Abstract
Long-term experience through development and evolution and shorter-term training in adulthood have both been suggested to contribute to the optimization of visual functions that mediate our ability to interpret complex scenes. However, the brain plasticity mechanisms that mediate the detection of objects in cluttered scenes remain largely unknown. Here, we combine behavioral and functional MRI (fMRI) measurements to investigate the human-brain mechanisms that mediate our ability to learn statistical regularities and detect targets in clutter. We show two different routes to visual learning in clutter with discrete brain plasticity signatures. Specifically, opportunistic learning of regularities typical in natural contours (i.e., collinearity) can occur simply through frequent exposure, generalize across untrained stimulus features, and shape processing in occipitotemporal regions implicated in the representation of global forms. In contrast, learning to integrate discontinuities (i.e., elements orthogonal to contour paths) requires task-specific training (bootstrap-based learning), is stimulus-dependent, and enhances processing in intraparietal regions implicated in attention-gated learning. We propose that long-term experience with statistical regularities may facilitate opportunistic learning of collinear contours, whereas learning to integrate discontinuities entails bootstrap-based training for the detection of contours in clutter. These findings provide insights in understanding how long-term experience and short-term training interact to shape the optimization of visual recognition processes.
Keywords: contour integration, shape perception, brain imaging, visual cortex
The ability to detect and identify meaningful targets in cluttered scenes is a fundamental skill for survival and social interactions. In fact, it is thought that the visual system is optimized through evolution and development for the detection of frequently occurring regularities that typically define shape contours in natural scenes (e.g., elements collinear to the contour path) (1–3). Previous studies have shown that human observers are indeed better at detecting collinear contours than contours defined by regularities (e.g., elements orthogonal to the contour path) that typically define discontinuities (e.g., texture boundaries) rather than coherent shape contours (4–6). However, recent computational approaches propose that experience with the statistics of natural environments in adulthood plays a critical role in enhancing our ability to interpret complex scenes (7, 8). Our previous work showed that observers learn to integrate image discontinuities (i.e., orthogonal elements) for contour detection, suggesting that short-term training may alter the utility of image regularities (9, 10). Despite accumulating computational and behavioral evidence for the role of experience in the interpretation of complex scenes, the brain plasticity mechanisms that mediate learning of statistical regularities in natural images remain largely unknown.
Here, we combine behavioral and functional MRI (fMRI) measurements to investigate the human-brain plasticity mechanisms that facilitate the detection of shape contours in cluttered scenes. Previous work (11) has suggested two different mechanisms for learning to segment objects from background clutter: an opportunistic mechanism that relates to the observers’ ability to exploit image cues (e.g., frequently occurring regularities) that enhance segmentation and a bootstrap-based mechanism that relates to learning new features for detecting targets in cluttered scenes. We ask whether discrete brain circuits are implicated in these different learning processes. We reasoned that learning of atypical contours (i.e., orthogonal contours) relates to bootstrap-based training mechanisms and may require supervised training with feedback, whereas learning of collinear contours relates to opportunistic learning mechanisms and may occur simply by frequent exposure that enhances the observers’ ability to exploit typical image regularities. We measured behavioral and fMRI responses to stimuli comprising of collinear and orthogonal contours embedded in background clutter (Fig. 1A and Fig. S1). Both contour types were comprised of widely separated Gabor elements, ensuring that the detection of collinear and orthogonal contours was equally difficult before training. We tested for the link between behavioral improvement and changes in brain-activation patterns after supervised training (i.e., contour-detection task with error feedback) or exposure (i.e., observers engaged in a contrast discrimination task irrelevant to contour detection) to the different contour types (i.e., collinear or orthogonal).
Fig. 1.
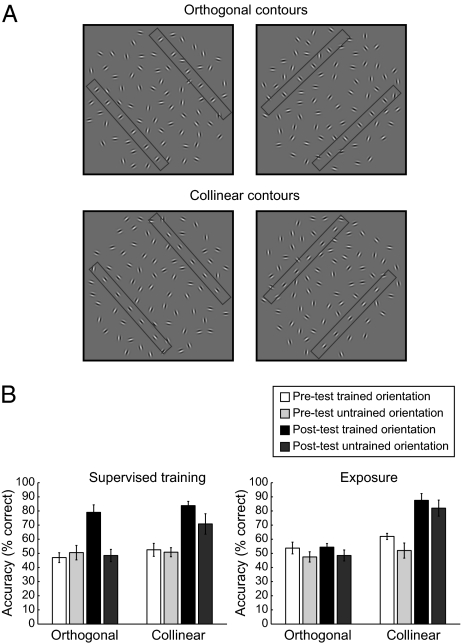
Stimuli and behavioral results. (A) Examples of collinear and orthogonal contours used for behavioral testing presented at two different contour orientations (45° and 135°). For demonstration purposes only, two rectangles illustrate the position of the two contour paths in each stimulus. (B) Average behavioral performance across subjects (percent correct) before and after supervised training or exposure. Error bars denote SEM.
Our findings provide evidence for discrete brain learning mechanisms that mediate our ability to detect shape contours in cluttered scenes. Specifically, learning to integrate discontinuities (i.e., elements orthogonal to contour paths) requires task-specific training, is stimulus-dependent, and enhances contour processing in intraparietal regions. In contrast, learning regularities typical in natural contours (i.e., collinearity) can occur simply through frequent exposure, generalizes across untrained stimulus features, and shapes contour processing in occipitotemporal regions. These findings provide insights into the interplay between short-term training and longer-term brain plasticity mechanisms that mediate our ability to interpret complex scenes.
Results
Behavioral Results.
We tested contour detection before and after training in a two-interval forced-choice task (i.e., observers judged which stimulus interval contained a contour). Analysis of the observers’ behavioral performance showed that training with feedback (i.e., supervised training) is necessary for improvement in the detection of orthogonal contours, whereas detection of collinear contours can be improved simply by exposure to the stimuli (Fig. 1B and Figs. S2 and S3). In particular, to quantify the observers’ performance in the contour-detection task, we measured accuracy (percent correct) when observers were presented with fully aligned contours (i.e., zero contour-element jitter). Before training, detection was difficult for both collinear (54.09 ± 2.07%) and orthogonal (49.77 ± 2.16%) contours. No significant differences were observed for contour types [collinear vs. orthogonal: F(1,21) = 2.79, P < 0.17], training procedures [supervised vs. exposure: F(1,21) = 1.08, P = 0.31], or global contour orientations [F(1,21) = 1.76, P = 0.20]. After training, the observers’ performance in the detection of orthogonal contours improved significantly after supervised training but not after mere exposure. A repeated measures ANOVA showed significantly higher accuracy in the detection of orthogonal contours after supervised training [F(1,4) = 18.75, P < 0.05] but not exposure [F(1,5) = 0.05, P = 0.84]. Specifically, after supervised training, observers’ detection performance was similar [i.e., no significant difference: F(1,10) = 0.66, P = 0.44] for orthogonal and collinear contours. This learning effect after supervised training was specific to the trained contour orientation, as indicated by an interaction between session (pretest vs. posttest) and global contour orientation [F(1,4) = 8.95, P < 0.05].
In contrast, for collinear contours, observers showed similar improvement in detection performance [i.e., no significant differences: F(1,10) = 1.08, P = 0.33] after supervised training or exposure. A repeated-measures ANOVA showed significantly higher accuracy in the detection of collinear contours after supervised training [F(1,5) = 26.63, P < 0.01] or exposure [F(1,4) = 35.35, P < 0.01]. Interestingly, these learning effects were evident for both trained [supervised training: F(1,5) = 22.81, P < 0.01; exposure: F(1,4) = 16.16, P < 0.05] and untrained [supervised training: F(1,5) = 12.97, P < 0.05; exposure: F(1,4) = 49.66, P < 0.01] orientations. However, detection performance was significantly higher for trained compared with untrained global orientations after supervised training [F(1,5) = 6.74, P < 0.05] but marginally different after exposure [F(1,6) =6.54, P = 0.06]. These results suggest at least partial transfer of learning for collinear contours to untrained global contour orientations.
Could the lack of a significant learning effect after exposure to orthogonal contours relate to the performance of individual participants before training (e.g., observers with higher performance before training may show stronger improvement after training)? Correlation analysis on the behavioral performance of participants trained or exposed to collinear or orthogonal contours did not show any significant relationship before and after training (R = 0.24, P = 0.12). Furthermore, no significant differences were observed in performance for collinear vs. orthogonal contours before exposure [F(1,21) = 2.29, P = 0.15]. These analyses suggest that behavioral improvement in the detection of collinear, but not orthogonal, contours after exposure-based learning could not be caused by any individual differences in task difficulty before training.
fMRI Results: Contour-Responsive Regions.
To identify brain regions involved in processing the contour stimuli, we compared activations with contour vs. random stimuli after training (postscan session). Consistent with previous findings (10), we observed significantly higher activations (random effect analysis, P < 0.001, cluster-size threshold corrected, 80 mm2) for contours than random patterns in higher occipitotemporal [V3A, V3B/ kinetic occipital (KO), and lateral occipital (LO)], intraparietal [ventral intraparietal sulcus (VIPS), parieto-occipital intraparietal sulcus (POIPS), and dorsal intraparietal sulcus (DIPS)], and premotor [premotor dorsal (PMd) and premotor ventral (PMv)] areas (Fig. S4). We used these contour-responsive regions as regions of interest (ROI) for further analysis. We also included V1 as an additional control ROI, although no significant differences for contours vs. random stimuli were observed in this region.
Learning-Dependent Changes in fMRI Signals.
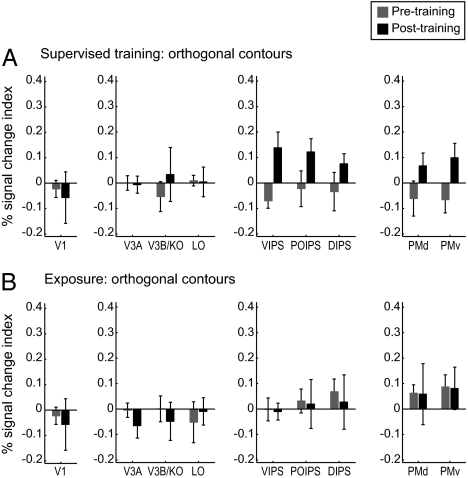
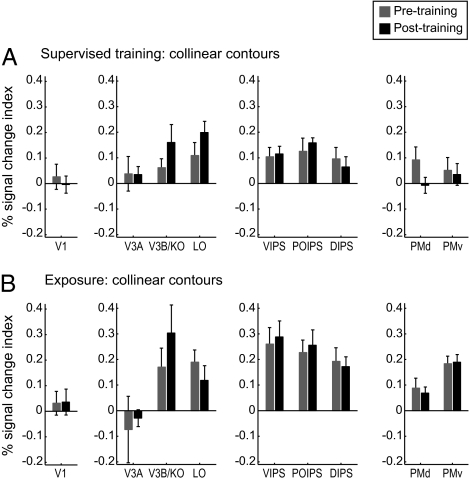
To investigate learning-dependent changes in fMRI activations related to behavioral improvement in contour detection, we compared fMRI signals in contour-responsive regions before vs. after supervised training or exposure to orthogonal and collinear contours. Note that comparing detection performance between the beginning and end of the pretraining test session did not show any significant differences [collinear: t(13) = 1.52, P = 0.15; orthogonal: t(13) = 0.77, P = 0.45], ensuring that the pretraining scanning session can serve as baseline for evaluating training-dependent fMRI changes. Our analysis showed that fMRI responses were significantly higher for collinear than orthogonal contours [F(1,21) = 12.81, P < 0.01], indicating overall higher responsiveness to collinear contours consistent with optimization for collinearity processing (1–3). Comparison of learning-dependent changes for collinear and orthogonal contours showed consistent results with the behavioral findings. In particular, for orthogonal contours, we observed enhanced fMRI responses after supervised training but not mere exposure. In contrast, for collinear contours, enhanced fMRI responses were evident after either supervised training or exposure. Importantly, the learning of different contour types implicated different cortical regions. Specifically, enhanced fMRI responses were observed after training in intraparietal regions for orthogonal contours, whereas in occipitotemporal regions for collinear contours.
We compared the percent signal change for trained orientations before and after training or exposure using the response to random stimuli as a baseline. For orthogonal contours (Fig. 2), a repeated measures ANOVA on fMRI responses for trained orientations showed a significant interaction between learning procedure (supervised training vs. exposure) and session (pretest vs. posttest) in intraparietal regions [e.g., VIPS: F(1,9) = 5.31, P < 0.05] but not in other contour-responsive areas [occipitotemporal areas: F(1,9) = 0.79, P = 0.40; premotor areas: F(1,9) = 0.92, P = 0.36] or early visual areas [e.g., V1: F(1,9) = 0.92, P = 0.36]. In contrast, no significant differences were observed for untrained orientations (Fig. S5) before vs. after supervised training [F(1,4) = 2.31, P = 0.20] or exposure [F(1,5) = 0.55, P = 0.49], consistent with the lack of behavioral improvement for untrained contours. These results suggest a link between behavioral improvement in the detection of orthogonal contours and fMRI changes in intraparietal regions after supervised training rather than exposure. This finding was further supported by a strong correlation between behavior (difference in detection accuracy for trained vs. untrained orientations before and after training) and the corresponding fMRI signals in intraparietal regions for supervised training (R = 0.97, P < 0.05) rather than exposure (R = −0.39, P = 0.44).
Fig. 2.
fMRI responses for observers trained with orthogonal contours, Signal-change index (percent signal change for collinear minus random contours) for each ROI. Data are shown for trained contour orientations before and after (A) supervised training and (B) exposure. Error bars denote SEM.
In contrast, for collinear contours, we observed enhanced fMRI responses in higher occipitotemporal (rather than intraparietal) regions for both supervised training and exposure (Fig. 3). In particular, for trained collinear contours, repeated measure ANOVAs showed significant interactions between session (pretest vs. posttest) and ROI for supervised training [F(12,60) = 2.12; P < 0.05] and exposure [F(8,32) = 2.86, P < 0.05]. Further analysis showed significantly higher fMRI responses after rather than before supervised training or exposure in occipitotemporal areas [e.g., V3B/KO: F(1,9) = 6.27, P < 0.05] but not intraparietal areas [F(1,9) = 0.13, P = 0.17], premotor areas [F(1,9) = 1.10, P = 0.32], or early visual areas [e.g., V1: F(1,9) = 0.12, P = 0.74]. Interestingly, for untrained orientations (Fig. S6), we observed significantly higher fMRI responses in intraparietal regions after vs. before supervised training [F(1,5) = 11.25, P < 0.05] or exposure [F(1,4) = 19.01, P < 0.05], consistent with the transfer of behavioral learning under both training protocols.
Fig. 3.
fMRI responses for observers trained with collinear contours. Signal change index (percent signal change for collinear minus random contours) for each ROI. Data are shown for trained contour orientations before and after (A) supervised training and (B) exposure. Error bars denote SEM.
Learning-Dependent Changes in fMRI Selectivity for Global Contour Orientation.
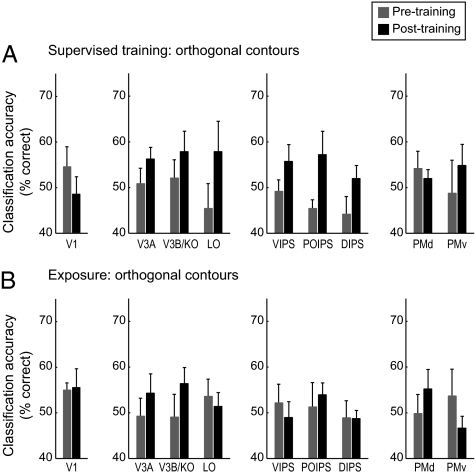
We tested whether learning changes fMRI-selective responses for trained compared with untrained contour orientations using multivoxel-pattern analysis methods (MVPA) (12–14). These methods take advantage of information across multivoxel patterns and have been shown to be more sensitive than conventional brain-imaging approaches that average across neural populations with differential selectivity within a given voxel. We exploit the sensitivity of these methods to discern differences in the processing of contour orientations before and after training. Training-dependent changes in the neural representations of contours may relate to changes in neural selectivity of single neurons or local population correlations enhanced through feedback. Although these hypotheses cannot be dissociated based on fMRI signals that represent the congregate activity of large neural populations, multivariate analyses allow us to understand these learning-dependent changes at the level of multivoxel patterns within ROIs. In particular, for each session (pre- or posttraining), we tested the accuracy of a linear-support vector machine in discriminating between fMRI activation patterns associated with the different contour orientations using a leave-one-run-out cross-validation procedure. We compared the average (across cross-validations and observers) classification accuracy before and after training for each ROI.
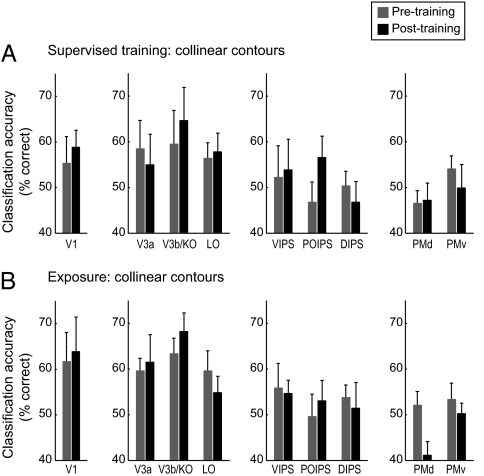
Similar to the behavioral learning effects, before training, there were no significant differences in classification accuracy for collinear vs. orthogonal contours [F(8,160) = 1.46, P = 0.18]. However, supervised training enhanced fMRI orientation selectivity for orthogonal contours in intraparietal regions (Fig. 4). In particular, a repeated measures ANOVA showed a significant interaction between session (pretest vs. posttest) and ROI [regions included early visual, occipitotemporal, intraparietal, and premotor areas; F(12,48) = 2.12, P < 0.05], with significantly higher classification accuracies after training in intraparietal regions [e.g., DIPS: F(1,4) = 8.27, P < 0.05], but not occipitotemporal areas [V3A, V3B/KO, and LO: F(1,4) = 1.82, P = 0.26; LO: F(1,4) = 1.29, P = 0.32], premotor areas [F(1,4) < 1, P = 0.71], or early visual areas [e.g., V1: F(1,4) < 1, P = 0.43]. In contrast, no significant differences were observed in classification accuracy between orientations [F(8,40) = 1.06, P = 0.41] after exposure to orthogonal stimuli, consistent with the lack of significant behavioral improvement after exposure to orthogonal contours.
Fig. 4.
MVPA for observers trained with orthogonal contours. MVPA accuracy (percent correct) per ROI for the classification of trained vs. untrained orientations of orthogonal contours. Data are shown for before and after (A) supervised training and (B) exposure. Error bars denote SEM.
The same analysis for collinear contours showed classification accuracies that matched the behavioral transfer of learning for untrained orientations (Fig. 5). In particular, classification accuracy of fMRI signals related to trained and untrained orientations was similar before and after supervised training [F(8,40) = 1.27, P = 0.28] or exposure [F(8,32) = 1.07, P = 0.41]. This could be because of the fact that collinear contour learning transferred across orientations, resulting in similar fMRI responses to trained and untrained orientations after training.
Fig. 5.
MVPA for observers trained with collinear contours. MVPA accuracy (percent correct) per ROI for the classification of trained vs. untrained orientations of collinear contours. Data are shown for before and after (A) supervised training and (B) exposure. Error bars denote SEM.
Is it possible that the differences in the classification accuracy before vs. after learning could be caused by differences in the signal-to-noise ratio across sessions and regions? Analysis of the functional signal-to-noise ratio across cortical regions showed no significant differences before and after training (Fig. S7), suggesting that differences in the classification accuracies could not be caused by differences in the overall fMRI signal across sessions. Furthermore, our choice of task during scanning (target detection) aimed to avoid possible confounds related to differences in task difficulty (i.e., higher fMRI responses for difficult conditions). This demanding task (70.90% accuracy for 414 ms mean response time) makes it unlikely that the observers continued performing the contour-detection task during scanning and ensured that observers’ attention was similar across training procedures, contour types, and sessions (SI Materials and Methods), suggesting that the learning-dependent fMRI changes could not be simply caused by differential allocation of attention. Moreover, we controlled for possible differences in general alertness across sessions (pretest vs. posttest) by comparing (i) normalized fMRI responses to contours using the signal to random stimuli per session as baseline and (ii) responses to trained and untrained orientations within the same scanning session. Furthermore, the differences in activation patterns for collinear compared with orthogonal contours could not be caused by differences in salience between contour types, because behavioral performance was similar for collinear and orthogonal contours after training. Finally, analysis of eye-movement data collected during scanning did not show any significant differences between scanning sessions in the eye position or number of saccades (Figs. S8 and S9), suggesting that differences in the fMRI activation patterns before and after training could not be significantly attributed to eye-movement differences.
Discussion
Our findings provide evidence for discrete brain plasticity mechanisms that mediate the flexible learning of statistical regularities for the detection of targets in cluttered scenes. We show that mere frequent exposure is adequate for the brain to extract regularities that typically signify shape contours (e.g., collinearity). However, when such regularities are lacking, the brain requires extensive training to reassign new functional roles to existing image statistics and group discontinuities for contour integration and detection. We propose that learning to detect collinear elements relies on opportunistic mechanisms that may facilitate the selection of behaviorally relevant features (i.e., collinear elements) for target detection. In contrast, learning to integrate discontinuities into coherent contours (i.e., orthogonal contours) requires bootstrap-based training on new features for target detection. This is further supported by the observation that learning for collinear contours occurred faster (i.e., fewer training trials) than learning for orthogonal contours (Figs. S2 and S3). Interestingly, bootstrap-based learning of atypical regularities is feature-dependent, whereas opportunistic learning of typical regularities generalizes across untrained features (i.e., collinear contours presented at untrained orientations).
Our study advances our understanding of the brain plasticity mechanisms that mediate our ability to detect targets in cluttered scenes in three main respects. First, it delineates discrete human-brain regions involved in opportunistic compared with bootstrap-based learning. We show that opportunistic learning may occur by frequent exposure and is mediated by occipitotemporal areas, whereas bootstrap-based learning requires extensive training and is mediated by intraparietal regions. This is consistent with the role of occipitotemporal areas in representing task-relevant shape features and global forms (15, 16), whereas the role of intraparietal regions is perceptual integration saliency (17) and attention-gated learning (18). Goal-directed attentional mechanisms facilitate the localization of salient image regions with local elements of similar alignment and may then optimize visual processing through feedback mechanisms (19, 20). Interestingly, our findings relate to work investigating the role of learning in language that—in a similar manner to visual recognition—entails integrating elements into coherent and meaningful structures. In particular, previous studies have shown that both infants and adults learn to exploit statistical regularities for parsing speech into meaningful language streams (21, 22). Furthermore, recent neuroimaging studies suggest that a ventral cortex region becomes specialized through experience and development for word recognition (23, 24), whereas dorsal parietal regions are recruited for recognizing words presented in unfamiliar formats (25). Taken together, these findings support the proposal that learning of statistical regularities shapes occipitotemporal processing, whereas learning new features for perceptual integration recruits parietal regions involved in the attentional gating of recognition processes.
It is interesting to note that we did not observe any significant learning-dependent changes in activations in primary visual areas. Furthermore, no significant differences were observed in V1 for collinear vs. orthogonal contours before [F(1,21) = 1.10, P = 0.31] or after training [F(1,21) = 1.92, P = 0.18]. This may seem surprising, because recurrent processing involving intrinsic connections between neurons with similar orientation preference and feedback from higher visual areas (26–29) has been suggested to facilitate perceptual integration and figure-ground segmentation as early as in V1 (30, 31). However, the evidence on experience-dependent plasticity in V1 is not yet conclusive. Some previous imaging studies show enhanced responses in V1 for oblique orientations after training (32), and some physiology studies show changes in orientation tuning (33). In contrast, it has been shown that learning does not alter the topography or basic receptive field properties (e.g., size, location, or orientation selectivity) in V1 (34, 35). Furthermore, training-dependent changes on orientation tuning are shown to be more pronounced in V4 (36, 37), whereas effects in V1 are shown to be task-dependent and may engage top-down facilitation mechanisms (35, 38–40). It is possible that the lack of significant learning effects in V1 in our study is caused by the large spacing between contour elements that may prevent integration within the small receptive fields of V1 neurons. Furthermore, during scanning, observers engaged in a target-detection rather than contour-detection task that may have gated feedback-based learning effects in V1. Further work manipulating stimulus scale and task requirements is needed for understanding the mechanisms that mediate learning-dependent changes in V1.
Second, our findings characterize the conditions under which task-irrelevant learning may occur in natural scenes. Most perceptual learning studies focus on extensive and feature-specific training (reviewed in refs. 41 and 42). However, recent work provides evidence that perceptual learning may also occur when attention is diverted away from the stimulus (43, 44) [e.g., when observers perform a task that is irrelevant to the stimulus of interest (reviewed in refs. 45 and 46)]. Our findings suggest that task-irrelevant learning may occur when statistical regularities that signify shape contours are present in natural scenes. In particular, detection performance improved significantly after sequential exposure to collinear contours while observers performed a contrast discrimination task. In contrast, in the absence of regularities typical of shape contours (i.e., orthogonal contours), significant improvement was observed only when observers received task-specific training (i.e., training on contour detection) rather than when they performed the contrast-discrimination task. However, it is possible that increasing the demands of the contrast-discrimination task would disrupt the learning of collinear contours. This manipulation would allow us to further explore the role of attention and task demands on learning of image regularities (e.g., ref. 47). Furthermore, although exposure-based learning lasted longer (observers used the maximum number of sessions: 16,000 trials) than supervised training, it is possible that exposure-based learning for orthogonal contours may entail longer time scales. The critical finding is that exposure-based learning occurred for collinear rather than orthogonal contours within the same time scale, suggesting different learning mechanisms for collinear rather than orthogonal contours in cluttered scenes.
Third, our findings provide insights in understanding how long-term plasticity honed through development and evolution and shorter-term training may interact in shaping the functional optimization of visual-recognition processes. In particular, previous work has shown an advantage in the detection of collinear contours compared with other local element arrangements, consistent with the long-term optimization of the visual system for regularities typical of shape contours in natural scenes (4–6). To investigate how this long-term plasticity contributes to the interpretation of scenes, we manipulated the local spacing among the contour elements so that the detection of collinear elements was as difficult as the detection of orthogonal contours. This manipulation revealed that sensitivity to collinearity—possibly acquired through long-term experience—affords the visual system the capability to improve in contour detection without the need to receive extensive training. However, long-term experience is not the only means to the optimization of visual processes. Although bootstrap-based learning is slower and necessitates task-specific training, it shapes the behavioral relevance of image statistics and affords integration and detection of contours defined by discontinuities. Interestingly, bootstrap-based training results in stimulus-specific learning, whereas long-term experience affords generalization across image changes (e.g., orientation) that preserve the stimulus identity.
In sum, our findings suggest that long-term experience with statistical regularities through development and evolution may facilitate opportunistic learning, whereas bootstrap-based training is necessary for the detection of atypical regularities in cluttered scenes. These different routes to visual learning have discrete brain plasticity signatures. In particular, opportunistic learning enhances processing and neural sensitivity in occipitotemporal regions involved in global-form processing, whereas bootstrap-based learning recruits intraparietal regions known to be involved in attention-gated learning. These findings provide insights in understanding how long-term plasticity and short-term training interact to shape the optimization of visual-recognition processes. Whether and when bootstrap-based learning results to features rendered as cues for opportunistic learning remains an open challenge for future research.
Materials and Methods
Stimuli.
Stimuli were Gabor fields consisting of 80 elements presented within a circular aperture that were generated using previously described methods (9). Contour stimuli contained parallel contours that were defined by Gabor elements placed along (collinear) or perpendicular (orthogonal) to straight paths and were embedded in a background of randomly positioned and oriented Gabor elements. All parameters (e.g., number and spacing of Gabor elements) were the same for collinear and orthogonal contours. More details on stimulus generation and presentation are described in SI Materials and Methods.
Psychophysical Design and Procedures.
Each observer was assigned to one of two experimental procedures. One group of participants (n = 14) was trained on contour detection with auditory feedback (tone of 600 Hz, duration = 0.15 s) on incorrect responses (supervised training). Another group (n = 14) was exposed to contour stimuli while performing a contrast-discrimination task unrelated to contour detection (exposure group). For each procedure, one-half of the observers trained on (or were exposed to) collinear contours, whereas the other one-half trained on (or were exposed to) orthogonal contours. One-half of the observers were trained with contours whose global orientation was near the left (135 ± 15°) diagonal, whereas the rest were trained with contour orientation near the right (45 ± 15°) diagonal. That is, different groups (supervised training and exposure) of observers were trained with (or exposed to) either collinear or orthogonal contours presented in one of the two sets of global contour orientations.
Observers participated in multiple psychophysical sessions and two fMRI sessions that were conducted on different days. The first and the last session were pretest and posttest sessions to evaluate the observers’ performance in detecting contours. The posttest session was always conducted the day after the final supervised training or exposure session. The intermediate sessions were either supervised training or exposure sessions (SI Materials and Methods has more details). Observers participated in minimum 5 and maximum 32 intermediate sessions on consecutive days. Interspersed within the intermediate sessions after every fourth session were short quick-test sessions (100 trials) to assess the improvement of the observers’ performance. When accuracy (percent correct) at zero jitter on these quick-test sessions reached above 80%, the posttest session was conducted, provided that at least 5 intermediate supervised training or 10 exposure sessions had been completed.
fMRI Scan Sessions.
Observers were scanned before training (after the pretest psychophysical session) and after training (after the posttest psychophysical session). Each scanning session comprised eight experimental runs, each of which lasted 5 min and 20 s. A run comprised 14 16-s long stimulus blocks, including the initial and final blocks, during which only the fixation cross was presented. The experimental blocks contained stimuli from six conditions: collinear contours near the left (135 ± 15°) diagonal, collinear contours near the right (45 ± 15°) diagonal, orthogonal contours near the left (135 ± 15°) diagonal, orthogonal contours near the right (45 ± 15°) diagonal, random-1, and random-2. For each observer, the contour type presented in the scanner was the same as in the psychophysical sessions. Random-1, and random-2 were two conditions of random stimuli (random fields without any embedded contours). For each stimulus in condition random-1, the 10 elements corresponding to the contour elements were presented at random positions and orientations. The set of stimuli presented in condition random-2 was generated by rotating these elements by 90°. Thus, stimuli in the two random conditions differed by 90° rotation of the local elements that matched the orientation difference between contours near the left and right diagonal.
Supplementary Material
Acknowledgments
We thank V. Chen, S. Rappaport, and D. S. Schwartzkopf for preliminary work. This work was supported by a Biotechnology and Biological Sciences Research Council Grant BB/D52199X/1 and the Cognitive Foresight Initiative BB/E027436/1 (to Z.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002506107/-/DCSupplemental.
References
- 1.Geisler WS. Visual perception and the statistical properties of natural scenes. Annu Rev Psychol. 2008;59:167–192. doi: 10.1146/annurev.psych.58.110405.085632. [DOI] [PubMed] [Google Scholar]
- 2.Sigman M, Cecchi GA, Gilbert CD, Magnasco MO. On a common circle: Natural scenes and Gestalt rules. Proc Natl Acad Sci USA. 2001;98:1935–1940. doi: 10.1073/pnas.031571498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simoncelli EP, Olshausen BA. Natural image statistics and neural representation. Annu Rev Neurosci. 2001;24:1193–1216. doi: 10.1146/annurev.neuro.24.1.1193. [DOI] [PubMed] [Google Scholar]
- 4.Bex PJ, Simmers AJ, Dakin SC. Snakes and ladders: The role of temporal modulation in visual contour integration. Vision Res. 2001;41:3775–3782. doi: 10.1016/s0042-6989(01)00222-x. [DOI] [PubMed] [Google Scholar]
- 5.Field DJ, Hayes A, Hess RF. Contour integration by the human visual system: Evidence for a local “association field.”. Vision Res. 1993;33:173–193. doi: 10.1016/0042-6989(93)90156-q. [DOI] [PubMed] [Google Scholar]
- 6.Geisler WS, Perry JS, Super BJ, Gallogly DP. Edge co-occurrence in natural images predicts contour grouping performance. Vision Res. 2001;41:711–724. doi: 10.1016/s0042-6989(00)00277-7. [DOI] [PubMed] [Google Scholar]
- 7.Fiser J, Aslin RN. Unsupervised statistical learning of higher-order spatial structures from visual scenes. Psychol Sci. 2001;12:499–504. doi: 10.1111/1467-9280.00392. [DOI] [PubMed] [Google Scholar]
- 8.Perruchet P, Pacton S. Implicit learning and statistical learning: One phenomenon, two approaches. Trends Cogn Sci. 2006;10:233–238. doi: 10.1016/j.tics.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Schwarzkopf DS, Kourtzi Z. Experience shapes the utility of natural statistics for perceptual contour integration. Curr Biol. 2008;18:1162–1167. doi: 10.1016/j.cub.2008.06.072. [DOI] [PubMed] [Google Scholar]
- 10.Schwarzkopf DS, Zhang J, Kourtzi Z. Flexible learning of natural statistics in the human brain. J Neurophysiol. 2009;102:1854–1867. doi: 10.1152/jn.00028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brady MJ, Kersten D. Bootstrapped learning of novel objects. J Vis. 2003;3:413–422. doi: 10.1167/3.6.2. [DOI] [PubMed] [Google Scholar]
- 12.Norman KA, Polyn SM, Detre GJ, Haxby JV. Beyond mind-reading: Multi-voxel pattern analysis of fMRI data. Trends Cogn Sci. 2006;10:424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Kamitani Y, Tong F. Decoding the visual and subjective contents of the human brain. Nat Neurosci. 2005;8:679–685. doi: 10.1038/nn1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haynes JD, Rees G. Predicting the orientation of invisible stimuli from activity in human primary visual cortex. Nat Neurosci. 2005;8:686–691. doi: 10.1038/nn1445. [DOI] [PubMed] [Google Scholar]
- 15.Ostwald D, Lam JM, Li S, Kourtzi Z. Neural coding of global form in the human visual cortex. J Neurophysiol. 2008;99:2456–2469. doi: 10.1152/jn.01307.2007. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Ostwald D, Giese M, Kourtzi Z. Flexible coding for categorical decisions in the human brain. J Neurosci. 2007;27:12321–12330. doi: 10.1523/JNEUROSCI.3795-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- 18.Roelfsema PR, van Ooyen A. Attention-gated reinforcement learning of internal representations for classification. Neural Comput. 2005;17:2176–2214. doi: 10.1162/0899766054615699. [DOI] [PubMed] [Google Scholar]
- 19.Itti L, Koch C. Computational modelling of visual attention. Nat Rev Neurosci. 2001;2:194–203. doi: 10.1038/35058500. [DOI] [PubMed] [Google Scholar]
- 20.Treue S. Visual attention: The where, what, how and why of saliency. Curr Opin Neurobiol. 2003;13:428–432. doi: 10.1016/s0959-4388(03)00105-3. [DOI] [PubMed] [Google Scholar]
- 21.Peña M, Bonatti LL, Nespor M, Mehler J. Signal-driven computations in speech processing. Science. 2002;298:604–607. doi: 10.1126/science.1072901. [DOI] [PubMed] [Google Scholar]
- 22.Saffran JR, Aslin RN, Newport EL. Statistical learning by 8-month-old infants. Science. 1996;274:1926–1928. doi: 10.1126/science.274.5294.1926. [DOI] [PubMed] [Google Scholar]
- 23.Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words: A proposal. Trends Cogn Sci. 2005;9:335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 24.McCandliss BD, Cohen L, Dehaene S. The visual word form area: Expertise for reading in the fusiform gyrus. Trends Cogn Sci. 2003;7:293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- 25.Cohen L, Dehaene S, Vinckier F, Jobert A, Montavont A. Reading normal and degraded words: Contribution of the dorsal and ventral visual pathways. Neuroimage. 2008;40:353–366. doi: 10.1016/j.neuroimage.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 26.Sigman M, Cecchi GA, Gilbert CD, Magnasco MO. On a common circle: Natural scenes and Gestalt rules. Proc Natl Acad Sci USA. 2001;98:1935–1940. doi: 10.1073/pnas.031571498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilbert CD, Sigman M, Crist RE. The neural basis of perceptual learning. Neuron. 2001;31:681–697. doi: 10.1016/s0896-6273(01)00424-x. [DOI] [PubMed] [Google Scholar]
- 28.Hochstein S, Ahissar M. View from the top: Hierarchies and reverse hierarchies in the visual system. Neuron. 2002;36:791–804. doi: 10.1016/s0896-6273(02)01091-7. [DOI] [PubMed] [Google Scholar]
- 29.Fitzpatrick D. Seeing beyond the receptive field in primary visual cortex. Curr Opin Neurobiol. 2000;10:438–443. doi: 10.1016/s0959-4388(00)00113-6. [DOI] [PubMed] [Google Scholar]
- 30.Zipser K, Lamme VA, Schiller PH. Contextual modulation in primary visual cortex. J Neurosci. 1996;16:7376–7389. doi: 10.1523/JNEUROSCI.16-22-07376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roelfsema PR. Cortical algorithms for perceptual grouping. Annu Rev Neurosci. 2006;29:203–227. doi: 10.1146/annurev.neuro.29.051605.112939. [DOI] [PubMed] [Google Scholar]
- 32.Furmanski CS, Schluppeck D, Engel SA. Learning strengthens the response of primary visual cortex to simple patterns. Curr Biol. 2004;14:573–578. doi: 10.1016/j.cub.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 33.Schoups A, Vogels R, Qian N, Orban G. Practicing orientation identification improves orientation coding in V1 neurons. Nature. 2001;412:549–553. doi: 10.1038/35087601. [DOI] [PubMed] [Google Scholar]
- 34.Ghose GM, Yang T, Maunsell JH. Physiological correlates of perceptual learning in monkey V1 and V2. J Neurophysiol. 2002;87:1867–1888. doi: 10.1152/jn.00690.2001. [DOI] [PubMed] [Google Scholar]
- 35.Crist RE, Li W, Gilbert CD. Learning to see: Experience and attention in primary visual cortex. Nat Neurosci. 2001;4:519–525. doi: 10.1038/87470. [DOI] [PubMed] [Google Scholar]
- 36.Yang T, Maunsell JH. The effect of perceptual learning on neuronal responses in monkey visual area V4. J Neurosci. 2004;24:1617–1626. doi: 10.1523/JNEUROSCI.4442-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raiguel S, Vogels R, Mysore SG, Orban GA. Learning to see the difference specifically alters the most informative V4 neurons. J Neurosci. 2006;26:6589–6602. doi: 10.1523/JNEUROSCI.0457-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li W, Piëch V, Gilbert CD. Learning to link visual contours. Neuron. 2008;57:442–451. doi: 10.1016/j.neuron.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li W, Piëch V, Gilbert CD. Perceptual learning and top-down influences in primary visual cortex. Nat Neurosci. 2004;7:651–657. doi: 10.1038/nn1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sigman M, et al. Top-down reorganization of activity in the visual pathway after learning a shape identification task. Neuron. 2005;46:823–835. doi: 10.1016/j.neuron.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fahle M. Perceptual learning: A case for early selection. J Vis. 2004;4:879–890. doi: 10.1167/4.10.4. [DOI] [PubMed] [Google Scholar]
- 42.Fine I, Jacobs RA. Comparing perceptual learning tasks: A review. J Vis. 2002;2:190–203. doi: 10.1167/2.2.5. [DOI] [PubMed] [Google Scholar]
- 43.Gutnisky DA, Hansen BJ, Iliescu BF, Dragoi V. Attention alters visual plasticity during exposure-based learning. Curr Biol. 2009;19:555–560. doi: 10.1016/j.cub.2009.01.063. [DOI] [PubMed] [Google Scholar]
- 44.Turk-Browne NB, Scholl BJ, Chun MM, Johnson MK. Neural evidence of statistical learning: Efficient detection of visual regularities without awareness. J Cogn Neurosci. 2009;21:1934–1945. doi: 10.1162/jocn.2009.21131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seitz AR, Watanabe T. The phenomenon of task-irrelevant perceptual learning. Vision Res. 2009;49:2604–2610. doi: 10.1016/j.visres.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sasaki Y, Nanez JE, Watanabe T. Advances in visual perceptual learning and plasticity. Nat Rev Neurosci. 2010;11:53–60. doi: 10.1038/nrn2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baker CI, Olson CR, Behrmann M. Role of attention and perceptual grouping in visual statistical learning. Psychol Sci. 2004;15:460–466. doi: 10.1111/j.0956-7976.2004.00702.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.