Abstract
Whole-genome searches have identified nicotinic acetylcholine receptor α5-α3-β4 subunit gene variants that are associated with smoking. How genes support this addictive and high-risk behavior through their expression in the brain remains poorly understood. Here we show that a key α5 gene variant Asp398Asn is associated with a dorsal anterior cingulate–ventral striatum/extended amygdala circuit, such that the “risk allele” decreases the intrinsic resting functional connectivity strength in this circuit. Importantly, this effect is observed independently in nonsmokers and smokers, although the circuit strength distinguishes smokers from nonsmokers, predicts addiction severity in smokers, and is not secondary to smoking per se, thus representing a trait-like circuitry biomarker. This same circuit is further impaired in people with mental illnesses, who have the highest rate of smoking. Identifying where and how brain circuits link genes to smoking provides practical neural circuitry targets for new treatment development.
Keywords: genetics, imaging, smoking, functional connectivity, nAChR
Smoking is influenced by both genetic and environmental factors, although the major factors associated with persistent smoking are genetics [heritability at 75% with minimal shared environmental contribution (1, 2)] and mental illnesses [smoking rate at 50–80% in many mental illnesses (3, 4)]. The brain mechanisms linking these factors to smoking behavior are not clear. In this context, the recent discovery of several polymorphisms at the 15q nicotinic acetylcholine receptor (nAChR) α5-α3-β4 gene cluster is important because they were found based on genome-wide searches (5–7), have been replicated a considerable number of times (SI Methods), and provide a starting point to dissect the neurobiological pathways leading to persistent smoking. The most replicated functional marker associated with smoking, and secondarily to lung cancer, is the α5 subunit gene (CHRNA5) rs16969968 or Asp398Asn polymorphism that changes aspartic acid (Asp) to asparagine (Asn) (5–7), with the Asn risk allele reducing α4β2α5 receptor function (6). The α4β2 nAChR is a key regulator of nicotine addiction (8–10), and the participation of the α5 subunit substantially impacts α4β2 function (11).
Identifying relevant genes is only the first step in finding more effective therapeutic agents. Identifying the brain circuit(s) linking the “risk alleles” to smoking is an important next step. Brain regions consistently associated with smoking-related behaviors and nicotine actions are the cingulate, striatum, orbitofrontal and prefrontal cortex, insula, and thalamus (12–16). The extant literature suggests that the α5 subunit is expressed cortically mainly in the cingulate; its mRNA expression is found in layer VIb in rats, with the highest concentration in the cingulate (17) and the cortical expression pattern of α5 seen in the cingulate and retrosplenial cortex (18). Subcortically, the α5 subunit is expressed in striatum, thalamus, hippocampus, and amygdala (SI Methods) (17, 19, 20). By using a resting-state functional connectivity (rsFC) approach, we previously searched the entire cingulate and showed that a dorsal anterior cingulate (dACC)–ventral striatal circuit is associated with nicotine addiction (21), suggesting that the dACC and these subcortical areas, all of which express the α5 subunit, represent candidate brain circuits that may mediate α5 genetic influence on nicotine addiction.
rsFC is a functional MRI method that measures the synchronization of intrinsic low-frequency fluctuations between brain regions in the absence of any specific task performance (22, 23), providing a tool to assay in vivo circuit-level functions. These synchronized circuits at “rest” are constrained by direct or polysynaptic connections between correlated regions (24), correspond closely to networks engaged during task performance (25), predict brain activation associated with behavioral performance (26, 27), and are significantly heritable (28). Communications among groups of neurons in the “resting” human brain may serve the function of predisposing brain networks to facilitate subsequent behavioral responses (29). Consistent with this notion, we propose that one or more smoking-related genes may influence specific resting neural communication between regions in which the gene is expressed, and predispose individuals carrying the risk allele vulnerable to partake in this high-risk behavior.
In addition to genetic influence, psychiatric conditions contribute substantially to smoking risks: people with mental illnesses are at the highest smoking risk (40–80%) (3, 4, 30–33) and more than 50% of lifetime smokers report psychiatric conditions (4), making the inclusion of a psychiatric population in etiological studies of smoking an important and relevant experimental approach. We therefore included people with psychiatric illnesses in our sample, and tested whether the high risk of smoking in psychiatric patients is related to the aforementioned genes or circuits associated with smoking. Many mental illnesses—similar to smoking—are highly genetic (34, 35), suggesting a potentially shared genetic influence. However, as initial data showed that 398Asn itself was not overrepresented in some psychiatric populations (36, 37), this raises an intriguing hypothesis that the high risk of smoking in mental illnesses may not necessarily be related to shared genetics, but rather may be a result of converging neural circuits related to smoking.
Results
We ascertained the smoking status of 311 subjects (149 smokers, 148 nonsmokers, 14 ex-smokers). Among this cohort, 93 smokers, 79 nonsmokers, and 12 ex-smokers (N = 184) completed a resting-state functional MRI scan session. Smoking assessment and subgroup information are described in Methods and Table S1. We genotyped Asp398Asn and two other SNPs from the neighboring α3 subunit gene (CHRNA3), rs578776, and rs1051730 (Table S2): rs578776 is a 3′ UTR variant that has been shown to represent an independent smoking-related signal in this gene cluster (6), and rs1051730 is a synonymous SNP that has been associated with smoking and diseases closely related to smoking (5–7). Additional gene selection rationale and genotyping information are provided in SI Methods.
All three SNPs were associated with smoking status (P ≤ 0.002, significant after Bonferroni correction) and remained significant after covarying for genetic ancestry (38), age, and sex in logistic regression. The associations were significant in the full sample and the imaging subsample (Fig. S1). The current sample included smokers with psychiatric illnesses (45.5%) who were frequency-matched to nonsmokers with psychiatric illnesses (32.5%, χ2 = 3.10, P = 0.075; Table S1). The primary psychiatric diagnoses in this sample were schizophrenia or schizoaffective disorder, substance dependence, and anxiety and depressive disorders; all of these conditions are known to be associated with increased rates of smoking (4, 30, 33). Previous studies suggest that 398Asn itself is not overrepresented in at least two studies of psychiatric populations (36, 37). It is also not overrepresented in our sample (psychiatric illness vs. genotype, n = 309; χ2 = 0.92, P = 0.630). Thus, we conducted genotype-imaging analyses using all subjects with genotype and imaging data available, given the similar genotype distribution and that all three CHRNA marker associations to smoking were found to be consistent with those in large genome-wide association study datasets (5–7).
Next, the dACC was manually segmented based on the boundaries described previously (21) in each of the 184 individuals that were scanned. We used this as the “seed” in a whole-brain voxel-wise regression analysis to identify dACC functional circuits associated with each SNP. rsFC of the dACC was correlated with multiple brain regions, including bilateral insula, medial frontal, middle frontal and temporal cortices, middle and posterior cingulate and precuneus, striatum, amygdale, hippocampus, thalamus, and brainstem (more details are provided in Fig. S2). The risk allele Asn of Asp398Asn was associated with reduced functional connectivity between dACC and eight brain regions (Pcorrected < 0.05; Fig. 1 A–C). The two largest clusters (clusters 1 and 2) were bilaterally located within a region bound by the ventral striatum, substantia innominata, amygdala, and hippocampus, overlapping closely with regions previously identified only by clinical nicotine addiction severity rating (21). These subcortical locations are consistent with the so-called ventral striatopallidal system, also known as the sublenticular extended amygdala (39). For brevity, we use the term “ventral striatum” to describe this area. The other significant connectivity associated regions are shown in Fig. 1A.
Fig. 1.
Functional connectivity circuits between dACC and brain regions associated with Asp398Asn (A). Colors in these regions are for illustration purpose and do not imply differences in statistical significance: they all passed the Pcorrected < 0.05 threshold such that more risk alleles are associated with less connectivity strength. Regions 1 and 2 are the two largest regions. They are located at the right (local maxima x, y, z: 24, −9, −19) and left (−23, −5, −16) ventral striatum/substantia innominata/extended amygdala/hippocampus; 3, orbitofrontal cortex (−19, 40, −14); 4, rostral cingulate (5, 29, 22); 5, middle cingulate (10, −14, 33); 6, middle temporal cortex (−53, −44, 7); 7, precuneus (−10, −57, 4); and 8, cuneus (2, −77, 14). (B) Coronal section of regions 1 and 2 at the level of the anterior commissure: their connectivity with the dACC (manually traced red area) are associated with Asp398Asn, smoking, and nicotine addiction severity. Arrows depict the functional connectivity between the dACC seed and these regions and do not imply directionality. L, left. Regions 3 through 8 were associated with Asp398Asn but not smoking or nicotine addiction severity. (C) The genotype effect on the eight circuits. (D and E) Significant relationships between connectivity strength and FTND in smokers in circuits 1 and 2. (F and G) Comparison of smokers (SK) and nonsmokers (NS) and show significantly reduced functional connectivity in circuit 1 in smokers compared with nonsmokers. “Connectivity” is the z score of the resting state functional connectivity strength. Error bars are SEs in all graphs. **Significant after Bonferroni correction. *Nominally significant.
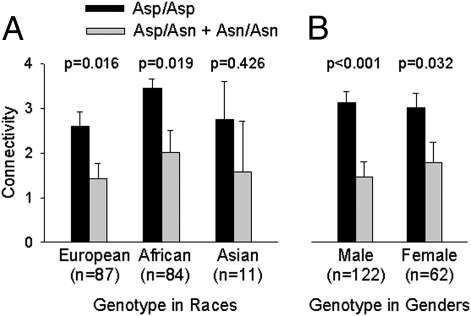
Post-hoc tests showed that, of the eight circuits identified, only the dACC–right ventral striatum circuit had significantly reduced functional connectivity strength in smokers compared with nonsmokers (P = 0.003; Fig. 1F). None of the other circuits were significant (all P ≥ 0.010) after Bonferroni correction for eight comparisons (P < 0.006). The genetic effect on this dACC–right ventral striatum circuit was independently present across ethnic backgrounds (Fig. 2A). Although the sample was skewed to have more male subjects, the genetic effect was significant in each sex (Fig. 2B). Overall, the genetic effect was replicable in multiple subgroups.
Fig. 2.
(A) Asp398Asn genotype effect is independently present across different ethnic backgrounds and is significant in subgroups of European and African ancestry. (B) The genetic effect is also independently significant in each sex. Connectivity refers to the functional connectivity strength between dACC–right ventral striatum/extended amygdala (circuit 1 in Fig. 1). Dark color bars are individuals with no risk allele; gray color bars are Asn risk allele carriers. Data of two subjects from other ethnic backgrounds are not shown.
We then used regression analyses to examine which, if any, circuits predicts nicotine addiction severity as measured by the Fagerström Test for Nicotine Addiction (FTND) in smokers. Reduced dACC–right ventral striatum (ΔR2 = 12.8%, F = 12.14, P < 0.001) and dACC–left ventral striatum (ΔR2 = 9.8%, F = 9.00, P = 0.004) circuit rsFC strength significantly predicted greater nicotine addiction severity (Fig. 1 D and E). The smoking variance explained by the allele-modulated circuits (9.8–12.8%) is much higher than the smoking variance explained by the genotype alone (approximately 1–4%; e.g., ref. 7). This is consistent with the more direct and thus expectedly higher penetrance from gene to circuit or from circuit to behavior, compared with the penetrance measured from gene to behavior. Given that many genes likely contribute to the genetic predisposition of smoking, with each expected to have only a small effect (7, 40), brain circuit measures may serve as an intermediate marker for the convergent effects of genes and thus have better explanatory power. Indeed, mediation analyses provide additional support: the right ventral striatum–dACC (Sobel test, z = 2.28, P = 0.022; bootstrapped 95% CI = 0.12–0.71) (41) and left ventral striatum–dACC (z = 2.35, P = 0.019; 95% CI = 0.12–0.64) rsFC strength significantly mediated gene (Asp398Asn) and behavior (i.e., FTND). None of the other circuits was significantly associated with FTND after Bonferroni correction.
Notwithstanding the aforementioned data, circuit impairment in the CHRNA5 398Asn carriers may simply be a secondary effect of smoking exposure, rather than a direct genetic effect per se. However, after covarying for chronic exposure (i.e., pack-years) and recent smoking before imaging (expired CO levels), we found that genotype still significantly contributed to both the right (t = −2.63, P = 0.010) and left (t = −2.89, P = 0.007) ventral striatum–dACC circuits. Nonsmoker Asn carriers also showed reduced rsFC compared with nonsmoker Asp homozygotes (no risk allele; right, t = −2.66, P = 0.009; left, t = −2.20, P = 0.031), confirming a genetic effect independent of smoking status. Asn/Asn and Asn/Asp were combined as the “Asn carriers” in these analyses. Together, these data strongly support a primary effect of gene rather than a secondary effect of smoking on connectivity strength.
These circuit findings were based on a dACC seed. We then undertook an extensive confirmatory study by segmenting the striatal–amygdale “terminal end” of the circuit into 16 anatomical seeds and repeating the whole-brain analyses. This “reverse” approach evaluates whether the dACC–ventral striatum is the only subcortical-based circuit associated with Asp398Asn and smoking. The segmented subregions included substantia innominata, amygdala, thalamus, hippocampus, and striatum; the latter was subdivided into caudate, putamen, globus pallidus, and nucleus accumbens (Fig. 3A). The segmentation methods are described in SI Methods. The imaging analyses followed that described for the dACC seed.
Fig. 3.
Significant findings from confirmatory subcortical seed analyses. (A) Partitions of subcortical regions (from anterior commissure: −5, 0, +5, and +20 mm). See SI Methods for partition procedures. (B) Seven of the functionally connected circuits (arrows) from subcortical “seeds” were significantly associated with both Asp398Asn and smoking. Middle: Manually painted subcortical seeds. The significant seeds were (1) right substantia innominata; (2) right amygdala; (3) right and (4) left nucleus accumbens; and (5) right and (6) left hippocampus. Left and Right: Significant functional connectivity destinations from these seeds. Colored clusters are areas significantly associated with Asp398Asn; those in red circles are also significantly associated with smoking. Blue color clusters refer to significantly reduced functional connectivity in Asn carriers compared with Asp homozygotes (statistics and P values and the complete results are shown in Fig. S3). Pink color clusters refer to the opposite effects. Note that six of the seven circuits that were significantly associated with both Asp398Asn and smokers are localized to the dACC such that the risk allele Asn reduced functional connectivity between the dACC and the right substantia innominata, right amygdala, bilateral nucleus accumbens, and bilateral hippocampus. The remaining circuit (pink arrow) was associated with smoking in the opposite direction. Arrows do not imply directionality of the path.
Many subcortical functional connectivity circuits were significantly associated with Asp398Asn [total of 58 from the 16 bilateral regions of interest (ROIs); Fig. S3]. Critically, only seven of these Asp398Asn-influenced circuits significantly separated smokers from nonsmokers (Fig. 3B), and are characterized by three convergent observations: (i) six of the seven were from subcortical regions to dACC such that the risk allele and smoking were both associated with reduced connectivity; (ii) the remaining circuit was a local circuit between nucleus accumbens and caudate; (iii) no other circuits were significantly and simultaneously associated with Asp398Asn and smoking. These “reverse” circuit findings strongly support the specificity of the dACC–ventral striatum/extended amygdala circuit.
Because 398Asn was not overrepresented in the psychiatric patient sample (psychiatric illness vs. genotype, n = 309, χ2 = 0.92, P = 0.630), a shared genetic etiology hypothesis for smoking and psychiatric conditions would not be supported here. Rather, there was a significant effect of diagnosis (presence or absence of psychiatric diagnosis, P = 0.026) and genotype (Asp/Asp or Asn carrier, P < 0.001; Fig. 4A), suggesting an additive disease load on the allele-influenced circuit. The diagnosis × genotype interaction term was not significant (P = 0.12) and was dropped, although from Fig. 4A the diagnosis effect appears more prominent in Asp homozygotes compared with Asn carriers, possibly as a result of a “ceiling” effect when disease and Asn effects were converged. We should emphasize that Asn carriers were associated with reduced rsFC in the nonpsychiatric group (P < 0.001) independently and also in the psychiatric group (P = 0.038) analyzed separately. Analyzing the effect of diagnosis versus smoking status showed that diagnosis (P = 0.008) and smoking (P = 0.013), but not their interaction, affected the identified circuit (Fig. 4B). Age and sex were covariates and were not significant in these analyses. Therefore, the genetic effect on the dACC–ventral striatum circuit is independently present and more robust in healthy controls. The correlation between FTND and circuit strength is also independent of disease and more robust in normal control smokers (Fig. 4 C and D).
Fig. 4.
Psychiatric disease effect on Asp398Asn-influenced dACC–right ventral striatum functional connectivity circuit. (A) Psychiatric illnesses (#P = 0.026) were associated with reduced connectivity strength in addition to the effect of 398Asn (##P < 0.001). Asn refers to Asn carriers (Asp/Asn and Asn/Asn). (B) Psychiatric illnesses (###P = 0.008) were associated with a reduced connectivity in addition to the effect of smoking (####P = 0.013). (C and D) The correlations between functional connectivity and FTND were numerically different in smokers with versus without psychiatric illnesses, although the correlation coefficients were not statistically different (P = 0.484).
Analyses of the rs1051730 SNP revealed a similar result to that seen for Asp398Asn, which is not surprising because of their strong linkage disequilibrium (r2 = 0.87; Table S2). Assuming that Asp398Asn is the functional locus, results from rs1051730 are not further reported. However, the relevance of this and other loci in LD with Asp398Asn cannot be ruled out.
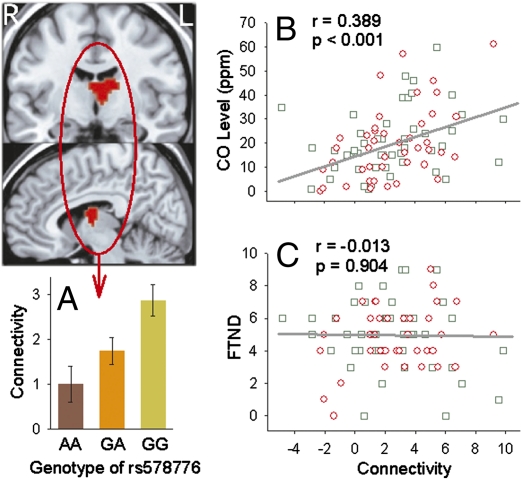
Finally, the rs578776 SNP represents an independent smoking-related signal in the α5-α3-β4 gene cluster (6). We found that its risk allele was associated with increased rsFC strength only between dACC and left anterior thalamus (Pcorrected < 0.05; Fig. 5A). The confluence of a nAChR α3 3′ UTR genetic effect on this location (as the only significant location) is intriguing because the α3 subunit has the highest expressed concentration in the thalamus in humans (42) and the medial habenula (located at the epithalamus) in rodents (43). However, this circuit was associated with recent smoking exposure (expired CO level; Fig. 5B) rather than addiction severity (Fig. 5C). Whether this effect is related to nicotine level, craving or withdrawal symptoms, or a potentially direct neural effect of CO (44) remains to be determined. This circuit also significantly differentiated smokers from nonsmokers (P = 0.024), but not healthy controls from psychiatric patient subjects (P = 0.774). We found no significant interaction between circuits under the influence of α3 rs578776 and α5 Asp398Asn.
Fig. 5.
The CHRNA3 3′ UTR rs578776 risk allele (G) was associated with increased dACC–left thalamus functional connectivity (Pcorrected < 0.05) (A). The significant cluster is centered at the anterior/medial dorsal thalamic nuclei but also involves the caudate head. The connectivity strength of this circuit related to CO levels measured before fMRI (B) but did not correlate with nicotine addiction severity (C). Green squares, healthy smokers; red circle, psychiatric patient smokers.
Discussion
We described evidence that the α5 Asp398Asn polymorphism affects a dACC–ventral striatum/extended amygdala functional circuit, such that the Asn “risk allele” is associated with reduced rsFC strength between these regions. Our findings suggest a plausible circuit-level explanation on why both α5 Asp398Asn and α3 rs578776 are associated with smoking, yet represent two independent smoking-related signals in the α5-α3-β4 gene cluster (6): the α3 rs578776-related, dACC–thalamus circuit appears sensitive to the “state” of smoking, whereas the Asp398Asn-influenced dACC–ventral striatum circuit is marking the “trait” aspects of nicotine addiction such that a weakened intrinsic synchronization in this circuit predisposes people to smoking and predicts addiction severity in those who do smoke.
Although multiple circuits appear to be affected by Asp398Asn, only bilateral dACC–ventral striatum circuits were associated with smoking status and addiction severity as measured by FTND, which is a heritable addiction marker (2), and is associated with a dACC–ventral striatum circuit (21). Although the previously identified circuit and the one isolated in the current study do not completely overlap, the convergence of a clinical phenotype analysis (21) and a genotype analysis onto what appear to be a single circuit provides a strong inference that this circuit functionally supports the effect of Asp398Asn on nicotine addiction. The centers of the current loci incorporate not just the ventral striatum, but also extend ventrally and posteriorly into the substantia innominata, amygdala, and hippocampus (Fig. 1A), coinciding with the sublenticular extended amygdala that is thought to be at the center of the cortical–subcortical reentrant pathways critically implicated in both addiction and psychiatric illnesses (39).
An arresting question is why Asp398Asn influences the functional connectivity of many circuits, yet most do not separate smokers from nonsmokers. CHRNA5 is evolutionally highly conserved (45), likely because it evolved to serve important functions long before cigarette smoking became prevalent in humans. Indeed, perhaps many of the allele-influenced circuits subserve functions unrelated to smoking; their potential pleiotropic effects on other behaviors would be of future interest. The current study shows that one of these circuits influences nicotine addiction. Note that we cannot rule out that Asp398Asn could be merely a marker of other CHRNA5 genetic changes in LD. However, in vitro experiments demonstrated that Asp398Asn is functional (6).
How the Asp398Asn polymorphism or other CHRNA5 variants in LD could modulate rsFC is an interesting and still open question. The α4β2 receptors are the main nAChRs on striatal dopaminergic terminals (46, 47) and by modulating dopamine release in the striatum and prefrontal cortical areas, including the anterior cingulate (8–10), they are thought to regulate the reinforcing effects and addictive properties of nicotine (48). The α5 participation in the α4β2 assembly (i.e., α4β2α5) impacts the α4β2 function (11). Deletion of the α5 gene in rodents reduces α4β2 receptor function (19, 49), which may resemble the effect of Asp398Asn amino acid substitution in humans (6). Whether such a nAChR–dopaminergic pathway mechanism in the ventral striatum/extended amygdale and anterior cingulate areas might have mediated the observed functional connectivity associated with nicotine addiction remains to be tested in future studies.
Inclusion of high-risk populations in etiopathophysiological studies is a classical approach for achieving new insight into medical conditions. The unique finding here is that psychiatric illnesses affect the same circuit independently from and in addition to the α5 genetic effect. 398Asn has repeatedly shown a signal for smoking (5, 7, 37, 50–54) but, thus far, not for increased smoking in other psychiatric diagnoses (36, 37). Because the sample sizes for psychiatric diagnoses in the present and many of these previous samples are relatively small, these disorders might potentially show a signal with still larger sample sizes.
The lack of 398Asn over-representation in the psychiatric population in the face of an additional disease effect on the identified Asn-influenced, smoking-related circuit raises an interesting hypothesis. Perhaps the higher risk of smoking in some mental illnesses is not necessarily a result of a shared genetic etiology, but rather shared neural circuits for smoking in the setting of maintained different genetic etiologies. Although most psychiatric patients were receiving psychotropic agents that may have confounded the gene–circuit relationships, the similar genetic effects found in the nonpsychiatric group (Fig. 4A) suggests that the findings are not primarily a result of the psychotropic agents. Taken together, these data suggest that the identified dACC–ventral striatum circuit may serve as a convergent path for nicotine addiction, in which the negative impact may come from the nAChR α5 risk allele and/or psychiatric disease–specific factors.
One obstacle in the discovery of more effective compounds for smoking cessation is the absence of preclinical animal model bioassays that have convincing predictive validity for human nicotine addiction (55). In this context, it is important to note that rsFC can be measured in anesthetized animals and revealed circuits similar to awake human subjects (23). Given the specificity of the circuit identified and the cross-species applicability of the assay method, this quantitative in vivo dACC–ventral striatum/extended amygdala measure could provide a valuable translational bioassay complimenting current animal models of human nicotine addition. Asp398Asn is robustly associated with nicotine dependence whereas multiple other genetic variants have been associated with smoking cessation (56). Comparative analyses of the convergence and divergence of neural circuits associated with these genes in future studies may yield new insight into mechanisms differentiating successful smoking cessation versus persistent smoking. Finally, results from this study by no means exhaust the possible genes or brain circuits that work together to support nicotine addiction. Our work describes one brain circuit that explains approximately 10% to 12% of the nicotine addiction behavioral variance (three to four times that of the gene itself) and differs in smokers, and whose function is coherently altered by a functional risk genotype, which may provide a tangible biomarker/target for new treatment development that may facilitate further progress on reducing smoking behavior.
Methods
Clinical Information.
Participants, between 18 and 58 y of age, gave written informed consent approved by Institutional Review Board panels. Healthy smokers and nonsmokers were identified through local media advertisements. Smokers were defined as those who smoked for at least 1 y and were currently smoking. The amount of their smoking before imaging was not uniformly controlled. Rather, the expired breath CO level was measured before fMRI, which provides an estimate of recent smoking level. Chronic exposure was estimated in pack-years. The FTND was used to measure nicotine addiction severity. Nonsmoker controls were defined by lifetime smoking of less than 20 cigarettes. Ex-smokers were past smokers who had quit smoking by self-report for any duration; their current nonsmoking status was verified by CO level before scan. Data from these individuals were only included in analyses that did not involve smoker-versus-nonsmoker comparisons. CO levels (ppm, mean ± SD) before scan were 20.08 ± 14.13 in smokers, 2.03 ± 2.82 in nonsmokers, and 3.11 ± 2.89 in past smokers. Self-reported ethnic descents were 131 European, 153 African, 20 Asian, and seven other. However, we used only genomically determined ethnicity as covariates in case-control association analyses.
The 191 individuals who underwent resting fMRI (184 included in analyses, plus seven in whom image quality assessment failed) were screened for urine toxicology (Triage), breath CO, and breath alcohol before fMRI. Positive breath alcohol, major medical conditions, and head injury history with cognitive sequelae were exclusionary. Nineteen of the 184 subjects were involved in a related imaging study (no genetic imaging analysis was involved) (21). Smokers and nonsmokers were similar in age (P = 0.80). There were proportionally more male smokers (χ2 = 8.62, P = 0.003).
All 311 subjects were screened by using Structured Clinical Interview for DSM-IV to identify psychiatric illnesses. The total sample included 208 subjects without DSM-IV Axis I diagnosis and 103 subjects with at least one diagnosis. Patients with psychiatric diagnoses were recruited from the Maryland Psychiatric Research Center clinics and neighboring mental health clinics. Table S1 provides sample size by smoking status and genotype. This full sample was used for genotype × diagnosis association analysis. Within the subjects with imaging data, 115 had no DSM-IV psychiatric diagnosis and 76 had at least one Axis I diagnosis. The primary psychiatric conditions in this sample were schizophrenia or schizoaffective disorder (n = 48), substance dependence (cocaine, alcohol, marijuana; n = 21), and anxiety and depressive disorders (n = 7). All these conditions are known to be associated with increased rates of smoking (4, 30, 33). Within smokers in the imaging group, nonpatient and patients were similar in pack-years (18.2 ± 14.5 vs. 17.3 ± 17.4, P = 0.78), FTND (4.8 ± 2.3 vs. 4.8 ± 1.8, P = 0.98), and CO level before scan (20.1 ± 13.0 vs. 19.4 ± 14.9, P = 0.80). Nonpatients and patients were not different in age (33.3 ± 9.4 y vs. 33.6 ± 10.2 y; P = 0.84) but were different in sex (male, 59.1% vs. 75.0%; P = 0.03). Age and sex were covariates in our analyses.
Gentotyping.
The three SNPs were genotyped using TaqMan genotyping assays. The 186 ancestrally informative genomic markers were genotyped with an Illumina SNP array. Additional gene selection rationale and genotyping information are provided in SI Methods.
Imaging.
Imaging data were collected on a 3-T Siemens Allegra scanner. Subjects underwent a minimum 5-min resting scan while they were instructed to rest, keep their eyes open, and not think of anything in particular. Functional images were processed to generate individual seed-based functional connectivity maps, as described elsewhere (21). Additional imaging and regional segmentation procedures are provided in SI Methods.
Statistical Analysis.
Details of statistical analysis are provided in SI Methods.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health Grants MH70644, MH79172, MH49826, MH77852, MH68580, M01-RR16500, and N01-DA-5-9909; the National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism Intramural Research Programs; and the Maryland Cigarette Restitution Fund Program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004745107/-/DCSupplemental.
References
- 1.Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine Tob Res. 1999;1(suppl 2):S51–S57. doi: 10.1080/14622299050011811. [DOI] [PubMed] [Google Scholar]
- 2.Vink JM, Willemsen G, Boomsma DI. Heritability of smoking initiation and nicotine dependence. Behav Genet. 2005;35:397–406. doi: 10.1007/s10519-004-1327-8. [DOI] [PubMed] [Google Scholar]
- 3.Regier DA, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- 4.Lasser K, et al. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 5.Saccone SF, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bierut LJ, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorgeirsson TE, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Picciotto MR. Nicotine as a modulator of behavior: Beyond the inverted U. Trends Pharmacol Sci. 2003;24:493–499. doi: 10.1016/S0165-6147(03)00230-X. [DOI] [PubMed] [Google Scholar]
- 9.Tapper AR, et al. Nicotine activation of alpha4* receptors: Sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- 10.Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- 11.Ramirez-Latorre J, et al. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- 12.Brody AL, et al. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- 13.Stein EA, et al. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry. 1998;155:1009–1015. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- 14.Rose JE, et al. Regional brain activity correlates of nicotine dependence. Neuropsychopharmacology. 2007;32:2441–2452. doi: 10.1038/sj.npp.1301379. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, et al. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci. 2007;27:14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grünwald F, Schröck H, Kuschinsky W. The effect of an acute nicotine infusion on the local cerebral glucose utilization of the awake rat. Brain Res. 1987;400:232–238. doi: 10.1016/0006-8993(87)90622-6. [DOI] [PubMed] [Google Scholar]
- 17.Wada E, McKinnon D, Heinemann S, Patrick J, Swanson LW. The distribution of mRNA encoded by a new member of the neuronal nicotinic acetylcholine receptor gene family (alpha 5) in the rat central nervous system. Brain Res. 1990;526:45–53. doi: 10.1016/0006-8993(90)90248-a. [DOI] [PubMed] [Google Scholar]
- 18.Winzer-Serhan UH, Leslie FM. Expression of alpha5 nicotinic acetylcholine receptor subunit mRNA during hippocampal and cortical development. J Comp Neurol. 2005;481:19–30. doi: 10.1002/cne.20357. [DOI] [PubMed] [Google Scholar]
- 19.Salminen O, et al. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- 20.Flora A, et al. Neuronal and extraneuronal expression and regulation of the human alpha5 nicotinic receptor subunit gene. J Neurochem. 2000;75:18–27. doi: 10.1046/j.1471-4159.2000.0750018.x. [DOI] [PubMed] [Google Scholar]
- 21.Hong LE, et al. Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66:431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 23.Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 24.Honey CJ, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci USA. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith SM, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Glahn DC, et al. Genetic control over the resting brain. Proc Natl Acad Sci USA. 2010;107:1223–1228. doi: 10.1073/pnas.0909969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 30.Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: Results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- 31.Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. Am J Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- 32.de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76:135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Ziedonis D, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine Tob Res. 2008;10:1691–1715. doi: 10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]
- 34.Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ. A population-based twin study of alcoholism in women. JAMA. 1992;268:1877–1882. [PubMed] [Google Scholar]
- 35.Purcell SM, et al. International Schizophrenia Consortium Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang JC, et al. COGEND collaborators and GELCC collaborators Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum Mol Genet. 2009;18:3125–3135. doi: 10.1093/hmg/ddp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grucza RA, et al. A risk allele for nicotine dependence in CHRNA5 is a protective allele for cocaine dependence. Biol Psychiatry. 2008;64:922–929. doi: 10.1016/j.biopsych.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Z, et al. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heimer L. A new anatomical framework for neuropsychiatric disorders and drug abuse. Am J Psychiatry. 2003;160:1726–1739. doi: 10.1176/appi.ajp.160.10.1726. [DOI] [PubMed] [Google Scholar]
- 40.Bergen AW, et al. Dopamine genes and nicotine dependence in treatment-seeking and community smokers. Neuropsychopharmacology. 2009;34:2252–2264. doi: 10.1038/npp.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 42.Rubboli F, et al. Distribution of neuronal nicotinic receptor subunits in human brain. Neurochem Int. 1994;25:69–71. doi: 10.1016/0197-0186(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 43.Whiteaker P, et al. Involvement of the alpha3 subunit in central nicotinic binding populations. J Neurosci. 2002;22:2522–2529. doi: 10.1523/JNEUROSCI.22-07-02522.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boehning D, Snyder SH. Novel neural modulators. Annu Rev Neurosci. 2003;26:105–131. doi: 10.1146/annurev.neuro.26.041002.131047. [DOI] [PubMed] [Google Scholar]
- 45.Couturier S, et al. Alpha 5, alpha 3, and non-alpha 3. Three clustered avian genes encoding neuronal nicotinic acetylcholine receptor-related subunits. J Biol Chem. 1990;265:17560–17567. [PubMed] [Google Scholar]
- 46.Zoli M, et al. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;22:8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grady SR, et al. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol. 2007;74:1235–1246. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- 49.Salas R, et al. The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol Pharmacol. 2003;63:1059–1066. doi: 10.1124/mol.63.5.1059. [DOI] [PubMed] [Google Scholar]
- 50.Bierut LJ, et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet. 2007;16:24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berrettini W, et al. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen X, et al. Variants in nicotinic acetylcholine receptors alpha5 and alpha3 increase risks to nicotine dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:926–933. doi: 10.1002/ajmg.b.30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le Marchand L, et al. Smokers with the CHRNA lung cancer-associated variants are exposed to higher levels of nicotine equivalents and a carcinogenic tobacco-specific nitrosamine. Cancer Res. 2008;68:9137–9140. doi: 10.1158/0008-5472.CAN-08-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sherva R, et al. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with ‘pleasurable buzz’ during early experimentation with smoking. Addiction. 2008;103:1544–1552. doi: 10.1111/j.1360-0443.2008.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lerman C, et al. Translational research in medication development for nicotine dependence. Nat Rev Drug Discov. 2007;6:746–762. doi: 10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- 56.Uhl GR, et al. Molecular genetics of successful smoking cessation: convergent genome-wide association study results. Arch Gen Psychiatry. 2008;65:683–693. doi: 10.1001/archpsyc.65.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.