Abstract
Embryonic stem cells (ESCs) can undergo unlimited self-renewal and retain the pluripotency to differentiate into all cell types in the body, thus holding great promise as a renewable source of cells for human therapy. The mechanisms that maintain self-renewal of ESCs remain unclear. Here we show that Nanog, a transcription factor crucial for the self-renewal of ESCs, is phosphorylated at multiple Ser/Thr-Pro motifs. This phosphorylation promotes the interaction between Nanog and the prolyl isomerase Pin1, leading to Nanog stabilization by suppressing its ubiquitination. Inhibition of Pin1 activity or disruption of Pin1–Nanog interaction in ESCs suppresses their capability to self-renew and to form teratomas in immunodeficient mice. Therefore, in addition to the stringent transcriptional regulation of Nanog, the expression level of Nanog is also modulated by posttranslational mechanisms.
Keywords: teratomas, self-renewal, ubiquitination, transcription
Embryonic stem cells (ESCs) can undergo unlimited self-renewal and retain the pluripotency to differentiate into all cell types in the body. Therefore, as a potentially unlimited source of various cell types, ESCs hold great promise for cell replacement therapy in many human diseases. To achieve their therapeutic potential, ESCs and their derivatives must be genetically stable to prevent tumorigenesis by the transplanted cells. Therefore, it is critical to elucidate the mechanisms that maintain the self-renewal and genomic stability of ESCs. Recent studies indicate that Nanog, an ESC-specific homeodomain protein, is required for the self-renewal of ESCs (1, 2). In this context, constitutive overexpression of Nanog suppresses the differentiation of ESCs induced by various stimuli such as RA or leukemia inhibitory factor withdrawl (1). In addition, decrease of Nanog expression in mouse and human ESCs leads to spontaneous differentiation of ESCs (2). Recent findings indicate that p53 directly suppresses the transcription of Nanog after DNA damage, contributing to the differentiation and elimination of DNA-damaged ESCs from the self-renewing pool (3). In addition, the important roles of p53 in maintaining the genomic stability of ESCs are further supported by the findings that p53−/− human ESCs are genetically unstable (4). Therefore, ESCs maintain their genomic stability by coordinating their self-renewal capability with DNA damage responses (5).
As a transcriptional factor, Nanog functions by activating or suppressing the expression of target genes. Although it remains unclear which of the transcription targets of Nanog are involved in maintaining the self-renewal of ESCs, recent genome-scale location analysis has identified a large panel of promoters that are bound by Nanog in human ESCs (6). Several studies have identified Nanog-interacting proteins that might collaborate with Nanog in the transcription of its target genes (7, 8). In addition, it has recently been reported that Nanog is cleaved by caspases upon induction of differentiation (9). However, in addition to stringent transcriptional regulation of Nanog, the posttranslational mechanisms that regulate the stability and activity of Nanog in ESCs remain largely unknown. Although there is evidence that Nanog is a phosphoprotein (10), the functional significance of this posttranslational modification has yet to be addressed.
The reversible phosphorylation of proteins on serine or threonine followed by proline residues (pS/pT-P), also called proline-directed phosphorylation, is critical in regulating numerous cellular events (11, 12). The peptidyl prolyl bond is characterized by a peculiar backbone conformation that allows the protein to adopt two slowly interconverting states; the switch between cis and trans conformation is a limiting step for protein folding and requires an isomerase to efficiently catalyze the process. Among the members of the prolyl isomerase family, Pin1 is the only one that can recognize the pS/pT-P motif and induce the cis/trans conversion of the proline bond (13, 14). Pin1 consists of an N-terminal protein–binding WW domain and a C-terminal domain (PPIase) that is responsible for the peptidyl-prolyl isomerase activity (13). The WW domain of Pin1 binds to phospho-Ser-Pro (pS-P) or phospho-Thr-Pro (pT-P)–containing peptide sequences (15). By isomerizing the pS/T-Pro bonds, Pin1 has been shown to induce conformational change in proteins, including cyclin D1, p73 and p53, thereby having profound impacts on their phosphorylation state, catalytic activity, protein–protein interactions, and turnover (16–19). In particular, recent results indicate that such Pin1 activity is correlated with a change in target protein stability through a ubiquitin-mediated mechanism (20–25). These data indicate that Pin1 dependent conformational changes are a unique signaling mechanism pivotal in regulating many cellular functions.
To investigate the regulation of Nanog by posttranslational modification, we identified several Ser/Thr-Pro motifs of Nanog that, when they have been phosphorylated, promote the interaction between Nanog and Pin1. This interaction is important for the stabilization of Nanog by reducing its ubiquitin dependent degradation and represents a mechanism critical for the self-renewal and the teratoma-forming potential of ESCs.
Results
Nanog Is Proline-Directed and Phosphorylated.
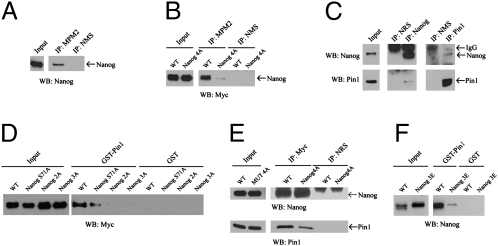
To identify the potential Ser/Thr-Pro motifs of Nanog that are phosphorylated, we inspected the protein sequence of the mouse, rat, and human Nanog and identified four conserved Ser/Thr-Pro motifs that, when they have been phosphorylated, may provide Pin1 binding sites (Fig. S1). To assess if some of these sites could be phosphorylated in ESCs, we used the MPM2 antibody that specifically recognizes the phosphorylated Ser/Thr-Pro motifs. Our findings indicate that Nanog is phosphorylated at Ser/Thr-Pro motifs in ESCs (Fig. 1A). To test whether these sites are indeed the phosphorylation sites, we introduced Ser/Thr to Ala mutations into these four sites (Ser-52, 65, and 71, and Thr-287) of the myc-tagged Nanog. The mutant Nanog protein is denoted Nanog4A. When the expression vectors expressing the myc-tagged WT Nanog and myc-tagged Nanog4A were independently transfected into ESCs and the expression of myc-tagged Nanog proteins were confirmed, MPM2 antibody was used to test whether Nanog4A is still phosphorylated at Ser/Thr-Pro motifs. The 4A mutation greatly reduces the phosphorylation of Nanog at Ser/Thr-Pro motifs in ESCs, indicating that these evolutionarily conserved sites are the major Ser/Thr-Pro motifs phosphorylated in ESCs (Fig. 1B). The residual levels of phosphorylation detected by MPM2 antibody suggested that other Ser/Thr-Pro motifs of Nanog might also be phosphorylated.
Fig. 1.
Nanog interacts with Pin1 through its phosphorylated Ser/Thr-Pro motifs. (A) The endogenous Nanog is phosphorylated at Ser/Thr-Pro motifs. Endogenous Nanog was immunoprecipitated with anti-MPM2 antibody that specifically recognizes phosphorylated Ser/Thr-Pro motifs, followed by Western blotting for the levels of Nanog protein in the immunoprecipitate. (B) Mapping of the phosphorylated Ser/Thr-Pro motifs of Nanog. Cell lysates derived from mouse ESCs expressing myc-tagged WT Nanog and myc-tagged Nanog4A were immunoprecipitated with MPM2 antibody, followed by Western blotting with anti-myc antibody. (C) The interaction of the endogenous Nanog and Pin1 in mouse ESCs shown by reciprocal coimmunoprecipitation analysis. NRS and NMS refer to normal rabbit serum and normal mouse serum, respectively, that are negative controls for the specificity of antibodies used in the immunoprecipitation. IP and WB refer to immunoprecipitation and Western blotting, respectively. (D) The phosphorylated Ser/Thr-Pro motifs at the N terminus of Nanog are responsible for binding to Pin1. GST-Pin1 pull-down assay were performed using lysates of HEK 293 cells expressing myc-tagged WT Nanog, NanogS71A, Nanog3A and 2A (D), and Nanog3E (E). GST was used as negative control for the pull down assay. (E) Phosphorylation of Nanog promotes its interaction with Pin1 in ESCs. The interaction between myc-tagged Nanog4A and Pin1 is greatly reduced in ESCs as shown by coimmunoprecipitation. (F) Nanog3E (Ser52, 65, 71 to Glu) mutation disrupts the interaction between Nanog and Pin1 as shown by GST-Pin1 pull-down assay.
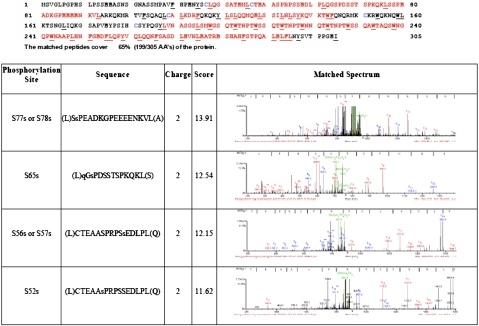
To further confirm that these Ser/Thr-Pro motifs of Nanog are phosphorylated in cells, myc-tagged Nanog was expressed in 293 cells because ESCs express low levels of endogenous and transgenic Nanog protein, making it difficult to analyze the phosphorylation of Nanog by MS. Myc-tagged Nanog protein purified from 293 cells was analyzed by MS, revealing that Nanog is phosphorylated at several residues, including Ser52 and Ser65, in 293 cells (Fig. 2).
Fig. 2.
Identification of the phosphorylation sites of Nanog by MS. Myc-tagged Nanog expressed in 293 cells were purified and analyzed by MS. Unique peptides from Nanog were identified with a protein sequence coverage of 65%. Four phosphorylation sites of Nanog were identified at S52, S56 or S57, S65, and S77 or S78. Phosphorylations of S52 and S65 were unambiguously identified, whereas the MS/MS data do not allow us to pinpoint the exact phosphorylation sites between S56/S57 and S77/S78.
Pin1 binds to phosphorylated Ser/Thr-Pro motifs, so we tested whether Pin1 interacts with Nanog in ESCs. Reciprocal coimmunoprecipitation experiments indicated that the endogenous Pin1 and Nanog interact in mouse ESCs (Fig. 1C). In addition, when myc-tagged Nanog was expressed in mouse ESCs, coimmunoprecipitation experiments indicated that it interacts with Pin1 (Fig. 1E). To confirm that the identified phosphorylated Ser/Thr-Pro motifs of Nanog are indeed the binding sites for Pin1, cell lysates from 293 cells expressing Nanog WT and the mutants Nanog S71A, Nanog3A (S52/65/71A), and Nanog2A (S52/65A) were subjected to a pull-down assay with GST-Pin1 or with GST alone as a negative control. The binding of Pin1 to Nanog3A and Nanog2A was dramatically reduced, indicating that the two phosphorylated motifs (Ser52, 65-Pro) of Nanog are important for its binding to Pin1 (Fig. 1D). Consistent with this notion, coimmunoprecipitation experiments showed that the interaction between Nanog4A and endogenous Pin1 was greatly reduced in mouse ESCs (Fig. 1E). To test whether the interaction between Nanog and Pin1 can be promoted by the negative charge conferred by phosphorylation, we used the GST-Pin1 pull-down assay to determine the interaction between Pin1and a Nanog mutant containing a phosphomimetic mutation (glutamic acid) at positions 52, 65, and 71 (denoted Nanog3E). Our findings indicate that these S-to-E mutations profoundly inhibited the binding of Nanog to Pin1 (Fig. 1F).
Inhibition of Pin1 Activity Suppresses ESC Self-Renewal by Reducing Nanog Protein Levels.
Pin1 is responsible for modulating the stability and activity of many transcription factors by isomerizing the phosphorylated Ser/Thr-Pro bonds (17, 26–28). Therefore, we speculate that Pin1 might regulate the stability and activity of Nanog. To test this hypothesis, we analyzed the expression of Nanog and Oct4 in mouse and human ESCs at different time points after treating with the Pin1 inhibitor PiB. PiB is a selective and reversible inhibitor of Pin1 isomerase activity (IC50 of approximately 1.5 μM) (29). Molecular modeling shows that PiB can bind Pin1 at the active site and inhibit its PPIase activity in a competitive manner by masking its substrate binding sites (29). A significant reduction of Nanog protein levels was detected in mouse ESCs within 8 h of PiB treatment (Fig. 3A). In contrast, protein levels of Oct4 were not affected by the same treatment, indicating that the reduction of Nanog protein levels after PiB treatment is not a result of the induction of differentiation by PiB (Fig. 3A). The mRNA levels of Nanog were normal whereas its protein levels were reduced at 4 h in mouse ESCs after PiB treatment, suggesting that Pin1 inhibitor decreases the stability of Nanog (Fig. 3A and Fig. S2A). In addition, Nanog-dependent transcription was normal at early time points after the treatment with Pin1 inhibitor (Fig. 3A and Fig. S2A). The impact of Pin1 inhibitor on the stability of Nanog was directly measured by treating ESCs with cycloheximide in the presence or absence of PiB; this showed that PiB decreases the stability of Nanog (Fig. 3B). These findings suggest that the isomerase activity of Pin1 is important for Nanog stabilization. Similarly to the findings in mouse ESCs, the protein levels of Nanog, but not Oct4, were significantly reduced in human ESCs within 8 h of PiB treatment, indicating an evolutionarily conserved role of Pin1 in stabilizing Nanog in ESCs (Fig. 3C).
Fig. 3.
Pin1 is important for the self-renewal of mouse and human ESCs. (A) Pin1 inhibitor reduces the protein levels of Nanog but not Oct4 in mouse ESCs. The protein levels of Nanog and Oct4 in mouse ESCs were analyzed at different time points after treatment with 20 μM PiB. The time points are indicated on the top. (B) Pin1 inhibitor reduces the stability of Nanog. The protein levels of Nanog in mouse ESCs were analyzed at different time points after the treatment with cycloheximide (CHX) in the presence or absence of Pin inhibitor (PiB). (C) Pin1 inhibitor reduces the protein levels of Nanog in human ESCs. Human ESCs were mock-treated or treated with 18 μM PiB and harvested 8 and 16 h after treatment for the analysis of the Nanog and Oct4 protein levels. The time points and treatment are indicated on the top. (D) Silencing of Pin1 expression via RNAi reduces the protein levels of Nanog in mouse ESCs. Pin1, Nanog, OCT4, and tubulin are indicated. (E) Pin1 inhibitor (PiB) suppresses the self-renewal of mouse and human ESCs. Clonogenic survival assay of mouse ESCs (Left) and human ESCs (Right). ESCs were trypsinized into single cells and plated on feeder layer at low density. Twenty-four hours after plating, the cells were mock treated or treated with increasing concentrations of PiB. The number of colonies are counted at 8 d (mouse ESC) or 14 d (human ESC) after treatment. Mean value from three independent experiments are presented with error bars. (F) Knockdown of Pin1 with RNAi in mouse ESCs reduces their self-renewal potential. The number of colonies was counted 8 d after plating. Mean values from three experiments are presented with error bars.
To confirm that Pin1 is responsible for stabilizing Nanog in ESCs, the expression of Pin1 in mouse ESCs was silenced by RNAi (Fig. 3D). When the protein levels of Pin1 were reduced in ESCs, the protein levels of Nanog were also reduced (Fig. 3D). In contrast, the protein levels of Oct4 and tubulin were not affected by Pin1 depletion (Fig. 3D). Therefore, we conclude that Pin1 stabilizes Nanog in ESCs.
Considering the critical role of Nanog in maintaining the self-renewal of ESCs, mouse and human ESCs were treated with increasing concentrations of PiB, and their self-renewal efficiency was evaluated by a clonal survival assay as previously described (30). The self-renewal of mouse and human ESCs were essentially abolished when treated with higher concentrations of PiB (Fig. 3E). To confirm that the observed self-renewal defect is a result of inhibition of Pin1 activity, the self-renewal of mouse ESCs in the presence of another Pin1 inhibitor Juglone (31) was ascertained. Juglone is a natural compound known to be a selective cell-permeable, irreversible inhibitor of parvulin-like PPIases, such as Escherichia coli parvulin, yeast Ptf1/Ess1, and human Pin1 (IC50 of approximately 1.5 μM) (31). Similarly to PiB, higher concentrations of Juglone suppressed the self-renewal of ESCs, indicating that Pin1 activity is important for ESC self-renewal (Fig. S2B). To exclude the possibility that the impaired self-renewal activity is a result of any cytotoxic effect of the Pin1 inhibitor on ESCs, ESCs treated with high concentrations of PiB were stained with Annexin V to detect apoptotic and dead cells. No significant difference in cell death was found between ESCs treated with PiB and mock-treated ESCs (Fig. S2C). In further support of a role of Pin1 in the self-renewal of ESCs, knockdown of Pin1 in ESCs with siRNA reduced their self-renewal (Fig. 3F). Therefore, functional Pin1 is required for the self-renewal of ES cells.
Pin1 Promotes Nanog Stabilization via a Ubiquitin Dependent Mechanism.
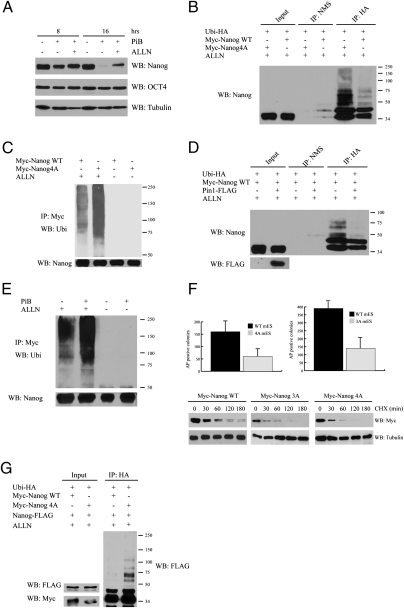
To understand how Pin1 regulates the protein stability of Nanog, we tested whether Pin1 inhibits the degradation of Nanog in a proteasome-dependent manner. The proteasome inhibitor ALLN could prevent the reduction of Nanog protein levels after PiB treatment, suggesting that Pin1 might inhibit the ubiquitin-proteasome–dependent degradation of Nanog (Fig. 4A). To investigate whether Pin1 affects Nanog ubiquitination, HEK293 cells were cotransfected with vectors expressing HA-tagged ubiquitin together with Myc-tagged WT Nanog or Myc-tagged Nanog4A, respectively. The ubiquitination levels of Nanog4A were much higher than those of WT Nanog, suggesting that Nanog is ubiquitinated in ESCs and the interaction between Pin1 and Nanog inhibits the ubiquitination of Nanog (Fig. 4B). Consistent with these findings, the ubiquitination levels of Nanog4A were much higher than WT Nanog in ESCs (Fig. 4C). In addition, overexpression of Pin1 reduced the ubiquitination of Nanog (Fig. 4D). As treatment with Pin1 inhibitor also increased the ubiquitination of Nanog in ESCs, the isomerase activity of Pin1 is important for inhibiting the ubiquitination of Nanog in ESCs (Fig. 4E). In summary, these findings suggest that Pin1-dependent isomerization of Nanog stabilizes Nanog in ESCs by inhibiting its ubiquitination.
Fig. 4.
Pin1 inhibits the ubiquitination of Nanog. (A) Proteosome-dependent pathway contributes to Nanog degradation. Mouse ESCs were mock-treated or treated with 20 μM PiB. Four hours before harvest, the PiB-treated cells were mock-treated or treated with the proteasome inhibitor ALLN. The treatments and time points are indicated on the top. The interaction between Pin1 and Nanog promotes the ubiquitination of Nanog in HEK293 cells (B) and in mouse ESCs (C). In B, HEK293 cells expressing HA-ubiquitin together with myc-tagged WT Nanog or Nanog4A were mock-treated or treated with ALLN. The ubiquitination of Nanog was revealed by immunoprecipitation with anti-HA antibody followed by immunoblotting with anti-Nanog antibody. In C, myc-tagged WT Nanog or Nanog4A was immunoprecipitated from ESCs expressing these proteins followed by immunoblotting with antiubiquitin antibody. The levels of unubiquitinated Nanog is also shown. (D) Increased Pin1 expression reduces the ubiquitination of Nanog in HEK293 cells. HEK293 cells expressing HA-ubiquitin and myc-tagged WT Nanog in the absence or presence of the overexpression of Flag-Pin1 were immunoprecipitated with anti-HA antibody followed by immunoblotting with anti-Nanog antibody. (E) Pin1 inhibitor greatly increases the ubiquitination of Nanog in mouse ESCs. Mouse ESCs expressing myc-tagged Nanog were mock-treated or treated with PiB for 6 h in the absence or presence of ALLN. The ubiquitination of Nanog was detected by immunoprecipitation with anti-myc antibody followed by immunoblotting with antiubiquitin antibody. The total amount of unubiquitinated Nanog was revealed by anti-Nanog antibody. (F) Self-renewal of ESCs expressing Nanog 4A and 3A is reduced compared with WT. ESCs expressing Nanog WT, 4A, and 3A were plated at clonal density and the colonies were stained with the alkaline phosphatase detection kit and counted (Top). Nanog4A and 3A are less stable than WT Nanog in ESCs (Bottom). ESCs expressing myc-tagged WT Nanog, Nanog4A, or Nanog3A were incubated with cycloheximide and harvested at the indicated time points. The protein levels of myc-tagged Nanog are revealed with anti-myc antibody. (G) The expression of Nanog4A triggers NanogWT to ubiquitin mediated degradation. HEK293 cells expressing HA-ubiquitin and Nanog-FLAG in the presence of NanogWT-Myc or Nanog4A-Myc, respectively, were immunoprecipitated by HA antibody and probed with an anti-FLAG antibody.
Compared with WT control, mouse ESCs expressing Nanog4A or Nanog3A were impaired in self-renewal with a decreased stability of mutant Nanog protein compared with WT, indicating the importance of these phosphorylation events of Nanog in maintaining its stability and the self-renewal of ESCs (Fig. 4F). As Nanog functions as a dimer (32, 33), this finding suggests that Nanog4A and Nanog3A could destabilize the endogenous WT Nanog through dimerization. In support of this notion, coexpression of WT Nanog and Nanog4A leads to their dimerization and to increased ubiquitination of WT Nanog (Fig. 4G).
Disruption of the Pin1-Nanog Pathway Impairs the Teratoma-Forming Potential of ESCs.
The unlimited self-renewal capability and pluripotency of ESCs enables undifferentiated ESCs to form teratomas after transplantation into immunodeficient hosts. Pin1 is required for the self-renewal of ESCs, so we tested the importance of Pin1 in the formation of teratomas by ESCs. Coinjection of mouse ESCs with the Pin1 inhibitor PiB greatly reduced the size of teratomas formed in immunodeficient mice compared with that of WT ESCs implanted in the same mice (Fig. 5A). Similarly, pretreatment of mouse ESCs with PiB for 24 h before implantation into immunodeficient mice also significantly reduced the size of teratomas (Fig. 5B). Importantly, the levels of Nanog and Oct4 were significantly reduced in the teratomas derived from ESCs treated with Pin1 inhibitor, suggesting that transient Pin1 inhibition can significantly reduce the potential to form teratomas by mouse ESCs (Fig. 5C); the cells that express Oct4 and Nanog are the self-renewing stem cells (34).
Fig. 5.
Pin1 and its interaction with Nanog is important for teratoma formation of ESCs in SCID mice. (A) Pin1 inhibitor (PiB) suppresses the teratomas formation when mixed with mouse ESCs. Mouse ESCs mixed with PiB (20 μM) were injected s.c. into right side of SCID mice. As an internal control for the potential of teratomasformation by the treated ESCs, the same number of mock-treated ESCs was implanted at the left side of the same SCID mice. Approximately 4 wk after implantation, tumors were excised and weighted. Representative image of one set of tumors derived from treated and control ESCs is shown. The ratio of the weight of the tumor derived from treated ESCs versus untreated control is shown below. Mean ratio from three independent experiments is shown with error bars. (B) Pretreatment of mouse ESCs with PiB for 24 h suppresses teratoma formation in SCID mice. Mouse ESCs were mock-treated or treated with PiB (20 μM) for 24 h before implantation. The treated ESCs were implanted on the right side of the SCID whereas the same number of control ESCs were implanted on the left side of the same mouse. The ratio of the weight of the tumor derived from treated ESCs versus untreated control is shown below. Mean ratio from three independent experiments is shown with error bars. (C) The levels of Nanog and Oct4 are dramatically reduced in the teratomas derived from PiB-treated ESCs. Protein extracts from control and PiB-treated teratomas were probed with Nanog, OCT4, and tubulin antibody. (D) The interaction between Pin1 and Nanog is important for teratomas formation by mouse ESCs. Mouse ESCs expressing Nanog4A or Nanog3A (as indicated) and control ESCs were implanted into left and right side of the same SCID mice, respectively. The ratio of the weight of the tumor-derived from treated ESCs versus untreated control is shown below. Mean values from three independent experiments are shown with error bars. (E) Pin1 inhibitor suppresses the teratomas formation of human ESCs in SCID mice. Human ESCs mixed with PiB and mock-treated control ESCs were implanted into the right and left sides of the same SCID mice. Six weeks later, the tumors were weighted and shown. The ratio of the weight of the tumor derived from treated ESCs versus untreated control is shown below.
Pin1 regulates the stability and activity of several proteins important for cellular proliferation and survival. To evaluate the importance of the Pin1-Nanog interaction in teratoma formation by ESCs, we assayed teratoma formation by mouse ESCs expressing Nanog4A and Nanog3A in immunodeficient mice. The sizes of the teratomas formed by ESCs expressing Nanog4A or Nanog3A were approximately 20% or 30% of that formed by control ESCs implanted in the same mice, supporting the notion that the Pin1-Nanog pathway is important for teratoma formation by ESCs (Fig. 5D). This finding also provides a functional link between the capability of ESCs to undergo self-renewal and teratoma formation in vivo. As the roles of Pin1 in Nanog stabilization and ESC self-renewal are evolutionarily conserved between mouse and human ESCs, we tested whether Pin1 inhibitor can also suppress teratoma formation by hESCs. Similar to the findings with mouse ESCs, treating human ESCs with Pin1 inhibitor suppressed the formation of teratomas by human ESCs in immunodeficient mice (Fig. 5E).
Discussion
The expression and activity of Nanog must be maintained to allow efficient self-renewal of ESCs. Like other important transcription factors such as p53, the stability and activity of Nanog might be efficiently modulated by posttranslational modifications (35). In support of this idea, we demonstrated that phosphorylation of Nanog at Ser/Thr-Pro motifs promotes its interaction with Pin1, leading to the inhibition of ubiquitin-proteasome–dependent degradation of Nanog. To our knowledge, this is the first reported posttranslational mechanism that is evolutionarily conserved to regulate the stability of Nanog in mouse and human ESCs. Therefore, in addition to stringent transcriptional regulation, posttranslational modification plays important roles in modulating the protein levels of pluripotency factors critical for the self-renewal of ESCs.
Several lines of evidence support the importance of this posttranslational mechanism in ESC self-renewal. First, Pin1 activity is important to stabilize Nanog in ESCs by inhibiting its ubiquitination. The prolyl isomerization activity of Pin1 is required for Nanog stabilization, suggesting that the Pin1-induced conformational change of Nanog might inhibit its ubiquitination. In support of this notion, recent studies have shown that Pin1 acts as a ubiquitination switch in regulating p53 and other transcription factors (23). Second, by using Nanog phosphorylation site mutants that cannot interact with Pin1, we have shown that the disruption of the interaction between Nanog and Pin1 suppresses ESC self-renewal. Pin1 is known to regulate the stability and activity of many transcription factors such as NF-κB and p53 that are involved in cell cycle regulation and survival, so this finding underscores the importance of the Nanog-Pin1 pathway in ESC self-renewal. Because Nanog functions as a dimer, our findings that unphosphorylatable Nanog is highly unstable and can promote the ubiquitination of coexpressed WT Nanog indicate a dynamic regulation of Nanog stability by phosphorylation. In this context, one might speculate that unphosphorylated Nanog could reduce the total amount of Nanog dimers in ESCs by ubiquitin-dependent proteolysis of both unphosphorylated Nanog and its dimerized phosphorylated partner.
The protein kinases that mediate the phosphorylation of Nanog at these Ser/Thr-Pro motifs remain to be identified. Based on the findings that the phosphorylation of Nanog at the Ser/Thr-Pro motifs is also evident when Nanog is ectopically expressed in differentiated 293 cells, we can conclude that the kinases that mediate the phosphorylation of Nanog at Ser/Thr-Pro motifs are not ESC-specific. Previous studies have shown that cyclin-dependent kinases and mitogen activated protein kinases are the major types of kinases involved in the phosphorylation of Ser/Thr-Pro motifs of other proteins (36). If these kinases are also involved in phosphorylating Nanog at the Ser/Thr-Pro motifs leading to its stabilization, it would suggest that the protein levels of Nanog could be regulated by cell cycle or mitogenic pathways. In this context, considering the critical roles of Nanog in the self-renewal of ESCs, this posttranslational modification could serve as a mechanism for ESCs to sense the cellular proliferation state and growth conditions to dictate whether to undergo self-renewal or to differentiate.
Materials and Methods
Detailed materials and methods can be found in SI Materials and Methods.
Expression Constructs.
Phosphorylation site mutations (Ser/Thr to Ala) of Nanog were generated by site-directed mutagenesis as previously described (3) and verified by sequencing. The myc-tagged Nanog cDNAs and the HA-tagged ubiquitin were cloned into a vector containing the chicken β-actin promoter. The Pin1 knockdown plasmid was generated by cloning the sequence specifically targeting the 21-nucleotide region (GAGACCTGGGTGCCTTCAGCA) conserved in mouse, rat, and human mRNA downstream an 1H promoter (37). The Pin-FLAG was constructed by attaching a FLAG tag at the C terminus of Pin. The Nanog-HA and its phosphorylation site(s) mutants were generated by adding a HA tag at the C terminus of Nanog or its mutants.
Supplementary Material
Acknowledgments
We thank Z. Rong for technical support and Dr. A. Varki for histological analysis of teratomas. This work was supported by Grant RC1-00148 from the California Institute of Regenerative Medicine (to Y.X.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005847107/-/DCSupplemental.
References
- 1.Chambers I, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 2.Mitsui K, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 3.Lin T, et al. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol. 2005;7:165–171. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]
- 4.Song H, Chung SK, Xu Y. Modeling disease in human ESCs using an efficient BAC-based homologous recombination system. Cell Stem Cell. 2010;6:80–89. doi: 10.1016/j.stem.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Zhao T, Xu Y. p53 and stem cells: New developments and new concerns. Trends Cell Biol. 2010;20:170–175. doi: 10.1016/j.tcb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Boyer LA, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, et al. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- 8.Liang J, et al. Nanog and Oct4 associate with unique transcriptional repression complexes in embryonic stem cells. Nat Cell Biol. 2008;10:731–739. doi: 10.1038/ncb1736. [DOI] [PubMed] [Google Scholar]
- 9.Fujita J, et al. Caspase activity mediates the differentiation of embryonic stem cells. Cell Stem Cell. 2008;2:595–601. doi: 10.1016/j.stem.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yates A, Chambers I. The homeodomain protein Nanog and pluripotency in mouse embryonic stem cells. Biochem Soc Trans. 2005;33:1518–1521. doi: 10.1042/BST0331518. [DOI] [PubMed] [Google Scholar]
- 11.Lu KP, Liou YC, Zhou XZ. Pinning down proline-directed phosphorylation signaling. Trends Cell Biol. 2002;12:164–172. doi: 10.1016/s0962-8924(02)02253-5. [DOI] [PubMed] [Google Scholar]
- 12.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 13.Ranganathan R, Lu KP, Hunter T, Noel JP. Structural and functional analysis of the mitotic rotamase Pin1 suggests substrate recognition is phosphorylation dependent. Cell. 1997;89:875–886. doi: 10.1016/s0092-8674(00)80273-1. [DOI] [PubMed] [Google Scholar]
- 14.Shen M, Stukenberg PT, Kirschner MW, Lu KP. The essential mitotic peptidyl-prolyl isomerase Pin1 binds and regulates mitosis-specific phosphoproteins. Genes Dev. 1998;12:706–720. doi: 10.1101/gad.12.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verdecia MA, Bowman ME, Lu KP, Hunter T, Noel JP. Structural basis for phosphoserine-proline recognition by group IV WW domains. Nat Struct Biol. 2000;7:639–643. doi: 10.1038/77929. [DOI] [PubMed] [Google Scholar]
- 16.Liou YC, et al. Loss of Pin1 function in the mouse causes phenotypes resembling cyclin D1-null phenotypes. Proc Natl Acad Sci USA. 2002;99:1335–1340. doi: 10.1073/pnas.032404099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zacchi P, et al. The prolyl isomerase Pin1 reveals a mechanism to control p53 functions after genotoxic insults. Nature. 2002;419:853–857. doi: 10.1038/nature01120. [DOI] [PubMed] [Google Scholar]
- 18.Mantovani F, et al. Pin1 links the activities of c-Abl and p300 in regulating p73 function. Mol Cell. 2004;14:625–636. doi: 10.1016/j.molcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Shaw PE. Peptidyl-prolyl cis/trans isomerases and transcription: Is there a twist in the tail? EMBO Rep. 2007;8:40–45. doi: 10.1038/sj.embor.7400873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brondani V, Schefer Q, Hamy F, Klimkait T. The peptidyl-prolyl isomerase Pin1 regulates phospho-Ser77 retinoic acid receptor alpha stability. Biochem Biophys Res Commun. 2005;328:6–13. doi: 10.1016/j.bbrc.2004.12.130. [DOI] [PubMed] [Google Scholar]
- 21.Luo Z, Wijeweera A, Oh Y, Liou YC, Melamed P. Pin1 facilitates the phosphorylation-dependent ubiquitination of SF-1 to regulate gonadotropin {beta}-subunit gene transcription. Mol Cell Biol. 2010;30:745–763. doi: 10.1128/MCB.00807-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakano A, et al. Pin1 down-regulates transforming growth factor-beta (TGF-beta) signaling by inducing degradation of Smad proteins. J Biol Chem. 2009;284:6109–6115. doi: 10.1074/jbc.M804659200. [DOI] [PubMed] [Google Scholar]
- 23.Siepe D, Jentsch S. Prolyl isomerase Pin1 acts as a switch to control the degree of substrate ubiquitylation. Nat Cell Biol. 2009;11:967–972. doi: 10.1038/ncb1908. [DOI] [PubMed] [Google Scholar]
- 24.Yeh E, et al. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat Cell Biol. 2004;6:308–318. doi: 10.1038/ncb1110. [DOI] [PubMed] [Google Scholar]
- 25.Zhou W, et al. Pin1 catalyzes conformational changes of Thr-187 in p27Kip1 and mediates its stability through a polyubiquitination process. J Biol Chem. 2009;284:23980–23988. doi: 10.1074/jbc.M109.022814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanya KJ, Liu Y, Means AR, Kao HY. Cdk2 and Pin1 negatively regulate the transcriptional corepressor SMRT. J Cell Biol. 2008;183:49–61. doi: 10.1083/jcb.200806172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryo A, Nakamura M, Wulf G, Liou YC, Lu KP. Pin1 regulates turnover and subcellular localization of beta-catenin by inhibiting its interaction with APC. Nat Cell Biol. 2001;3:793–801. doi: 10.1038/ncb0901-793. [DOI] [PubMed] [Google Scholar]
- 28.Li H, et al. Pin1 contributes to cervical tumorigenesis by regulating cyclin D1 expression. Oncol Rep. 2006;16:491–496. [PubMed] [Google Scholar]
- 29.Uchida T, et al. Pin1 and Par14 peptidyl prolyl isomerase inhibitors block cell proliferation. Chem Biol. 2003;10:15–24. doi: 10.1016/s1074-5521(02)00310-1. [DOI] [PubMed] [Google Scholar]
- 30.Kang J, Bronson RT, Xu Y. Targeted disruption of NBS1 reveals its roles in mouse development and DNA repair. EMBO J. 2002;21:1447–1455. doi: 10.1093/emboj/21.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hennig L, et al. Selective inactivation of parvulin-like peptidyl-prolyl cis/trans isomerases by Juglone. Biochemistry. 1998;37:5953–5960. doi: 10.1021/bi973162p. [DOI] [PubMed] [Google Scholar]
- 32.Mullin NP, et al. The pluripotency rheostat Nanog functions as a dimer. Biochem J. 2008;411:227–231. doi: 10.1042/BJ20080134. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Levasseur DN, Orkin SH. Requirement of Nanog dimerization for stem cell self-renewal and pluripotency. Proc Natl Acad Sci USA. 2008;105:6326–6331. doi: 10.1073/pnas.0802288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wernig M, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 35.Xu Y. Regulation of p53 responses by post-translational modifications. Cell Death Differ. 2003;10:400–403. doi: 10.1038/sj.cdd.4401182. [DOI] [PubMed] [Google Scholar]
- 36.Schutkowski M, et al. Role of phosphorylation in determining the backbone dynamics of the serine/threonine-proline motif and Pin1 substrate recognition. Biochemistry. 1998;37:5566–5575. doi: 10.1021/bi973060z. [DOI] [PubMed] [Google Scholar]
- 37.Becker EB, Bonni A. Pin1 mediates neural-specific activation of the mitochondrial apoptotic machinery. Neuron. 2006;49:655–662. doi: 10.1016/j.neuron.2006.01.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.