Abstract
Arbuscular mycorrhizal (AM) fungi are obligate biotrophs that acquire carbon (C) solely from host plants. AM fungi can proliferate hyphae in, and acquire nitrogen (N) from, organic matter. Although they can transfer some of that N to plants, we tested the hypothesis that organic matter is an important N source for the AM fungi themselves. We grew pairs of plants with and without the AM fungus Glomus hoi in microcosms that allowed only the fungus access to a 15N/13C-labeled organic patch; in some cases, one plant was shaded to reduce C supply to the fungus. The fungal hyphae proliferated vigorously in the patch, irrespective of shading, and increased plant growth and N content; ∼3% of plant N came from the patch. The extraradical mycelium of the fungus was N-rich (3–5% N) and up to 31% of fungal N came from the patch, confirming the hypothesis. The fungus acquired N as decomposition products, because hyphae were not 13C-enriched. In a second experiment, hyphae of both G. hoi and Glomus mosseae that exploited an organic material patch were also better able to colonize a new host plant, demonstrating a fungal growth response. These findings show that AM fungi can obtain substantial amounts of N from decomposing organic materials and can enhance their fitness as a result. The large biomass and high N demand of AM fungi means that they represent a global N pool equivalent in magnitude to fine roots and play a substantial and hitherto overlooked role in the nitrogen cycle.
Keywords: nutrient capture, organic patches, stable isotopes, fitness
Arbuscular mycorrhizal (AM) symbioses, formed by fungi in the Glomeromycota with the majority of land plants (1), are central to the phosphorus cycle: AM fungi (AMF) capture poorly mobile phosphate ions that would be otherwise unavailable to plants via an extensive hyphal network outside the nutrient depletion zone around the root (2). However, they are held to play only a minor role in the N cycle, because they are obligate biotrophs lacking the saprotrophic capability needed to acquire N from organic sources and because both ammonium and especially nitrate ions are relatively mobile in soil, reducing the potential benefit to the plant of fungal-mediated uptake (3). Here we show that AMF proliferate preferentially in a patch of organic material and can acquire a third of their N from that source. We also show that, because AMF hyphae are N-rich in comparison with plants, they are likely to play a large but hitherto unconsidered role in the global N cycle.
Nitrogen is a limiting nutrient in many terrestrial ecosystems (4). AMF can transfer inorganic N (NO3− or NH4+) to their host plant (5), but because these ions can readily move to the root via diffusion, it has been assumed that roots would not require mycorrhizal assistance to capture inorganic N, particularly because plants that form the AM symbiosis frequently occur in ecosystems with high nitrification rates (6). However, N limitation can occur even in these ecosystems (7) and nitrate acquisition via AMF might offer an advantage to the host plant in highly competitive environments, as in the analogous case of root proliferation in patches of organic material (8). Nevertheless, because most N in soils is in complex organic form and AMF possess no saprotrophic capability, their access to available N must be limited.
AMF can enhance decomposition of organic matter, although the mechanism is unknown, and can transfer N acquired from a patch of organic material to host plants in proportion to hyphal density in the patch (9). AMF also have the ability to detect and respond to soluble organic compounds, which induce specific transcriptional responses in the external mycelium of Glomus intraradices but not in the intraradical mycelium or the asymbiotic phase (10). These fungi can therefore detect decomposing patches of organic matter, proliferate hyphae in them, acquire inorganic N and transport it in the mycelium, apparently as arginine (5). Moreover, a plant ammonium transporter that is mycorrhiza-specific and preferentially activated in arbusculated cells has recently been discovered (11), suggesting that N transfer to the plant may operate in a similar manner to P transfer.
Despite the accumulating evidence that AMF can capture N for plants, as they do P, it remains unknown whether N acquisition is – as is the case for P - a central part of symbiotic function; if so, there should be a relationship between N transfer and C supply from the host. Alternatively, the strong response that the AMF show to organic N sources may reflect large fungal demand for N, in which case observed N transfer to the plant may be an incidental result of processes that have evolved to regulate C and P fluxes.
Whatever the role of N in symbiotic function, if AMF acquire substantial amounts of N from organic matter in soil, then they play a hitherto unsuspected but potentially major role in the N cycle, because they are among the most abundant soil microbes in many soils (3). We therefore tested the hypothesis that an AMF acquires N from complex organic N sources in soil to meet its own metabolic demands, and independently of the amount of (i) carbon supplied by the host plant and (ii) inorganic N supplied to both the fungus and the host plant. Moreover, as AMF are known to proliferate extensively within organic patches (9, 12, 13), a second experiment tested whether this increase in hyphal growth enhanced fungal resource acquisition and hence made the AMF more aggressive colonizers of new host plants.
In both experiments, we used compartmented microcosm units. First (experiment A) we grew pairs of plants of Plantago lanceolata L. in two-compartment microcosm units. One (plant) compartment was further divided into two by a 0.25-mm stainless steel mesh to allow separation of the two root systems. A patch of organic material (1 g of oven-dried Lolium perenne L. leaves dual-labeled with 15N and 13C) was added to the other (hyphal) compartment, which was separated from the planted compartments by a double layer of 20-μm mesh, permeable to hyphae but not roots. In 50 of the units (AM), 50 g of colonized root inoculum of the AMF (Glomus hoi) was added to each of the two plant compartments; a further ten units (non-AM) received sterilized inoculum and unsterilized microbial filtrate of the inoculum to equalize starting microbial communities. All non-AM units were harvested at the end of the experiment.
To measure the impact of plant carbon supply to the fungus, we shaded some plants. In half of both AM and non-AM units, one of the two plants was enclosed in horticultural shading mesh, which reduced photosynthetically active radiation (PAR) flux to the shaded plant by 60% and to its unshaded neighbor by 29% (Fig. S1A). In contrast to the shaded plants, which grew more slowly, the 29% reduction in PAR to unshaded neighbors of shaded plants had no adverse effect: they had the same specific leaf area, dry weight (DW), N and C contents and concentrations, and C:N ratios as unshaded plants with unshaded neighbors. There were five replicates per treatment and the experiment ran for 63 d between 30 July and 1 October, 2006, in a heated, lit glasshouse. The mean temperature over the duration of experiment A was 17.5 °C (SE ± 0.2). PAR flux was recorded weekly at noon at plant level for five randomly selected plants from the unshaded (U/U) treatment, and both the unshaded and shaded plants in the partial shaded treatment (U/S).
In the second experiment (experiment B) we used a microcosm unit with three compartments in series (Fig. S1B). The two end compartments contained a single P. lanceolata plant and we added 100 g of either a colonized root inoculum of G. hoi or granular inoculum of G. mosseae (Biorhize) to one of these planted compartments. One plant was therefore inoculated, whereas the other became colonized by mycelial extension from that first host. In half the units a patch of 1.5 g of the same organic material as in experiment A was added to the middle (hyphal) compartment. The middle compartment was separated from each of the two planted compartments by a double layer of 20-μm mesh, permeable to hyphae but not roots. The other half of the units contained only the background sand:TerraGreen medium. To measure the impact of N supply to the host plants on fungal exploitation of an organic patch, both plants in half the units received a 50-mL high N (2.1 mg N) feed twice weekly, whereas the other half were fed with low N (0.21 mg N) nutrient solution. There were four replicates per treatment and the experiment ran for 120 d after patch addition.
Results and Discussion
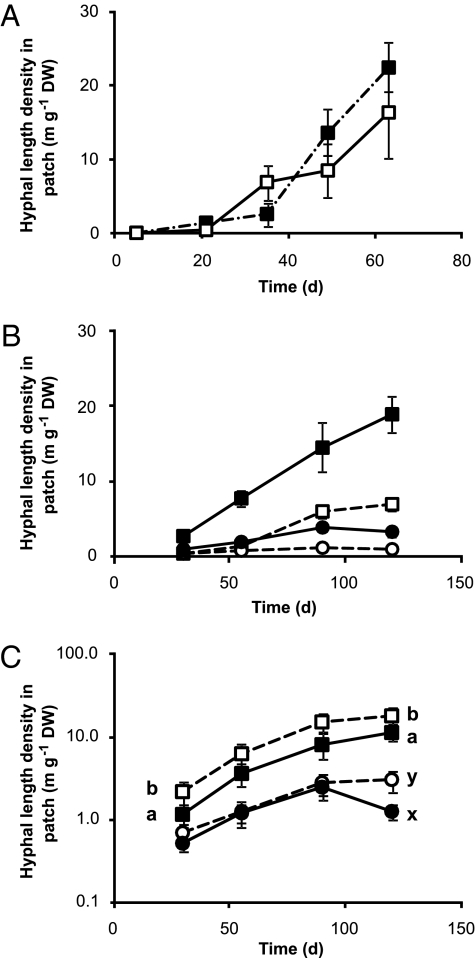
AM hyphae grew prolifically in the organic patch. In both experiments hyphae of G. hoi reached a density of ca. 20 m g−1 patch DW by the end of the experimental period (Fig. 1 A and B), much higher than hyphal length densities commonly reported in soil (9, 14 but see ref. 15) but comparable to some other artificial systems (16). Hyphal densities of G. mosseae in the patch were lower but still enhanced at all harvests (experiment B, Fig. 1B); overall hyphal length densities were three times higher (Z = −5.478, P < 0.001) in the patch (6.61 ± 0.92 m g−1 DW) compared with the equivalent area in units without an organic patch (2.14 ± 0.34 m g−1 DW). G. hoi produced greater (Z = −5.733, P < 0.001) hyphal length densities than G. mosseae in this compartment (7.29 ± 0.92 m g−1 DW G. hoi vs. 1.54 ± 0.19 m g−1 DW G. mosseae). In contrast, low N supply in the plant compartment increased hyphal length density of G. hoi in the patch compartment only at days 30 and 120 and of G. mosseae only at day 120 (Fig. 1C).
Fig. 1.
Development of extraradical hyphal length densities (m g−1 DW) patch with time. (A) Experiment A, units were plants were partially shaded are shown by the closed symbols, whereas those where both were unshaded are shown by the open symbols. There were no significant differences between the shaded treatments. Values are means ± SE (n = 5). (B and C) Development of hyphal length density in the organic patch with time in experiment B for G. hoi (square symbols) and G. mosseae (circles) in the presence (filled symbols) or absence (open symbols) of an organic patch (B) and under high (filled symbols) and low (open symbols) N (C). At all harvests the hyphal length densities were higher in the presence of an organic patch in both AMF species (not shown on graph for clarity). In contrast, N level only influenced hyphal length density of G. hoi at days 30 and 120 and at day 120 for G. mosseae when hyphal length densities were higher under low N as indicated by the different letters on the graph. For B and C values are means ± SE (n = 8 except for at day 30 in the G. hoi no patch and low N treatments when n = 7).
The growth medium in both experiments was a sand:TerraGreen mixture with a restricted microbial community and few saprotrophs: there were almost no fungal hyphae in the patch in the non-AM treatments (experiment A). The extensive hyphal proliferation in the organic patches, particularly for G. hoi, is remarkable for fungi with no saprotrophic ability (3) and raises the question as to what benefit the fungus received from growth in the patch. It was also surprising that hyphal proliferation did not differ at any time between unshaded and shaded treatments (Fig. 1A): because AMF are obligate biotrophs, we expected that reducing the C supply from one of the plants would restrict hyphal proliferation. One explanation would be that the fungus was acquiring a limiting resource—presumably N—from the decomposing patch and using it for its own growth.
Shading did not affect hyphal growth. If that were because the unshaded neighbor was supporting the fungus and compensating for the reduction in C supply, then unshaded plants next to a shaded neighbor would have had reduced biomass, C content or concentration, or an increased proportion of root length colonized compared with plants in units with no shading applied. None of these results were found: there were no significant differences between the unshaded plants in the two shading treatments in any parameter measured. The shaded plants, however, did have reduced root length colonized and arbuscule frequency compared with the unshaded plants (Table S1). Thus, even though shaded plants had reduced colonization, with no accompanying increase in colonization of their unshaded neighbor, the ability of the AMF to proliferate in the organic material was undiminished (Fig. 1A), a finding consistent with the sustained C supply to G. intraradices observed by Olsson et al. (17) even when its host Trifolium subterraneum was shaded.
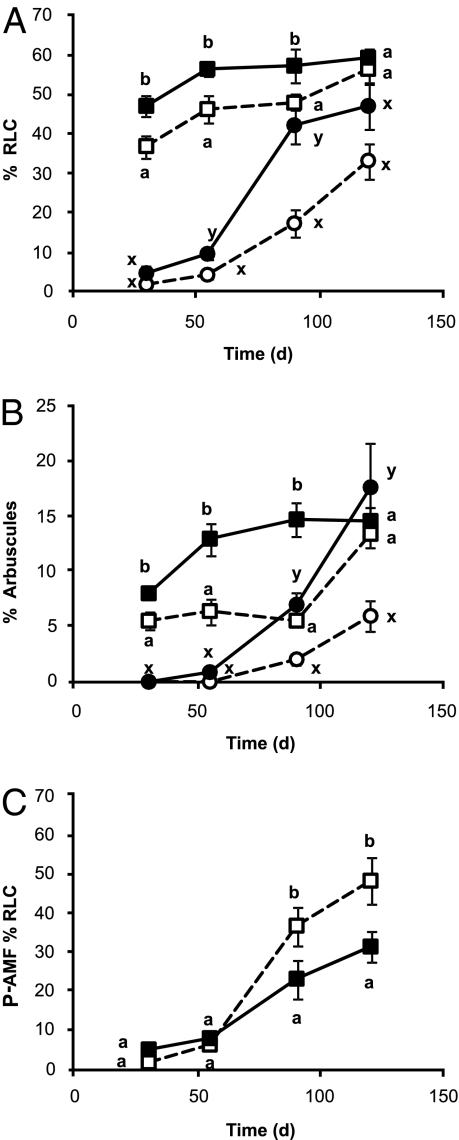
The presence of an organic patch also made AMF more aggressive colonizers of roots both of their existing plant partner and of new potential hosts (experiment B), increasing both root length colonization and arbuscule frequency in inoculated plants (P+AMF) at all harvests except the last (Fig. 2 A and B). N level had no such effect. G. mosseae colonized roots more rapidly than G. hoi but after day 30 there were no differences in colonization between the two fungi.
Fig. 2.
Root length colonization (% RLC) (A) and % arbuscule frequency (B) in the presence (filled symbols) or absence (open symbols) of an organic patch in the middle, unplanted compartment for the plants initially with the AMF inoculum (P+AMF, squares) and those initially without the AMF inoculum (P-AMF, circles) with time. P+AMF and P-AMF plants, and differences at each harvest, were analyzed separately. Data shown are means ± SE (n = 16 except for at day 30 in the no patch treatment when n = 15). Different letters indicate significant differences with the plant treatments. (C) The influence of N on root length colonization of the P-AMF plants. %RLC under high N (filled symbols) or low N (open symbols). Differences were analyzed for each time point separately. Different letters represent significant differences (P < 0.05) between treatments at each time point. Data shown are means ± SE bars (n = 16 except for at day 30 in the low N and G. hoi treatments when n = 15).
For the plants initially without AMF inoculum (P-AMF), the presence of an organic patch increased root length colonized at days 55 and 90 and arbuscule frequency at days 90 and 120 (Fig. 2 A and B). Increased N supply reduced the percentage of root length colonization (RLC) from day 90 (Fig. 2C). Increased colonization when N is limiting has previously been shown (18, 19) and may interact with phosphate availability (18, 20). In contrast to the P+AMF plants, G. hoi colonized the roots more rapidly (days 30 and 55) but G. mosseae colonized the roots to a greater extent thereafter (90 and 120 d; Fig. S2).
The external mycelium, especially that of G. hoi, was N-rich (Tables S2 and S3). In experiment B, N concentration was higher in G. hoi than in G. mosseae mycelium (5.2 ± 0.1%N G. hoi v 3.2 ± 0.1%N G. mosseae) and similar to that in experiment A (5.3 ± 0.1%). In contrast, plant shoots generally had low N concentrations (< 1% N) similar to those of field-grown plants (21). The hyphae were also more 15N enriched than the plant material. Assuming that capture of 14N from the patch was proportional to the amount of 15N capture in fungal tissue and to the 15N:14N ratio in the patch, nearly a third (31.2 ± 4.2%) of the total N detected in the AMF hyphae came from the organic patch, irrespective of shading treatment in experiment A. The contribution of patch N to total AMF N was lower in experiment B, 16.5 ± 0.9% for G. mosseae and 12.0 ± 0.7% for G. hoi, possibly because the greater soil volume for the fungi may have increased nonpatch N availability and diluted patch N in the AMF tissue. These values are high compared with those reported for plant root N capture from comparable organic material sources, where 10% is common (22). Our data demonstrate both that these fungi have a high N demand and that the organic patch was an important source of N.
The other sources of N in the system were the same for fungus and plant (i.e., bonemeal and inorganic N). Although the inorganic N added to the plant compartment was readily available to the fungus, AM hyphae grew preferentially into the patch compartment in both experiments: hyphal densities in the unshaded planted compartments at day 63 were only 3.8 m g−1 DW in experiment A (compare with Fig. 1). Moreover, this increase in hyphal growth in the organic patch made the AMF more rapid colonizers of a second host plant.
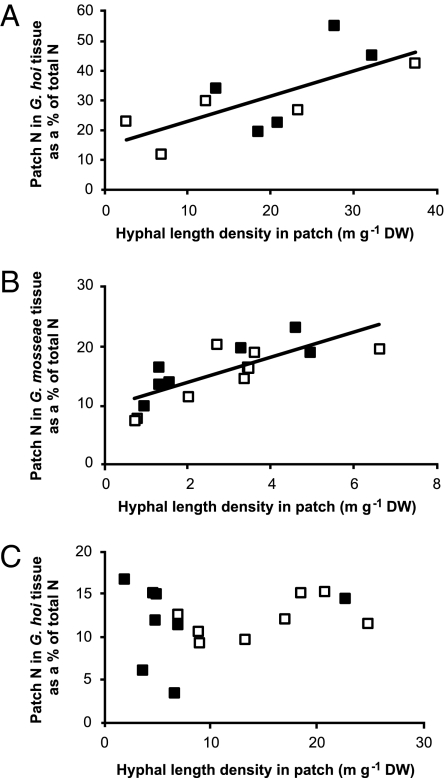
Proliferation in the patch increased N capture by the symbiosis: the percentage of total fungal N derived from the patch increased with hyphal length density in the patch (Fig. 3 A and B). Moreover, the ability of the fungus to incorporate N from the organic material into its own tissue was unaffected by shading (Fig. 3A) as was total hyphal N concentration (P = 0.280). There was also a positive relationship between the percentage of total fungal N derived from the patch and hyphal length density in the patch for G. mosseae (but not for G. hoi; Fig. 3C) in experiment B, which was unaffected by N supply to the planted compartments (Fig. 3B). These data demonstrate that the patch was an important N source for the AMF and that neither shading nor N supply to the planted compartments altered this dependence.
Fig. 3.
The relationship between hyphal length density in the patch and N from the patch (as a % of total fungal N) in extracted AM fungal tissue outside the patch zone. (A) Data for G. hoi from experiment A shading study at day 63. The data are fitted by a significant regression (N from patch as a percentage of total AM fungal N = 14.7 + 0.844 m hyphae g−1 growth medium; P = 0.021, F1,9 = 8.15, r2 = 50.5%). Units where plants were partially shaded are shown by the closed symbols, whereas those where both were unshaded are shown by the open symbols. (B and C) data from experiment B, high or low N experiment. (B) Data for G, mosseae for day 55–90 harvests fitted by a significant regression (N from patch as a percentage of total AM fungal N = 9.449 + 2.129 m hyphae g−1 growth medium; P < 0.001, F1,14 = 20.86, r2 = 59.8%). (C) Data for G. hoi for days 55–90 harvests showing no significant relationship. (B and C) Units where plants received high N are shown by the closed symbols, whereas those where plants received low N are shown by the open symbols.
The total amount of patch N captured by the two plants together in both experiments was related to the hyphal proliferation within the organic patch zone (Fig. S3), supporting previous findings (9). The earlier study examined root and shoot N content; here we use shoot N only because that must represent transfer from the AMF, whereas N detected in the colonized root material may still be held in AMF tissue. The amount of N transferred was unrelated to either shading or N supply (Fig. S3 A–C).
Despite the relationship between AM hyphal proliferation and patch N capture by the plant overall, the benefit of being mycorrhizal differed between the two experiments. In experiment A, colonization by G. hoi increased shoot growth and both root and shoot N content of the unshaded plants (Tables S4 and S5). Nearly 2% of the N in the shoots and 4.5% of the N in the roots of colonized plants came from the organic patch material, compared with only 0.1% in both roots and shoots of the non-AM plants, showing that diffusion of decomposition products from the patch to the roots was not an important N capture pathway.
In experiment B, plants provided with AM inoculum had significantly lower biomass and shoot N contents than the plants that became colonized during the experiment (Tables S6 and S7) presumably due to the additional cost of having to establish the AMF partner at the start of the experiment. The presence of an organic patch did not increase either plant biomass or total N content in experiment B (Table S6). However, inoculated plants had more N from the patch in their shoots than the plants that became colonized (i.e., 0.94 ± 0.10 vs. 0.53 ± 0.05 mg patch N) irrespective of N treatment. The amount of patch N in the shoot for both plants was the same with both AMF species. After 120 d nearly 4% of the N in the shoots and 5% of N in the roots of plants grown under low N came from the organic patch. In contrast, only 2% in the shoots and 4% in the roots came from the patch under high N. This difference arose because of the extra N acquired by the plants from the nutrient solution: the total amount of patch N in the plant material did not differ between N treatments.
Reynolds et al. (23) have suggested that AM symbiosis is not an important plant N capture mechanism because too little N is transferred to the plant to satisfy plant demand. However, we found that AM colonization did significantly increase both biomass and the amount of N acquired by the plants from both the organic patch and other sources of N, compared with non-AM controls in experiment A (Tables S4 and S5). The increased N content appears to have been a direct result of access by the fungus to a new N source, rather than a result of increased plant growth driven by greater mycorrhizal P uptake (23). Increasing N or P supply can reduce AM colonization (18, 24); whether mycorrhizal colonization is affected by N supply may depend on whether the N is first acquired by fungus or plant: application of N to the roots decreased carbon allocation to the mycorrhizal mycelium more than when N was supplied to the external mycelium (25).
Irrespective of its role in transferring N to the plant, our results reveal organic material as an important source of N for the AMF. Indeed, most of the N captured by the symbiosis in this study remained in the fungus: extraradical hyphae of both AMF, but especially G. hoi, are shown here to be N-rich and to acquire a large proportion of their N from the organic material. In contrast, plants acquired only ∼3% of their N from the patch via the fungus, compared with >10% when roots alone had access to similar patches of organic matter (22). An unknown fraction of the “root” N will be in the internal mycelium of the fungus, but because shoots were also enriched with 15N, some N was transferred from fungus to plant. Surprisingly, shading did not restrict the amount of N that plants obtained from the patch (Tables S4 and S5).
These findings suggest that the fungus was using the organic patch as a principal source of N for its own metabolism and growth. Little attention has been given to the sources of N for the growth of AMF mycelia. The reduction in AM colonization associated with high N availability (23) and the lack of impact of mycorrhizal colonization on plant N capture when both fungus and roots have access to the N (14, 22) must be viewed in the light of the effective N capture strategy of the fungus and its high N concentration (3–5% vs. <1% in the plant shoots).
We have shown in two experiments under contrasting experimental conditions that AMF used N from organic material to stimulate their own growth and acquired a large proportion of that N to meet their own nutritional demands. It is surprising that biotrophic fungi should so effectively exploit an organic N source. Although some N was transferred to the plants, the one that supported the fungus the most (as measured by colonization) was not the main recipient (experiment A). Further, the patch N transferred to the plant shoots did not alter with the N supplied to the host plant (experiment B). C is not therefore directly exchanged for N in the AM symbiosis, as proposed for ectomycorrhizal fungi (26). Because no 13C enrichment was detected in fungal or plant tissues, the fungus cannot be transferring simple organic N forms intact and mineralization must occur before N transfer. Govindarajulu et al. (5) suggested that transfers of N and phosphate may be linked but did not propose a site for the transfer, although a plant ammonium transporter that is mycorrhizal specific is preferentially activated in arbusculated cells (11). These findings are consistent with a model of N/C exchange similar to that proposed for P/C exchange (27).
Although our microcosms were artificial and the grass leaves used as organic matter were less complex than much soil organic matter, our results suggest that AMF can acquire substantial quantities of N from organic sources and may play a previously unappreciated role in the nitrogen cycle, intercepting inorganic N released from decomposing organic matter before roots can acquire it and passing some of it on to plants. Evidence both from our experiments and from the literature shows that this role may be quantitatively important. Measurements of AMF biomass vary widely, but the global total of the intraradical mycelium (IRM) biomass has been estimated as 1.4 Pg dry weight (28), whereas the biomass of extraradical mycelium (ERM) may be even greater (up to 10 times that of the IRM) (29). Jackson et al. (30) estimated global live fine root biomass as 40.8 Pg; discounting boreal, tundra and cultivated systems, in none of which are AMF abundant, live fine root biomass in AM-dominated systems may therefore be ∼34 Pg (30), of which 1.4 Pg (i.e., 4%) would be AMF biomass. At a conservative estimate, ERM biomass will be at least equal to the IRM and total AMF biomass therefore around 3 Pg, or 7% of global fine root biomass. This figure may well be an underestimate because AMF appear typically to account for 5–10% of total photosynthetic C fixation (31) and up to 20% of root C flux.
AMF hyphae in these experiments were shown to be N-rich, in common with other fungal mycelia: ERM hyphae had 4–7 times the N concentration of the plant shoots, and therefore probably at least 10 times that of the roots. This high N concentration may explain the observation that AMF acquire significant amounts of N from the soil for their own growth and do not transfer it to the plant in the same proportion as phosphate. The high biomass (at least 7% of global fine root biomass) and N concentration (10× that in roots) of AMF mycelia suggests that the global AM fungal N pool may be at least 70% (i.e., 10 × 7%) of that in the root pool, which was estimated by Jackson et al. to be 0.48 Pg (30). This figure is confirmed by an estimate of the N content of roots and hyphae in the shading experiment. If the sample taken was representative of AM hyphal density within the patch compartment, the total N content of the hyphae in the patch compartment alone was 8.0 mg, whereas that in the roots was 9.8 mg, in both cases averaged across treatments. In other words, total hyphal N content in just one compartment and ignoring the intraradical mycelium, was 82% of that in the roots. Root and AMF N pools appear therefore to be comparable in magnitude.
AMF have no known saprotrophic capability (3) and are assumed to acquire N from inorganic sources. However, the data in this paper show that AMF acquire N from decomposing organic matter and use this N principally for their own growth and metabolism, which would place them in competition with host plants. The N pool in AMF mycelia is similar in magnitude to that in roots and probably turns over rapidly (32). Given their ubiquity, we believe that AMF play an unappreciated role in N cycling and that these findings have implications for global N cycling models.
Materials and Methods
Microcosms.
Microcosm units were constructed by joining two (experiment A) or three (experiment B) plastic containers (each 13.5 × 14 × 14 cm3) via a double-mesh (20-μm mesh) barrier through which hyphae, but not plant roots, could grow. In experiment A, one of the compartments (plant compartment) was further divided into two by a stainless steel mesh (0.25-mm mesh size) inserted into a Perspex sheet to separate the root systems of the two P. lanceolata L. plants grown. In experiment B, the two end compartments were each planted with one P. lanceolata seedling. In experiment A, 60 (50 AM and 10 non-AM) microcosm units were established, whereas in experiment B 64 (32 G. hoi and 32 G. mosseae) units were established.
The plant compartments were filled with 1.8 kg mixture (1:1 vol/vol) of quartz sand and TerraGreen (a calcined attapulgite clay soil conditioner, Oil-Dri), 100 g of the AM inoculum, and 0.3 g L−1 bonemeal (as a slow-release nitrogen and phosphorus source). In experiment A, the mixture was split evenly between the divided sections of the plant compartment. The non-AM plants in experiment A were set-up as above but received a sterilized AM inoculum (121 °C; 30 min) and a microbial filtrate to equalize starting microbial communities (33). In experiment B, the second planted compartment was also set-up as above but received no AM inoculum. The AM inoculum was G. hoi (isolate no. UY 110) added as colonized roots of P. lanceolata in a sand and TerraGreen growth medium (experiment A and B) or G. mosseae (Biorhize) added as a granular inoculum (experiment B only).
In experiment A, in half the microcosm units both plants were unshaded (U/U), but in the other half one of the two plants was shaded (U/S). To create shading, green Rokelene (East Riding Horticulture) mesh was suspended from looped metal rods (1 m × 1.6 mm). Shading commenced at the time of organic patch addition.
The second (or middle compartment in experiment B) compartment (patch) was filled with 1.8 kg mixture (1:1 vol/vol) of quartz sand and TerraGreen and 0.3 g L−1 bonemeal. This growth medium, commonly used in AM research, was chosen to enable extraction of clean AMF hyphae at the end of the experimental period. A section of PVC pipe (length 15 cm, i.d. 6.5 cm) was placed centrally within the patch compartment to a depth of 6 cm from the top of the microcosm unit to enable placement of organic material once the seedlings had developed and to keep disturbance of the unit to a minimum.
In experiment A seeds of P. lanceolata were sown into the plant compartments on 2 July 2006. After a further 28 d, the organic material patches were added and the shading treatment installed. The experiment was set up in a randomized block design in a heated, lit glasshouse and ran for 63 d between 30 July and 1 October 2006. Microcosm units with AM plants were destructively harvested at days 5, 21, 35, 49, and 63 following patch addition and start of shading. There were five replicate microcosms for each of the U/U and U/S treatments at each harvest. At the final harvest (day 63) the 10 units containing nonmycorrhizal plants (five U/U and five U/S), were also destructively harvested to determine the difference between the fungal contribution to N acquired from the patch and that acquired simply by diffusion processes. All compartments of the microcosm units were watered daily. Each plant received 50 mL of a low phosphorus, low nitrogen (0.21 mg N per 50 mL) nutrient solution weekly commencing on 24 July 2006. The bonemeal supplied a further 7.78 mg N L−1 in each plant compartment and in the organic patch compartment.
Experiment B was also set up in a randomized block design in a heated, lit glasshouse Seeds of P. lanceolata were sown into the plant compartments on May 11, 2004 and feed twice weekly with 50 mL of nutrient solution (34) but with reduced (1/10th) phosphorus. After a further 41 d, the organic material patches were added to half the units, whereas the other half received a sand (control) patch. In addition, the nitrogen levels in the nutrient solution to half the microcosm units were reduced to 1/10th (0.21 mg N per 50 mL) that of the original solution in a full factorial design. The experiment ran for 120 d between June 21 and October 19, 2004. Microcosm units were destructively harvested at 30, 55, 90, and 120 d following patch addition. There were four replicate microcosms for each of the AMF and two N levels treatments at each harvest. The mean temperature over the duration of experiment was 20.5 °C (SE ± 0.1) and PAR flux, recorded weekly, averaged 243 (SE ± 35) μmol m−2 s−1 at plant level.
Organic Patch Material.
In both experiments. the organic material added as patches was oven-dried finely milled L. perenne L. shoot material labeled with both 15N and 13C (35). In experiment B, half the units received 1.5 g of sand only (control patch). The patch material was placed in the space created by removal of the PVC tube and was added as a thin, concentrated layer (ca. 6.5 cm diameter, 1 mm depth) at 6-cm depth in the microcosm unit. The remainder of the space was filled with the sand:TerraGreen mix. In experiment A, 1.0 g of the organic patch added to all units contained 20.83 mg N (4.71 mg 15N) and 396.8 mg C (5.79 mg 13C) with a C:N ratio of 19:1. In experiment B, 1.5 g of the organic patch added to half the units contained 33.47 mg N (8.52 mg 15N) and 648.4 mg C (17.49 mg 13C) with a C:N ratio of 19.4:1.
AM Hyphal Extraction.
Extraradical AM hyphae were carefully collected by picking the hyphae between the mesh window and the patch boundary in the patch compartment of the microcosm unit with fine forceps under 100× magnification. Contamination of hyphal material by patch material, which would have invalidated stable isotope analyses can be ruled out because of the procedure adopted: hyphae were extracted near the mesh window and outside the patch boundary, carefully washed several times in deionized water and checked microscopically before analysis. The hyphae extracted had a clear translucent appearance, septa were largely absent or infrequent and spores were still attached in many cases. We have previously observed that AMF tend to sporulate in such organic patches. Once enough hyphae were collected the hyphal material was dried until a constant weight in predried and preweighed tin capsules ready for mass spectrometry analysis. The absence of 13C enrichment in hyphal and plant tissue confirms that the extraction procedure was effective and that no contamination had occurred.
Analyses.
A subsample of root material was used for mycorrhizal assessment as in ref. 36 but excluding phenol. AMF hyphal length was measured using a modified membrane filter technique (37). Shoot, root, and hyphal samples were dried (70 °C, 48 h) and plant material milled in a ball mill to a fine powder for mass-spectrometric analysis performed on a continuous flow isotope ratio mass spectrometer (CF-IRMS). Data presented are for 13C and 15N in excess of the natural abundances of 15N and 13C.
For statistical analysis data were checked and transformed appropriately to normalize skewed distributions before statistical analysis. Kolmogorov-Smirnov and Levene tests were used to test for normality and homogeneity of variances. For experiment A, data are both unshaded plants in the units added together and divided by 2 and the unshaded plants in the partially shaded treatments (Tables S4 and S5).
Although the PAR received by the unshaded plants in the U/S treatment was ca. 71% that received by unshaded plants in the U/U treatment, there were no significant differences between unshaded plants in either the U/U or U/S treatments that indicated that growing next to a shaded plant had little effect on the unshaded neighbor in this study. For the percent root length colonized and arbuscule frequency data, differences as a result of shading were tested by the following:
where U-S represents the difference between unshaded and shaded plants grown together (U/S treatment), and U-U that between two unshaded plants grown together (U/U). To calculate, U-U, the unshaded plant grown at the same side of the microcosm unit as those in the shaded treatment was subtracted from the other unshaded plant. The difference data for each treatment were tested to determine if they differed significantly from zero using t-Tests. U-U values did not differ significantly from zero that indicates that there was no environmental gradient in the glasshouse that might have created a spurious difference in the U-S comparisons. In contrast, the U-S % RLC and % arbuscules data did differ significantly from zero showing in both cases the shaded plant had reduced mycorrhizal parameters than the unshaded plants (Table S1).
Supplementary Material
Acknowledgments
We thank C. Scrimgeour and W. Stein for conducting the mass spectrometry analysis. This work was supported by the Biotechnology and Biological Sciences Research Council United Kingdom.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005874107/-/DCSupplemental.
References
- 1.Fitter AH, Moyersoen B. Evolutionary trends in root-microbe symbioses. Phil Trans R Soc Lond B Biol Sci. 1996;351:1367–1375. [Google Scholar]
- 2.Sanders FE, Tinker PB. Mechanism of absorption of phosphate from soil by Endogone mycorrhizas. Nature. 1971;233:278–279. doi: 10.1038/233278c0. [DOI] [PubMed] [Google Scholar]
- 3.Smith SE, Read DJ. Mycorrhizal symbiosis. San Diego: Academic Press; 2008. [Google Scholar]
- 4.Vitousek PM, Howarth RW. Nitrogen limitation on land and in the sea; how can it occur? Biogeochemistry. 1991;13:87–115. [Google Scholar]
- 5.Govindarajulu M, et al. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature. 2005;435:819–823. doi: 10.1038/nature03610. [DOI] [PubMed] [Google Scholar]
- 6.Read DJ. Mycorrhizas in ecosystems. Experientia. 1991;47:376–396. [Google Scholar]
- 7.Tilman D. Secondary succession and the pattern of plant dominance along experimental nitrogen gradients. Ecol Monogr. 1987;57:189–214. [Google Scholar]
- 8.Hodge A, Robinson D, Griffiths BS, Fitter AH. Why plants bother: Root proliferation results in increased nitrogen capture from an organic patch when two grasses compete. Plant Cell Environ. 1999;22:811–820. [Google Scholar]
- 9.Hodge A, Campbell CD, Fitter AH. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature. 2001;413:297–299. doi: 10.1038/35095041. [DOI] [PubMed] [Google Scholar]
- 10.Cappellazzo G, Lanfranco L, Bonfante P. A limiting source of organic nitrogen induces specific transcriptional responses in the extraradical structures of the endomycorrhizal fungus Glomus intraradices. Curr Genet. 2007;51:59–70. doi: 10.1007/s00294-006-0101-2. [DOI] [PubMed] [Google Scholar]
- 11.Guether M, et al. A mycorrhizal-specific ammonium transporter from Lotus japonicus acquires nitrogen released by arbuscular mycorrhizal fungi. Plant Physiol. 2009;150:73–83. doi: 10.1104/pp.109.136390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St John TV, Coleman DC, Reid CPP. Association of vesicular-arbuscular mycorrhizal hyphae with soil organic particles. Ecology. 1983;64:957–959. [Google Scholar]
- 13.Leigh J, Hodge A, Fitter AH. Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol. 2009;181:199–207. doi: 10.1111/j.1469-8137.2008.02630.x. [DOI] [PubMed] [Google Scholar]
- 14.Hodge A. Arbuscular mycorrhizal fungi influence decomposition of, but not plant nutrient capture from, glycine patches in soil. New Phytol. 2001;151:725–734. doi: 10.1046/j.0028-646x.2001.00200.x. [DOI] [PubMed] [Google Scholar]
- 15.Joner EJ, Jakobsen I. Growth and extracellular phosphatase activity of arbuscular mycorrhizal hyphae as influenced by soil organic matter. Soil Biol Biochem. 1995;27:1153–1159. [Google Scholar]
- 16.Cavagnaro TR, Smith FA, Smith SE, Jakobsen I. Functional diversity in arbuscular mycorrhizas: Exploitation of soil patches with different phosphate enrichment differs among fungal species. Plant Cell Environ. 2005;28:642–650. [Google Scholar]
- 17.Olsson PA, Rahm J, Aliasgharzad N. Carbon dynamics in mycorrhizal symbiosis is linked to carbon costs and phosphorus benefits. FEMS Microbiol Ecol. 2010;72:123–131. doi: 10.1111/j.1574-6941.2009.00833.x. [DOI] [PubMed] [Google Scholar]
- 18.Blanke V, et al. Nitrogen supply affects arbuscular mycorrhizal colonization of Artemisia vulgaris in a phosphate-polluted field site. New Phytol. 2005;166:981–992. doi: 10.1111/j.1469-8137.2005.01374.x. [DOI] [PubMed] [Google Scholar]
- 19.Mosse B, Phillips JM. The influence of phosphate and other nutrients on the development of vesicular-arbuscular mycorrhizal in culture. J Gen Microbiol. 1971;69:157–166. [Google Scholar]
- 20.Sylvia DM, Neal LH. Nitrogen affects the phosphorus response of VA mycorrhizal. New Phytol. 1990;115:303–310. doi: 10.1111/j.1469-8137.1990.tb00456.x. [DOI] [PubMed] [Google Scholar]
- 21.Thompson K, Parkinson JA, Band SR, Spencer RE. A comparative study of leaf nutrient concentrations in a regional herbaceous flora. New Phytol. 1997;136:679–689. doi: 10.1046/j.1469-8137.1997.00787.x. [DOI] [PubMed] [Google Scholar]
- 22.Hodge A. The plastic plant: Root responses to heterogeneous supplies of nutrients. New Phytol. 2004;162:9–24. [Google Scholar]
- 23.Reynolds HL, Hartley AE, Vogelsang KM, Bever JD, Schultz PA. Arbuscular mycorrhizal fungi do not enhance nitrogen acquisition and growth of old-field perennials under low nitrogen supply in glasshouse culture. New Phytol. 2005;167:869–880. doi: 10.1111/j.1469-8137.2005.01455.x. [DOI] [PubMed] [Google Scholar]
- 24.Treseder KK, Allen MF. Direct nitrogen and phosphorus limitation of arbuscular mycorrhizal fungi: A model and field test. New Phytol. 2002;155:507–515. doi: 10.1046/j.1469-8137.2002.00470.x. [DOI] [PubMed] [Google Scholar]
- 25.Olsson PA, Burleigh SH, van Aarle IM. The influence of external nitrogen on carbon allocation to Glomus intraradices in monoxenic arbuscular mycorrhiza. New Phytol. 2005;168:677–686. doi: 10.1111/j.1469-8137.2005.01532.x. [DOI] [PubMed] [Google Scholar]
- 26.Klironomos JN, Hart MM. Food-web dynamics. Animal nitrogen swap for plant carbon. Nature. 2001;410:651–652. doi: 10.1038/35070643. [DOI] [PubMed] [Google Scholar]
- 27.Fitter AH. What is the link between carbon and phosphorus fluxes in arbuscular mycorrhizas? A null hypothesis for symbiotic function. New Phytol. 2006;172:3–6. doi: 10.1111/j.1469-8137.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 28.Treseder KK, Cross A. Global distributions of arbuscular mycorrhizal fungi. Ecosystems (N.Y., Print) 2006;9:305–316. [Google Scholar]
- 29.Olsson PA, Thingstrup I, Jakobsen I, Bååth E. Estimation of the biomass of arbuscular mycorrhizal fungi in a linseed field. Soil Biol Biochem. 1999;31:1879–1887. [Google Scholar]
- 30.Jackson RB, Mooney HA, Schulze ED. A global budget for fine root biomass, surface area, and nutrient contents. Proc Natl Acad Sci USA. 1997;94:7362–7366. doi: 10.1073/pnas.94.14.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bryla D, Eissenstat D. In: Plant Respiration. Lambers H, Ribas-Carbo M, editors. Dordrecht, The Netherlands: Springer; 2005. pp. 207–224. [Google Scholar]
- 32.Staddon PL, Ramsey CB, Ostle N, Ineson P, Fitter AH. Rapid turnover of hyphae of mycorrhizal fungi determined by AMS microanalysis of 14C. Science. 2003;300:1138–1140. doi: 10.1126/science.1084269. [DOI] [PubMed] [Google Scholar]
- 33.Koide RT, Li M. Appropriate controls for vesicular arbuscular mycorrhiza research. New Phytol. 1989;111:35–44. [Google Scholar]
- 34.Thornton B, Bausenwein U. Seasonal protease activity in storage tissue of the deciduous grass Molinia caerulea. New Phytol. 2000;146:75–81. [Google Scholar]
- 35.Hodge A, Stewart J, Robinson D, Griffiths BS, Fitter AH. Root proliferation, soil fauna and plant nitrogen capture from nutrient-rich patches in soil. New Phytol. 1998;139:479–494. doi: 10.1046/j.1469-8137.2000.00602.x. [DOI] [PubMed] [Google Scholar]
- 36.Kormanik PP, McGraw A-C. In: Methods and Principles of Mycorrhizal Research. Schenck NC, editor. St Paul, Minnesota: American Phytolpathol Soc; 1982. pp. 37–46. [Google Scholar]
- 37.Staddon PL, Fitter AH, Graves JD. Effect of elevated atmospheric CO2 on mycorrhizal colonization, external mycorrhizal hyphal production and phosphorus inflow in Plantago lanceolata and Trifolium repens in association with the arbuscular mycorrhizal fungus Glomus mosseae. Glob Change Biol. 1999;5:347–358. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.