Abstract
Thymus organogenesis requires coordinated interactions of multiple cell types, including neural crest (NC) cells, to orchestrate the formation, separation, and subsequent migration of the developing thymus from the third pharyngeal pouch to the thoracic cavity. The molecular mechanisms driving these processes are unclear; however, NC-derived mesenchyme has been shown to play an important role. Here, we show that, in the absence of ephrin-B2 expression on thymic NC-derived mesenchyme, the thymus remains in the cervical area instead of migrating into the thoracic cavity. Analysis of individual NC-derived thymic mesenchymal cells shows that, in the absence of ephrin-B2, their motility is impaired as a result of defective EphB receptor signaling. This implies a NC-derived cell-specific role of EphB–ephrin-B2 interactions in the collective migration of the thymic rudiment during organogenesis.
Keywords: collective cell migration, thymus organogenesis, ephrin-B2, Eph receptor, neural crest
Thymus development in the mouse begins at E9.5 with the formation of the third pharyngeal pouches and concomitant expression of transcription factors including Hoxa3, Pax1/9, Eya1, and Six1/4 within the third pharyngeal pouch endoderm (1–4). Soon after, neural crest (NC)-derived mesenchymal cells (NCCs) from the posterior hindbrain undergo epithelial-to-mesenchymal transition (EMT), delaminate, and migrate to and surround the pouch, which simultaneously begins to grow into a separate structure containing the thymus and parathyroid primordia. By E12.5, the thymic primordium is completely detached from both the pharyngeal pouch and parathyroid. Subsequently, the bilateral lobes descend into the thoracic cavity, where they finally settle in the upper mediastinum above the heart.
NCCs, which contribute to various tissues in the mouse, are also key players in thymus organogenesis. NC-derived cells have been shown to regulate patterning of the third-pouch endoderm into thymus and parathyroid-specific domains (5) and stimulate thymic epithelial cell (TEC) proliferation and maturation through production of FGFs (6). NC-derived cells also stabilize blood-vessel structures by differentiating into perivascular cells (7, 8).
The mechanisms that control the various morphogenetic events during early thymus organogenesis are still largely unknown, although some genes have been identified that affect these events. Hox3 and Pax1/9 transcription factors act in a pathway that is required for proper separation and/or migration of the developing thymic and parathyroid primordia from the pharynx, although the cell-type specificity of these functions is poorly understood (2, 9, 10). Splotch embryos, which have a null allele of Pax3 and are largely deficient of NCCs, also exhibit pharyngeal-pouch defects, including an ectopic thymus (5, 11). This NCC deficiency resulted in delayed separation of the thymus and parathyroid from the pharynx, and the boundary between thymus and parathyroid-fated domains was abnormal. These results strongly implicated NCC migration to the third pharyngeal pouch in patterning and morphogenesis of the thymus and parathyroids (5). Interestingly, embryos that have deleted the TGF-β type-1 receptor, ALK5, in NC-derived cells also have ectopically located thymi as a result of delayed separation of the parathyroid from the thymic rudiment without histological or other differentiation defects. Moreover, this defect was not caused by defective NC migration to the third pharyngeal pouch but was thought to be caused by increased apoptosis in postmigratory NCCs (12). However, to date, no mutants with ectopic thymi have been verified to have migration defects without additional defects in separation from the pharynx.
Eph receptors and their ligands, ephrins, are required for a number of developmental processes [e.g., blood-vessel formation through capillary sprouting (13), demarcation of arteries and veins (13, 14), and NCC migration to the pharyngeal arches (15–18)]. The mechanisms by which Eph/ephrins control these processes are believed to involve regulation of actin cytoskeleton dynamics, cell-substrate adhesion, intercellular junctions, cell shape, and cell movement (reviewed in refs. 19 and 20).
A significant number of Eph receptors and ephrin ligands are expressed in the thymus (21–25) and are known to have immuno-regulatory properties (26). The defects seen in thymocyte maturation in Eph- or ephrin-deficient thymi are thought to result from abnormal development of the stromal cell compartments (27) and modulation of T cell responses (28). For example, EphB2- and/or EphB3-deficient mice exhibit decreased numbers of thymocyte subsets (29), the lack of EphA4 expression results in hypoplastic thymi and decreased numbers of double-positive (CD4+ CD8+) thymocytes (27), and blocking fetal thymic organ cultures with EphB2/Fc or ephrin-B1/Fc fusion proteins decreases double-positive and single-positive T cell populations (30).
To investigate the role of EphB ephrin-B2 interactions in thymus development, we have used Cre-Lox genetic tools to specifically ablate ephrin-B2 expression on TECs or NC-derived cell populations. Mice with LoxP sites flanking exon 2 of the Efnb2 gene (31) were crossed with mice expressing Cre recombinase in TECs under the control of the IL7 (32) or in NCCs under the control of the Wnt1 (33) promoter and regulatory elements. Cre activity was reported through activation of a silent enhanced YFP (eYFP) expressed from the Rosa26 locus (34).
Results
Ephrin-B2 Expression on NC-Derived Cells Is Required for Correct Positioning of the Thymus but Not for Thymocyte Development.
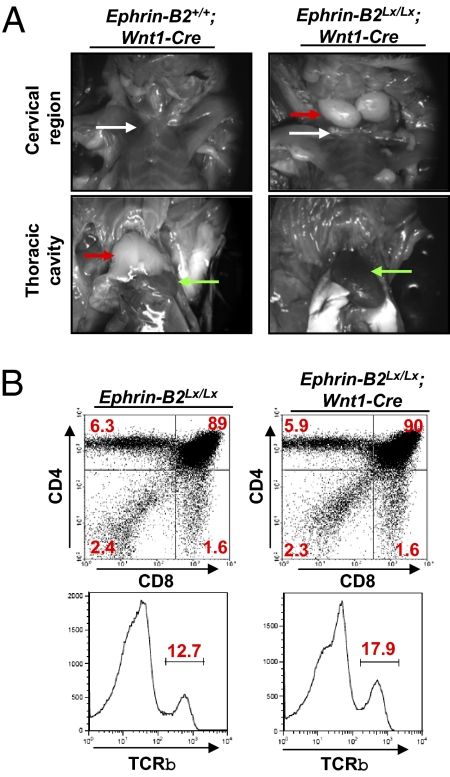
Analysis of TECs and NC-derived thymic mesenchyme showed high levels of ephrin-B2 and EphB4 expression in adult and fetal tissues using flow cytometry and confocal microscopy (Fig. S1 A–C); a similar and widespread expression of ephrin-B2 in embryos has previously been observed (35). To study the role of ephrin-B2 expression on NC-derived cells and TECs, we used Cre recombinase under the control of Wnt1 (NC-specific) or IL-7 (TEC-specific) promoters. In mice that had deleted ephrin-B2 specifically from NC-derived cells, the thymus was absent from its normal anatomical location above the heart (Fig. 1A Lower Right). Instead, it was ectopically located in the cervical region (Fig. 1A Upper Right). In a small number of embryos, one ectopic thymus lobe was located in the cervical region, and the other was within the superior mediastinum above the heart. In contrast, deletion of ephrin-B2 on TECs showed no requirement for normal thymus development and function (Fig. S2) or in peripheral T cell populations (Fig. S3).
Fig. 1.
Expression of ephrin-B2 on NC-derived cells is required for the normal anatomical position of the thymus. (A) Three-month-old adult control Ephrin-B2+/+;Wnt1-Cre;Rosa26eYfp (Left) and mutant Ephrin-B2Lx/Lx;Wnt1-Cre;Rosa26eYfp (Right) mice were dissected to reveal the cervical region (Upper) and thoracic cavity (Lower). White arrows, clavicle; red arrows, thymus; green arrows, heart. (B) Thymi from 3-mo-old control Ephrin-B2Lx/Lx and mutant Ephrin-B2Lx/Lx;Wnt1-Cre;Rosa26eYfp mice were dissected, digested with collagenase, stained with antibodies recognizing CD4, CD8 (Upper), and TCRβ (Lower), and analyzed by flow cytometry. Similar results were obtained from more than three experiments consisting of 14 Ephrin-B2Lx/Lx;Wnt1-Cre mice.
To determine the cellular composition of ephrin-B2–deficient ectopic thymi, they were digested with collagenase. The resulting cell suspension was counted and analyzed after staining with antibodies detecting CD4, CD8 (Fig. 1B Upper), and TCRβ (Fig. 1B Lower) to label T cells and CD19 (Fig. S4) to label B cells. Expression patterns of T cell markers and a lack of B cell markers indicated that the ectopic structures were thymus lobes and not lymph nodes, and no changes were observed in neuronal innervation in the thymus (Fig. S5). Moreover, ectopic thymi exhibited very similar cellularity as thoracic thymi (252 × 106 compared with 224 × 106). Analysis of spleens from controls and mice with ectopic thymi showed no difference in peripheral T cell numbers (1.35 × 108 in spleens of mutant mice and 1.5 × 108 in control mice) (Fig. S6) or ability of T cells to proliferate in response to mitogenic stimulation (Fig. S7). Thus, the absence of ephrin-B2 from NCCs does not affect T cell development, but rather, it results in ectopic thymus structures in the cervical region.
NCCs Colonize and Differentiate Normally Within the Thymus in the Absence of Ephrin-B2.
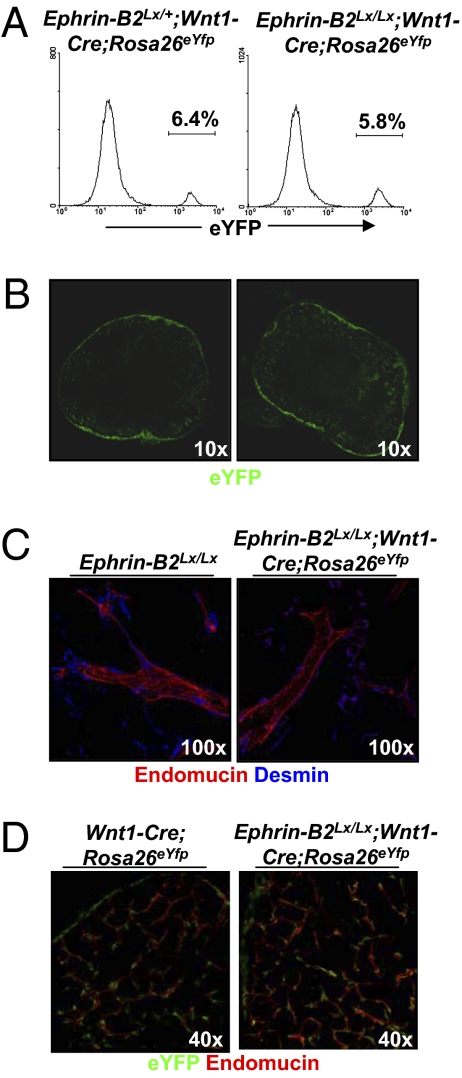
To determine whether there is a defect in NCC migration to the third pouch, control Wnt1-Cre;Rosa26eYfp and mutant Ephrin-B2Lx/Lx;Wnt1-Cre;Rosa26eYfp E10.5 embryos were analyzed by in situ hybridization with a Crabp1 probe to determine if there was a delay in NCC colonization. We show that there is no significant difference in NCCs migrating to and colonizing the rudiment by E10.5 in mutants compared with controls (Fig. S8A). Thymi from E15.5 control Ephrin-B2+/+;Wnt1-Cre;Rosa26eYfp (Fig. 2 A Left and B Left) and mutant Ephrin-B2Lx/Lx;Wnt1-Cre;Rosa26eYfp (Fig. 2 A Right and B Right) embryos were imaged by confocal and flow cytometry. No significant differences were observed in eYFP+ NC-derived cells [5.8% (Fig. 2B Right) from mutants compared with 6.4% in controls (Fig. 2B Left)]. To determine if loss of ephrin-B2 expression leads to changes in factors involved in positional information, we analyzed Hoxa3 expression in situ. Hoxa3 RNA was readily detected in the 3′ pharyngeal region of both mutant and wild-type mice, suggesting that absence of Eph/Ephrin signaling does affect the expression of this gene (Fig. S8B).
Fig. 2.
NC-derived mesenchymal cells associate normally with the blood vasculature in the ectopic thymus. (A and B) Thymi from 3-mo-old adult control Ephrin-B2Lx/+;Wnt1-Cre;Rosa26eYfp (A Left and B Left) and mutant Ephrin-B2Lx/Lx;Wnt1-Cre;Rosa26eYfp mice (A Right and B Right) were dissected, digested with collagenase, and analyzed by flow cytometry (A) or fixed, stained with an anti-YFP antibody, and analyzed by confocal microscopy (B). (C and D) One hundred-micrometer sections of thymi from 3-mo-old adult control Ephrin-B2Lx/Lx (Left) and mutant Ephrin-B2Lx/Lx;Wnt1-Cre;Rosa26eYfp (Right) mice were stained with antibodies recognizing (red) endomucin and (blue) desmin (C) or (green) eYFP and (red) endomucin (D) and analyzed by confocal microscopy.
NC-derived cells in the thymus normally differentiate into cells characteristic of pericytes and smooth-muscle cells associated with blood vessels (11, 12). Ephrin-B2 and its preferred receptor EphB4 are known to play a role in normal vasculature development, and deficiency in these molecules is embryonic lethal (24). To determine whether ephrin-B2 expression is required for NC-derived mesenchyme attachment to the walls of vessels, we have analyzed 100-μm vibratome sections of 3-mo-old adult control Ephrin-B2+/+;Wnt1-Cre;Rosa26eYfp and mutant Ephrin-B2Lx/Lx;Wnt1-Cre;Rosa26eYfp thymi. The sections were stained with antibodies detecting eYFP, the pericyte marker desmin, and the endothelial marker endomucin. These analyses revealed that vessels in the mutant thymus develop normally with desmin+ perivascular cells surrounding endomucin+ vascular endothelium (Fig. 2C Right) compared with control thymi (Fig. 2C Left). In addition, detection of eYFP+ perivascular cells in the adult thymus suggests that they are NC-derived and are not replaced by cells of other mesenchymal origin (Fig. 2D). Thus, ephrin-B2 is not necessary for NC association with the developing thymus or their mesenchymal differentiation.
Deletion of Ephrin-B2 Does Not Disrupt Separation of the Thymus/Parathyroid Rudiment from the Third Pharyngeal Pouch.
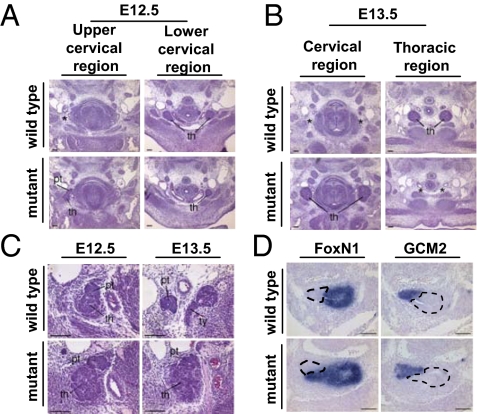
The ectopic thymus location in the mutant mice could be the result of a delay in the separation of the organ primordia from the pharynx and subsequent inability of the thymus rudiment to reach the superior mediastinum before the thoracic cavity closes. The primordium, which starts forming at E11, detaches from the third-pouch endodermal epithelium by E12. Because Eph/ephrins have been shown to play a role in boundary formation and maintenance between discrete structures in other tissues, it is possible that they could also be involved in this process. To assess whether the defect seen in Ephrin-B2Lx/Lx;Wnt1-Cre mice is caused by a failure in forming a boundary between the rudiment and pharyngeal pouch, leading to delayed separation, serial sections through the neck and thoracic cavity from E12.5 and E13.5 control Ephrin-B2+/+ and mutant Ephrin-B2Lx/Lx;Wnt1-Cre;Rosa26eYfp embryos were examined. This analysis revealed that the rudiment does separate fully from the third pharyngeal pouch with normal timing (Fig. 3 A and B).
Fig. 3.
Histological analyses and Foxn1 and Gcm2 expression in E11.5–E13.5 wild-type and Ephrin-B2Lx/Lx;Wnt1-Cre;Rosa26eYfp mutant embryos. (A and B) Transverse sections through E12.5 (A) and E13.5 (B) wild-type [Ephrin-B2+/+; (Upper Left) upper cervical region; (Upper Right) lower cervical region/thoracic region] and mutant embryos [Ephrin-B2Lx/Lx;Wnt1-Cre;Rosa26eYfp; (Lower Left) upper cervical region; (Lower Right) lower cervical region/thoracic region]. Upper and Lower are at equivalent positions along the anterior–posterior axis; thus, Left panels are higher in the cervical region than Right panels, indicating the slightly more anterior location of the mutant thymus lobes. Asterisk in Upper Left indicates the area corresponding to the location of the mutant thymus lobe in Lower Left. Asterisk in Lower Right indicates the equivalent position of the normal thymus in Upper Right. (C) Histological analyses of the cervical region in (Left) E12.5 and (Right) E13.5 (Upper) wild-type (Ephrin-B2+/+) and (Lower) mutant (Ephrin-BLx/Lx;Wnt1-Cre;Rosa26eYfp) embryos, indicating parathyroid position. Upper indicates the normal location of the parathyroids, attached to the thymus at E12.5 (Left) and detached from the thymus and adjacent to the thyroid gland at E13.5 (Right). (Lower) In mutant embryos, the parathyroids remain attached to the thymus lobes at both E12.5 (Left) and E13.5 (Right). th, thymus; pt, parathyroid; ty, thyroid. (D) Sagittal sections through the common thymus–parathyroid primordium at E11.5 showing prospective organ domains marked by (Left) Foxn1 (thymus) and (Right) Gcm2 (parathyroid). (Upper) Wild type. (Lower) Mutant. Dotted line in Left indicates thymic rudiment and in Right, parathyroid rudiment. (Scale bar: 100 μm.)
In addition to separating from the third pharyngeal pouch, the combined thymus and parathyroid rudiments also divide from each other into two separate structures; the thymus migrates into the thoracic cavity, whereas the parathyroid remains in the neck adjacent to the thyroid gland. We examined whether deletion of ephrin-B2 disrupts the separation between the thymus and parathyroid domains. Analysis of serial transverse sections of E12.5–13.5 mutant embryos showed a defect in separation of the thymus and parathyroid domains compared with control embryos (Fig. 3A). This phenotype was caused by a delay rather than failure, because adult mutant mice showed complete separation of the two organs (Fig. S9A). To investigate whether this delayed separation was caused by disruption of the boundary between thymus and parathyroid, in situ hybridization for Foxn1 (thymus) (Fig. 3D Right) and Gcm2 (parathyroid) (Fig. 3D Left) was carried out on sagittal sections of E11.5 wild-type Ephrin-B2+/+ and mutant Ephrin-B2Lx/Lx;Wnt1-Cre embryos. Despite the failure in thymus and parathyroid migration (Fig. 3C), there was no mixing of parathyroid and thymus cells in the absence of ephrin-B2 expression on NCCs (Fig. 3D Lower) compared with littermate controls (Fig. 3D Upper), and no apparent defects associated with parathyroid dysfunction were observed.
Together, these results indicate that deletion of ephrin-B2 from NC-derived cells does not affect or delay the separation of the thymus/parathyroid-combined rudiment from the third pharyngeal pouch, but it does affect the rate of separation of the thymus and parathyroid rudiments from each other.
Eph/Ephrin Signaling Is Required for Normal Migration of NC-Derived Mesenchymal Cells.
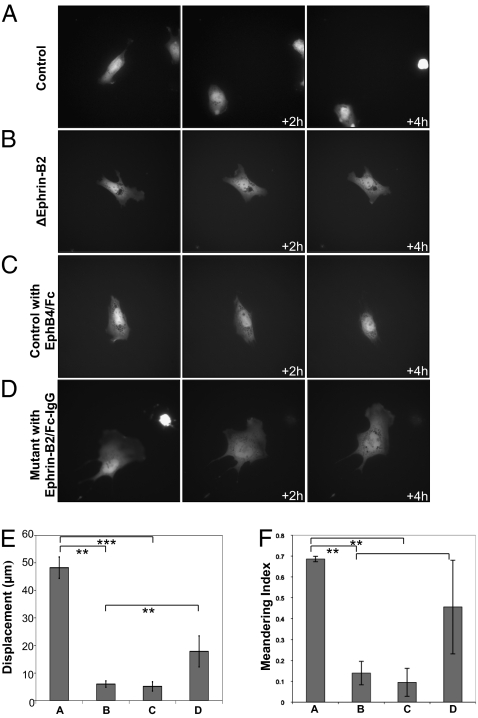
The normal separation from the pharynx in mutant mice suggested that thymus migration itself may be delayed. To examine whether ephrin-B2 expression on NCCs is required for normal mobility, we digested mutant E13.5 Ephrin-B2Lx/Lx;Wnt1-Cre;Rosa26eYfp and control Ephrin-B2+/+;Wnt1-Cre;Rosa26eYfp embryonic thymi with collagenase and incubated the resulting cell suspension in conditions described in the legend. eYFP+ cell movements were imaged over a period of 4 h. Deletion of ephrin-B2 resulted in marked changes in cell motility and behavior. They appeared to form many unstable lamellipodial protrusions, were unable to retract these protrusions, and had frequent changes in overall direction of movement (Fig. 4B). Tracking these cells indicated a displacement of 6.4 μm over 4 h (Fig. 4E) and low meandering index (a measure of directionality where 1 is migration in one direction and 0 is totally random movement) of 0.19 (Fig. 4F). In contrast, control cells appeared polarized, migrated a total of 45.5 μm over 4 h, and exhibited a meandering index of 0.67 (Fig. 4 A, E, and F and Movies S1 and S2).
Fig. 4.
NC-derived cells deficient in ephrin-B2 exhibit abnormal polarization and migration. (A–D) Cells from E13.5 control Wnt1-Cre;Rosa26eYfp or mutant Ephrin-B2Lx/Lx;Wnt1-Cre;Rosa26eYfp thymi were incubated with or without ephrin-B2/Fc cross-linked with IgG or EphB4/Fc for 2 h in serum-free IMDM, as indicated on the figure, and imaged by fluorescence microscopy. Images of cells are shown at 0-, 2-, and 4-h time points. (Magnification: ×60.) (E) Total displacement of the cells shown in A–D. t test: (A and B) P = 0.000315, (A–C) P = 0.000374, and (B–D) P = 0.0228. (F) Meandering index was calculated for each cell shown in A–D; 1 indicates directional movement, and 0 indicates random movement. t test: (A and B) P = 0.00101, (A–C) P = 0.00134, and (B–D) P = 0.0769. Similar results were obtained from at least three experiments for each condition.
It was possible to induce cell behavior in control cells similar to that seen in the mutants using an EphB4 fusion protein that blocks receptor signaling induced by ephrin-B2. Under these conditions, control cells became unable to polarize, acquired many unstable lamellipodial protrusions, exhibited a displacement of 5.89 μm over 4 h, and had a decreased meandering index of 0.16 (Fig. 4 C, E, and F). In contrast, incubation of mutant cells with an ephrin-B2 fusion protein cross-linked with anti-IgG, which binds and activates the EphB receptors, restored polarization and directional migration, showing a 23.6-μm displacement over 4 h and an increased meandering index of 0.55 (Fig. 4 D, E, and F and Movies S3 and S4). It is possible that this is the result of a cell autonomous function, or it could be because of either previous cell–cell interactions or cross-talk with other receptor signaling pathways in the same cell. Similar results were obtained when cells were incubated with EphB4/Fc fusion protein or IgG cross-linked ephrin-B2/Fc fusion protein for 2 h or overnight before imaging. These results indicate that deletion of ephrin-B2 from NC-derived cells changes their polarization and migration capacity, possibly by abolishing signaling pathways downstream of EphB receptors within these cells.
Discussion
During embryogenesis, the thymic rudiment migrates down into the superior mediastinum, resting above the heart. Shortly after thymus migration is complete, the thoracic cavity closes by midline fusion of paired cartilage bars. The mechanism leading to the collective migration of the thymus primordium is unknown. Several different gene families have been shown to have important roles in the migration and cellular organization during neural development and angiogenesis. One of these families includes the Eph receptors and ephrin ligands, which are expressed on multiple cell lineages in the thymus, including thymocytes, TECs, NC-derived mesenchyme, and endothelial cells (this report and refs. 21–25). Eph-receptor expression varies during the different stages of T cell maturation and the different regions of the thymus (24, 36), suggesting a role in the regulation of thymus organization and function. However, little is known about the role of ephrin ligands in thymus organogenesis.
Eph/ephrins are bidirectional signaling molecules that are involved in cellular repulsion between receptor- and ligand-expressing cells, driving them into distinct domains. Eph/ephrins are also known to play a key role in the migration of NCCs, dictating the timing and patterning of their migration. Specifically, ephrin-B2 has been shown to be important for NCC migration into the second pharyngeal arch after NCC delamination from the fourth and sixth rhombomeres (17). Because of the essential role of ephrin-B2 in other developmental processes, we examined mice that lack ephrin-B2 specifically on NCCs or TECs. Absence of ephrin-B2 from TECs had no effect on thymus development. Deletion of ephrin-B2 in NCCs also had no effect on thymocyte development or the migration of NCCs to the developing thymic rudiment, indicating that an alternative ephrin ligand may be responsible for their delamination and migration to this region.
We found an essential role for ephrin-B2 in determining the anatomical location of the thymus in adult mice. Our data indicate that, in the absence of ephrin-B2 on NC-derived cells, the epithelial bud forms normally from the third pharyngeal pouch, detaches from the endodermal epithelium at the correct embryonic stage, and contains fully functional thymus- and parathyroid-fated domains. The thymic rudiment, however, fails to migrate into the thoracic cavity. In E13.5 embryos that lack ephrin-B2 expression on NC-derived cells, the thymus and parathyroid rudiments remain attached to each other, whereas in adult mice, they can be isolated as individual structures, indicating that there is a delay in separation of these two organs.
Our data suggest that a primary defect in the rate of migration is the main mechanism for the ectopic location of the thymus in adult NCC-specific ephrin-B2−/− mutant mice. It is possible that during its descent, the rudiment may leave behind fragments in some strains, leading to the cervical thymi described before, an extreme manifestation of which are the mutants we present here (37, 38). However, in mice deficient for ephrin-B2 expression on NCCs, two contralateral thymic structures were present, along with lymph nodes. Importantly, we found no additional lymphoid structures containing double-positive T cells that would indicate the presence of extra cervical thymic lobes. The ectopic thymi exhibited a very similar cellularity to the thoracic thymus of wild-type mice, unlike the cervical thymic structures that were much reduced in size (Fig. 1). Moreover, H&E-stained sections of ectopic thymus indicated the presence of multiple thymic lobes similar to the thoracic thymus and in contrast to the cervical thymus that was previously published (Fig. S9A). Thus, we think it unlikely that the ectopic structures observed in the mice described in this report are, in fact, enlarged cervical thymi such as those described before.
Signaling mutants, which only block reverse signaling by disrupting the postsynaptic density protein/Drosophila discs large tumour supresssor/Zonula occludens-1 protein (PDZ) domain or phosphotyrosine docking sites of ephrin-B2, showed no disruption in thymus positioning, indicating that forward signaling through EphB receptors is probably responsible for the observed phenotype (Fig. S9B). Signaling molecules downstream of Eph receptors include small GTPases of the Rho and Ras family, focal adhesion kinase, and the JAK/STAT and PI3K pathways (39, 40). Rho family molecules such as Rac mediate actin dynamics, which control cell shape and movement by promoting the formation of lamellipodia, filopodia, and stress fibers (41–43). In vitro experiments described here showed that, in the absence of ephrin-B2 expression, polarization and migration of NC-derived cells are defective. This phenotype was rescued by exposure of the mutant cells to soluble ephrin-B2/Fc cross-linked with IgG, thus providing a substitute ligand to activate the receptor. Together, these results strongly suggest that signaling downstream of EphB receptors is required for normal migratory behavior of thymic mesenchyme and correct positioning of the thymus.
These data raise the possibility that the behavior of individual cells might control the migration of the whole thymic rudiment in a process analogous to collective cell migration described in other organisms. During morphogenesis, a number of developmental steps require collective migration: cells of the inner blastocyst during morphogenesis (43), branching of epithelial sprouts (44), migration of border cells (45), movement of epithelial cells at the rim of the optic and thyroid placodes (46), and migration of the lateral line in zebrafish (47). This process requires that the migrating mass comprising cells that are tightly adhered to each other by cell–cell junctions is dragged forward by the cells at the leading edge, whereas the relative position of the cells within the group is maintained (48). Thus, it is possible that signaling downstream of Eph receptors contributes to the formation of lamelipodia by NC-derived cells that protrude into the surrounding extracellular matrix. Subsequent shortening of actin filaments then results in retraction of the trailing edge and movement of the cell body, which drags behind it the whole rudiment. It remains unknown how the contractile force of the NCCs that surround the thymic rudiment are sufficient to move the whole anlagen. Thus, it will be interesting to identify in the future the molecular cues that provide directionality and mechanical forces in these processes.
In summary, our data show that ephrin-B2 expression on NC-derived cells is required for the migration of the thymic primordium to its normal position in the thoracic cavity. However, the loss of ephrin-B2 on NC-derived cells or TECs has no functional consequence for T cell development. Individual NC-derived cells from mutant thymuses show perturbed migration, showing a key role of EphB receptors in cell motility. Furthermore, although previously shown to be important for various functions in embryonic development, here we show a role for Eph/ephrin signaling in the process of collective cell migration.
Experimental Procedures
Mice.
Ephrin-B2Lx/Lx (31), Wnt1-Cre (33), Rosa26eYfp (34), IL7-Cre (32), Foxn1-Cre (49), and C57BL/6 mice were bred at National Institute of Medical Research (London). Ephrin-B2ΔV/ΔV and Ephrin-B25Y/5Y (50) mice were bred at Cancer Research UK. Experiments were carried out according to UK Home Office regulations.
Stereo Microscopy.
eYFP expression was analyzed using a Zeiss M2Bio (Carl Zeiss) microscope. Pictures were obtained in Open Lab (Improvision), and contrast was enhanced in Photoshop (Adobe).
Whole-Mount Immunohistochemistry.
Embryos and organs were prepared for imaging as previously described (7). Briefly, tissues were fixed, cut into 100-μm sections, and stained with antibodies recognizing eYFP (Alexa Fluor 647; Invitrogen), Endomucin (D. Vestweber, Max Planck Institute for Molecular BioMedicine, Muenster, Germany), Desmin (Abcam), and appropriate secondary antibodies against rat IgG and rabbit IgG (Alexa Fluor 594; Invitrogen).
Confocal Microscopy.
For detection of immunofluoresence, samples were analyzed using a Leica SP2 confocal (Leica). Confocal images are presented as single sections or a 3D rendering of many serial sections in Volocity (Improvision).
Video Microscopy.
Thymic rudiments from E13.5 Wnt1-Cre;Rosa26eYfp control or Ephrin-B2Lx/Lx;Wnt1-Cre;Rosa26eYfp mutant embryos were dissected, digested with collagenase D (Roche), seeded in glass-bottomed dishes (MatTek), and incubated for 2 h or overnight in serum-free Iscove's Modified Dulbecco Medium with or without ephrin-B2/Fc cross-linked with IgG or EphB4/Fc as indicated on the figure. Images (Fig. 4) were obtained at 5-min intervals on a Deltavision microscope. Images were analyzed in SoftWorx (Applied Precision) and Volocity (Improvision).
Flow-Cytometric Cell Sorting.
NC-derived cells and TECs were analyzed by digesting the thymus with collagenase D. The resulting cell suspension was stained with antibodies against ephrin-B2, EphB4 (R&D Systems), CD45, CD4, CD8, TCRβ, and CD19 (eBiosciences). Samples were acquired on a FACS Calibur (BD Biosciences) and analyzed in FlowJo (TreeStar).
In Situ Hybridization.
In situ hybridization for Crabp1, Foxn1, and Gcm2 was performed on 10-μm paraffin sections as previously described (51).
H&E Sections.
Whole embryos were processed for paraffin sectioning and stained with H&E.
Supplementary Material
Acknowledgments
We thank K. Roderick, A. Patel, T. Norton, K. Williams, A. Rae, G. Preece, Y. Gu, and K. Sullivan for assistance. This work was funded by the Medical Research Council (MRC). T.M. is supported by Cancer Research UK. C.B. is supported by the Leukaemia Research Fund. R.A. is supported by the Max-Planck Institute. M.C. is supported by MRC Grant G0601156. J.G., K.C., E.R., and N.M are supported by National Institutes of Health grants.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003747107/-/DCSupplemental.
References
- 1.Wallin J, et al. Pax1 is expressed during development of the thymus epithelium and is required for normal T-cell maturation. Development. 1996;122:23–30. doi: 10.1242/dev.122.1.23. [DOI] [PubMed] [Google Scholar]
- 2.Su D, Ellis S, Napier A, Lee K, Manley NR. Hoxa3 and pax1 regulate epithelial cell death and proliferation during thymus and parathyroid organogenesis. Dev Biol. 2001;236:316–329. doi: 10.1006/dbio.2001.0342. [DOI] [PubMed] [Google Scholar]
- 3.Peters H, Neubüser A, Kratochwil K, Balling R. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 1998;12:2735–2747. doi: 10.1101/gad.12.17.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou D, et al. Patterning of the third pharyngeal pouch into thymus/parathyroid by Six and Eya1. Dev Biol. 2006;293:499–512. doi: 10.1016/j.ydbio.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffith AV, et al. Increased thymus- and decreased parathyroid-fated organ domains in Splotch mutant embryos. Dev Biol. 2009;327:216–227. doi: 10.1016/j.ydbio.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Revest J-M, Suniara RK, Kerr K, Owen JJT, Dickson C. Development of the thymus requires signaling through the fibroblast growth factor receptor R2-IIIb. J Immunol. 2001;167:1954–1961. doi: 10.4049/jimmunol.167.4.1954. [DOI] [PubMed] [Google Scholar]
- 7.Foster K, et al. Contribution of neural crest-derived cells in the embryonic and adult thymus. J Immunol. 2008;180:3183–3189. doi: 10.4049/jimmunol.180.5.3183. [DOI] [PubMed] [Google Scholar]
- 8.Müller SM, et al. Neural crest origin of perivascular mesenchyme in the adult thymus. J Immunol. 2008;180:5344–5351. doi: 10.4049/jimmunol.180.8.5344. [DOI] [PubMed] [Google Scholar]
- 9.Hetzer-Egger C, et al. Thymopoiesis requires Pax9 function in thymic epithelial cells. Eur J Immunol. 2002;32:1175–1181. doi: 10.1002/1521-4141(200204)32:4<1175::AID-IMMU1175>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 10.Manley NR, Capecchi MR. Hox group 3 paralogs regulate the development and migration of the thymus, thyroid, and parathyroid glands. Dev Biol. 1998;195:1–15. doi: 10.1006/dbio.1997.8827. [DOI] [PubMed] [Google Scholar]
- 11.Epstein JA, et al. Migration of cardiac neural crest cells in Splotch embryos. Development. 2000;127:1869–1878. doi: 10.1242/dev.127.9.1869. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, et al. Defective ALK5 signaling in the neural crest leads to increased postmigratory neural crest cell apoptosis and severe outflow tract defects. BMC Dev Biol. 2006 doi: 10.1186/1471-213X-6-51. 10.1186/147-213X-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams RH, et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: Demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 15.Holder N, Klein R. Eph receptors and ephrins: Effectors of morphogenesis. Development. 1999;126:2033–2044. doi: 10.1242/dev.126.10.2033. [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson DG. Eph receptors and ephrins: Regulators of guidance and assembly. Int Rev Cytol. 2000;196:177–244. doi: 10.1016/s0074-7696(00)96005-4. [DOI] [PubMed] [Google Scholar]
- 17.Adams RH, et al. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104:57–69. doi: 10.1016/s0092-8674(01)00191-x. [DOI] [PubMed] [Google Scholar]
- 18.Davy A, Aubin J, Soriano P. Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev. 2004;18:572–583. doi: 10.1101/gad.1171704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murai KK, Pasquale EB. “Eph”ective signaling: Forward, reverse and crosstalk. J Cell Sci. 2003;116:2823–2832. doi: 10.1242/jcs.00625. [DOI] [PubMed] [Google Scholar]
- 20.Noren NK, Pasquale EB. Eph receptor-ephrin bidirectional signals that target Ras and Rho proteins. Cell Signal. 2004;16:655–666. doi: 10.1016/j.cellsig.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Fox GM, et al. cDNA cloning and tissue distribution of five human EPH-like receptor protein-tyrosine kinases. Oncogene. 1995;10:897–905. [PubMed] [Google Scholar]
- 22.Gurniak CB, Berg LJ. A new member of the Eph family of receptors that lacks protein tyrosine kinase activity. Oncogene. 1996;13:777–786. [PubMed] [Google Scholar]
- 23.Luo H, Yu G, Wu Y, Wu J. EphB6 crosslinking results in costimulation of T cells. J Clin Invest. 2002;110:1141–1150. doi: 10.1172/JCI15883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vergara-Silva A, Schaefer KL, Berg LJ. Compartmentalized Eph receptor and ephrin expression in the thymus. Gene Expr Patterns. 2002;2:261–265. doi: 10.1016/s1567-133x(02)00054-6. [DOI] [PubMed] [Google Scholar]
- 25.Yu G, Luo H, Wu Y, Wu J. Ephrin B2 induces T cell costimulation. J Immunol. 2003;171:106–114. doi: 10.4049/jimmunol.171.1.106. [DOI] [PubMed] [Google Scholar]
- 26.Wu J, Luo H. Recent advances on T-cell regulation by receptor tyrosine kinases. Curr Opin Hematol. 2005;12:292–297. doi: 10.1097/01.moh.0000166497.26397.9f. [DOI] [PubMed] [Google Scholar]
- 27.Muñoz JJ, et al. Thymic alterations in EphA4-deficient mice. J Immunol. 2006;177:804–813. doi: 10.4049/jimmunol.177.2.804. [DOI] [PubMed] [Google Scholar]
- 28.Freywald A, Sharfe N, Rashotte C, Grunberger T, Roifman CM. The EphB6 receptor inhibits JNK activation in T lymphocytes and modulates T cell receptor-mediated responses. J Biol Chem. 2003;278:10150–10156. doi: 10.1074/jbc.M208179200. [DOI] [PubMed] [Google Scholar]
- 29.Alfaro D, et al. Alterations in the thymocyte phenotype of EphB-deficient mice largely affect the double negative cell compartment. Immunology. 2008;125:131–143. doi: 10.1111/j.1365-2567.2008.02828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alfaro D, et al. EphrinB1-EphB signaling regulates thymocyte-epithelium interactions involved in functional T cell development. Eur J Immunol. 2007;37:2596–2605. doi: 10.1002/eji.200737097. [DOI] [PubMed] [Google Scholar]
- 31.Grunwald IC, et al. Hippocampal plasticity requires postsynaptic ephrinBs. Nat Neurosci. 2004;7:33–40. doi: 10.1038/nn1164. [DOI] [PubMed] [Google Scholar]
- 32.Repass JF, et al. IL7-hCD25 and IL7-Cre BAC Transgenic Mouse Lines: New Tools for Analysis of IL-7 Expressing Cells. Genesis. 2009;47:281–287. doi: 10.1002/dvg.20497. [DOI] [PubMed] [Google Scholar]
- 33.Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- 34.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001 doi: 10.1186/1471-213X-1-4. 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davy A, Soriano P. Ephrin-B2 forward signaling regulates somite patterning and neural crest cell development. Dev Biol. 2007;304:182–193. doi: 10.1016/j.ydbio.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muñoz JJ, et al. Expression and function of the Eph A receptors and their ligands ephrins A in the rat thymus. J Immunol. 2002;169:177–184. doi: 10.4049/jimmunol.169.1.177. [DOI] [PubMed] [Google Scholar]
- 37.Dooley J, Erickson M, Gillard GO, Farr AG. Cervical thymus in the mouse. J Immunol. 2006;176:6484–6490. doi: 10.4049/jimmunol.176.11.6484. [DOI] [PubMed] [Google Scholar]
- 38.Terszowski G, et al. Evidence for a functional second thymus in mice. Science. 2006;312:284–287. doi: 10.1126/science.1123497. [DOI] [PubMed] [Google Scholar]
- 39.Knöll B, Drescher U. Src family kinases are involved in EphA receptor-mediated retinal axon guidance. J Neurosci. 2004;24:6248–6257. doi: 10.1523/JNEUROSCI.0985-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parri M, et al. EphrinA1 activates a Src/focal adhesion kinase-mediated motility response leading to rho-dependent actino/myosin contractility. J Biol Chem. 2007;282:19619–19628. doi: 10.1074/jbc.M701319200. [DOI] [PubMed] [Google Scholar]
- 41.Shamah SM, et al. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- 42.Irie F, Yamaguchi Y. EphB receptors regulate dendritic spine development via intersectin, Cdc42 and N-WASP. Nat Neurosci. 2002;5:1117–1118. doi: 10.1038/nn964. [DOI] [PubMed] [Google Scholar]
- 43.Penzes P, et al. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- 44.Trinkaus JP, Trinkaus M, Fink RD. On the convergent cell movements of gastrulation in Fundulus. J Exp Zool. 1992;261:40–61. doi: 10.1002/jez.1402610107. [DOI] [PubMed] [Google Scholar]
- 45.Zegers MM, O'Brien LE, Yu W, Datta A, Mostov KE. Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol. 2003;13:169–176. doi: 10.1016/s0962-8924(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 46.Montell DJ. Developmental regulation of cell migration. Insight from a genetic approach in Drosophila. Cell Biochem Biophys. 1999;31:219–229. doi: 10.1007/BF02738240. [DOI] [PubMed] [Google Scholar]
- 47.Hilfer SR, Marrero L, Sheffield JB. Patterns of cell movement in early organ primordia of the chick embryo. Anat Rec. 1990;227:508–517. doi: 10.1002/ar.1092270414. [DOI] [PubMed] [Google Scholar]
- 48.Gilmour DT, Maischein HM, Nüsslein-Volhard C. Migration and function of a glial subtype in the vertebrate peripheral nervous system. Neuron. 2002;34:577–588. doi: 10.1016/s0896-6273(02)00683-9. [DOI] [PubMed] [Google Scholar]
- 49.Hegerfeldt Y, Tusch M, Bröcker EB, Friedl P. Collective cell movement in primary melanoma explants: Plasticity of cell–cell interaction, beta1-integrin function, and migration strategies. Cancer Res. 2002;62:2125–2130. [PubMed] [Google Scholar]
- 50.Gordon J, et al. Specific expression of lacZ and cre recombinase in fetal thymic epithelial cells by multiplex gene targeting at the Foxn1 locus. BMC Dev Biol. 2007 doi: 10.1186/1471-213X-7-69. 10.1186/1471-213X-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mäkinen T, et al. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.