Abstract
During normal development and in disease, cohesive tissues undergo rearrangements that require integration of signals from cell adhesions to neighboring cells and to the extracellular matrix (ECM). How a range of cell behaviors is coordinated by these different adhesion complexes is unknown. To analyze epithelial cell motile behavior in response to combinations of cell–ECM and cell–cell adhesion cues, we took a reductionist approach at the single-cell scale by using unique, functionalized micropatterned surfaces comprising alternating stripes of ECM (collagenIV) and adjustable amounts of E-cadherin-Fc (EcadFc). On these surfaces, individual cells spatially segregated integrin- and cadherin-based complexes between collagenIV and EcadFc surfaces, respectively. Cell migration required collagenIV and did not occur on surfaces functionalized with only EcadFc. However, E-cadherin adhesion dampened lamellipodia activity on both collagenIV and EcadFc surfaces and biased the direction of cell migration without affecting the migration rate, all in an EcadFc concentration-dependent manner. Traction force microscopy showed that spatial confinement of integrin-based adhesions to collagenIV stripes induced anisotropic cell traction on collagenIV and migration directional bias. Selective depletion of different pools of αE-catenin, an E-cadherin and actin binding protein, identified a membrane-associated pool required for E-cadherin–mediated adhesion and down-regulation of lamellipodia activity and a cytosolic pool that down-regulated the migration rate in an E-cadherin adhesion-independent manner. These results demonstrate that there is crosstalk between E-cadherin– and integrin-based adhesion complexes and that E-cadherin regulates lamellipodia activity and cell migration directionality, but not cell migration rate.
Keywords: cell migration, cell–cell adhesion, alpha-E-catenin, micropattern, traction forces
During development, cohesive tissues exhibit extensive rearrangements that range from en masse migration, such as in wound healing (1), to complex local cell rearrangements, such as cell intercalation (2). In extreme cases in development (3) and in diseases such as metastatic cancers (4), tissue cohesion is lost and single-cell migration enabled, which results in cells populating distant sites. These morphogenetic processes reveal the importance of a fine coregulation, or crosstalk, between tissue cohesion (cadherin-based cell–cell adhesion) and cell migration [integrin-based extracellular matrix (ECM) adhesion] in the maintenance of tissue integrity and function.
Interest in the crosstalk between cell–cell adhesion and cell migration dates back to the pioneering studies of Abercrombie and Heaysman in the 1950s (5, 6) and even earlier (7). Abercrombie coined the term “contact inhibition” to describe how cell–cell interactions between fibroblasts initially inhibited and then redirected their migration. Whether cell–cell contact inhibition of cell migration results from cell–cell contact-dependent spatial redistribution or down-regulation of the cell migration machinery, or both remains unknown.
A major component of intercellular adhesion in epithelia is the E-cadherin/catenin complex (8), through which control of the actin cytoskeleton machinery is an important, albeit poorly understood, determinant of tissue morphogenesis (9). ECM-based cell migration results from the transformation of actomyosin cytoskeleton activity into cell translocation by force transmission to the ECM through integrin-based Focal Adhesions (FAs) (10). Crosstalk between intercellular adhesion and cell migration is suggested from observations of spatiotemporal regulation of lamellipodia activity upon cell–cell contact (11) and redistribution of FAs away from cell–cell contacts during initial cell–cell adhesion (12). However, most of our knowledge about ECM-based cell migration comes from studies of individual cells. It is not known how cellular mechanisms involved in individual cell migration are affected by intercellular adhesion and whether integrin–ECM adhesion is the only mechanism that supports cell migration during cell rearrangements in multicellular sheets. This limited knowledge is due in great part to the difficulty in experimentally controlling two different adhesive environments and dissecting their individual and ensemble effects on cell motile behavior in a multicellular setting.
To address these problems, we used unique surface functionalization to expose single Madin–Darby Canine Kidney (MDCK) epithelial cells to alternating stripes of ECM (collagenIV) and either a control surface (PEG, Fc) or the functional extracellular domain of the primary epithelial cell–cell adhesion protein E-cadherin tagged with the human Fc fragment [EcadFc (13)] (Fig. S1A). By varying EcadFc surface density and choosing an appropriate striped geometry, we could monitor the motile behavior of individual cells as a function of the combinations of these different adhesion cues. The results reveal different roles for E-cadherin– and integrin-based adhesion in controlling membrane dynamics, cell migration rate, and directional migration bias.
Results
MDCK cells are well-characterized normal epithelial cells that recapitulate in culture the structures and functions of simple (single-layered) epithelial tissues, exhibit contact inhibition of proliferation, and carry out apical-basal polarized transport. Cell–cell adhesion is mediated principally by cadherins, which form Ca2+-dependent trans-interactions between opposed extracellular domains on adjacent cells and bind a cytoplasmic complex of β-catenin and α-catenin (14). Adhesion of E-cadherin–expressing MDCK epithelial cells to surfaces functionalized with EcadFc mimics this interaction, because the bridging chemistry correctly orients the N terminus of the E-cadherin extracellular domain outward while still allowing rotation and short-range lateral clustering of the protein (13, 15).
To simultaneously mimic cell binding to ECM and other cells, we used unique, functionalized micropatterned surfaces comprised of alternating stripes of ECM (collagenIV) and adjustable amounts of EcadFc. Recruitment of cellular proteins to different stripes was monitored with GFP-tagged proteins that had been previously well characterized (8, 16). On collagenIV:EcadFc surfaces, cellular E-cadherin-GFP (EcadGFP) was preferentially recruited to EcadFc stripes in an EcadFc concentration-dependent manner, and not to adjacent collagenIV stripes or combinations of either collagenIV:PEG or collagenIV:Fc (Fig. 1 A and B). β-Catenin-GFP and αE-catenin-GFP were also preferentially recruited to EcadFc stripes (Fig. 1C), and F-actin [labeled with UtrCH-GFP (17)] organized into thin radial bundles on EcadFc and into thick bundles parallel to the cell edge abutting the collagenIV stripes (Fig. 1D). Fluorescence recovery after photobleaching of GFP-tagged proteins over EcadFc surfaces showed that the turnover rates of E-cadherin, β-catenin, αE-catenin, and F-actin (Fig. 1E) were within ranges observed at normal MDCK cell–cell junctions (8, 18).
Fig. 1.
(A) EcadGFP-expressing MDCK cells on collagenIV:EcadFc, collagenIV:Fc, or collagenIV:PEG (the collagenIV stripe is represented in red, and the opposing stripe in black) seen in widefield epifluorescence. (B) Left axis: EcadGFP recruitment to the stripe opposing collagenIV as a function of the EcadFc/Fc ratio (or PEG). IE, IC are the fluorescence intensities of EcadGFP on EcadFc/Fc (or PEG stripes) and collagenIV stripes, respectively. Right axis: cell spread area on EcadFc/Fc (EcadFc %) or PEG, and collagenIV patterns. SE, SC are cell surface areas on EcadFc/Fc (or PEG) stripes and collagenIV stripes, respectively. A positive value denotes a larger area spread on noncollagenIV stripes than on collagenIV stripes. Data show mean ± SEM; line is a smooth fit to guide the eye. n = 12 (PEG), 32 (Fc), 14 (EcadFc 25%), 13 (EcadFc 33%), 21 (EcadFc 50%), and 44 (EcadFc 100%) for Ecad-GFP recruitment. n = 10 (PEG), 25 (Fc), 11 (EcadFc 25%), 9 (EcadFc 33%), 15 (EcadFc 50%), and 42 (EcadFc 100%) for contact area. *P < 0.05, **P < 0.01, ***P < 0.001, with respect to Fc data; Mann–Whitney test (two-tailed). (C) β-Catenin-GFP and α-E-catenin-GFP–expressing cells on collagenIV:EcadFc, seen in total internal reflection fluorescence (TIRF). (D) F-actin organization on collagenIV:EcadFc, seen in widefield epifluorescence and TIRF. (E) E-cadherin (n = 19), β-catenin (n = 14), α-E-catenin (n = 10), and UtrCH (n = 13, 13, 10; see D) mobility on collagenIV:EcadFc assessed by fluorescence recovery after photobleaching. Bar: 10 μm.
Because cells are deformable, the cell contact area can be used as a measure of adhesion strength (adhesion energy per unit area) on a given substrate. Significantly, the cell contact area on EcadFc stripes increased with increasing EcadFc density compared with the cell contact area on collagenIV (Fig. 1B). Notably, contact area and EcadGFP recruitment follow the same trend, saturating at 50% EcadFc. Hence, the strength of E-cadherin–mediated adhesion increased with cellular E-cadherin recruitment to EcadFc, similar to that between MDCK cell–cell contacts (19).
CollagenIV is the primary protein of epithelial ECM. Collagen supports MDCK cell migration and scattering better than laminin or fibronectin, and the MDCK cell migration rate is less dependent on collagen surface concentration than other ECM proteins (20), which allowed us to keep the amount of collagenIV constant while varying the amount of EcadFc.
Cell adhesion to collagenIV occurs at FAs formed by integrin receptors and actin-associated cytoskeletal proteins, including vinculin and paxillin (21). On collagenIV:EcadFc surfaces, FAs marked by vinculin-GFP (VinGFP) or paxillin-GFP formed preferentially on collagenIV stripes (Fig. 2 A–D), and we found some vinculin staining on EcadFc stripes in small and rare foci (Fig. 2 A–D and Discussion). Vinculin was more stable in FAs on collagenIV than in foci on EcadFc, and vinculin turnover on collagenIV was unaffected by cell adhesion on adjacent EcadFc stripes (Fig. 2E). FAs formed only on collagenIV stripes on collagenIV:PEG surfaces, but on both collagenIV and Fc stripes on collagenIV:Fc surfaces (Fig. 2F). In addition, FAs did not form on stripes coated with E-cadherin in which the Fc link was exchanged for biotin (Fig. 2C). Thus, on collagenIV:EcadFc surfaces, FAs were excluded from sites of homotypic E-cadherin adhesion specifically, and not by the Fc component of EcadFc. FA exclusion from E-cadherin adhesions is similar to that found in cell–cell contacts between MDCK cells (12).
Fig. 2.
(A–D) Vinculin–GFP (A) or paxillin–GFP (C) recruitment on collagenIV versus EcadFc (A) or EcadBiot (E-cadherin extracellular fragment fused to biotin) (C). Note that the preferential localization of FAs on collagenIV did not depend on the FA marker used or the E-cadherin chimeric protein on the functionalized surface. Vinculin recruitment to EcadFc (in foci) versus collagenIV in FAs per cell (fluorescence intensities weighted by surface area covered by all foci and all FAs, respectively) (B: n = 3) and per focus and FA (fluorescence intensities unweighted) (D: EcadFc n = 6; collagenIV n = 7). The difference between B and D reflects the larger surface covered by all FAs on collagenIV than covered by all vinculin foci on EcadFc in a given cell. (E) Vinculin mobility on collagenIV with (circle, n = 7) and without (square, n = 8) juxtaposed EcadFc stripes, as assessed by fluorescence recovery after photobleaching. Vinculin mobility on EcadFc stripes (cross, n = 6). (F) VinGFP-expressing MDCK cells on collagenIV:EcadFc, collagenIV:Fc, or collagenIV:PEG. (Maximum intensity projection over time. Left, original fluorescence intensity signal; Right, FA localization by edge detection.) Bar: 10 μm. Data show mean ± SEM. *P < 0.05; ***P < 0.001, with respect to indicated controls; Student's t test.
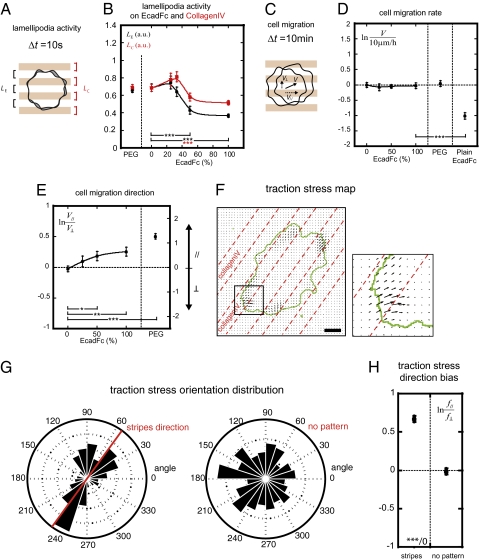
We first assessed the effect of E-cadherin adhesion on lamellipodia activity (Fig. 3A), a measure of peripheral membrane protrusions and retractions mediated by the dynamic assembly and disassembly of actin filaments (22). On collagenIV:Fc and collagen:PEG surfaces, lamellipodia activity was equally high on collagenIV, Fc, or PEG stripes (Fig. 3B and Movie S1). Significantly, replacing PEG or Fc with EcadFc decreased lamellipodia activity on both collagenIV and EcadFc stripes in an EcadFc concentration-dependent manner, with the greatest effect on the EcadFc stripes (Fig. 3B and Movie S2). This effect mimicked the decrease in lamellipodia activity at both cell–cell contacting and noncontacting membranes observed during intercellular adhesion between MDCK cells (11).
Fig. 3.
(A) Measurement of lamellipodia activity (average area of cell surface difference between 10 s intervals; Movie S1 and Movie S2). (B) Lamellipodia activities LC and LE on collagenIV (red) and EcadFc/Fc or PEG stripes (black), respectively, as a function of non-collagenIV stripe functionalization. n = 10 (PEG), 26 (Fc), 11 (EcadFc 25%), 9 (EcadFc 33%), 15 (EcadFc 50%), and 42 (EcadFc 100%). (C) Measurement of cell migration (average cell velocity sampled at 10 min intervals; Movie S3, Movie S4, and Movie S5). (D) Migration rate on patterned surfaces as a function of non-collagenIV stripe functionalization and on plain EcadFc surfaces. (E) Migration direction on patterned surfaces as a function of non-collagenIV stripe functionalization. A positive migration direction index denotes that the migration rate component parallel to pattern direction V// is higher than perpendicular component V┴. n = 43 (PEG), 24 (Fc), 23 (EcadFc 25%), 15 (EcadFc 50%), 12 (EcadFc 100%), and 16 (plain EcadFc) for D and E. (F–H) Traction force microscopy. (F) Traction stress map: local traction stress magnitude and orientation (black vectors, a.u.) exerted by a single cell (delineated by the green line) on collagenIV-printed (inside dashed lines) polyacrylamide gel sheet. (G) Traction stress orientation distribution: count of local traction stress vectors as a function of their orientation on collagen stripes (left, n = 1,431) and on nonpatterned collagen (right, n = 1,518). (H) Quantification of traction stress direction bias. A positive value denotes a stronger traction in the direction of the stripes than in the perpendicular direction (collagen stripes: n = 1,431; non-patterned collagen: n = 1,518). Bar: 10 μm. Scatter plots show mean ± SEM; line is a smooth fit to guide the eye. *P < 0.05, *P < 0.01, ***P < 0.001, with respect to indicated control; Mann–Whitney test (two-tailed).
Next we analyzed the effects of different combinations of EcadFc and collagenIV on cell migration rate and directionality (Fig. 3C). Despite dampening of lamellipodia activity with increasing EcadFc surface density (Fig. 3B), cell migration rate was similar with all combinations of collagenIV, Fc, PEG, and EcadFc (10 μm/h; Fig. 3D, Movie S3, and Movie S4) and independent of stripe width (Fig. S1B). Cell migration was dependent on cell adhesion to ECM because cells were essentially immobile on surfaces coated only with EcadFc (Fig. 3D and Movie S5).
Although the rate of ECM-mediated cell migration was independent of E-cadherin adhesion, migration was biased parallel to the stripes (Fig. 3E). Above a threshold density of EcadFc, the parallel component was ∼30% higher than the perpendicular component. This effect was even stronger on collagenIV:PEG surfaces where PEG confined FAs to the collagenIV stripes or where the collagenIV stripes were even thinner (Fig. S1C). Thus, E-cadherin-mediated adhesion biased the direction of cell migration without altering the migration rate by spatially confining FAs to collagenIV.
To examine how spatial confinement of FAs resulted in migration bias, we used Traction Force Microscopy (TFM) to measure the direction of traction forces exerted by cells on a polyacrylamide gel surface functionalized with stripes of collagenIV to spatially confine FAs (23, 24). Cells exerted traction stresses exclusively on collagenIV stripes at the cell periphery (Fig. 3F) and the forces oriented in the direction of the stripes (Fig. 3 G and H). In contrast, the distribution of cell traction forces orientation was isotropic when cells adhered to nonpatterned collagen (Fig. 3 G and H). Thus, spatial confinement of FAs induces anisotropic cell traction on ECM and migration directional bias.
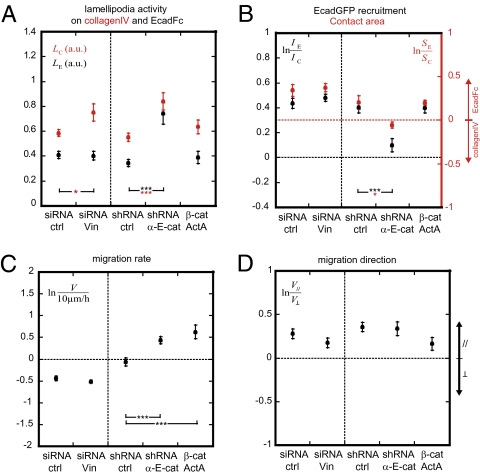
Vinculin and αE-catenin are two actin-binding paralogs that are major components of integrin- and E-cadherin–mediated adhesion complexes, respectively (Figs.1 and 2) (25, 26). To investigate the molecular mechanisms underlying regulation of cell motile behavior by E-cadherin adhesion, we depleted either vinculin or αE-catenin. siRNA depletion of vinculin led to a loss of 75% of total vinculin content and 50% of vinculin from FAs (Fig. S2); excess cytoplasmic vinculin may buffer vinculin depletion at FAs, as observed by others (27, 28). At this level of vinculin depletion, lamellipodia activity increased slightly over collagenIV stripes on collagenIV:EcadFc surfaces (Fig. 4A). However, vinculin depletion did not affect lamellipodia activity on EcadFc stripes (Fig. 4A), which remained dampened, and did not affect E-cadherin recruitment to EcadFc or cell spreading on EcadFc (Fig. 4B). Vinculin depletion had no effect on cell migration rate (Fig. 4C). Note that the relationship between the vinculin expression level and epithelial cell migration remains complex (29). Vinculin depletion did not affect the migration directional bias, which remained parallel to the stripes (Fig. 4D). Neither did vinculin depletion affect αE-catenin levels (Fig. S3) or E-cadherin recruitment to EcadFc stripes (Fig. 4B).
Fig. 4.
(A) Effects of vinculin and αE-catenin depletions on lamellipodia activity and (B) on EcadGFP recruitment and contact area. n = 20 (siRNA ctrl), 11 (siRNA Vin), 10 (shRNA ctrl), 9 (shRNA α-E-cat), and 11 (β-cat ActA) for A; n = 23 (siRNA ctrl), 13 (siRNA Vin), 11 (shRNA ctrl), 11 (shRNA a-cat), and 11 (β-cat ActA) for EcadGFP recruitment; n = 18 (siRNA ctrl), 10 (siRNA Vin), 10 (shRNA ctrl), 8 (shRNA α-E-cat), and 11 (β-cat ActA) for contact area. (C and D) Effects of vinculin and αE-catenin depletions on cell migration (C) rate and (D) direction. Note that control clone (expressing VinGFP) for αE-catenin depletion migrates faster than control clone (EcadGFP) for vinculin depletion. n = 16 (siRNA Vin), 22 (siRNA ctrl), 39 (shRNA α-E-cat), and 9 (β-cat ActA). *P < 0.05, **P < 0.01, ***P < 0.001, with respect to controls; Mann–Whitney test (two-tailed).
αE-Catenin is an allosteric protein that can either bind the E-cadherin/β-catenin complex on the plasma membrane or form a homodimer that has a higher affinity for actin and inhibits the Arp2/3 complex in the cytoplasm (30). shRNA-mediated depletion of all pools of αE-catenin resulted in a 90% decrease of total αE-catenin (Fig. S4 A–C). αE-Catenin depletion partially reduced the amount of cellular E-cadherin recruited to EcadFc stripes (Fig. 4B), which decreased the cell contact area (spreading) on EcadFc (Fig. 4B). There was no effect on the level or distribution of vinculin (Fig. S3). Significantly, αE-catenin depletion resulted in an increase in lamellipodia activity on both collagenIV and EcadFc stripes to levels similar to those on surfaces lacking EcadFc, such as collagenIV:Fc (Fig. 4A).
To distinguish roles of different pools of αE-catenin, we selectively sequestered the cytoplasmic pool to mitochondria using a chimeric protein containing the αE-catenin binding site of β-catenin fused to ActA (β-cat ActA; Fig. S4 E–G) (31). On collagenIV:EcadFc, depletion of the cytoplasmic pool of αE-catenin did not affect lamellipodia activity, E-cadherin recruitment or contact area compared with the control (Fig. 4 A and B). Since shRNA-mediated depletion of αE-catenin affected both the membrane and cytoplasmic pools of αE-catenin, we conclude that in the presence of E-cadherin–mediated cell–cell adhesion the plasma membrane pool of αE-catenin mediates the down-regulation of lamellipodia activity.
To address whether αE-catenin affects cell migration rate and directional bias, we depleted all pools of αE-catenin using shRNA. Cell migration rate increased from 10 to ∼16 μm/h (Fig. 4C). Significantly, depletion of only the cytosolic pool of αE-catenin in β-cat ActA cells also increased the cell migration rate (Fig. 4C). Depletion of all pools of αE-catenin, however, did not significantly affect migration bias (Fig. 4D), an effect resulting from partial E-cadherin recruitment to EcadFc stripes (Fig. 4B) and spatial restriction of FAs to collagenIV stripes (Fig. S4D). These results indicate that the cytosolic pool of αE-catenin regulates cell migration rate in an E-cadherin adhesion-independent manner.
We next investigated the effects of E-cadherin adhesion and different levels of pools of αE-catenin on cell rearrangements in multicellular epithelial sheets. We analyzed how individual cells migrated relative to their neighbors (migration coordination) in groups of either β-cat-ActA cells depleted of only the cytosolic pool of αE-catenin, total αE-catenin–depleted cells, or control cells (Fig. 5A, Movie S6, and Movie S7). Significantly, migration coordination was greatly reduced between cells that were depleted of total αE-catenin (about two-thirds coordination loss beyond one to two cell diameters; ξ ∼25μm), whereas migration coordination was high in cohesive sheets of β-cat-ActA cells or control cells (about two-thirds coordination loss beyond four cell diameters; ξ ∼50 μm) (Fig. 5B). Cell migration rate, which is regulated by the cytoplasmic pool of αE-catenin (Fig. 4C), was higher in β-cat-ActA and total αE-catenin–depleted cells compared with control cells (Fig. 5C). Thus, similar to the directional migration of single cells on micropatterned collagenIV:EcadFc surfaces, E-cadherin–mediated adhesion within an epithelial sheet is permissive for cell migration and required for migration coordination, and the cytosolic pool of αE-catenin regulates cell migration rate.
Fig. 5.
(A–C) Coordination and rate of cell migration in an epithelial sheet (Materials and Methods). (A) α is the angle between velocity vectors of two cells and r the distance between these cells. (B) Coordination index as a function of the distance between cells. <cos α> is the coordination index between all cells at a distance r from each other at a given time, averaged over time. Lines are fits of experimental data (dots) with an exponential decay function <cos α> = exp(−r/ξ), where ξ is the coordination length beyond which cells have lost about two-thirds of their coordination. (C) Average migration rate in the epithelial sheet. Data are displayed as a box-whisker plot (50% of the data within the box, 100% within the whiskers). ***P < 0.001, Mann–Whitney test (two-tailed). (D). Model of functional regulation of cell motile behavior by crosstalk between E-cadherin– and integrin-mediated adhesions (see text for details).
Discussion
Mechanisms involved in coordinated cell migration in a cohesive epithelial sheet are difficult to elucidate because of the molecular complexity and spatial separation of cell–cell and cell–ECM adhesions. The present study provides a proof of principle for a unique approach to quantitatively dissect the effects of cell–ECM and cell–cell adhesion cues on cell motile behavior. The design of the striped geometry of the micropatterns was tailored to be small enough so that individual cells simultaneously adhered to both ECM and E-cadherin, thereby providing a cell with a local interface between ECM and E-cadherin wherever it migrated. The dual-patterned surfaces used here provide an experimental approach to isolate the effects of cell–cell adhesion specific to E-cadherin. This method also could be used to reconstruct step-by-step the complexity of cell–ECM and cell–cell contacts and to test the effects on the behaviors of other cell types as well as primary cells.
On micropatterned surfaces, EcadFc specifically recruited cellular E-cadherin, αE-catenin, and β-catenin (Fig. 1), all of which had mobilities within the ranges of these proteins at bona fide cell–cell contacts in MDCK cells (8, 18). Thus despite the relative immobility of the EcadFc substrate, the cellular E-cadherin–catenin complex appeared to be as dynamic as that at normal cell–cell adhesions.
Vinculin was preferentially recruited to FAs on collagenIV, and to a lesser extent to EcadFc. Vinculin has been reported to bind to β-catenin (8, 28), although vinculin binding to actin is not activated (8). However, the recruitment pattern of vinculin on EcadFc did not resemble that of β-catenin or αE-catenin (Figs. 1 and 2), and vinculin depletion did not affect lamellipodia activity on EcadFc (Fig. 4A). In addition, vinculin depletion did not impair recruitment of cellular E-cadherin to EcadFc patterns or at cell–cell contacts (Fig. 4B and Fig. S2A), unlike a recent study with another cell type (28). Therefore, our results do not implicate a role for vinculin at E-cadherin adhesion sites, at least under these conditions.
Although it is known that epithelial cells transmit forces through intercellular contacts (32), it is unclear whether this induces a form of migration that is cadherin-based. We showed, however, that MDCK cells migrated only in the presence of collagenIV and not on pure EcadFc surfaces (Fig. 3D). Note that because the time courses of these experiments were similar, ECM deposition on EcadFc, either from the medium or locally by cells, must be insignificant in all conditions because cells did not migrate on EcadFc.
Because cell migration on only EcadFc is prevented, it might be expected on patterned surfaces that strengthening of E-cadherin adhesion, by increased E-cadherin recruitment and cell contact area on EcadFc, would resist or down-regulate ECM-based cell migration. However, the cell migration rate remained independent of EcadFc surface concentration on patterned surfaces (Fig. 3D). Similarly, maintenance of E-cadherin–mediated adhesion in cells lacking cytoplasmic αE-catenin did not decrease the migration rate compared with cells lacking all pools of αE-catenin and E-cadherin adhesion (Fig. 4C). That cells are able to strongly adhere to, but still slide over, surfaces as they migrate is likely permitted by rapid and continuous turnover of the membrane-bound E-cadherin–catenin complex (Fig. 1E).
We found that cell migration was biased in the direction of the stripes on collagenIV:EcadFc and collagenIV:PEG patterned surfaces (Fig. 3E). Both EcadFc and PEG prevented FA formation, which resulted in FA confinement to collagenIV (Fig. 2). We observed that traction forces were exerted by cells through this anisotropic distribution of FAs, which oriented the direction of cell migration (Fig. 3 F–H). Thus anisotropic distribution of FAs on collagenIV is sufficient to induce migration directional bias.
Lamellipodia activity was equivalently high on collagenIV and PEG surfaces that do not assemble FAs, but decreased on those surfaces in the presence of an adjacent EcadFc stripe (Fig. 3B). It is thought that lamellipodial protrusion occurs by resistance of FAs to actin retrograde flow between the leading edge and the lamella (33). Loss of FA resistance to retrograde flow may be compensated by faster flow and a higher actin polymerization rate at the leading edge, resulting in similar lamellipodia activity on adherent (collagenIV) and nonadherent (PEG) substrates, as recently proposed by Renkawitz et al. (34). Therefore, it is unlikely that lack of FAs on EcadFc surfaces is responsible for the loss of lamellipodia activity on those surfaces.
Previous studies showed that lamellipodia activity decreases during cell–cell adhesion and that this might be important to allow weak, transient cell–cell adhesions to become stabilized (11). Using micropatterned surfaces, our results show clearly that lamellipodia dampening is a direct and concentration-dependent effect of E-cadherin adhesion. Depletion of total αE-catenin, including the membrane-bound pool, decreased E-cadherin–mediated adhesion and subsequent lamellipodia activity dampening, whereas selective depletion of the cytoplasmic pool of αE-catenin, which did not affect E-cadherin adhesion, did not (Fig. 4 A and B). Hence, the membrane-bound pool, and not the cytoplasmic pool of αE-catenin, is required for dampening lamellipodia activity upon E-cadherin adhesion. That E-cadherin adhesion decreased lamellipodia activity, but did not affect migration rate (Fig. 3 B and D), supports recent studies showing that lamellipodia activity is dispensable for epithelial cell migration but may allow local exploration (22, 35).
We sought to place these insights into the crosstalk between integrin- and E-cadherin–based adhesion complexes in a multicellular context. In a confluent, cohesive epithelial sheet, cells exhibit an active migratory behavior with a high degree of coordination (Fig. 5). Thus, intercellular adhesion is permissive for cell migration, but appears to affect the directional coordination of cells, as expected from our results of cell migration on EcadFc:collagenIV (Fig. 3E). A possible mechanism for migration coordination between adherent neighboring cells could be intercellular friction, although E-cadherin adhesion itself does not appear to provide sufficient friction between cells because it did not impede cell migration on patterned surfaces (Fig. 3D). As observed on EcadFc:collagenIV surfaces, E-cadherin–mediated adhesion repelled FAs and oriented cell traction onto the ECM, which might allow neighbor cells to move in a coordinated fashion in an epithelial sheet.
The higher migration rate of cells in an epithelial sheet depleted of αE-catenin is likely due to an intercellular adhesion-independent increase in cell migration rate, as observed on patterned surfaces (Fig. 4C). Interestingly, total αE-catenin depletion significantly reduced cell migration coordination in epithelial sheets, whereas cells still migrated with a bias on patterned surfaces. This difference may reflect varying levels of E-cadherin recruitment in cells on micropatterned surfaces compared with cells in confluent monolayers. Cellular E-cadherin recruitment and adhesion strength (Fig. 4B) on EcadFc stripes were only partially reduced in total αE-catenin–depleted cells, because the high density of immobile EcadFc likely traps some cellular E-cadherin to a level that inhibits FA formation (Fig. S4D). In confluent monolayers, however, decreased recruitment of E-cadherin on both sides of the cell–cell contacts may cooperatively impair E-cadherin–mediated effects on directional bias.
It is noteworthy that epithelial sheets with normal intercellular adhesion (control and β-cat-ActA) exhibited larger cell density fluctuations over time while maintaining their cohesion (Movie S6), whereas cell sheets with reduced intercellular adhesion (αE-catenin shRNA) did not (Movie S7). This suggests that cells are subject to higher strains in cohesive sheets, pointing toward a possible regulatory role of intracellular viscosity on cell migration rate in cohesive epithelial sheets.
In summary, our results provide insights into the ensemble effects of cadherin- and integrin-based adhesion complexes on epithelial cell motile behavior (Fig. 5D). First, E-cadherin engagement did not decrease the rate of ECM-mediated cell migration, despite global dampening of lamellipodia activity. Second, E-cadherin–mediated adhesion highly constrained integrin-based FA assembly and strongly biased ECM-based traction forces and cell migration along the direction of E-cadherin/collagenIV interfaces. Hence, cell–cell (E-cadherin) adhesion redirects but does not down-regulate cell migration, as originally postulated by Abercrombie and Heaysman (6). Third, E-cadherin adhesion and lamellipodia activity are regulated by the membrane pool of αE-catenin and cell migration by the cytoplasmic pool. That αE-catenin is a common regulator of E-cadherin adhesion and cell migration may be important during specific morphogenetic processes or pathological conditions involving uncoordinated single-cell migration. Indeed, in vivo studies have shown that loss of αE-catenin compromises epithelium integrity and that these effects can be more severe than those caused by loss of E-cadherin and intercellular adhesion (36, 37).
Materials and Methods
Micropatterned surfaces were prepared in a two-step process. First, the ECM was microcontact-printed using standard protocols (38). Then, non-ECM–coated surfaces were functionalized with EcadFc, Fc, or PEG as previously reported (13, 39).
Normal MDCK type II G cells stably or transiently expressing fluorescently tagged proteins of interest were monitored on a widefield epifluorescence inverted microscope, and depending on the experiment, 2–4 d after siRNA/shRNA protein depletion. Fluorescence signal from the cells and the micropatterns was used to assess protein recruitment, cell-contour fluctuations at short time scales for measurement of lamellipodia activity, and cell position at long time scales for measurement of cell migration. TFM was performed as previously described (24) on polyacrylamide gel sheets functionalized with micropatterns of ECM (23).
Image analysis was performed with Image J, Metamorph, and MATLAB softwares. Statistical analysis was performed using a two-tailed Mann–Whitney test or a Student's t test, as indicated (SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank the Stanford Nanofabrication Facility and the Boxer laboratory (Stanford University, Stanford, CA) for materials and reagents; Yunxiang Zhang, Vikram Mukundan, Cyrus Wilson, Erin Barnhart, and Julie Theriot for technical advice; Paige Cooper for contributing preliminary vinculin-GFP fluorescence recovery after photobleaching experiments; Benedikt Sabass and Ulrich Schwarz (University of Heidelberg, Heidelberg, Germany) for providing the MATLAB script for TFM; Feng-Chiao Tsai (Stanford University, Stanford, CA) for providing the MATLAB script for cell tracking in epithelial sheets; Adam Kwiatkowski for establishing the αE-catenin shRNA cell line and with Dan Dickinson for critically reading the manuscript; and members of the W.J.N. laboratory for valuable discussions. This work was supported by National Institutes of Health Grants GM35527 (to W.J.N.) and DP10D00354 (to M.L.G.), postdoctoral fellowship SPE20060407147 (to N.B.) from the Fondation Recherche Médicale, Cancer Biology Program Training Grant 5 T32 CA009151-34 (to M.L.), a Burroughs Wellcome Fund Career Award at the Scientific Interface (M.L.G.), and a grant from the Stanford Center for Integrated Science.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 13199.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002662107/-/DCSupplemental.
References
- 1.Wood W, et al. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol. 2002;4:907–912. doi: 10.1038/ncb875. [DOI] [PubMed] [Google Scholar]
- 2.Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- 3.Duband JL, Monier F, Delannet M, Newgreen D. Epithelium-mesenchyme transition during neural crest development. Acta Anat (Basel) 1995;154:63–78. doi: 10.1159/000147752. [DOI] [PubMed] [Google Scholar]
- 4.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Abercrombie M, Heaysman JE. Observations on the social behaviour of cells in tissue culture. II. Monolayering of fibroblasts. Exp Cell Res. 1954;6:293–306. doi: 10.1016/0014-4827(54)90176-7. [DOI] [PubMed] [Google Scholar]
- 6.Abercrombie M, Heaysman JEM. Observations on the social behaviour of cells in tissue culture. I. Speed of movement of chick heart fibroblasts in relation to their mutual contacts. Exp Cell Res. 1953;5:111–131. doi: 10.1016/0014-4827(53)90098-6. [DOI] [PubMed] [Google Scholar]
- 7.Loeb L. Amoeboid movement, tissue formation and consistency of protoplasam. Am J Physiol. 1921;56:140–167. doi: 10.1126/science.53.1368.261. [DOI] [PubMed] [Google Scholar]
- 8.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borghi N, James Nelson W. Intercellular adhesion in morphogenesis: Molecular and biophysical considerations. Curr Top Dev Biol. 2009;89:1–32. doi: 10.1016/S0070-2153(09)89001-7. [DOI] [PubMed] [Google Scholar]
- 10.Lauffenburger DA, Horwitz AF. Cell migration: A physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 11.Ehrlich JS, Hansen MDH, Nelson WJ. Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell-cell adhesion. Dev Cell. 2002;3:259–270. doi: 10.1016/s1534-5807(02)00216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol. 2007;178:517–527. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drees F, Reilein A, Nelson WJ. Cell-adhesion assays: Fabrication of an E-cadherin substratum and isolation of lateral and Basal membrane patches. Methods Mol Biol. 2005;294:303–320. doi: 10.1385/1-59259-860-9:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pokutta S, Weis WI. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu Rev Cell Dev Biol. 2007;23:237–261. doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Sivasankar S, Nelson WJ, Chu S. Resolving cadherin interactions and binding cooperativity at the single-molecule level. Proc Natl Acad Sci USA. 2009;106:109–114. doi: 10.1073/pnas.0811350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams CL, Nelson WJ, Smith SJ. Quantitative analysis of cadherin-catenin-actin reorganization during development of cell-cell adhesion. J Cell Biol. 1996;135:1899–1911. doi: 10.1083/jcb.135.6.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burkel BM, von Dassow G, Bement WM. Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophin. Cell Motil Cytoskeleton. 2007;64:822–832. doi: 10.1002/cm.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Beco S, Gueudry C, Amblard F, Coscoy S. Endocytosis is required for E-cadherin redistribution at mature adherens junctions. Proc Natl Acad Sci USA. 2009;106:7010–7015. doi: 10.1073/pnas.0811253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu Y-S, et al. Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J Cell Biol. 2004;167:1183–1194. doi: 10.1083/jcb.200403043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Rooij J, Kerstens A, Danuser G, Schwartz MA, Waterman-Storer CM. Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J Cell Biol. 2005;171:153–164. doi: 10.1083/jcb.200506152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Cohen DM, Choudhury DM, Kioka N, Craig SW. Spatial distribution and functional significance of activated vinculin in living cells. J Cell Biol. 2005;169:459–470. doi: 10.1083/jcb.200410100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponti A, Machacek M, Gupton SL, Waterman-Storer CM, Danuser G. Two distinct actin networks drive the protrusion of migrating cells. Science. 2004;305:1782–1786. doi: 10.1126/science.1100533. [DOI] [PubMed] [Google Scholar]
- 23.Damljanović V, Lagerholm BC, Jacobson K. Bulk and micropatterned conjugation of extracellular matrix proteins to characterized polyacrylamide substrates for cell mechanotransduction assays. Biotechniques. 2005;39:847–851. doi: 10.2144/000112026. [DOI] [PubMed] [Google Scholar]
- 24.Sabass B, Gardel ML, Waterman CM, Schwarz US. High resolution traction force microscopy based on experimental and computational advances. Biophys J. 2008;94:207–220. doi: 10.1529/biophysj.107.113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 26.Nagafuchi A, Takeichi M, Tsukita S. The 102 kd cadherin-associated protein: Similarity to vinculin and posttranscriptional regulation of expression. Cell. 1991;65:849–857. doi: 10.1016/0092-8674(91)90392-c. [DOI] [PubMed] [Google Scholar]
- 27.Shiraishi I, et al. Vinculin is an essential component for normal myofibrillar arrangement in fetal mouse cardiac myocytes. J Mol Cell Cardiol. 1997;29:2041–2052. doi: 10.1006/jmcc.1997.0438. [DOI] [PubMed] [Google Scholar]
- 28.Peng X, Cuff LE, Lawton CD, DeMali KA. Vinculin regulates cell-surface E-cadherin expression by binding to beta-catenin. J Cell Sci. 2010;123:567–577. doi: 10.1242/jcs.056432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson KJ, et al. Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nat Cell Biol. 2008;10:1027–1038. doi: 10.1038/ncb1762. [DOI] [PubMed] [Google Scholar]
- 30.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamin JM, et al. AlphaE-catenin regulates actin dynamics independently of cadherin-mediated cell-cell adhesion. J Cell Biol. 2010;189:339–352. doi: 10.1083/jcb.200910041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trepat X, et al. Physical forces during collective cell migration. Nat Phys. 2009;5:426–430. [Google Scholar]
- 33.Ji L, Lim J, Danuser G. Fluctuations of intracellular forces during cell protrusion. Nat Cell Biol. 2008;10:1393–1400. doi: 10.1038/ncb1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renkawitz J, et al. Adaptive force transmission in amoeboid cell migration. Nat Cell Biol. 2009;11:1438–1443. doi: 10.1038/ncb1992. [DOI] [PubMed] [Google Scholar]
- 35.Gupton SL, et al. Cell migration without a lamellipodium: Translation of actin dynamics into cell movement mediated by tropomyosin. J Cell Biol. 2005;168:619–631. doi: 10.1083/jcb.200406063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 2001;104:605–617. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 37.Benjamin JM, Nelson WJ. Bench to bedside and back again: Molecular mechanisms of alpha-catenin function and roles in tumorigenesis. Semin Cancer Biol. 2008;18:53–64. doi: 10.1016/j.semcancer.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishizawa M, Takoh K, Matsue T. Micropatterning of HeLa cells on glass substrates and evaluation of respiratory activity using microelectrodes. Langmuir. 2002;18:3645–3649. [Google Scholar]
- 39.Cuvelier D, Rossier O, Bassereau P, Nassoy P. Micropatterned “adherent/repellent” glass surfaces for studying the spreading kinetics of individual red blood cells onto protein-decorated substrates. Eur Biophys J. 2003;32:342–354. doi: 10.1007/s00249-003-0282-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.