Abstract
Everyday circumstances require efficient updating of behavior. Brain systems in the right inferior frontal cortex have been identified as critical for some aspects of behavioral updating, such as stopping actions. However, the precise role of these neural systems is controversial. Here we examined how the inferior frontal cortex updates behavior by combining reversible cortical interference (transcranial magnetic stimulation) with an experimental task that measures different types of updating. We found that the right inferior frontal cortex can be functionally segregated into two subregions: a dorsal region, which is critical for visual detection of changes in the environment, and a ventral region, which updates the corresponding action plan. This dissociation reconciles competing accounts of prefrontal organization and casts light on the neural architecture of human cognitive control.
Keywords: cognitive control, dual-tasking, inhibition, prefrontal cortex, transcranial magnetic stimulation
We are confronted every day with multitask situations in which different courses of action can be followed. When the environment changes, actions need to be updated to comply with the new task demands. Cognitive models assume that an executive control system updates behavior by manipulating the activation of task goals that drive subordinate processes (1, 2). For instance, when updating behavior requires stopping a motor response, the control system activates a stop goal to countermand planned or ongoing motor processes (3–5).
One cortical region in humans that is critical for behavioral updating is the right inferior frontal cortex (rIFC). In particular, studies using response-inhibition paradigms, such as the stop-signal task and the go/no-go task, have shown that rIFC is important for stopping actions (6). This has led some researchers to conclude that rIFC has a predominant inhibitory role in updating (7–9). However, the inhibitory role of rIFC is intensely debated and there are other explanations for the critical involvement of rIFC in updating.
A first alternative explanation is that rIFC could have an attentional role (10–13). The attentional hypothesis assumes that rIFC mediates detection of stimulus change, whereas other cortical regions control inhibition and updating of actions. Specifically, several authors have argued that the presupplementary motor area (preSMA) is the primary site for motor inhibition and action updating (10–14). Note, however, that it is also possible that rIFC could mediate both attention and inhibition. Results of a recent neuroimaging study suggest that the more dorsal right inferior frontal junction (rIFJ) is engaged during attention, whereas the more ventral right inferior frontal gyrus (rIFG) is activated during inhibition (15).
A second alternative explanation is that rIFC could have a general role in updating action plans. Evidence from human neuroimaging has shown that rIFC is involved in reprogramming a motor response when a “go” stimulus is replaced by another go stimulus (16). This suggests that rIFC could be important for the selection of an action in the face of a concurrently activated action plan. In some situations, updating or reprogramming could involve the selection of the plan to execute an alternative motor response, whereas in other situations, updating could require the selection of the plan not to respond (4). Thus, the key difference between the inhibition and updating accounts is that the inhibition account assumes that rIFC is selectively crucial for stopping, whereas the updating account assumes that rIFC is crucial for different forms of updating (including stopping).
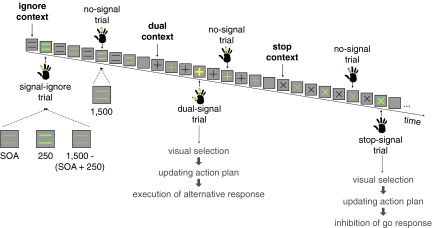
To investigate how the rIFC and preSMA update behavior, we applied continuous theta-burst stimulation as a causal neurological intervention. This form of repetitive transcranial magnetic stimulation is delivered for a brief duration (<1 min) to produce a disruptive suppression of neural function for up to 1 h (17). We combined continuous theta-burst stimulation with the context-cuing paradigm (Fig. 1; ref. 18). On each trial, the identity of a cue (=, +, x) indicated one of three possible task contexts (ignore, dual, and stop, respectively). When the cue changed color, subjects initiated a go response. On a minority of trials, the colored cue turned bold after a variable delay: in the ignore context (signal-ignore trials), subjects had to ignore the change and execute the go response; in the dual-task context (dual-signal trials), subjects had to execute an additional response following the go response by pressing an alternate key; and in the stop context (stop-signal trials), subjects tried to withhold the go response.
Fig. 1.
Typical trial sequence illustrating the different trial types in the three contexts and the required responses. The color-response mapping was counterbalanced across subjects. On signal trials, the colored cue turned bold for 250 ms after a variable delay (SOA). On signal-ignore and dual-signal trials, SOAs were fixed (100, 250, or 400 ms); on stop-signal trials, SOA was dynamically adjusted (Materials and Methods). Time intervals for signal and no-signal trials are in milliseconds. Presumed control processes for dual- and stop-signal trials are indicated in gray.
The context-cuing paradigm dissociates several control processes involved in updating behavior. First, stop-signal trials but not dual-signal trials require response inhibition. Therefore, if a region is selectively crucial for stopping actions, then disruption of this region should lengthen the time it takes to stop on stop-signal trials while leaving the time it takes to execute an additional response on dual-signal trials unaffected. Second, correct performance on dual- and stop-signal trials requires visual detection of a relevant change in the stimulus features (i.e., the cue turning bold). Therefore, if a region is crucial for visual detection of a relevant change in the environment, then disruption of this region should increase both the stop and dual-response latency. Third, dual- and stop-signal trials each require the updating of a global action plan. Specifically, these trials require programming of an alternative action in the face of the more dominant plan to execute a single response: on dual-signal trials, subjects must decide to execute a second response, whereas on stop-signal trials, they must decide not to respond. Therefore, if a region is crucial for updating actions plans then cortical disruption should, again, increase both the stop and dual-response latency.
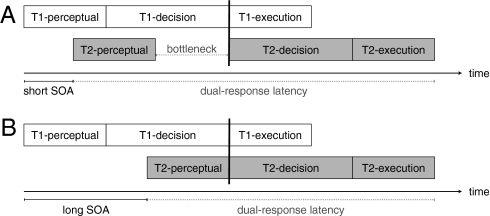
The visual-detection account and the action-updating account make the same behavioral predictions: they both predict that stop and dual-response latencies should increase after continuous theta-burst stimulation. To distinguish between these two accounts, we exploited the well-established properties of the psychological refractory period (for reviews, see refs. 19, 20). The refractory-period paradigm was originally developed to investigate the timing of dual-task performance (21). In most reaction time tasks, there are three general processing stages: a perceptual stage, a central decision stage that includes response selection, and a motor execution stage (e.g., refs. 22, 23). A substantial body of evidence indicates that in dual-task situations, the decision stages of two tasks do not overlap, unlike the perceptual and motor stages (19, 20). This lack of overlap creates a processing bottleneck when the delay between two tasks (stimulus onset asynchrony, SOA) is short because the decision stage of task 2 is postponed until the decision stage of task 1 is finished (Fig. 2A). When the SOA is long, the decision stage of task 1 is more likely to be completed by the time the perceptual stage of task 2 is finished (Fig. 2B). Therefore, the decision stage of task 2 can start immediately and no bottleneck is observed at long SOAs.
Fig. 2.
At short delays (SOA; Upper), the decision stage for task 2 is postponed until the decision stage for task 1 is finished (indicated by the vertical black line). This creates a bottleneck period. At longer SOAs (Lower), the decision stage of task 1 is completed when the perceptual-processing stage of task 2 is finished. Therefore, the decision stage of task 2 can start immediately at longer SOAs.
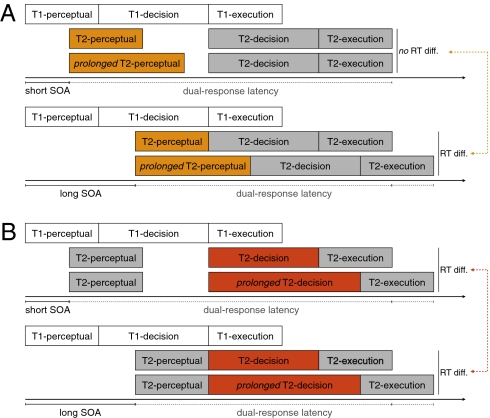
Over many years, the bottleneck at short SOAs has been used to determine which processing stages are influenced by experimental manipulations (e.g., refs. 24, 25). Most of these studies used the locus-of-slack procedure (25). This approach is based on two key predictions of the bottleneck model that have been confirmed by a long history of research in experimental psychology (e.g., refs. 26–28). First, the bottleneck model predicts that factors that influence task 2 processing at the prebottleneck (perceptual) stage should have a larger effect at longer SOAs than at shorter SOAs. This is because, at short SOAs, the prolongation of the perceptual stage can be absorbed in the bottleneck period (Fig. 3A Upper). At longer SOAs, there is no bottleneck, so prolongation of the perceptual stage should be detected (Fig. 3A Lower). Second, the bottleneck model predicts that factors that influence task 2 processing at or after the bottleneck should produce a uniform effect that does not depend on the SOA (Fig. 3B). This is because the prolongation cannot be absorbed in the bottleneck period at short SOAs (Fig. 3B Upper). Thus, analyzing the latency of the dual response as a function of SOA enables one to identify which processing stages are influenced by an experimental factor.
Fig. 3.
(A) At short SOAs (Upper), prolongation of the perceptual stage of task 2 is absorbed within the bottleneck period. At longer SOAs (Lower) there is no bottleneck, so the prolongation of the perceptual stage of task 2 should be detected. Consequently, interfering with prebottleneck processes such as visual detection should produce a RT deficit that increases with SOA. (B) Prolongation of stages at or after the bottleneck should be detected both at short (Upper) and long (Lower) SOAs. Consequently, interfering with processes at or after the bottleneck, such as action updating, should produce a uniform RT deficit that does not depend on SOA.
Here, we used the locus-of-slack procedure to determine the locus of the disruptive effect of theta-burst stimulation. To do this, we analyzed the latency of the dual response as a function of the delay between the go signal (i.e., when the cue changed color) and dual-task signal (i.e., when the cue turned bold). Visual detection occurs before the bottleneck stage (19). Therefore, if a region is important for detection of changes in stimulus features, then disruption of this region should produce a deficit of dual-response latency that increases with SOA (Fig. 3A). By contrast, selection and implementation of the alternative action plan occurs at or after the bottleneck stage. Therefore, if a region is important for action updating, then disruption of this region should produce a uniform deficit in dual-response latency that does not depend on SOA (Fig. 3B).
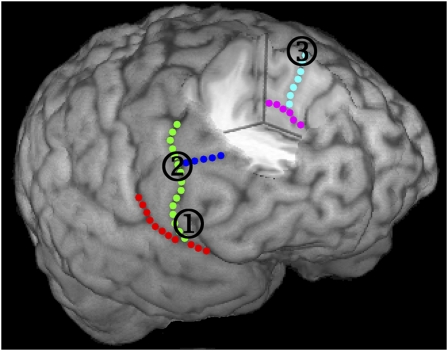
On different days, we stimulated the three cortical regions that previous research has identified as most important for inhibition and updating: rIFG, rIFJ, and preSMA (Fig. 4). This resulted in four stimulation sessions: the three sites and a Sham condition (baseline). During Sham, the coil was oriented away from the scalp to mimic the auditory artifacts of stimulation without stimulating the cortex. In the hour following continuous theta-burst stimulation, 18 subjects undertook multiple blocks of the context-cuing paradigm. Data were analyzed using linear mixed effects, an optimal method for considering time-series data (29). For each behavioral measure, we tested the main effect of stimulation site (see SI Text for full details). If the main effect of site was significant, we undertook pairwise mixed-effect contrasts comparing each site with Sham. To eliminate confounding effects of cue encoding or context switching (18), all analyses included context-repetition trials only (see SI Text for an analysis of switch trials). Signal-ignore trials were included in the design to test whether subjects followed the instructions. These trials are not discussed further as the data clearly indicated that subjects followed the instructions in all sessions (SI Text).
Fig. 4.
Cortical regions that were disrupted with continuous theta-burst stimulation. Right inferior frontal gyrus (rIFG), right inferior frontal junction (rIFJ), and presupplementary motor area (preSMA) shown in one participant. All sites were defined from individual neuroanatomical landmarks (Materials and Methods) (1) rIFG, (2) rIFJ, and (3) preSMA. Sulci: red, lateral sulcus; green, precentral sulcus; dark blue, inferior frontal sulcus; magenta, cingulate sulcus; light blue, dorsal cingulate branch.
Results
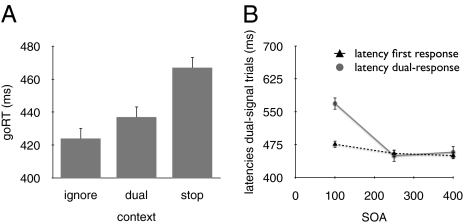
The baseline (Sham) condition replicates previous findings (Fig. 5), which validate our behavioral paradigm. First, mean stop latency in the Sham condition was 268 ms, which was within the normal range (30). For no-signal trials, we found significant differences between the latencies of the go response (goRT) in the three contexts (Fig. 5A), consistent with previous studies (18, 31). The difference between the ignore context and the dual-task and stop contexts likely arose due to increased working memory demands (18). Furthermore, the difference between the dual-task and stop context suggests that subjects made additional proactive response-strategy adjustments in the stop context (18). For dual-signal trials, we found that the dual-response latency decreased substantially when SOA increased, whereas the latency of the first response on dual-signal trials was influenced by SOA only to a small extent (Fig. 5B). Consistent with many previous studies (19, 20), these findings confirm the presence of a decision bottleneck and validate the application of the refractory-period methodology to contrast the stopping, visual-detection, and action-updating accounts.
Fig. 5.
Data of the linear mixed model for the baseline (Sham) condition. (A) A goRT difference between the three contexts [F(2, 1006) = 433.3, P < 0.001]. goRTs were slower in the stop context than in the dual-task context and ignore context (t = 20.2, P < 0.001, and t = 28.6, P < 0.001, respectively). In addition, goRTs were longer in the dual-task context than in the ignore context (t = 8.3, P < 0.001). (B) The dual-response latency substantially decreased when SOA increased [F(2,1006) = 1267.8, P < 0.0001]. There was also a smaller effect of SOA on the latency of the first response on dual-signal trials [F(2,1006) = 39.202, P < 0.0001].
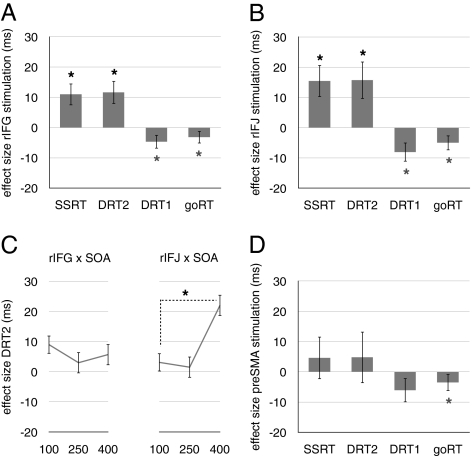
Following disruption of rIFG, both stop- and dual-signal performance were impaired relative to Sham (stop latency: t = 3.09, P = 0.002; dual-response latency: t = 3.02, P = 0.003; Fig. 6A). This common effect of rIFG stimulation on stopping and dual-tasking shows that rIFG is not selectively crucial for inhibitory control. Further, the dual-task effect did not interact with SOA, t = 0.84, P = 0.40 (Fig. 6C), which is inconsistent with the hypothesis that rIFG is crucial for visual detection. Instead, the results indicate that rIFG is crucial for updating action plans. On no-signal trials, goRT was not impaired and instead decreased, t = 2.5, P = 0.01; a similar trend was observed for the latency of the first response on dual-signal trials, t = 2.0, P = 0.04. This facilitation of goRT was possibly due to a general increase in arousal or alertness after theta-burst stimulation. Stimulation of rIFG did not influence the goRT difference between the task contexts.
Fig. 6.
Results of linear mixed effect analyses. Error rates were minimal (SI Text). Disruption of rIFG and rIFJ increased the stop latency (SSRT) and the dual-response latency (DRT2) (A and B). By contrast, goRT and the latency of the first response on dual-signal trials (DRT1) decreased. Crucially, the roles of rIFG and rIFJ were dissociated by the SOA analysis: the effect of stimulation for DRT2 depended on SOA for rIFJ, but not for rIFG (C). The interaction between site and SOA was significant (SI Text). Finally, stimulation of preSMA did not reliably influence signal performance (D). Asterisks indicate that the LME contrast with baseline (Sham) condition was significant (α = 0.05; black asterisks indicate site-specific effects; gray asterisks indicate site-nonspecific effects). Error bars indicate ±1 SEM of the LME estimates.
Following disruption of rIFJ, stop- and dual-signal performance were impaired relative to Sham (stop latency: t = 2.93, P = 0.004; dual-response latency: t = 2.54, P = 0.01; Fig. 6B), showing that rIFJ, like rIFG, is not selectively crucial for inhibitory control. Now however, the dual-task effect did interact strongly with SOA, t = 4.85, P = 0.0001 (Fig. 6C), reliably dissociating rIFJ from the nearby rIFG. These results indicate that, rather than updating action plans, rIFJ is crucial for visual detection of changes in stimulus features. As with rIFG, disruption of rIFJ also facilitated goRT (goRT: t = 3.9, P = 0.0001; latency first response dual-signal trials: t = 2.57, P = 0.01), but did not influence the goRT difference between the task contexts (SI Text).
Finally, disruption of preSMA did not influence signal performance (stop latency: t = 0.66, P = 0.55; dual-response latency: t = 0.56, P = 0.57; Fig. 6D). Consistent with the other sites, stimulation facilitated goRT, t = 2.79, P = 0.005, while the trend for the latency of the first response on dual-signal trials did not reach significance, t = 1.52, P = 0.12. Stimulation of preSMA did not influence the goRT difference between task contexts (SI Text). The absence of behavioral effects may be explained by our adherence to safe limits for the intensity of theta-burst stimulation, and by the fact that preSMA lies deep within the medial wall.
Discussion
The right inferior frontal cortex is crucial for behavioral updating, and recent clinical studies have put it forward as a strong candidate endophenotype for cognitive control (32, 33). However, there is much controversy concerning the precise neurocognitive roles of rIFC in updating (e.g., refs. 7, 10, 13, 15, 34, 35). In the present study, we contrasted three accounts of rIFC function by combining continuous theta-burst stimulation with the context-cuing paradigm. Our findings isolated two subregions in rIFC that play a distinct critical role in updating behavior.
Consistent with previous studies, we found that disruption of rIFG impaired stop performance (35, 36), which shows that rIFG is critical for stopping a response. However, we also observed an effect of rIFG disruption on dual-task performance. The dual-task deficit demonstrates that the cognitive role of rIFG is not purely one of inhibitory control. The SOA analyses subsequently showed that the role of rIFG also is not attentional, which is consistent with the observation that rIFG is activated not by the detection of infrequent events but when responding to such events (37). On the basis of these findings, we propose that the posterior rIFG implements cognitive control by updating action plans following changes in behaviorally relevant stimuli (see also ref. 16). In both the dual-task and stop context, the dominant action plan is to execute a single go response, but subjects must activate an alternative (nondominant) plan when a signal is presented. More specifically, on stop-signal trials, subjects must activate the plan not to respond. The go response could then be countermanded via connections with the subthalamic nucleus (38, 39) or preSMA (34). On dual-signal trials, subjects must instead activate the plan to execute an additional response, which could be triggered via connections with the premotor cortex (40). Importantly, the role of rIFG seems to be restricted to situations in which controlled forms of updating or response selection are required. On no-signal trials, goRTs decreased, which shows that rIFG is not critically involved in the selection of dominant responses (see also refs. 35, 41). Therefore, we propose that rIFG is important for higher-order (i.e., executive) control of updating and selection of actions in the face of alternative, competing plans. This is consistent with the view that posterio-inferior frontal cortex is critical for contextually controlled forms of action selection in situations where the dominant action may not be appropriate (42, 43).
In addition to demonstrating the role of rIFG in action updating, our findings also dissociate rIFG from the more dorsal rIFJ. They show, in particular, that rIFJ is crucial for visual detection of infrequent changes in task-relevant stimulus features rather than action planning. This conclusion is consistent with a recent fMRI investigation of response inhibition in which a similar dissociation between rIFJ and rIFG was observed (15). Furthermore, several neuroimaging studies have shown that the dorsal extension of rIFC is engaged in stimulus- and change-detection tasks (e.g., refs. 44, 45). We found that rIFJ stimulation slowed the detection of the dual and stop signals, without impairing detection of the more frequent go signals. This suggests that rIFJ is especially crucial for detecting changes in stimulus features or the detection of infrequent but relevant events. Although our paradigm does not enable further delineation of these attentional mechanisms, we ensured that the go, dual, and stop signals appeared in one sensory modality (vision) and in the same foveal spatial location; thus our results enable us to rule out a selective critical role of rIFJ in either visuospatial orienting (46) or cross-modal attention (47).
It is important to consider three alternative explanations for the disruptive effect on rIFC observed here. First, the rIFC could be important for rule switching and/or maintaining task rules (48). However, stimulation of the rIFC did not influence the cost associated with switching between the task contexts, or the slowing on signal-ignore trials (SI Text). Thus, subjects experienced no additional difficulties in switching between rules and, subsequently, applying the correct task rules after stimulation of rIFC. Possibly, the network for rule maintenance is more left lateralized (49). Second, previous work suggests that successful stopping depends on a monitoring mechanism (18). This mechanism adjusts response speed in the go task to balance between going quickly and stopping. It is possible that rIFC has such a monitoring role (50). The goRT difference between the dual and stop contexts suggests that subjects did make control adjustments; crucially, however, these goRT differences were not influenced by stimulation of rIFC. Therefore, the goRT data do not support an explanation of our findings in terms of monitoring. Finally, rIFC could be involved in advance task preparation. The goRT data also eliminate this explanation because they show that subjects prepared for upcoming dual- and stop-signal performance (indicated by the slowing; Fig. 5A), and that these preparation effects were not influenced by stimulation of rIFC.
In conclusion, our results illustrate how the rIFC updates behavior when the environment changes. Specifically, we propose that the rIFJ visually selects the behaviorally relevant stimulus features, whereas the more ventral rIFG updates the corresponding action plan. By demonstrating the necessity of these regions for dissociable functions, these findings help to reconcile competing accounts of how the cognitive control system in human inferior frontal cortex updates behavior.
Materials and Methods
Behavioral Task.
Eighteen naive right-handed adults with normal color vision (9 females; aged 20–38 y, average age = 25.9 y) participated for monetary compensation (£10 per hour; £110–140 in total). The study was approved by the local Psychology Research Ethics Committee at Cardiff University.
Stimuli were presented on a 21-in CRT monitor against a gray background. Gaze was monitored online with a 250-Hz Video Eyetracker Toolbox (Cambridge Research Systems). The task was programmed in Matlab (Mathworks) using Psychophysics Toolbox (51). The primary task was to respond as quickly as possible to the color of the cue by pressing “J” or “K” on a keyboard with the right index or middle finger, respectively (Fig. 1). Cues were green or yellow. The color-response mapping was held constant within subjects, and was counterbalanced across the sample. One-third of the trials were signal trials (Fig. 1). On signal-ignore trials, subjects pressed J or K. On dual-signal trials, subjects pressed the space bar with the right thumb in addition to J or K. On stop-signal trials, subjects attempted to refrain from responding.
Task contexts switched randomly every four trials and occurred with equal probability. Context-run repetitions (i.e., two consecutive runs of the same context) were excluded from the design. The first trial of a context started with the presentation of a black cue. After 500 ms, the cue changed color. On trials 1–3 of a run, the cue turned black again after 1,500 ms; on trial 4, the cue was removed after 1,500 ms. The interval between trials was 250 ms. Response registration started when the first color cue appeared and ended when the last cue of a run disappeared. On signal trials, the cue turned bold for 250 ms after a variable delay. On signal-ignore and dual-signal trials, the delay was 100, 250, or 400 ms; these delays occurred with equal probability and were chosen randomly. On stop-signal trials, the delay was initially set at 200 ms and continuously adjusted according to a tracking procedure to obtain an approximate probability of stopping of 0.50: SOA increased by 50 ms each time inhibition was successful, and decreased by 50 ms each time inhibition failed (52). Subjects were instructed not to wait for the signal to occur. For dual-signal trials, they were told to execute the two responses independently, as quickly as possible, and not to group responses. For stop-signal trials, subjects were informed that it would be easy to stop on some trials and difficult or impossible to stop on other trials because the SOA varied.
The experiment consisted of six sessions on separate days. The first session started with a safety screening according to standard safety guidelines (53, 54), followed by 18 training blocks. The second session started with 6 practice blocks, followed by the motor-threshold procedure. Sessions 3–6 were counterbalanced experimental sessions (Sham, rIFG, rIFJ, preSMA); they started with a screening for adverse effects after the previous TMS session, and a screening for sleep deprivation, and alcohol, drug, and caffeine consumption. Then, subjects received 6 practice blocks, followed by continuous theta-burst stimulation, followed by 24 experimental blocks. In all sessions, each block consisted of 72 trials and lasted 2.5 min; subjects received a 1-min break after every three blocks.
TMS Procedure.
Before the motor-threshold session, we obtained anatomical magnetic resonance (MR) brain scans from each subject using a GE 3T system. To enable TMS/MR coregistration, we scanned subjects with contrast markers (vitamin E capsules) attached to known scalp locations. Anatomical sites for TMS were localized on the basis of individual neuroanatomy (Fig. 4). For each subject, we localized the stimulation sites (rIFG, rIFJ, and preSMA) according to the same anatomical landmarks (see SI Text for mean coordinates for the three sites). For rIFG, we identified the lateral sulcus (LS), the inferior frontal sulcus (IFS), and the precentral sulcus (preCS). rIFG was directly anterior to the preCS (35); the vertical distance between the stimulation site and the LS was ≈20% of the total distance between the LS and the IFS. For rIFJ, the stimulation site was defined as the junction of the preCS and IFS (48). For preSMA, we considered brain activations across several fMRI studies of response inhibition, and found a convergence of MNI coordinates on approximately x = 4, y = 33, z = 62 for the nearest surface coordinate. We then identified the nearest anatomical landmark to this location in each individual brain, which was slightly posterior to the superior termination of a clearly identifiable branch of the cingulate sulcus on the medial wall, and anterior to the paracentral sulci. For each subject, the TMS coil was placed adjacent to the dorsal termination of this branch. During Sham, the TMS coil was oriented away from the scalp, mimicking the auditory artifacts that accompany magnetic discharge without stimulating the cortex.
Before stimulation, scalp locations were calculated using a magnetic tracking device (miniBird 500; Ascension Tech) and MR coregistration software (MRIReg). Continuous theta-burst stimulation (17) was administered using a Magstim Rapid2 system (2.2T, Magstim) and 70-mm figure-of-eight induction coil. For rIFG and rIFJ, the coil was positioned with the handle in an upward vertical orientation, whereas for preSMA, it was oriented with the handle in a posterior direction. The output intensity was calibrated according to the maximum level of comfortable stimulation, expressed as a proportion of resting motor threshold (average = 51.5% of maximum stimulator output), and corrected for differences in scalp–cortex distance between brain regions (55, 56). This protocol yielded an average stimulation output of 70% distance-adjusted motor threshold (range 51–80%) and an average stimulator output of 30% (range 20–39%). The average distance gradient was 2.3% per millimeter. This indicates that for every extra millimeter in coil-scalp distance, stimulator output had to increase by 2.3% to induce a twitch in the contralateral hand on 50% of the trials.
Order of stimulation sites was counterbalanced across subjects and consecutive testing sessions were separated by at least 24 h. No subjects reported minor or major adverse effects after continuous theta-burst stimulation.
Data Analysis.
We estimated stop latency using the integration method (ref. 52; see SI Text), obtaining one estimate per 6 blocks (36 signal trials per estimation). To investigate the effect of stimulation over time (i.e., “moment”), we used a moving window to estimate stop latencies and the other measures (blocks 1–6, blocks 2–7, …, blocks 19–24; 19 windows in total).
All data were analyzed using LME models in R (www.r-project.org). The LME model is becoming increasingly popular in biological and social sciences (57) and was developed as an extension of the general linear model (GLM) to handle data with a correlated outcome variable. It does so by adding subject-specific random effects to the population-level fixed effects that are also present in the GLM. By adding these random effects one reduces the within-subject correlations in the model's error term. In addition to the random effects, one can select a pattern for the residual correlation that best matches the data (29). For our data, LME is the most appropriate analysis technique because the within-subject correlations did not have a compound symmetry structure. The LME model is also more appropriate than a repeated measures MANOVA as it can model the within-subject correlations with far fewer parameters. In our experiment, “site” and “moment” were fixed effects; individual differences in baseline performance and in moment were random effects. Quite often, the covariance of the residuals had an autoregressive structure—correlations were lower for observations that were further apart in time—which is commonly used to fit data models with equally spaced longitudinal observations (57).
We used the top-down model building approach for fitting LME models for each behavioral measure, and we selected the most parsimonious model that, at the same time, performed best at predicting the dependent variables (57). When the main effect of stimulation site was significant for the final model, we used LME contrasts to compare rIFG, rIFJ, and preSMA with the Sham baseline. The models are presented in the SI Text.
Supplementary Material
Acknowledgments
We thank Russ Poldrack, Fred Boy, Petroc Sumner, and three anonymous reviewers for their comments on this manuscript. This research was supported by the Biotechnology and Biological Sciences Research Council (BBSRC), Wales Institute of Cognitive Neuroscience, and grant BOF07/24J/077 by the research council of Ghent University. F.V. is a Postdoctoral Fellow of the Research Foundation-Flanders (FWO-Vlaanderen). C.D.C. is a BBSRC David Phillips Fellow.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1001957107/-/DCSupplemental.
References
- 1.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 2.Logan GD, Gordon RD. Executive control of visual attention in dual-task situations. Psychol Rev. 2001;108:393–434. doi: 10.1037/0033-295x.108.2.393. [DOI] [PubMed] [Google Scholar]
- 3.Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychol Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- 4.Verbruggen F, Schneider DW, Logan GD. How to stop and change a response: The role of goal activation in multitasking. J Exp Psychol Hum Percept Perform. 2008;34:1212–1228. doi: 10.1037/0096-1523.34.5.1212. [DOI] [PubMed] [Google Scholar]
- 5.Boucher L, Palmeri TJ, Logan GD, Schall JD. Inhibitory control in mind and brain: An interactive race model of countermanding saccades. Psychol Rev. 2007;114:376–397. doi: 10.1037/0033-295X.114.2.376. [DOI] [PubMed] [Google Scholar]
- 6.Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev. 2009;33:631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- 9.Chevrier AD, Noseworthy MD, Schachar R. Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Hum Brain Mapp. 2007;28:1347–1358. doi: 10.1002/hbm.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: Inhibition and attentional control. Neuroimage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mostofsky SH, Simmonds DJ. Response inhibition and response selection: Two sides of the same coin. J Cogn Neurosci. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- 12.Li CSR, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: Neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharp DJ, et al. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci USA. 2010;107:6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nachev P, Wydell H, O'neill K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. Neuroimage. 2007;36(Suppl 2):T155–T163. doi: 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chikazoe J, et al. Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cereb Cortex. 2009;19:146–152. doi: 10.1093/cercor/bhn065. [DOI] [PubMed] [Google Scholar]
- 16.Mars RB, Piekema C, Coles MGH, Hulstijn W, Toni I. On the programming and reprogramming of actions. Cereb Cortex. 2007;17:2972–2979. doi: 10.1093/cercor/bhm022. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 18.Verbruggen F, Logan GD. Proactive adjustments of response strategies in the stop-signal paradigm. J Exp Psychol Hum Percept Perform. 2009;35:835–854. doi: 10.1037/a0012726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pashler H. Dual-task interference in simple tasks: Data and theory. Psychol Bull. 1994;116:220–244. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- 20.Pashler HE, Johnston. JC. Attentional limitations in dual-task performance. In: Pashler HE, editor. Attention. Hove, UK: Psychology Press; 1998. pp. 155–180. [Google Scholar]
- 21.Welford AT. The “psychological refractory period” and the timing of high speed performance: A review and a theory. Br J Psychol. 1952;43:2–19. [Google Scholar]
- 22.Ratcliff R. Modeling response signal and response time data. Cognit Psychol. 2006;53:195–237. doi: 10.1016/j.cogpsych.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sternberg S. The discovery of processing stages: Extensions of Donders’ method. Acta Psychol (Amst) 1969;30:276–315. [Google Scholar]
- 24.Ruthruff E, Johnston JC, Van Selst M. Why practice reduces dual-task interference. J Exp Psychol Hum Percept Perform. 2001;27:3–21. [PubMed] [Google Scholar]
- 25.Miller J, Reynolds A. The locus of redundant-targets and nontargets effects: Evidence from the psychological refractory period paradigm. J Exp Psychol Hum Percept Perform. 2003;29:1126–1142. doi: 10.1037/0096-1523.29.6.1126. [DOI] [PubMed] [Google Scholar]
- 26.De Jong R. Multiple bottlenecks in overlapping task performance. J Exp Psychol Hum Percept Perform. 1993;19:965–980. doi: 10.1037//0096-1523.19.5.965. [DOI] [PubMed] [Google Scholar]
- 27.Pashler H, Johnson L. Chronometric evidence for central postponement in temporally overlapping tasks. Q J Exp Psychol. 1989;41A:19–45. [Google Scholar]
- 28.McCann RS, Johnston JC. Locus of the single-channel bottleneck in dual-task interference. J Exp Psychol Hum Percept Perform. 1992;18:471–484. [Google Scholar]
- 29.Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-PLUS. New York: Springer; 2000. [Google Scholar]
- 30.Logan GD. On the ability to inhibit thought and action: A user's guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory Processes in Attention, Memory and Language. San Diego: Academic; 1994. pp. 189–239. [Google Scholar]
- 31.Verbruggen F, Logan GD. Aftereffects of goal shifting and response inhibition: A comparison of the stop-change and dual-task paradigms. Q J Exp Psychol. 2008;61:1151–1159. doi: 10.1080/17470210801994971. [DOI] [PubMed] [Google Scholar]
- 32.Barch DM, Braver TS, Carter CS, Poldrack RA, Robbins TW. CNTRICS final task selection: Executive control. Schizophr Bull. 2009;35:115–135. doi: 10.1093/schbul/sbn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menzies L, et al. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007;130:3223–3236. doi: 10.1093/brain/awm205. [DOI] [PubMed] [Google Scholar]
- 34.Duann JR, Ide JS, Luo X, Li CS. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci. 2009;29:10171–10179. doi: 10.1523/JNEUROSCI.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chambers CD, et al. Executive “brake failure” following deactivation of human frontal lobe. J Cogn Neurosci. 2006;18:444–455. doi: 10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- 36.Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 37.Shulman GL, et al. Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia-cortical networks. J Neurosci. 2009;29:4392–4407. doi: 10.1523/JNEUROSCI.5609-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kühn AA, et al. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 2004;127:735–746. doi: 10.1093/brain/awh106. [DOI] [PubMed] [Google Scholar]
- 40.Koechlin E, Jubault T. Broca's area and the hierarchical organization of human behavior. Neuron. 2006;50:963–974. doi: 10.1016/j.neuron.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 41.Chambers CD, et al. Dissociable mechanisms of cognitive control in prefrontal and premotor cortex. J Neurophysiol. 2007;98:3638–3647. doi: 10.1152/jn.00685.2007. [DOI] [PubMed] [Google Scholar]
- 42.Badre D, D'Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci. 2007;19:2082–2099. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- 43.Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends Cogn Sci. 2007;11:229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Downar J, Crawley AP, Mikulis DJ, Davis KD. The effect of task relevance on the cortical response to changes in visual and auditory stimuli: An event-related fMRI study. Neuroimage. 2001;14:1256–1267. doi: 10.1006/nimg.2001.0946. [DOI] [PubMed] [Google Scholar]
- 45.Vossel S, Weidner R, Thiel CM, Fink GR. What is “odd” in Posner's location-cueing paradigm? Neural responses to unexpected location and feature changes compared. J Cogn Neurosci. 2009;21:30–41. doi: 10.1162/jocn.2009.21003. [DOI] [PubMed] [Google Scholar]
- 46.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 47.Macaluso E. Orienting of spatial attention and the interplay between the senses. Cortex. 2010;46:282–297. doi: 10.1016/j.cortex.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Derrfuss J, Brass M, Neumann J, von Cramon DY. Involvement of the inferior frontal junction in cognitive control: Meta-analyses of switching and Stroop studies. Hum Brain Mapp. 2005;25:22–34. doi: 10.1002/hbm.20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD. Neural circuits subserving the retrieval and maintenance of abstract rules. J Neurophysiol. 2003;90:3419–3428. doi: 10.1152/jn.00910.2002. [DOI] [PubMed] [Google Scholar]
- 50.Picton TW, et al. Effects of focal frontal lesions on response inhibition. Cereb Cortex. 2007;17:826–838. doi: 10.1093/cercor/bhk031. [DOI] [PubMed] [Google Scholar]
- 51.Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- 52.Verbruggen F, Logan GD. Models of response inhibition in the stop-signal and stop-change paradigms. Neurosci Biobehav Rev. 2009;33:647–661. doi: 10.1016/j.neubiorev.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMS Consensus Group Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 55.Stokes MG, et al. Simple metric for scaling motor threshold based on scalp-cortex distance: Application to studies using transcranial magnetic stimulation. J Neurophysiol. 2005;94:4520–4527. doi: 10.1152/jn.00067.2005. [DOI] [PubMed] [Google Scholar]
- 56.Stokes MG, et al. Distance-adjusted motor threshold for transcranial magnetic stimulation. Clin Neurophysiol. 2007;118:1617–1625. doi: 10.1016/j.clinph.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 57.West BT, Welch KB, Galecki AT. Linear Mixed Models: A Practical Guide Using Statistical Software. Boca Raton, FL: Chapman and Hall/CRC; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.