Abstract
Signal transduction pathways that regulate longevity, immunity, and stress resistance can profoundly affect organismal survival. We show that a signaling module formed by the G protein alpha subunit, Gqα, and one of its downstream signal transducer phospholipase C β (PLCβ) can differentially affect these processes. Loss of Gqα and PLCβ functions result in increased sensitivity to pathogens and oxidative stress but confer life span extension. Gqα and PLCβ modulate life span and immunity noncell autonomously by affecting the activity of insulin/IGF1 signaling (IIS). In addition, Gqα and PLCβ function cell autonomously within the intestine to affect the activity of the p38 MAPK pathway, an important component of Caenorhabditis elegans immune and oxidative stress response. p38 MAPK activity in the intestine is regulated by diacylglycerol levels, a product of PLCβ’s hydrolytic activity. We provide genetic evidence that life span is largely determined by IIS, whereas p38 MAPK signaling is the primary regulator of oxidative stress in PLCβ mutants. Pathogen sensitivity of Gqα and PLCβ mutants is a summation of the beneficial effects of decreased IIS through reduced neuronal secretion and the detrimental effects of reduced activity of intestinal p38 MAPK. We propose a model whereby Gqα signaling differentially regulates pathogen sensitivity, oxidative stress, and longevity through cell autonomous and noncell autonomous effects on p38 MAPK and insulin/IGF1 signaling, respectively.
Keywords: insulin/IGF-1 signaling, p38 MAPK signaling, infection, aging
Appropriate responses to endogenous and exogenous threats at the organismal level are achieved by the coordination and integration of both cell-intrinsic and systemic signaling events. The conserved insulin/IGF1 signaling (IIS) pathway regulates life span, stress responses, and immunity. In Caenorhabditis elegans, activation of the insulin/IGF1-like receptor (IGFR) DAF-2 results in phosphorylation and retention of the forkhead transcription factor DAF-16 in the cytoplasm (1). Reduction in IGFR function results in increased life span and oxidative stress resistance in C. elegans (2, 3), Drosophila (4), and mice (5). Oxidative stress response is also regulated by the p38 MAPK cascade, which in C. elegans comprises of a three-tiered kinase cascade leading to phosphorylation by a MAPK kinase homolog SEK-1 and activation of the p38 MAPK homolog PMK-1 (6). Loss of either sek-1 or pmk-1 function results in increased sensitivity to oxidative stress (7). The p38 MAPK pathway affects oxidative stress resistance cell autonomously in part through regulation of the Nrf family transcription factor SKN-1 in the intestine (8, 9).
IIS and p38 MAPK signaling contribute to C. elegans immunity. Diminished IIS confers pathogen resistance because of derepression of DAF-16 transcriptional activity and increased immune-effector gene expression (10, 11), whereas diminished p38 MAPK signaling confers increased pathogen sensitivity (12); these pathways regulate distinct sets of immune-related genes (13–15). Regulation of neuronal dense core vesicle fusion by Goα signaling also modulates immunity noncell autonomously through its effect on IIS in the intestine. Reduced secretion of INS-7, an insulin-like agonist, results in decreased IIS within the intestine, nuclear translocation of DAF-16, and higher expression of immune-related genes. By contrast, sustained increased neurosecretion due to loss of Goα signaling results in increased IIS and depressed immune function (16). Gqα signaling antagonizes Goα signaling through its effects on presynaptic levels of diacylglycerol (DAG) (17). Gqα signaling is mediated by the heterotrimeric G protein α q subunit (EGL-30), which stimulates phospholipase C β (EGL-8) to produce DAG. egl-30 or egl-8 mutants have decreased DAG and decreased neuronal secretion (17). In addition to EGL-8, EGL-30 also signals independently through UNC-73 (Trio RhoGEF) to activate Rho signaling and influence growth, locomotion, and egg laying (18). Reduced neuronal signaling mediated by EGL-30 through activation of EGL-8 influences adult life span in an IIS-dependent manner (19). Goα signaling is restricted to the neurons, but Gqα signaling components are also expressed in the intestine (17, 20, 21). Whether Gqα signaling plays a tissue-specific role in modulating distinct aspects of organismal physiology is unknown.
Here, we focus on the molecular basis for life span extension but diminished resistance to oxidative stress and infection due to the loss of Gqα-PLCβ signaling. EGL-30/Gqα and EGL-8/PLCβ influence longevity noncell autonomously, primarily through neuroendocrine effects on intestinal IIS but function cell autonomously in the intestine to affect primarily the activity of PMK-1 and oxidative stress response. Pathogen resistance is an aggregate of the systemic and cell intrinsic effects of Gqα signaling on the activity of the insulin/IGF1 and p38 MAPK pathways, respectively.
Results
Loss of Gqα and PLCβ Functions Result in Decreased IIS and Increased Life Span.
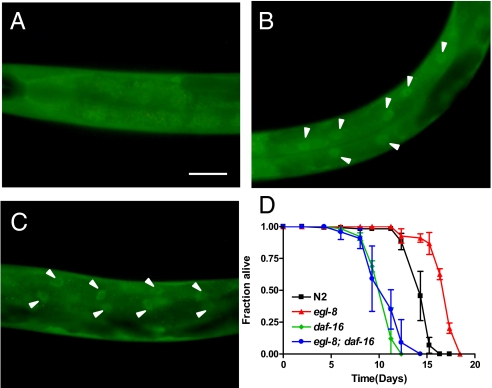
Given that Gqα signaling is required for neuronal secretion, we hypothesized that the loss of EGL-30/Gqα and EGL-8/PLCβ would result in reduced secretion of neuropeptides, including INS-7, leading to decreased stimulation of IIS, increased nuclear localization of DAF-16, and increased expression of DAF-16–regulated longevity-promoting genes (22, 23). To determine the effects of Gqα signaling on IIS, we examined the subcellular localization of DAF-16 in egl-30(n686) loss-of-function and egl-8(n488) null mutants by using a functional DAF-16::GFP fusion protein as a reporter (22). In wild-type animals, DAF-16::GFP is evenly distributed in all cells under standard growth conditions (Fig. 1A), whereas decreased IIS results in nuclear accumulation of DAF-16::GFP (22). In egl-30(n686) and egl-8(n488) under standard growth conditions, DAF-16::GFP was constitutively nuclear, most notably in the intestine (Fig. 1 B and C), suggesting diminished IIS signaling. Decreased IIS is associated with increased life span. As expected, both egl-30(n686) and egl-8(n488) exhibited increased mean life span relative to wild-type animals (15.5 ± 0.2, 15.2 ± 0.1, and 14.2 ± 0.1 d, respectively; log rank, P < 0.001). In agreement with a prior report (19), life span extension of egl-8(n488) depended on daf-16: Both the mean and maximal life span of egl-8(n488); daf-16(mu86) was indistinguishable from daf-16(mu86) (Fig. 1D). Enhanced nuclear localization of DAF-16::GFP due to the loss of egl-30 and egl-8 and complete suppression of life span extension of egl-8(n488) by daf-16(mu86) suggests that Gqα-PLCβ signaling functions upstream of IIS to regulate life span.
Fig. 1.
Constitutive down-regulation of IIS and increased life span of Gqα and PLCβ mutants. (A–C) Nuclear accumulation of DAF-16 in Gqα and PLCβ mutants. Localization of DAF-16 is monitored by fluorescence of DAF-16::GFP in wild-type (A), egl-8(n488) (B), and egl-30(n686) (C) at 25 °C. Arrowhead points to localization of DAF-16::GFP in intestinal cells (anterior to the left) (Scale bar: 100 μm.) (Original magnification, 200×.) (D) Life span extension of egl-8(n488) animals is suppressed by daf-16(mu86) at 25 °C. The fraction of worms alive is plotted as a function of time for a representative of three independent experiments, with 100–120 animals per strain.
Loss of Gqα and PLCβ Functions Conferred Enhanced DAF-16–Regulated Immune Gene Expression but Decreased Pathogen Resistance.
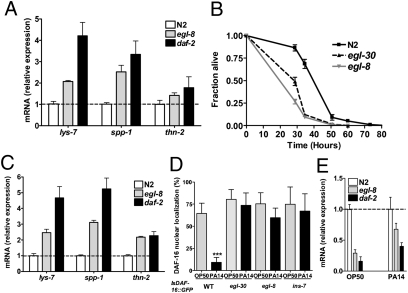
Decreased IIS is associated with increased basal expression of DAF-16–regulated immune effectors and enhanced resistance to pathogens (15, 23). Consistent with constitutive nuclear localization of DAF-16, expression of antimicrobial genes lys-7, thn-2, and spp-1 was significantly elevated in egl-8(n488), egl-30(n686), and egl-8(md1971), an independent loss of function allele of egl-8, relative to wild-type (Fig. 2A and Fig. S1A). Unexpectedly, egl-30(n686), egl-8(n488), and egl-8(md1971) were more sensitive to killing by Pseudomonas aeruginosa, which primarily infects the C. elegans intestine (Fig. 2B and Fig. S1B). Both egl-30(n686) and egl-8(n488) showed increased P. aeruginosa colonization (Fig. S2A) and cleared pathogen less effectively than wild-type (Fig. S2B). egl-8(n488) is defective in the defecation motor program (DMP) (24). To rule out a defect in DMP as a contributor to increased pathogen sensitivity of egl-8 and egl-30 mutants, we determined their defecation rates by measuring the intervals between successive expulsion of gut contents (Exp). We confirmed previous reports that egl-8(n488) had severely extended Exp interval (24). The Exp intervals of egl-30(n686) were, however, indistinguishable from wild-type (Fig. S3 A and B), indicating that enhanced pathogen sensitivity is not due to defects in defecation.
Fig. 2.
Gqα and PLCβ mutants are sensitive to pathogen despite reduced IIS. (A) Elevated expression of IIS-regulated immune genes in egl-8 mutants. mRNA levels of immune genes in egl-8(n488) and wild-type (N2) under conditions of normal growth on E. coli as measured by qRT-PCR are shown. (B) Gqα and PLCβ mutants are sensitive to pathogen. Fraction of egl-30(n686), egl-8(n488), and wild-type (N2) alive are plotted as a function of time of exposure to P. aeruginosa. Shown is a representative of three experiments with 100–120 age-matched adults for each strain, mean time to death of 128.5 ± 4.8, 83.7 ± 2.5, and 77.6 ± 2.7 h for N2, egl-8(n488), and egl-30(n686), respectively. Log rank P < 0.001 relative to N2. (C–E) egl-8 mutants maintain reduced IIS upon infection. mRNA levels of lys-7, spp-1, and thn-2 (C) and ins-7 (E) in egl-8(n488) and wild-type (N2) after 12 h exposure to P. aeruginosa as measured by qRT-PCR. In A, C, and E, mRNA level of each gene was compared with N2, which was set at 1. Shown is the mean ± SD for a representative of three independent experiments. daf-2(e1370) used for comparison was generated previously (15). All datasets were significantly different from wild-type (P < 0.05; ANOVA Dunnett's test). egl-8 was not significantly different from daf-2 in A, B, and E (P > 0.05, Dunnett's test). (D) DAF-16::GFP localization in wild-type, egl-8(n488), egl-30(n686), and ins-7(tm1907) after exposure to P. aeruginosa. The number of nuclei showing distinct DAF-16 localization was enumerated under control (OP50) and infection (PA14) condition. Graph shows a representative of two independent experiments with 20 worms per strain after 21 h of exposure to P. aeruginosa. *P < 0.001 (Student's t test).
Given that reduced IIS confers increased pathogen resistance, we asked why the egl-30 and egl-8 mutants were sensitive to P. aeruginosa. First, we asked whether reduced IIS under normal growth conditions in egl-8 mutants is maintained after infection. P. aeruginosa is able to suppress host immunity by activating IIS, delocalizing DAF-16 from the nucleus, and down-regulating DAF-16–regulated immune effectors, such as lys-7, thn-2, and spp-1 (15). daf-2 mutants are resistant to P. aeruginosa in part because they are able to maintain the expression of lys-7, thn-2, and spp-1 at significantly higher levels than wild-type (15). Similar to daf-2 mutants, expression of lys-7, thn-2, and spp-1 was significantly higher in the egl-8 and egl-30 mutants compared with wild-type (Fig. 2C and Fig. S1C). Next, we assessed the ability of P. aeruginosa infection to delocalize nuclear DAF-16 in egl-30 and egl-8 mutants. ins-7(tm1907), which is resistant to P. aeruginosa and to DAF-16 delocalization by P. aeruginosa, was used as a control (15, 16). Similar to ins-7(tm1907), egl-30 and egl-8 mutants resisted P. aeruginosa-mediated DAF-16 delocalization (Fig. 2D). P. aeruginosa suppresses C. elegans immunity in part by inducing the expression of ins-7 (15). In mutants with reduced neuronal secretion, ins-7 is expressed, and presumably secreted, at lower levels relative to wild-type (16). Compared with wild-type animals, ins-7 transcripts were lower in egl-8 and egl-30 mutants under basal and infection condition (Fig. 2E and Fig. S1D). Taken together, these observations indicate that abrogation of Gqα-PLCβ signaling results in diminished IIS, leading to increased longevity, resistance to P. aeruginosa-mediated immune suppression, and increased basal and induced expression of DAF-16–regulated immune effectors.
EGL-30 and EGL-8 Are Required in the Intestine for Immunity but not for Longevity.
To account for the paradoxical observation that the Gqα and PLCβ mutants were more sensitive to P. aeruginosa, we investigated the role of Gqα-PLCβ signaling in different tissues. In addition to the neurons, Gqα signaling components are also expressed in the intestine (17, 21). We asked whether the activity of Gqα-PLCβ signaling in intestinal tissues could affect immune function and whether Gqα-PLCβ signaling in different tissues has distinct effects on organismal physiology. To address these questions, we knocked down the expression of egl-8 or egl-30 by RNAi either only in the intestine or in the entire animal and determined the life span and pathogen resistance of these animals. Reduced neuronal secretion due to the loss of neuronal egl-8 or egl-30 function (17) could be effectively determined by resistance to paralysis induced by aldicarb, an acetylcholinesterase inhibitor. RNAi-mediated knockdown of egl-30 or egl-8 in the entire animal conferred aldicarb resistance, indicating that egl-30 and egl-8 were effectively knocked down in the entire animal, including neurons (Table 1, rows 1–5 and Table S1), extended life span (Table 1, rows 6–10) but enhanced pathogen sensitivity (Table 1, rows 11–15). To knockdown the expression of either egl-30 or egl-8 only in the intestine, we used the VP303 strain (24), in which RNAi is active only in the intestine. The intestine-specific role for egl-8 in DMP (24) and the neuron-specific role of egl-8 and egl-30 in neurosecretion (17) were used as readouts to confirm that the target gene was effectively knocked down only in the intestine but not in the neurons in VP303. Whereas DMP defects were detected (Fig. S3C), aldicarb sensitivities were indistinguishable from controls (Table 1, rows 1–5), indicating that intestinal but not neuronal egl-30 or egl-8 was knocked down. Intestine-specific knockdown of egl-8 or egl-30 resulted in enhanced sensitivity to pathogen (Table 1, rows 11–15) but normal life span (Table 1, rows 6–10). The egl-8 gene is not known to be expressed in the epidermis (21). As expected, feeding egl-30 or egl-8 dsRNA to the NR222 strain, which restricts RNAi to the epidermis (25), had no effect on pathogen sensitivity, life span, or aldicarb resistance (Table S2). These results are consistent with the predominant role of egl-30 and egl-8 in the intestine for immune function and in the neurons for life span regulation.
Table 1.
Distinct contributions of intestinal and neuronal Gqα and PLCβ to pathogen sensitivity, oxidative stress sensitivity, and life span
| RNAi | Whole-animal gene knockdown (N2) | Intestine-restricted gene knockdown (VP303) |
| Sensitivity to paralysis by 0.7 mM aldicarb | ||
| Vector | 7.9 ± 0.7 (88/89) | 6.8 ± 0.8 (90/90) |
| egl-8 | 10.3 ± 1.0* (91/91) | 6.2 ± 0.5 (92/92) |
| egl-30 | 11.4 ± 1.0* (87/88) | 6.4 ± 0.6 (89/89) |
| sek-1 | 7.2 ± 0.7 (90/90) | 7.4 ± 1.0 (90/90) |
| Life span at 25 °C | ||
| Vector | 182.2 ± 2.7 (103/116) | 179.3 ± 3.1 (105/120) |
| egl-8 | 199.5 ± 2.7* (115/126) | 173.4 ± 3.1 (101/119) |
| egl-30 | 201.6 ± 3.1* (116/123) | 177.9 ± 4.2 (99/118) |
| sek-1 | 180.3 ± 4.1 (105/124) | 176.7 ± 2.9 (109/118) |
| Sensitivity to P. aeruginosa-mediated killing | ||
| Vector | 59.8 ± 1.9 (107/114) | 59.6 ± 2.0 (109/110) |
| egl-8 | 46.7 ± 1.7* (85/86) | 45.9 ± 1.3* (87/88) |
| egl-30 | 42.5 ± 1.4* (82/87) | 41.6 ± 1.9* (89/95) |
| sek-1 | 31.5 ± 1.0* (66/69) | 38.4 ± 1.3* (82/82) |
| Oxidative stress sensitivity (3 mM arsenite) | ||
| Vector | 54.4 ± 3.7 (82/84) | 40.3 ± 1.2 (84/87) |
| egl-8 | 35.4 ± 1.8* (79/84) | 19.4 ± 1.2* (79/79) |
| egl-30 | 36.8 ± 2.0* (84/88) | 18.3 ± 2.0* (85/89) |
| sek-1 | 15.5 ± 1.1* (67/85) | 11.0 ± 0.3* (84/84) |
Table shows the effect of egl-8 and egl-30 knockdown by RNAi in the entire animal (whole-animal gene knockdown) or only in the intestinal tissue (intestine-restricted gene knockdown) on various phenotypes. Shown is TDmean, the mean time to death (or mean time to paralysis for the aldicarb analysis) in hours as calculated by using Kaplan–Meier nonparametric analysis. In parentheses are number of animals used; first number represents the number of animals analyzed, and the second represents the number of animals at the start of experiment. *, log rank P < 0.001 relative to vector.
Gqα and PLCβ Modulate p38 MAPK Activity and Oxidative Stress Response by Affecting the Levels of DAG.
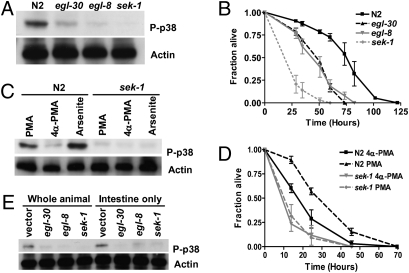
In C. elegans, DAG is a product of EGL-8 (17). DAG alone or with calcium can activate MAPK signaling through protein kinase C (PKC) (26). To ascertain whether the activity of p38 MAPK is altered in the egl-8(n488) and egl-30(n686), we determined the level of phosphorylated PMK-1 by using an anti-phospho p38-specific antibody that recognizes the doubly phosphorylated-activated form of PMK-1. In egl-30(n686) and egl-8(n488), the extent of PMK-1 phosphorylation was reduced by 76% and 89%, respectively, as compared with wild-type (Fig. 3A). By contrast, sek-1(km4) had undetectable PMK-1 phosphorylation, indicating that p38 MAPK signaling was reduced but not completely abrogated in the Gqα and PLCβ mutants. The p38 MAPK signaling-defective sek-1 and pmk-1 mutants are hypersensitive to treatment with oxidative stress generators, such as arsenite and paraquat (8). Consistent with reduced phosphorylation of PMK-1, both egl-8(n488) and egl-30(n686) mutants were sensitive to arsenite- (Fig. 3B) and paraquat-triggered oxidative stress (Fig. S4A).
Fig. 3.
Gqα-PLCβ signaling modulates PMK-1 activity cell autonomously through DAG. (A) Decreased activation of PMK-1 in egl-30(n686) and egl-8(n488). Western blot analysis was performed with a PMK-1-phosphospecific antibody. (B) Gqα-PLCβ signaling protects C. elegans from arsenite toxicity. Survival of wild-type (N2), egl-30(n686), and egl-8(n488) is shown after exposure to 3 mM arsenite. Shown is representative of a minimum of three experiments with 100–120 age-matched adults for each strain, with a mean time to death of 64.2 ± 2.0, 46.4 ± 1.2, and 40.5 ± 1.0 h for N2, egl-8(n488), and egl-30(n686), respectively; log rank P < 0.001 relative to N2. (C) Activation of PMK-1 by PMA is SEK-1–dependent. Wild-type (N2) and sek-1(km4) animals were treated with 100 ng/mL PMA or 4α-PMA for 6 h or with 3 mM arsenite for 6 h, and the extent of PMK-1 phosphorylation was determined by Western blot. (D) DAG level alters oxidative stress sensitivity. Shown is arsenite sensitivity of wild-type (N2) and sek-1(km4) adults following treatment with 100 ng/mL PMA or 4α-PMA. (E) Gqα-PLCβ signaling functions in the intestine to modulate PMK-1 activity. Shown are levels of phosphorylated PMK-1 in wild-type (N2) and VP303 animals following knockdown of egl-30, egl-8, sek-1, or vector RNAi control as detected by a PMK-1-phosphospecific antibody (P-p38). Anti-actin antibody was used as a loading control for protein levels (A, C, and E).
To determine whether the effect of Gqα-PLCβ signaling on p38 MAPK activity is mediated by DAG, we repeated the immunoblot analysis after treatment with a DAG mimetic, phorbol 12-myristate 13-acetate (PMA). After a 6-h treatment with either 100 ng/mL PMA or an inactive analog 4α-PMA, lysates from wild-type and sek-1(km4) were blotted with the anti-phospho p38-specific antibody. Arsenite, a known activator of PMK-1, was used as a control (8). Similar to arsenite treatment, PMA treatment activated PMK-1 in a SEK-1–dependent manner (Fig. 3C). To assess whether PMA-induced phosphorylation of PMK-1 resulted in a functionally active MAPK, we assayed the ability of PMA-treated adult C. elegans to survive oxidative stress. At the concentration of PMA that increased PMK-1 phosphorylation (Fig. 3C), PMA-treated, but not 4α-PMA-treated, wild-type worms showed sek-1–dependent increased resistance to arsenite-mediated oxidative stress (Fig. 3D). Together, the data suggest that DAG acts upstream of SEK-1 to activate PMK-1 and could be a mediator of Gqα-PLCβ signaling to p38 MAPK.
EGL-8 Is Required for p38 MAPK-Dependent Gene Expression.
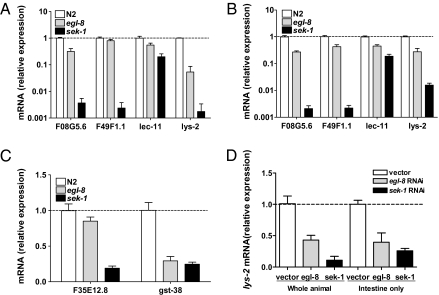
The p38 MAPK pathway regulates the expression of immune and oxidative stress genes (14, 27). We quantified transcript levels of four PMK-1–regulated immune genes (27) in wild-type and egl-8(n488) by qRT-PCR; sek-1(km4) served as a control. Consistent with reduced PMK-1 activation, basal expression of F08G5.6, lec-11, and lys-2 was significantly lower in egl-8(n488) than wild-type (Fig. 4A). After infection, all four transcripts were significantly lower in egl-8(n488) compared with wild-type (Fig. 4B). In addition, the basal expression level of two PMK-1–dependent genes, F35E12.8 and gst-38, which are required for protection from oxidative stress but not infection (27), was lower in egl-8(n488) relative to wild-type (Fig. 4C). A similar pattern of reduced p38 MAPK-regulated gene expression was observed in egl-8(md1971) and egl-30(n686) (Fig. S5). Thus, increased susceptibility of egl-8 to pathogen and oxidative stress is associated with reduced PMK-1 activation and reduced expression of p38 MAPK-regulated immune and oxidative stress protective genes.
Fig. 4.
PLCβ mutants have reduced levels of p38 MAPK-regulated immune genes. (A–C) Decreased expression of p38 MAPK-regulated immune and oxidative stress response genes in egl-8 mutants. mRNA levels of p38 MAPK-regulated immune genes in egl-8(n488) relative to wild-type (N2) adults during normal growth on E. coli (A) and after exposure to P. aeruginosa (B). (C) mRNA levels of p38 MAPK-regulated oxidative stress protective genes in egl-8(n488) relative to wild-type (N2) adults during normal growth on E. coli. mRNA levels in sek-1(km4) are shown for comparison and are from the dataset generated previously (15). (D) PLCβ and p38 MAPK signaling function cell autonomously in the intestine to regulate expression of an immune gene lys-2. Shown is quantification of lys-2 expression after mock knockdown (vector) or RNAi-mediated knockdown of egl-8 or sek-1 in N2 (whole organism) and VP303 (intestine only). For each graph, mRNA level of immune or stress genes is shown relative to the respective N2 or vector RNAi control, which was set at 1. Shown are relative mean ± SD of three replicates and are representative of three independent experiments. All datasets except C were significantly different from wild-type (P < 0.05, ANOVA Dunnett's test). egl-8 was significantly different from sek-1 in A and B (P < 0.05, Dunnett's test).
Regulation of Immunity and Oxidative Stress Resistance by Gqα-PLCβ Signaling Is Primarily Through the Intestinal p38 MAPK Pathway.
The C. elegans p38 MAPK module functions in the intestine for oxidative stress resistance (8) and immunity (28). In agreement with the reconstitution experiments, intestine-restricted knockdown of sek-1 by RNAi caused increased sensitivity to pathogens and arsenite (Table 1, rows 11–20). Intestine-restricted egl-8 or egl-30 knockdown also resulted in increased sensitivity to pathogens and arsenite similar to egl-8 or egl-30 knockdown in the entire animal (Table 1, rows 11–20). We hypothesized that Gqα and PLCβ signals through p38 MAPK within the intestinal cells to regulate immunity and oxidative stress. Indeed, intestine-restricted loss of egl-30, egl-8, and sek-1 resulted in decreased levels of phosphorylated MAPK that was similar to the respective RNAi-mediated gene knockdown in the entire animal (Fig. 3E) and the loss-of-function mutants (Fig. 3A). We next quantified the PMK-1–regulated lys-2 transcript, which is expressed primarily in the intestine (27), following RNAi-mediated knockdown of egl-8 or sek-1 either in the whole animals or only in the intestine. Similar to the egl-8(n488) and sek-1(km4) (Fig. 4A), knockdown of egl-8 and sek-1 in the entire animal or only in the intestine decreased expression of lys-2 (Fig. 4D), indicating that egl-8 and sek-1 function in the intestinal cells to regulate intestinal immune gene expression.
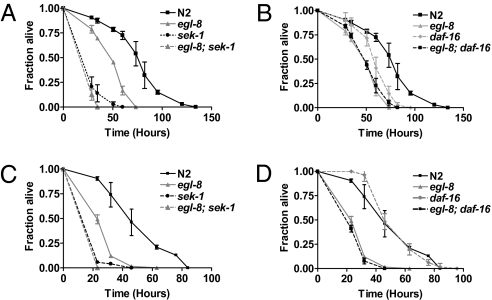
Reduced IIS confers life span extension, increased oxidative stress, and pathogen resistance in C. elegans. For example, the long-lived daf-2(e1370) are resistant to arsenite and pathogens, and these traits depend on daf-16 (3). Yet, despite reduced IIS signaling, egl-8(n488) was more sensitive to arsenite and pathogens, suggesting that reduction in p38 MAPK signaling in egl-8(n488) could negate the protective effects of reduced IIS. To test this idea, we generated double mutants between egl-8(n488) and null mutants in the IIS and p38 MAPK pathways. Consistent with the hypothesis that egl-8 and sek-1 function in the same pathway in the intestine to regulate oxidative stress response, arsenite sensitivity of egl-8(n488); sek-1(km4) was indistinguishable from sek-1(km4) (Fig. 5A). Arsenite sensitivity of egl-8(n488); daf-16(mu86) and egl-8(n488) was indistinguishable, suggesting that daf-16 does not contribute to oxidative stress sensitivity of egl-8(n488) (Fig. 5B). For immunity, similar results were obtained; egl-8(n488); sek-1(km4) was indistinguishable from sek-1(km4) for sensitivity to P. aeruginosa (Fig. 5C). Loss of daf-16 did not further enhance pathogen sensitivity of egl-8(n488) (Fig. 5D). These results provide genetic support that a defect in p38 MAPK signaling is a major contributor to oxidative stress and pathogen sensitivity observed in egl-8(n488) animals. Collectively, the results are consistent with egl-30 and egl-8 affecting p38 MAPK signaling cell autonomously in the intestine to influence oxidative stress and innate immunity.
Fig. 5.
PLCβ influences immunity and oxidative stress resistance primarily through the p38 MAPK pathway. p38 MAPK is the primary mediator of oxidative stress (A and B) and pathogen (C and D) sensitivity of egl-8(n488). Graphs show survival of wild-type (N2), egl-8(n488) compared with sek-1(km4), egl-8(n488); sek-1(km4) (A and C) and to daf-16(mu86) and egl-8(n488); daf-16(mu86) (B and D) after exposure to 3 mM arsenite (A and B) or P. aeruginosa (C and D). Shown is a representative of two independent experiments for a cohort of 100–120 age-matched adults for each strain.
The p38 MAPK pathway regulates nuclear localization and activation of intestinal SKN-1 in response to oxidative stress (8, 9). Under both normal growth and dietary-restriction conditions, SKN-1 contributes to longevity downstream of IIS and in parallel to DAF-16 (29). skn-1 is also essential for embryonic development (30). To determine whether SKN-1 mediates immune and oxidative stress responses as well as longevity in adult animals, we investigated the effect of loss of skn-1 by inactivating the gene by RNAi only in the adult stage (13). Knockdown of skn-1 expression only in the adult resulted in increased arsenite sensitivity and reduced life span but did not affect pathogen sensitivity (Table 2 and Fig. S6), indicating that skn-1 plays a role in intestinal oxidative stress regulation downstream of the p38 MAPK pathway and life span regulation downstream of the IIS pathway but is dispensable for immune response affected by both of these pathways.
Table 2.
SKN-1 is required in adults for the regulation of oxidative stress resistance and longevity but not for immune function
| RNAi | TDmean ± SEM, h | No. scored /Total* |
| Sensitivity to P. aeruginosa-mediated killing | ||
| Vector | 52.6 ± 2.4 | 112/113 |
| skn-1 | 49.3 ± 2.4 | 113/115 |
| Oxidative stress sensitivity (3 mM arsenite) | ||
| Vector | 26.1 ± 2.6 | 97/122 |
| skn-1 | 16.8 ± 2.3† | 89/114 |
| Life span at 25 °C | ||
| Vector | 259.4 ± 4.1 | 113/113 |
| skn-1 | 239.8 ± 5.0† | 115/115 |
*Number of animals tested. “No. scored” represents the number of animals analyzed, and “Total” represents the total number of animals at the start of experiment.
†Log rank P < 0.005 relative to vector.
TDmean is the mean time to death in hours as calculated by using Kaplan–Meier nonparametric analysis.
Discussion
Our results demonstrate that the EGL-30/Gqα-EGL-8/PLCβ signaling cascade influences pathogen sensitivity, oxidative stress resistance, and longevity through both systemic and cell-intrinsic effects (Fig. S7). EGL-30 and EGL-8 activity in the neurons contributes to activation of IIS in the intestine and longevity. EGL-30 and EGL-8 activity within the intestine contributes primarily to activation of p38 MAPK signaling and protection from pathogens and oxidative stress. Our observations suggest that within an intact multicellular organism, certain genes could engage distinct downstream signal transduction pathways in a tissue-specific manner.
The p38 MAPK module is critical for appropriate responses to biotic and abiotic stresses and development, but the molecular inputs appear to be distinct. For example, the calcium-dependent calmodulin kinase II, UNC-43, acts upstream of the p38 MAPK module to regulate odorant receptor asymmetry and osmotic stress response but is dispensable for immunity and oxidative stress response (8, 12, 31). We show that EGL-30 and EGL-8 are required for protection from oxidative stress and pathogens through their effects on PMK-1 activity. This effect is likely to be mediated by DAG generated by EGL-8 because PMA, a DAG mimetic, could functionally activate PMK-1 and rescue the reduced PMK-1 phosphorylation in egl-8 and egl-30 mutants (Fig. S4B). DAG interacts with C1 domain-containing proteins, including protein kinase C (PKC) and protein kinase D (32). That the PKCδ isoform, TPA-1, and a protein kinase D, DKF-2, are required to regulate PMK-1 and immune function (33) further support our observations that DAG regulates PMK-1 activation. EGL-8 mediates the generation of DAG from phosphatidylinositol biphosphate. Yet, residual activity of PMK-1 could be detected in the egl-8 null mutant, suggesting the possible existence of either an EGL-8-independent pathway or another intestinal phospholipase C in maintaining the activity of PMK-1. How upstream signals specifically regulate distinct signal transduction pathways to coordinate insult-specific responses remains to be fully elucidated. The requirement of EGL-30 in PMK-1 activation implicates participation of G protein-coupled receptor (GPCR) and the sensing of extrinsic signals in the regulation of p38 MAPK activity. A challenge for the future is to identify the GPCR that functions in Gqα-PLCβ-PMK-1 cascade in the intestine.
Using tissue-specific gene knockdown experiments, we show that the Gqα-PLCβ-MAPK signaling module functions cell autonomously in the intestine to regulate immune and oxidative stress responses. This observation is consistent with a recent report based on tissue-specific rescue experiments that TIR-1-NSY-1-SEK-1 function within the intestine to activate PMK-1 and regulate immune function (28). DKF-2 is also expressed in the intestine (33), raising the possibility that DAG generated by Gqα-PLCβ signaling activates DKF-2 and, subsequently, the p38 MAPK module cell autonomously in the intestine. The p38 MAPK module also functions in the neurons to regulate egg-laying behavior (28). Similar to sek-1 and nsy-1 mutants, the egl-30 and egl-8 mutants are defective in egg laying, raising the possibility that the Gqα-PLCβ-MAPK module might be recruited for regulation of egg laying in neurons just as it is recruited in the intestinal cells for the regulation of oxidative stress and pathogen response.
Intestinal egl-8 contributes to expression of immune effector genes and protection from P. aeruginosa. egl-8 also appears to contribute to the response of epidermal tissues to infection by Microbacterium nematophilum (34) and Drechmeria conidispora (35). M. nematophilum adheres to the postanal cuticle and rectum of C. elegans, and the host induces a defense response that involves swelling of the underlying hypodermal tissues. The swelling response requires EGL-8 and the extracellular signal-regulated (ERK) MAPK cascade (34), but whether EGL-8 acts through or in parallel to the ERK MAPK pathway remains to be determined. Upon D. coniospora infection, the host's underlying epidermis responds by up-regulating the expression of antimicrobial genes, including nlp-29. nlp-29 induction requires EGL-8 and the p38 MAPK signaling cascade (35). Given that both reporter GFP and antibody analyses did not detect egl-8 expression in the epidermis (21), it remains to be determined whether the contribution of EGL-8 to nlp-29 expression is via a cell autonomous or noncell autonomous mechanism.
Whether a causal relationship exists between oxidative stress resistance and life span extension remains to be fully resolved (36). We show that egl-30 and egl-8 mutants are long-lived despite being sensitive to oxidative stress. Thus, although the ability to protect from oxidative damage can influence organismal longevity, it is not the main determinant of longevity under normal conditions. Longevity and immunity are also highly correlated and often coordinately regulated. For example, elevated expression of antimicrobial genes in daf-2 mutants was proposed to contribute to increased longevity (23). It remains unclear whether antiaging mechanisms result in enhanced immunity or enhanced antimicrobial mechanisms result in increased longevity. That egl-30 and egl-8 mutants are sensitive to pathogen despite being long-lived argues against the premise that increased immunity of IIS mutants is merely a consequence of their increased longevity (10, 37). Instead, they suggest that longevity and immunity are distinctly regulated at the level of pathway activation in response to extrinsic factors. Additional support for the decoupling of longevity and immunity comes from the observation that these traits are separable within the components of IIS downstream of daf-2 (11).
Oxidative stress and pathogen resistance are thought to be linked because insulin/IGF1 and p38 MAPK signaling mutants showed altered oxidative stress and pathogen resistance (3, 7, 10, 12). For example, mutants in genes within IIS, such as daf-2, age-1, and akt-1 are resistant to pathogens and oxidative stress. These observations have led to the notion that these signaling pathways might influence pathogen and oxidative resistance through coregulated mechanisms (37). We also decoupled oxidative stress response and pathogen sensitivity in the context of SKN-1, a transcription factor downstream of the MAPK pathway; loss of SKN-1 in adults results in shortened life span and an increased sensitivity to oxidative stress without a detectable defect in immunity. Together, our observations indicate that longevity, immunity, and oxidative stress response are distinctly regulated at the level of signal initiation and downstream mediators, and collective effects of tissue intrinsic and systemic signaling impact organismal physiology.
Materials and Methods
Details for all experimental procedures are given in SI Materials and Methods. C. elegans survival assays were performed as described (38). Phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich) treatment was performed as described, and aldicarb resistance assay was used to determine efficacy for PMA treatment (16). Oxidative stress sensitivity was assayed using standard NGM plates coated with a final concentration of 3 mM sodium arsenite (Sigma-Aldrich) or M9 buffer containing 100 mM paraquat (methyl viologen; Sigma-Aldrich). RNA extraction and qRT-PCR analysis of antimicrobial gene targets was performed as described (16). DAF-16 nuclear delocalization assay was performed as described (11). For immunoblot analysis, phospho p38 antibody (Promega), p38 antibody (Cell Signaling Technology), and the anti-actin antibody (Sigma-Aldrich) were used.
Supplementary Material
Acknowledgments
We thank the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis) and the Japanese National BioResource Project (Tokyo) for strains, Dr. Charles Rubin, Albert Einstein College of Medicine, for help with the immunoblot protocol, and Dr. Fanglian He for critical reading of the manuscript. Funding was provided by US National Institutes of Health Grant GM66269 (to M.-W.T.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0914715107/-/DCSupplemental.
References
- 1.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 2.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 3.Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- 4.Clancy DJ, et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- 5.Holzenberger M, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 6.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 7.Mizuno T, et al. The Caenorhabditis elegans MAPK phosphatase VHP-1 mediates a novel JNK-like signaling pathway in stress response. EMBO J. 2004;23:2226–2234. doi: 10.1038/sj.emboj.7600226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoue H, et al. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 2005;19:2278–2283. doi: 10.1101/gad.1324805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.An JH, et al. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proc Natl Acad Sci USA. 2005;102:16275–16280. doi: 10.1073/pnas.0508105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garsin DA, et al. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science. 2003;300:1921. doi: 10.1126/science.1080147. [DOI] [PubMed] [Google Scholar]
- 11.Evans EA, Chen WC, Tan M-W. The DAF-2 insulin-like signaling pathway independently regulates aging and immunity in C. elegans. Aging Cell. 2008;7:879–893. doi: 10.1111/j.1474-9726.2008.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DH, et al. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science. 2002;297:623–626. doi: 10.1126/science.1073759. [DOI] [PubMed] [Google Scholar]
- 13.Shapira M, et al. A conserved role for a GATA transcription factor in regulating epithelial innate immune responses. Proc Natl Acad Sci USA. 2006;103:14086–14091. doi: 10.1073/pnas.0603424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Troemel ER, et al. p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. 2006;2:e183. doi: 10.1371/journal.pgen.0020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans EA, Kawli T, Tan M-W. Pseudomonas aeruginosa suppresses host immunity by activating the DAF-2 insulin-like signaling pathway in Caenorhabditis elegans. PLoS Pathog. 2008;4:e1000175. doi: 10.1371/journal.ppat.1000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawli T, Tan M-W. Neuroendocrine signals modulate the innate immunity of Caenorhabditis elegans through insulin signaling. Nat Immunol. 2008;9:1415–1424. doi: 10.1038/ni.1672. [DOI] [PubMed] [Google Scholar]
- 17.Lackner MR, Nurrish SJ, Kaplan JM. Facilitation of synaptic transmission by EGL-30 Gqalpha and EGL-8 PLCbeta: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron. 1999;24:335–346. doi: 10.1016/s0896-6273(00)80848-x. [DOI] [PubMed] [Google Scholar]
- 18.Williams SL, et al. Trio's Rho-specific GEF domain is the missing Galpha q effector in C. elegans. Genes Dev. 2007;21:2731–2746. doi: 10.1101/gad.1592007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ch'ng Q, Sieburth D, Kaplan JM. Profiling synaptic proteins identifies regulators of insulin secretion and lifespan. PLoS Genet. 2008;4:e1000283. doi: 10.1371/journal.pgen.1000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bastiani CA, Gharib S, Simon MI, Sternberg PW. Caenorhabditis elegans Galphaq regulates egg-laying behavior via a PLCbeta-independent and serotonin-dependent signaling pathway and likely functions both in the nervous system and in muscle. Genetics. 2003;165:1805–1822. doi: 10.1093/genetics/165.4.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller KG, Emerson MD, Rand JB. Goalpha and diacylglycerol kinase negatively regulate the Gqalpha pathway in C. elegans. Neuron. 1999;24:323–333. doi: 10.1016/s0896-6273(00)80847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol. 2001;11:1975–1980. doi: 10.1016/s0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- 23.Murphy CT, et al. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 24.Espelt MV, Estevez AY, Yin X, Strange K. Oscillatory Ca2+ signaling in the isolated Caenorhabditis elegans intestine: Role of the inositol-1,4,5-trisphosphate receptor and phospholipases C beta and gamma. J Gen Physiol. 2005;126:379–392. doi: 10.1085/jgp.200509355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qadota H, et al. Establishment of a tissue-specific RNAi system in C. elegans. Gene. 2007;400:166–173. doi: 10.1016/j.gene.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 27.Nandakumar M, Tan M-W. Gamma-linolenic and stearidonic acids are required for basal immunity in Caenorhabditis elegans through their effects on p38 MAP kinase activity. PLoS Genet. 2008;4:e1000273. doi: 10.1371/journal.pgen.1000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shivers RP, Kooistra T, Chu SW, Pagano DJ, Kim DH. Tissue-specific activities of an immune signaling module regulate physiological responses to pathogenic and nutritional bacteria in C. elegans. Cell Host Microbe. 2009;6:321–330. doi: 10.1016/j.chom.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tullet JMA, et al. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowerman B, Eaton BA, Priess JR. skn-1, a maternally expressed gene required to specify the fate of ventral blastomeres in the early C. elegans embryo. Cell. 1992;68:1061–1075. doi: 10.1016/0092-8674(92)90078-q. [DOI] [PubMed] [Google Scholar]
- 31.Troemel ER, Sagasti A, Bargmann CI. Lateral signaling mediated by axon contact and calcium entry regulates asymmetric odorant receptor expression in C. elegans. Cell. 1999;99:387–398. doi: 10.1016/s0092-8674(00)81525-1. [DOI] [PubMed] [Google Scholar]
- 32.Colón-González F, Kazanietz MG. C1 domains exposed: From diacylglycerol binding to protein-protein interactions. Biochimica Biophys Acta. 2006;1761:827–837. doi: 10.1016/j.bbalip.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Ren M, et al. Protein kinase D is an essential regulator of C. elegans innate immunity. Immunity. 2009;30:521–532. doi: 10.1016/j.immuni.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yook K, Hodgkin J. Mos1 mutagenesis reveals a diversity of mechanisms affecting response of Caenorhabditis elegans to the bacterial pathogen Microbacterium nematophilum. Genetics. 2007;175:681–697. doi: 10.1534/genetics.106.060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziegler K, et al. Antifungal innate immunity in C. elegans: PKCdelta links G protein signaling and a conserved p38 MAPK cascade. Cell Host Microbe. 2009;5:341–352. doi: 10.1016/j.chom.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 37.Bolm M, Chhatwal GS, Jansen WT. Bacterial resistance of daf-2 mutants. Science. 2004;303:1976–1976. doi: 10.1126/science.303.5666.1976a. [DOI] [PubMed] [Google Scholar]
- 38.Shapira M, Tan MW. Genetic analysis of Caenorhabditis elegans innate immunity. Methods Mol Biol. 2008;415:429–442. doi: 10.1007/978-1-59745-570-1_25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.