however, rIFG exerts a facilitatory influence over the M1 that corresponds to the responding hand.
Fig. 2.
Time course of frontal/M1 interactions. ppTMS and spTMS MEP ratios are plotted for each SOA between central fixation color change and TMS delivery. (A) MEP ratios for switch (gray) and stay (black) trials shown for rIFG/M1 interactions pooled over both hands. (B) Similar information for pre-SMA data from Mars et al. (12), adapted with permission of the Society for Neuroscience. (C) rIFG/M1 interactions separately for trials in which right and left hand responses were executed. Asterisks indicate significant inhibition compared with the single-pulse baseline. (D) rIFG/M1 interactions before and after 15 min of 1-Hz TMS over pre-SMA.
The exact same task and parallel procedure were used by Mars et al. (12) to investigate interactions between pre-SMA and M1 during action reprogramming. To compare influences exerted by pre-SMA and rIFG on M1 excitability during action reprogramming, we conducted an ANOVA on FDI switch trial MEP ratios from the current rIFG/M1 experiment and the previous pre-SMA/M1 experiment (12) with the within-subjects factors hand (left vs. right) and SOA (75, 125, and 175 ms) and the between-subjects factor “area” (pre-SMA vs. rIFG). The ANOVA revealed a significant between-subjects effect of area [F(1,19) = 6.410; P = 0.02] and a significant within-subjects effect of SOA [F(2,38) = 8.049; P = 0.002], a significant area × hand interaction [F(1,19) = 4.640; P = 0.044], and an area × SOA interaction [F(2,38) = 3.398; P = 0.047]. This indicates that although both areas, pre-SMA and rIFG, seem to exert an influence on M1 excitability during action reprogramming, the influence differs in time course and character (Fig. 2 A and B). Whereas pre-SMA, on switch trials, facilitates the unexpected and unprepared but correct response 125 ms after instruction cue onset, rIFG inhibits the prematurely activated but incorrect response 175 ms after cue onset (SI Text Section 2).
Effects at Different IPLs and Associated White Matter Pathways.
The aim of the next pair of experiments (performed on a single group of 16 participants in a counterbalanced order) was to investigate interactions between rIFG and M1 [“rIFG/M1 interpulse latency (IPL) experiment”] and interactions between pre-SMA and M1 (“pre-SMA/M1 IPL experiment”) at different latencies. Although the earlier experiments had investigated pre-SMA/M1 and rIFG/M1 interactions at different time points following visual cue onset, the interval between the conditioning and test pulses was always fixed. By contrast, in the current experiments, TMS was delivered at the postvisual cue time point of maximum interaction (125 ms for pre-SMA and 175 ms for rIFG) with variable interpulse latencies (IPLs = 3, 6, 9, 12, and 18 ms). We hypothesized that different pathways might mediate interactions between pre-SMA and M1 and between rIFG and M1 occurring at different IPLs. To relate patterns of functional connectivity to the anatomical white matter tracts by which they were mediated, we correlated individual differences in ppTMS effect sizes with individual differences in diffusion-weighted MRI (DW-MRI)–derived fractional anisotropy (FA) (SI Text Sections 5 and 6). Hence, the second aim of these experiments was to localize pathways mediating interactions between pre-SMA/M1 and rIFG/M1 connectivity during action reprogramming within the brain's white matter.
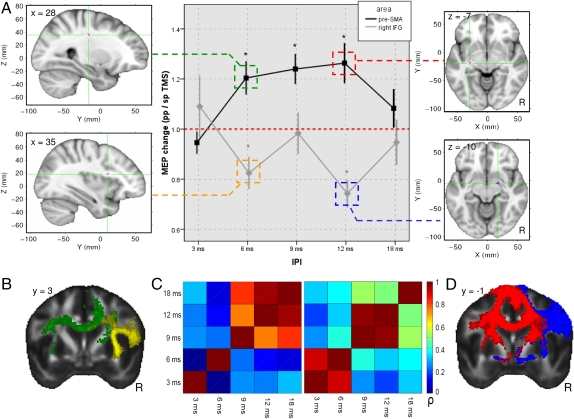
To test whether pre-SMA and M1 functional connectivity differed significantly from functional connectivity between rIFG and M1, we conducted an ANOVA with the within-subjects contrasts of hand (left vs. right), “IPL” (3, 6, 9, 12, and 18 ms), and area (pre-SMA vs. rIFG). The ANOVA showed a main effect of area [F(1,15) = 10.233; P = 0.006] and significant area × IPL [F(4,60) = 6.166; P < 0.001] and area × IPL × hand [F(4,60) = 3.208; P = 0.018] interactions. This suggests that both areas differently influence M1 excitability during action reprogramming (Fig. 3A). To investigate rIFG/M1 interactions further during action reprogramming at different IPLs, we conducted an ANOVA on the data from this session alone. We found a significant effect of IPL [F(4,64) = 6.160; P < 0.001]. Post hoc two-tailed one-sample t tests against baseline revealed a significant inhibition of M1 excitability attributable to rIFG conditioning pulses in right hand responses with an IPL of 6 ms [t(15) = −2.740; P = 0.015], and 12 ms [t(15) = −4.740; P < 0.00)] and in left hand responses with an IPL of 6 ms [t(15) = −2.873; P = 0.011] and 9 ms [t(15) = −3.071; P = 0.007]. A similar analysis of pre-SMA/M1 data identified a significant effect of hand [F(1,16) = 5.748; P = 0.029] and interaction of hand × IPL [F(4,64) = 3.063; P = 0.023]. Post hoc two-tailed one-sample t tests against baseline revealed significant facilitation of M1 excitability attributable to pre-SMA conditioning pulses in right hand responses with IPLs of 6 ms [t(15) = 3.077; P = 0.007], 9 ms [t(15) = 3.996; P = 0.001], and 12 ms [t(15) = 3.286; P = 0.005]. An additional analysis confirmed that conditioning pulse effects differed at short and long IPLs. The matrix of cross-correlation values for conditioning pulse effects was calculated, and it was clear that there was no correlation between the size of the conditioning pulse effect at short latencies (3 and 6 ms) and longer latencies (9, 12, and 18 ms) (Fig. 3C).
Fig. 3.
(A) Pre-SMA/M1 (black) and rIFG/M1 (gray) MEP ratios during action reprogramming at different IPLs, plotted for right hand responses only (asterisks indicate significant modulation from the single-pulse baseline). Clusters showing significant correlations between individual FA and MEP effect sizes are displayed on the MNI brain [pre-SMA: green (6 ms) and red (12 ms); rIFG: yellow (6 ms) and blue (12 ms)]. (B) Comparison of two connectivity networks derived from the pre-SMA (green) and the rIFG (yellow) at 6-ms IPI, showing dorsomedial cortical white matter. (C) Cross-correlation matrices for pre-SMA/M1 (Left) and rIFG/M1 (Right) functional connectivity effects at different IPLs. ppTMS and spTMS MEP effects are correlated across different IPLs and plotted with their Pearson correlation coefficient. The 6- and 12-ms effects are sufficiently uncorrelated so as to be separate regressors in a multiple regression model. (D) Comparison of two composite connectivity networks derived from pre-SMA (red) and rIFG (blue) at 12-ms IPL, showing white matter in the vicinity of the global pallidus and STN.
To assess whether there was a relationship between these functional interactions and white matter microstructure across participants, we used tract-based spatial statistics (TBSS) to investigate local correlations between FA and ppTMS/spTMS MEP ratio effect size (16). We used MEP ratios with 6- and 12-ms IPLs from right hand response trials in the pre-SMA/M1 IPL experiment and the rIFG/M1 IPL experiment. Therefore, we had four different and uncorrelated regressors in the TBSS multiple regression analysis. Effects were reported as being significant at a one-tailed statistical threshold of P < 0.001 (uncorrected) and a cluster size of >10 voxels (16). We found 2 clusters significantly correlated with the TMS effect in the pre-SMA/M1 experiment at an IPL of 6 ms. These clusters lay in dorsal white matter likely to connect pre-SMA, premotor, motor, and parietal areas. At an IPL of 12 ms, we found 6 clusters significantly correlated with pre-SMA/M1 interactions. Again, some of these clusters lay in white matter near pre-SMA, premotor, and motor areas, but others were now seen in the white matter surrounding the basal ganglia. We found 3 clusters significantly correlated with the TMS effect in the rIFG/M1 experiment at an IPL of 6 ms. These clusters were found in the white matter underlying anterior and posterior rIFG and motor cortex. At an IPL of 12 ms, we found 14 clusters significantly correlated with rIFG/M1 interactions. Once again, these clusters were found in white matter underlying rIFG and adjacent ventrolateral prefrontal cortex, but others were also now found in the vicinity of STN. Additional clusters were also seen near pre-SMA and in the superior longitudinal fascicle (Fig. 3; full list of regions is found in SI Text).
To elucidate the white matter tracts in which local clusters of FA correlation were located and the gray matter target areas to which they most likely projected, we carried out a second stage of DW-MRI analysis and used the respective areas of conditioning pulse stimulation as seed masks and the clusters as waypoints for multifiber probabilistic tractography (17). This allowed us to estimate the areas that had a high probability of interconnection, via the area of correlated FA, with the area of conditioning pulse application, either pre-SMA or rIFG. We used a mask of right pre-SMA as a seed mask for tractography via the clusters significantly correlated with TMS effects in the pre-SMA/M1 IPL experiment and a rIFG seed mask for tractography via the clusters significantly correlated with TMS effects in the rIFG/M1 IPL experiment. Clusters significantly correlated with TMS effects in the pre-SMA/M1 experiment with an IPL of 6 ms generated tracts within dorsomedial frontal and parietal white matter connecting pre-SMA and premotor areas with motor and parietal areas. However, tracts derived from clusters significantly correlated with TMS effects in the pre-SMA/M1 experiment with an IPL of 12 ms connected pre-SMA with ventral and dorsal premotor areas; areas in the rIFG, M1, and parietal areas; and subcortical areas in the vicinity of STN. Similarly, clusters significantly correlated with TMS effects in the rIFG/M1 experiment with an IPL of 6 ms generated tracts connecting rIFG with M1 and temporoparietal areas, whereas tracts derived from clusters significantly correlated with TMS effects at an IPL of 12 ms connected ventral premotor cortex with more anterior areas in rIFG, adjacent ventrolateral prefrontal cortex, medial frontal cortex and pre-SMA; M1 and areas in the parietal lobe; and STN.
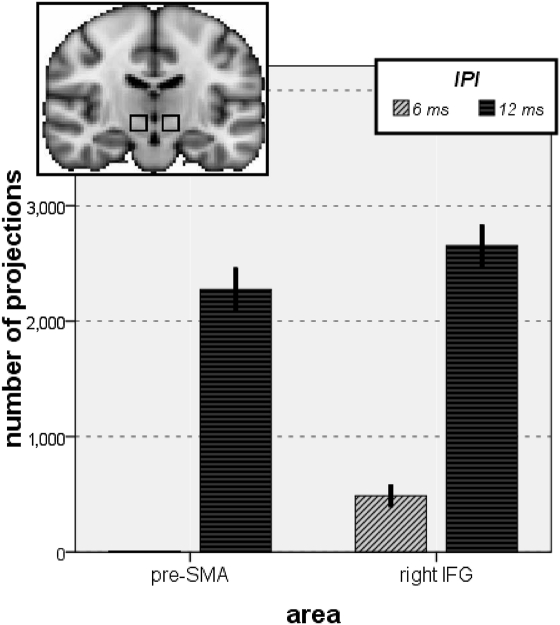
The evidence that longer latency pathways might be mediated by the basal ganglia, including STN, is important because it has been argued that STN is a particularly critical part of the circuit for action inhibition (2, 3) and that a pathway running from the striatum via the globus pallidus and STN might be critical for learning when to withhold actions that are unrelated to reward (18). Because tractography only provides probabilistic evidence about the existence of interconnections among brain regions, it might be argued that only limited importance can be ascribed to the evidence that any single FA region is likely to be connected to STN. We therefore quantified the number of tracts derived from all FA clusters correlated with the rIFG and pre-SMA 12-ms IPL effects that reached a region of interest (ROI) for STN, defined as a box sized 10 × 10 × 10 mm, centered at MNI coordinates (x = ±10, y = −15, z = −5) (2) and compared them with the numbers of tracts derived from FA clusters correlated with rIFG and pre-SMA 6-ms IPL effects (Fig. 4); an ANOVA on the tractography-derived voxels within the ROI with the within-subjects contrast of IPL (6 ms, 12 ms) and area (rIFG, pre-SMA) revealed a significant effect of IPL [F(1,15) = 252.084; P < 0.001] and of area [F(1,15) = 12.108; P = 0.003]. To investigate further whether there was a significantly greater chance of tracts ending up in STN at 6- or 12-ms IPLs, we performed paired-samples t tests. These confirmed that tractography derived from rIFG 12-ms IPL clusters reached the STN ROI significantly more often than tractography derived from rIFG 6-ms IPL clusters [t(15) = 10.236; P < 0.001] and that tractography derived from pre-SMA 12-ms IPL clusters reached the STN ROI significantly more often than tractography derived from pre-SMA 6-ms IPL clusters [t(15) = 11.767; P < 0.001]. The observed differences were still significant after correcting for the number of clusters revealed in each condition.
Fig. 4.
Bars show the number connections from probabilistic diffusion tractography that passed through the STN ROIs (Upper). Tracts were derived from clusters of significant correlation between FA and TMS effect size in the IPL experiments (pre-SMA, Left; rIFG, Right) and the two different IPLs (light gray = 6 ms, dark gray = 12 ms). It can be seen that tracts were significantly more likely to pass through or near STN at the 12-ms IPL only.
Influence of Pre-SMA Interference on rIFG/M1 Interactions.
The first experiments, which manipulated the time between visual cue presentation and TMS, demonstrated that pre-SMA/M1 interactions were modulated at 125 ms, which is before the time (175 ms) at which rIFG/M1 interactions were initially significantly modulated. The aim of the final experiment was to investigate whether functional interactions between rIFG and M1 depend on activity in pre-SMA. Activity in cortical areas can be decreased by a 15-min period of 1-Hz rTMS (14). We compared functional connectivity between rIFG and M1 at an SOA of 175 ms after cue onset before and after such a transient disruption of pre-SMA. An ANOVA on the pre-SMA rTMS data with “pre/post” and trial type (switch, stay) as within-subjects factors shows a significant trial type × pre/post interaction [F(1,7) = 11.918; P = 0.011]. Paired-samples t tests among MEP ratios revealed a significant difference between rIFG/M1 interactions on switch and stay trials [t(7) = −4.134; P = 0.004; stay > switch] only before rTMS application. This difference disappeared or, if anything, was even reversed after 15 min of 1-Hz rTMS over pre-SMA (switch > stay; P > 0.15). These results suggest that although rTMS over pre-SMA did not significantly change behavioral performance (SI Text), it did change the pattern of interactions between rIFG and M1. Identical rTMS over a parietal control site (electrode position Pz) did not induce any change in rIFG/M1 functional connectivity (SI Text).
Discussion
In the current set of studies, we investigated the interactions among regions of the human brain during a behavior that is one of the hallmarks of cognitive control, the adaptation of a planned movement to suit a change in the environment. We focused first on how regions in the frontal lobe influence M1 when a prepared action must be inhibited and an alternative selected. The rIFG has a preeminent role in a network for action inhibition because it inhibits M1 corticospinal activity during action reprogramming 175 ms after a visual cue, indicating that a change in action is needed (SI Text Section 2). It has previously been suggested that rIFG might exert its influence over other brain areas by inhibiting physiological activity (8), but this has been difficult to test empirically. The current results support this claim. At 175 ms, the timing of rIFG's effect in the current experiments is reminiscent of the timing of changes in synchrony recorded in this area during action inhibition (8). Despite the evidence that rIFG induces inhibition at a physiological level when cognitive control is needed, it should also be emphasized that the part of rIFG we investigated here (SI Text Section 1) exerted an excitatory influence on M1 corticospinal activity during routine action selection on “stay” trials. That rIFG might have such a facilitatory influence over action selection when action selection proceeds in a routine fashion has received less emphasis in neuroimaging experiments.
In contrast, pre-SMA's influence is earlier, at 125 ms, and its direct effect is to facilitate the M1 associated with the correct action on switch trials (12). There is evidence that pre-SMA plays a critical role in inhibiting actions in stop signal RT tasks (19, 20) at a behavioral level, but this was previously difficult to reconcile with an absence of evidence of direct physiological inhibition of M1 (12). The results of the final rTMS experiments reported here allow us to reconcile these positions. Consistent with previous suggestions that there is an increase in functional connectivity between rIFG and pre-SMA during action inhibition (5), we investigated rIFG/M1 interactions before and after a transient interruption of pre-SMA functioning. Interruption of pre-SMA rather than rIFG was chosen because the earlier paired-pulse experiments showed that pre-SMA effects precede those of rIFG. Following pre-SMA inactivation, the rIFG/M1 interactions broke down, indicating a role for pre-SMA in mediating rIFG/M1 interactions. The current findings thus suggest a route by which pre-SMA activity may result in inhibition of M1. Other researchers have also argued that medial frontal cortical activity is a determinant of lateral frontal activity when cognitive control must be asserted (11, 21).
It has been repeatedly suggested that interactions between frontal and motor areas during action inhibition involve a subcortical route (2, 3, 22). However, the IPLs of 6 or 8 ms used in previous ppTMS studies made it unlikely that the effects were mediated by such a route (12, 23). To investigate this issue further, the present experiment assessed pre-SMA/M1 and rIFG/M1 interactions using a range of IPLs and looked for correlations between TMS effect sizes and white matter integrity. Not only did we look at the location of regions of white matter correlation, but we used tractography to examine whether the regions were likely to be interconnected with STN. At short latencies, there was evidence only for cortical route mediation of pre-SMA/M1 and rIFG/M1 interactions. However, at longer latencies of 12 ms, additional routes that include STN are taken. These results provide direct evidence that there are multiple routes involved in frontal/M1 interactions during action inhibition: a subcortical route being used at longer latencies and a direct cortical route.
Together, these results provide direct evidence concerning the existence, timing, physiological nature, and causal direction of interactions in a network of frontal cortical regions during a key component of cognitive control: action reprogramming. In addition, the study provided information about two major sets of anatomical pathways that might mediate such interactions.
Methods
Participants and Experimental Setup.
Twenty-two participants (10 female) participated in the 28 rIFG/M1 experiments, 16 (10 female) in the 32 IPL and DW-MRI experiments and 11 (3 female) in the 14 rTMS experiments. All participants had no personal or familial history of neurological or psychiatric disease, were right handed, gave written informed consent, were screened for adverse reactions to TMS and risk factors by means of a safety questionnaire, and received monetary compensation for their participation. During the TMS experiments, participants were seated in a darkened room and wore a tight-fitting EEG cap, on which TMS sites were marked, and earplugs to protect against TMS noise. A chin rest was used to minimize head movement. The experimental procedures were approved by local research ethics committees, and the experiments were conducted in accordance with the Declaration of Helsinki. Experiments were controlled using a personal computer running Windows XP (Microsoft Corporation) and Presentation 0.53 (Neurobehavioral Systems, Inc.).
TMS Parameters.
In all rIFG/M1 ppTMS experiments, TMS pulses were delivered on 30 of 180 trials per block. There were two types of TMS trials. On half of the TMS trials, spTMS trials, a single TMS “test pulse” was delivered over the left M1 representation of the right FDI. The intensity of this TMS test pulse was such that an MEP of 1–1.5 mV was evoked in the relaxed contralateral FDI. On the other half of the trials, ppTMS trials, the M1 test pulse was preceded by a “conditioning pulse” over the rIFG. The intensity of the preceding conditioning pulse was set at 110% of the resting motor threshold (RMT, defined as the minimum stimulator output required to elicit a 50-μV MEP in 5 of 10 trials) of the left FDI muscle (right M1), and the IPL was 8 ms (23, 24). Several studies have used similar interhemispheric TMS coil configuration and conditioning and test pulse intensities, because the size of the head precludes placing two TMS coils over frontal and M1 areas in the same hemisphere in some participants and an interhemispheric configuration produces similar results, albeit with a slightly higher intensity conditioning pulse, to an intrahemispheric configuration (16, 23). We hypothesized that the preceding conditioning pulse would change the MEP amplitude elicited by the test pulse, depending on the type of the trial (switch vs. stay trial) and the time after center cue color onset (75, 125, or 175 ms) (Fig. 1B). TMS pulses were delivered through two 50-mm diameter figure-of-eight coils directly connected to two high-power Magstim 200 MonoPulse machines (The Magstim Company) (Fig. 1C). The location for the conditioning coil was determined using an MRI-aligned frameless stereotaxic neuronavigation system (Brainsight; Rogue Research, Inc.). When testing the influence of pre-SMA interference on rIFG/M1 interactions, 15 min of 1-Hz rTMS [900 pulses at 90% RMT through a 70-mm diameter figure-of-eight coil connected to a biphasic Magstim Super Rapid stimulator (The Magstim Company)] was applied over pre-SMA. PpTMS was used to investigate functional connectivity between rIFG and M1 at 175-ms SOA and with 8-ms IPL both at baseline and after the rTMS (the order of baseline and post-rTMS testing sessions was counterbalanced across subjects). A similar rTMS protocol was used over Pz in a control experiment. These procedures were repeated for all subsequent TMS experiments except for details described in the relevant sections.
DW-MRI and White Matter Pathways.
DW-MRI data were acquired for all participants using a 3.0-T Siemens Trio MR scanner. White matter FA values for each subject were calculated and regressed against each subject's MEP ratio in the rIFG/M1 IPL experiment and pre-SMA/M1 IPL experiment for right hand responses with 6- and 12-ms IPLs in a four-regressor multiple regression analysis (additional analyses are found in SI Text).
Supplementary Material
Acknowledgments
This work was supported by the German National Academic Foundation (to F.-X.N.), a Marie Curie Intra-European Fellowship within the Sixth European Community Framework Programme (to R.B.M.), a Royal Society International Incoming Short Visits grant (to E.O. and M.F.S.R.), a Bourse d'excellence Wallonie-Bruxelles International (to E.O.), and the Medical Research Council of the United Kingdom (to R.B.M. and M.F.S.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000674107/-/DCSupplemental.
References
- 1.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 2.Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: Role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dosenbach NU, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duann JR, Ide JS, Luo X, Li CS. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci. 2009;29:10171–10179. doi: 10.1523/JNEUROSCI.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mars RB, Piekema C, Coles MG, Hulstijn W, Toni I. On the programming and reprogramming of actions. Cereb Cortex. 2007;17:2972–2979. doi: 10.1093/cercor/bhm022. [DOI] [PubMed] [Google Scholar]
- 7.Forstmann BU, et al. Function and structure of the right inferior frontal cortex predict individual differences in response inhibition: A model-based approach. J Neurosci. 2008;28:9790–9796. doi: 10.1523/JNEUROSCI.1465-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swann N, et al. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J Neurosci. 2009;29:12675–12685. doi: 10.1523/JNEUROSCI.3359-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brass M, Haggard P. To do or not to do: The neural signature of self-control. J Neurosci. 2007;27:9141–9145. doi: 10.1523/JNEUROSCI.0924-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers CD, et al. Executive “brake failure” following deactivation of human frontal lobe. J Cogn Neurosci. 2006;18:444–455. doi: 10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 12.Mars RB, et al. Short-latency influence of medial frontal cortex on primary motor cortex during action selection under conflict. J Neurosci. 2009;29:6926–6931. doi: 10.1523/JNEUROSCI.1396-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res. 2002;43:111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 14.Walsh V, Cowey A. Transcranial magnetic stimulation and cognitive neuroscience. Nat Rev Neurosci. 2000;1:73–79. doi: 10.1038/35036239. [DOI] [PubMed] [Google Scholar]
- 15.Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nat Neurosci. 2007;10:240–248. doi: 10.1038/nn1830. [DOI] [PubMed] [Google Scholar]
- 16.Boorman ED, O'Shea J, Sebastian C, Rushworth MF, Johansen-Berg H. Individual differences in white-matter microstructure reflect variation in functional connectivity during choice. Curr Biol. 2007;17:1426–1431. doi: 10.1016/j.cub.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 17.Behrens TE, Johansen-Berg H, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank MJ. Hold your horses: A dynamic computational role for the subthalamic nucleus in decision making. Neural Netw. 2006;19:1120–1136. doi: 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Chen CY, Muggleton NG, Tzeng OJ, Hung DL, Juan CH. Control of prepotent responses by the superior medial frontal cortex. NeuroImage. 2009;44:537–545. doi: 10.1016/j.neuroimage.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Nachev P, Wydell H, O'Neill K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. NeuroImage. 2007;36(Suppl 2):T155–T163. doi: 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci. 2009;12:939–945. doi: 10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- 22.Isoda M, Hikosaka O. Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J Neurosci. 2008;28:7209–7218. doi: 10.1523/JNEUROSCI.0487-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buch ER, Mars RB, Boorman ED, Rushworth MF. A network centered on ventral premotor cortex exerts both facilitatory and inhibitory control over primary motor cortex during action reprogramming. J Neurosci. 2010;30:1395–1401. doi: 10.1523/JNEUROSCI.4882-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davare M, Lemon R, Olivier E. Selective modulation of interactions between ventral premotor cortex and primary motor cortex during precision grasping in humans. J Physiol. 2008;586:2735–2742. doi: 10.1113/jphysiol.2008.152603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.