Abstract
The clustered protocadherins (Pcdhs) are a large family of cadherin-like transmembrane proteins expressed in the nervous system. Stochastic expression of Pcdh genes and alternative splicing of their pre-mRNAs have the potential to generate enormous protein diversity at the cell surface of neurons. At present, the regulation and function of Pcdh proteins are largely unknown. Here, we show that Pcdhs form a heteromeric signaling complex(es), consisting of multiple Pcdh isoforms, receptor tyrosine kinases, phosphatases, and cell adhesion molecules. In particular, we find that the receptor tyrosine kinase rearranged during transformation (Ret) binds to Pcdhs in differentiated neuroblastoma cells and is required for stabilization and differentiation-induced phosphorylation of Pcdh proteins. In addition, the Ret ligand glial cell line-derived neurotrophic factor induces phosphorylation of Pcdhγ in motor neurons and phosphorylation of Pcdhα and Pcdhγ in sympathetic neurons. Conversely, Pcdh proteins are also required for the stabilization of activated Ret in neuroblastoma cells and sympathetic ganglia. Thus, Pcdhs and Ret are functional components of a phosphorylation-dependent signaling complex.
Keywords: intracellular domain, signal transduction, TAP tag, protein–protein interactions, protein purification
The clustered protocadherins (Pcdhs) are a large family of single-pass transmembrane proteins bearing six cadherin-like extracellular domains and unique intracellular domains (1, 2). Pcdhs are predominantly expressed in the nervous system and localize in puncta in neuronal cell bodies, along processes, and in synaptic regions (1, 3, 4). The mammalian Pcdhs are encoded in tandem in three closely linked gene clusters, designated Pcdhα, Pcdhβ, and Pcdhγ, each encoding 14–22 protein isoforms in the murine gene clusters. Individual isoforms are encoded by a single variable exon that includes the extracellular and transmembrane domains and a short intracellular domain. In the cases of the Pcdhα and Pcdhγ subclusters, each variable exon is spliced to three cluster-specific constant exons that encode the remainder of the intracellular domain (5). The distal intracellular domains encoded by constant exons are identical between the Pcdhα and Pcdhγ proteins but distinct between the Pcdhα and Pcdhγ proteins.
Differential expression of the more than 50 different Pcdh isoforms can create an enormous combinatorial diversity on neuronal cell surfaces and might allow the distinction of otherwise similar cells from each other. Distinct subsets of Pcdh isoforms are produced in individual neurons by differential promoter activation and alternative splicing (5, 6). In addition, Pcdh proteins have been reported to form cis-homodimers, cis-heterodimers (reviewed in 7), and large protein complexes of up to 1,000 kDa (8). In contrast to classic cadherins, individual Pcdh isoforms seem to have only weak adhesive properties (reviewed in 7), possibly because of intracellular retention (9). Pcdhs could function together in a large adhesive complex or as signaling and recognition molecules. Interestingly, the formation of specific connections in complex neuronal circuits has been hypothesized to require the expression of combinatorial arrays of adhesive cell surface proteins, which, together, could constitute a molecular code or address (10). Their wide variety, combinatorial expression, and potential adhesive function make the Pcdhs attractive candidates for defining this molecular code (2).
Mutational studies suggest that Pcdhs might indeed function as components in a neuronal code but could also have additional functions. Pcdhα mutant mice that lack the common cytoplasmic domain show defects in the targeting of olfactory sensory neurons into glomeruli (11) and abnormal arborization of serotonergic projections (12). Deletion of the Pcdhγ gene cluster causes excessive apoptosis of interneurons in the spinal cord and retina and a decrease in synapse number of spinal cord interneurons (13–15).
The molecular mechanisms by which Pcdhs regulate these cellular functions are not known. Although several Pcdhα- and Pcdhγ-binding candidates have been identified in yeast two-hybrid screens, most of these interactions have not been confirmed in primary cells or tissue, and the functional significance of most of these interactions (reviewed in 16) is yet to be demonstrated (17). The limited success of yeast two-hybrid screens suggests that posttranslational modifications or multidomain binding might be required for their endogenous interactions with other proteins.
Using affinity purification methods in the mouse central nervous system catecholaminergic cell line, the CAD (Cath.a-differentiated) cell line, we have identified a large number of interactions among distinct Pcdh isoforms and between the Pcdhs and a variety of adhesion molecules, kinases, and phosphatases. In particular, we find that the receptor tyrosine kinase rearranged during transformation (Ret) regulates Pcdhα and Pcdhγ phosphorylation and stability in CAD cells. Ret also regulates phosphorylation of Pcdhγ in motor neurons (MNs) and Pcdhα and Pcdhγ phosphorylation in sympathetic neurons. Conversely, Pcdhα and Pcdhγ also regulate Ret activation and turnover in CAD cells and sympathetic neurons. We conclude that the interaction between Pcdhs and Ret leads to mutual stabilization and the formation of a stable membrane complex for efficient signaling. These studies identify cellular proteins that interact functionally with Pcdh proteins in neuronal cells and suggest that Pcdhs have a primary function in cell signaling.
Results
Identification of Proteins That Interact with Pcdhα4 Using Tandem Affinity Purification.
To identify proteins that interact with Pcdhs in neuronal cells, the coding sequence of Pcdhα4 was fused to a tandem affinity purification tag (Pcdhα4-TAP) (Fig. 1A), which was stably expressed in CAD cells. CAD cells can be efficiently differentiated by serum withdrawal in cell culture into cells that display morphological and biochemical characteristics similar to those of primary neurons (18). The purified proteins were then analyzed by MS (details provided in SI Materials and Methods). Several distinct Pcdh isoforms representing all three Pcdh gene clusters were found to associate with Pcdhα4-TAP. The interactions between endogenous Pcdhγ and Pcdhα proteins were confirmed by coimmunoprecipitation and Western blot analysis (Fig. S1A). In addition to the Pcdh protein isoforms, a number of transmembrane proteins were found to associate with Pcdhα4-TAP. These Pcdh-interacting proteins include the receptor tyrosine kinase discoidin domain receptor 2 (DDR2), Ret, the receptor protein tyrosine phosphatase α (RPTPα), the leukocyte antigen-related receptor tyrosine phosphatase (LAR), and several cell adhesion molecules that include the CD98 heavy chain and metadherin. Several of the interacting proteins are known to be involved in neurite outgrowth and axonal pathfinding, for example, the microtubule destabilizing proteins stathmin-like 2 and stathmin-like 3, LAR, and Ret (19–21).
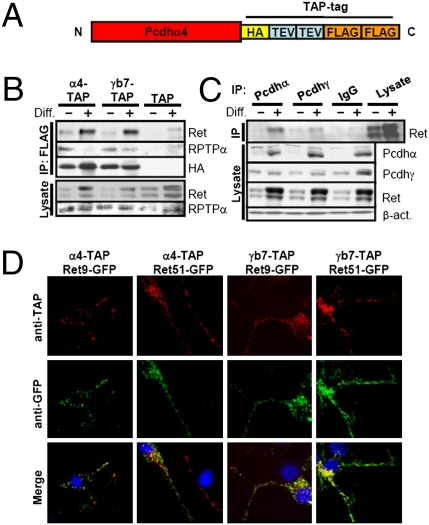
Fig. 1.
Identification of Pcdhα4-TAP–associated proteins. (A) Schematic representation of the Pcdhα4-TAP construct. The carboxyl terminus of the Pcdhα4 coding sequence was fused to one HA epitope tag, followed by two tobacco etch virus (TEV) cleavage sites and two FLAG tags. (B) Anti-FLAG (TAP) immunoprecipitates (IPs) of undifferentiated (Diff. −) or differentiated (Diff. +) CAD cells expressing Pcdhα4-TAP (α4-TAP), Pcdhγb7-TAP (γb7-TAP), or empty TAP vector (TAP) were blotted with anti-pan-Ret antibody or with an anti-RPTPα antibody. The anti-Ret blot was reprobed with an anti-HA (TAP) antibody. Cell lysates were blotted with anti-Ret and anti-RPTPα antibodies for total protein levels. (C) IPs from undifferentiated and differentiated CAD cells generated using anti-Pcdhα and anti-Pcdhγ antibodies or control rabbit serum (IgG) were blotted with anti-pan-Ret antibody. Cell lysates were blotted with anti-Pcdhα, anti-Pcdhγ, anti-Ret, and β-actin (β-act.) antibodies. (D) Pcdhα4-TAP and Pcdhγb7-TAP partially colocalize with Ret9-GFP and Ret51-GFP. Differentiated CAD cells that stably express Pcdhα4-TAP, Pcdhγb7-TAP, or TAP vector were transfected with Ret9-GFP or Ret51-GFP; fixed; stained with anti-HA (TAP) (red), anti-GFP (green), and DAPI (blue); and imaged by confocal microscopy.
Coimmunoprecipitation and Western blot analysis were used to confirm the interaction between Pcdhα4-TAP and five transmembrane domain-containing proteins identified as strong candidates by MS (Fig. S1B). All five tested candidate proteins [DDR2, metadherin, CD98, RPTPα, and two alternative splice forms of Ret (Ret9 and Ret51)] were detected at much higher levels in immunoprecipitates (IPs) from Pcdhα4-TAP–expressing samples relative to vector control samples (Fig. S1B). Our data, together with the previous observation that Pcdhα and Pcdhγ are present in an up to 1,000-kDa complex (8), suggest that Pcdhα4 interacts with different Pcdh isoforms, signaling molecules and adhesion proteins to form a high molecular weight complex or complexes.
Pcdhα and Pcdhγ Interact with Ret in Differentiated CAD Cells.
The receptor tyrosine kinase Ret is required for normal development of the kidneys, and the enteric, sympathetic, and parasympathetic nervous systems (reviewed in 21). Ret autophosphorylation and subsequent downstream signaling are activated by binding to a complex consisting of a glial cell line-derived neurotrophic factor (GDNF) family ligand and a glycosyl-phosphatidyl inositol (GPI)-anchored co-receptor of the GDNF receptor alpha (GFRα) family. Interestingly, Pcdhα is expressed at high levels in most tissues in which Ret plays an important cellular role, including MNs and sensory and sympathetic ganglia (21, 22). In addition, both the Pcdhs and Ret contain conserved cadherin-like extracellular domains (2, 23). Given these observations, we focused our attention on Ret and carried out experiments to determine whether the interaction between Pcdhs and Ret is functionally significant. Because Pcdhα4-TAP can associate with Pcdhγ isoforms, we also included the Pcdhγ isoform b7 in our analysis and generated a CAD cell line that stably expresses Pcdhγb7-TAP. Using a pan-Ret antibody that recognizes both of the major splice forms of Ret (Ret9 and Ret51), we found that both Pcdhα4-TAP and Pcdhγb7-TAP interact with endogenous Ret in both undifferentiated and differentiated CAD cells (Fig. 1B). The two Ret splice forms differ in their extreme C termini and migrate as overlapping doublets of bands of 150 kDa and 200 kDa in CAD whole-cell lysate (Fig. 1 B and C). The amount of Ret that interacts with Pcdhα4-TAP and Pcdhγb7-TAP was higher in differentiated cells, likely because of the higher levels of Ret expression in these cells (Fig. 1B). Ret binding is also observed when endogenous Pcdh protein is immunoprecipitated from CAD cell lysates (Figs. 1C and 2B). Pcdhα4-TAP, Pcdhγb7-TAP, and endogenous Pcdh proteins appear to interact more strongly with the slower migrating 200-kDa form of the Ret isoforms, which is thought to be the mature, glycosylated, membrane-bound form of both Ret9 and Ret51 (24). We also detect the association of Pcdhα4-TAP and Pcdhγb7-TAP with the tyrosine phosphatase RPTPα in both undifferentiated and differentiated CAD cells (Fig. 1B). We conclude that Pcdhs are components of protein complexes present in CAD cells before and after differentiation and that these complexes contain the active form of the receptor tyrosine kinase Ret, RPTPα, and distinct Pcdhα and Pcdhγ isoforms. We also note that endogenous protein levels of Ret and Pcdhα increase substantially in differentiated CAD cells, whereas Pcdhγ protein levels remain largely constant (Fig. 1 B and C). Thus, the expression of Ret and Pcdhα but not Pcdhγ increases upon CAD cell differentiation.
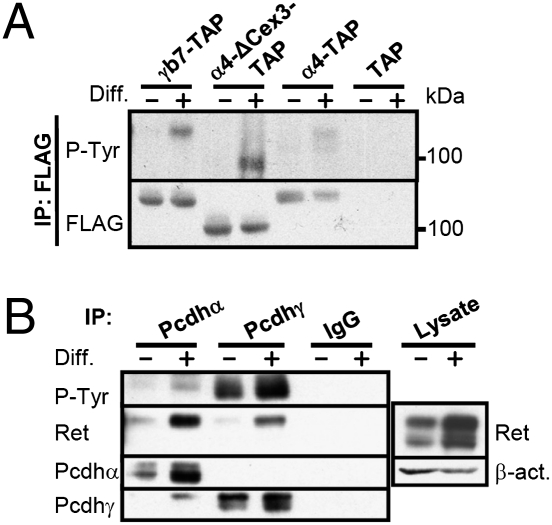
Fig. 2.
Pcdhα and Pcdhγ undergo differentiation-induced phosphorylation in CAD cells. (A) Anti-FLAG (TAP) immunoprecipitates (IPs) of undifferentiated (Diff. −) and differentiated (Diff. +) CAD cells expressing Pcdhα4-TAP (α4-TAP), Pcdhα4-ΔC3-TAP (ΔC3-TAP), Pcdhγb7-TAP (γb7-TAP), or TAP vector (TAP) were blotted with anti-P-Tyr. The blot was reprobed with an anti-FLAG (TAP) antibody. (B) Anti-Pcdhα and anti-Pcdhγ IPs from undifferentiated or differentiated CAD cells were blotted with anti-P-Tyr or anti-pan-Ret antibody. The blot was reprobed with anti-Pcdhα or anti-Pcdhγ antibody. Total cell lysates were blotted with anti-pan-Ret and β-actin (β-act.) antibodies.
We next mapped the domains of Pcdhα4-TAP required for its interaction with Ret. Deletion of the third constant exon (Pcdhα4-ΔCex3) or even the entire cytoplasmic domain (Pcdhα4-ΔCD) of Pcdhα4-TAP did not eliminate the interaction between Pcdhα4-TAP and Ret (Fig. S2B). Thus, the interaction between Pcdhα4-TAP and Ret requires the extracellular and/or transmembrane domain of the Pcdhα4 protein. We note that Pcdhα4-ΔCD interacts preferentially with the faster migrating but incompletely processed form of Ret. Pcdhα4-ΔCD might be less efficiently transported to the membrane and preferentially associate with the lower molecular weight but not completely processed form of Ret. Interestingly, we detect stronger Pcdhα4-ΔCD immunolocalization in internal compartments of CAD cells as compared with WT Pcdhα4 (Fig. S2E). The Pcdhα4 C-terminal domain might be required for proper intracellular trafficking, as has already been reported for the Pcdhγ C-terminal domain (9).
Next, we investigated whether Ret colocalizes with Pcdh proteins in CAD cells. We transfected CAD cells that stably express Pcdhα4-TAP or Pcdhγb7-TAP with GFP fusions of the two different Ret splice forms (Ret9-GFP and Ret51-GFP). The transfected CAD cells were differentiated and stained with an anti-FLAG antibody that recognizes the FLAPG epitope of the TAP tag and with an anti-GFP antibody that detects Ret-GFP fusions (Fig. 1D). Pcdhα4-TAP, Pcdhγb7-TAP, and Ret colocalized in a punctate pattern in cell bodies, at the plasma membrane and along the processes (Fig. 1D).
Our data suggest that Pcdhα4-TAP and Pcdhγb7 interact with different signaling molecules depending on the differentiation status of CAD cells. We carried out additional experiments to investigate the possible functional significance of these interactions.
Differentiation-Induced Phosphorylation of Pcdhα and Pcdhγ in CAD Cells.
The association of Pcdhα4-TAP with several tyrosine kinases and phosphatases led us to consider the possibility that the Pcdhs are regulated by tyrosine phosphorylation. Consistent with a role for tyrosine phosphorylation in Pcdh function, we detected significant tyrosine phosphorylation of immunoprecipitated Pcdhα4-TAP and Pcdhγb7-TAP in differentiated but not undifferentiated CAD cells (Fig. 2A). We also detected differentiation-induced tyrosine phosphorylation of endogenous Pcdhα and endogenous Pcdhγ (Fig. 2B). Interestingly, endogenous Pcdhγ is more tyrosine-phosphorylated than endogenous Pcdhα in differentiated CAD cells, although endogenous Pcdhα interacts more efficiently with Ret in differentiated CAD cells. These data indicate that although the Pcdhαs and Pcdhγs contain distinct cytoplasmic domains, both appear to be phosphorylated upon differentiation. A mutant form of Pcdhα4 lacking the third constant exon (Pcdhα4-dΔC3-TAP) is also tyrosine-phosphorylated in differentiated CAD cells (Fig. 2A), indicating that tyrosine phosphorylation is of Pcdhα4-TAP itself and not of an associated protein of similar size. These data provide evidence that Pcdhs are involved in a signaling pathway that is regulated during neuronal differentiation.
Phosphorylation of Pcdhα and Pcdhγ by the Ret Tyrosine Kinase.
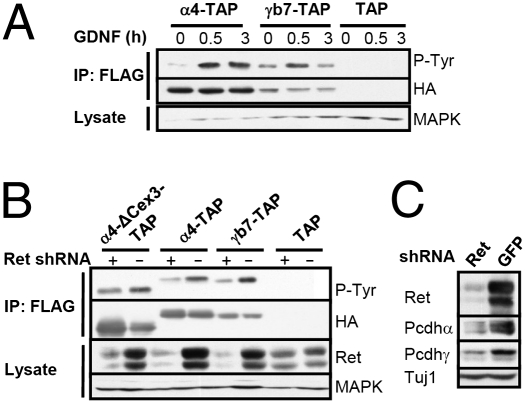
As shown above, the amount of Ret associated with Pcdhα and Pcdhγ and the tyrosine phosphorylation of Pcdhα and Pcdhγ increase in differentiated CAD cells. We therefore asked whether phosphorylation of Pcdhs depends on Ret signaling. Treatment of differentiated CAD cells with the Ret ligand GDNF and the recombinant soluble coreceptor GFRα1 for 30 min increased phosphorylation of Pcdhα4-TAP and Pcdhγb7-TAP (Fig. 3A) above differentiation-induced levels of phosphorylation. Phosphorylation of Pcdhα4-TAP remained elevated after 3 h of GDNF/GFRα1 treatment, whereas phosphorylation of Pcdhγb7-TAP decreased after 3 h (Fig. 3A). The level of phosphorylation detected with Pcdhγb7-TAP is less than that observed for Pcdhα4-TAP, likely attributable to the significantly lower expression levels of Pcdhγb7-TAP in CAD cells.
Fig. 3.
Ret regulates phosphorylation and protein levels of Pcdhα and Pcdhγ. (A) Differentiated CAD cells expressing Pcdhα4-TAP (α4-TAP), Pcdhγb7-TAP (γb7-TAP), or empty TAP vector (TAP) were left untreated or stimulated with GDNF/GFRα1 for 0.5 or 3 h. Anti-FLAG (TAP) immunoprecipitates (IPs) were blotted with anti-phosphotyrosine (anti-P-Tyr). The blot was reprobed with an anti-HA (TAP) antibody. Total cell lysates were blotted with anti-MAPK. (B) CAD cells expressing Pcdhα4-ΔC3-TAP (ΔC3-TAP), Pcdhα4-TAP, Pcdhγb7-TAP, or empty TAP vector were infected with anti-Ret shRNA or control anti-GFP shRNA lentivirus. Anti-FLAG (TAP) IPs of differentiated cells were blotted for P-Tyr, and the blot was reprobed with an anti-HA (TAP) antibody. Total lysate was blotted with an anti-Ret antibody. (C) Total lysate of differentiated CAD cells expressing lentiviral shRNA plasmids targeting Ret or GFP was blotted for Ret9 and Ret51, Pcdhα, Pcdhγ, and TuJ1.
To determine whether Ret is required for the phosphorylation of Pcdh proteins, we knocked down Ret protein levels using a lentiviral shRNA. We note that Ret knockdown dramatically reduced the levels of endogenous Pcdhα and Pcdhγ and overexpressed Pcdhα4-TAP, Pcdhα4-ΔC3-TAP, and Pcdhγb7-TAP (Fig. 3C and Fig. S3). Next, we adjusted the amount of material used during immunoprecipitation to give equivalent levels of precipitated Pcdhα4-TAP, Pcdhα4-ΔC3-TAP, and Pcdhγb7-TAP (Fig. 3B). As shown in Fig. 3B, shRNA-mediated knockdown of Ret reduced the levels of GDNF/GFRα1-induced tyrosine phosphorylation of Pcdhα4-TAP, Pcdhα4-ΔC3-TAP, and Pcdhγb7-TAP in differentiated CAD cells (Fig. 3B). The phosphorylation of Pcdhα4-TAP, Pcdhα4ΔC3-TAP, and Pcdhγb7-TAP was unaffected by either control siRNA or siRNA against c-Src, a tyrosine kinase that is activated downstream of Ret (21) (Fig. S3). Using Pcdhα4 cytoplasmic domain truncation mutants and tyrosine residue point mutants, we found that Tyr-750 and Tyr-763 are the most likely Ret-dependent phosphorylation sites (Fig. S2 A, C, and D).
These data show that Ret is required for GDNF/GFRα1-induced phosphorylation of Pcdhα and Pcdhγ in CAD cells. Ret also appears to regulate the stability of Pcdhα and Pcdhγ. Although Ret may directly phosphorylate the Pcdh proteins, we cannot rule out the possibility that Ret activates another kinase, which, in turn, phosphorylates Pcdhs.
Ret Is Required for the Phosphorylation of Pcdhα and Pcdhγ in MNs and Superior Cervical Ganglia Cells.
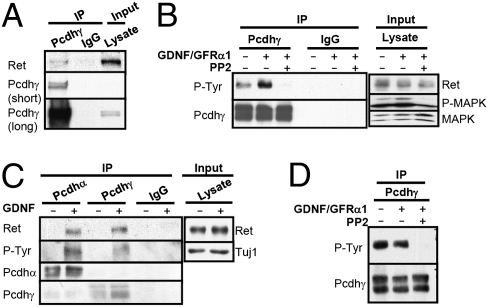
Next, we asked whether the interactions between Pcdhα or Pcdhγ and Ret and phosphorylation of the Pcdhs by Ret are general regulatory mechanisms for Pcdhs in Ret-expressing cells in the nervous system. Because both Ret and Pcdhs are highly expressed in MNs and superior cervical ganglia (SCG) (21, 22), we investigated the relationship between Ret and Pcdhs in ES cell-derived MNs and in primary sympathetic neuron cultures derived from SCGs. In agreement with our CAD cell results (Fig. 1C), endogenous Ret and Pcdhγ can be coimmunoprecipitated from MN lysates (Fig. 4A). Induction of Ret signaling with GDNF and GFRα1 induced tyrosine phosphorylation of immunoprecipitated Pcdhγ (Fig. 4B). Pretreatment of the cells with PP2, a selective inhibitor of the Src family of tyrosine kinase that also inhibits Ret (25), reduced Pcdhγ tyrosine phosphorylation below basal levels (Fig. 4B). We were unable to detect significant binding of Pcdhα to Ret or significant GDNF-induced phosphorylation of Pcdhα in MNs likely attributable to the lower expression levels of Pcdhα and Ret in MNs (Fig. S4C). Thus, Pcdhγ but not Pcdhα appears to be regulated by GDNF in MNs.
Fig. 4.
Pcdhγ undergoes GDNF-induced phosphorylation and interacts with Ret in MNs and sympathetic neurons. (A) Anti-Pcdhγ or control rabbit serum (IgG) immunoprecipitates (IPs) of MN lysate were blotted with anti-Ret and anti-P-Tyr antibodies. The blot was reprobed with an anti-Pcdhγ antibody. (B) MNs were left untreated, stimulated for 30 min with GDNF/GFRα1, or treated for 20 min with PP2 prior to 30 min of GDNF/GFRα1 stimulation. Anti-Pcdhγ or rabbit serum control (IgG) IPs were blotted with anti-P-Tyr and anti-Pcdhγ. Total cell lysate was blotted with anti-Ret, anti-P-MAPK, and anti-MAPK. (C) Sympathetic neurons were left untreated or stimulated for 30 min with GDNF. Anti-Pcdhα and anti-Pcdhγ IPs were blotted with anti-P-Tyr and anti-pan-Ret. The blots were reprobed with anti-Pcdhα and anti-Pcdhγ antibody. Total cell lysate was blotted with anti-Ret and anti-Tuj1. (D) Cortical glia were left untreated, stimulated with GDNF/GFRα1 for 30 min, or treated with PP2 before 30 min of GDNF/GFRα1 stimulation. Anti-Pcdhγ or rabbit control serum (IgG) IPs were blotted with anti-P-Tyr antibody. The blot was reprobed with an anti-Pcdhγ antibody.
Sympathetic neurons are commonly used to study Ret signaling, and treatment of SCG-derived neuronal cultures with GDNF induces robust autophosphorylation of Ret (Fig. S4A). GDNF treatment also induced the interaction between Pcdhα, Pcdhγ, and Ret and the phosphorylation of Pcdhα and Pcdhγ (Fig. 4C and Fig. S4A). We conclude that Pcdhα and Pcdhγ associate with Ret and are tyrosine-phosphorylated upon GDNF treatment of sympathetic neurons.
Because Pcdhγ is also highly expressed in glial cells (26), we investigated whether Pcdhγ is tyrosine-phosphorylated in cultures of total primary glia from postnatal day 0 (P0) cerebral cortex, consisting primarily of astrocytes (27). As shown in Fig. 4D, Pcdhγ is tyrosine-phosphorylated in glia in the absence of GDNF/GFRα1 stimulation, and this phosphorylation was unaffected by GDNF/GFRα1 treatment. This constitutive phosphorylation is sensitive to the Src family tyrosine kinase inhibitor PP2 (Fig. 4D). As expected, we did not detect significant expression of Ret in glial cells (Fig. S4B). These data suggest that Pcdhs associate with and are phosphorylated by different tyrosine kinases, depending on the cell type and maturation status of the cells.
Pcdhs Regulate Ret Activation and Stability.
We have shown that Ret is required for the phosphorylation and could influence the stability of Pcdhα and Pcdhγ proteins (Fig. 3 and Fig. S3). We next asked if the converse is true; that is, do Pcdhs affect Ret function or stability? In particular, the Ret51 splice form has been shown to undergo rapid ubiquitin-dependent degradation upon GDNF-induced phosphorylation in sympathetic neurons, and this effect can be inhibited by an NGF-dependent pathway (28).
We speculated that binding of Pcdhα and Pcdhγ to Ret might stabilize Ret. First, we investigated whether overexpression of Pcdhα4-TAP or Pcdhγb7-TAP affects expression levels or turnover of Ret51. In the vector control CAD cells, 30 min of GDNF/GFRα1 treatment induces robust Ret51 phosphorylation, which decreases after 3 h of stimulation (Fig. 5A). Total Ret51 protein levels are significantly reduced after only 30 min of GDNF/GFRα1 treatment and remain low after 3 h of treatment (Fig. 5A), suggesting that the protein is rapidly turned over. Pretreatment with the proteasome inhibitor MG132 rescues the decrease in phosphorylated Ret51 but does not prevent the overall decrease in Ret51 levels (Fig. 5A). Ret51 likely undergoes lysosomal degradation in the presence of MG132 (28). In Pcdhα4-TAP–overexpressing cells, the basal level of Ret51 protein is elevated (Fig. 5A and Fig. S5A). However, quantification of Ret51 levels suggests that it turns over with similar kinetics in Pcdhα4-TAP–overexpressing and control cell lines (Fig. S5A). In contrast to the vector control cells, GDNF/GFRα1 treatment in the Pcdhα4-TAP–overexpressing cell line leads to high levels of phosphorylated Ret51 that continue past 3 h (Fig. 5A and quantification in Fig. S5B). We also detect increased levels of total and phosphorylated Ret51 associated with Pcdhα4-TAP and Pcdhαb7-TAP (Fig. 5A). Expression of Pcdhγb7-TAP does not have a significant effect on Ret51 protein levels and turnover, likely attributable to the much lower expression levels when compared with Pcdhα4-TAP (Fig. 5A). Our data suggest that on GDNF/GFRα1 stimulation, Pcdhα4-TAP interacts with the phosphorylated form of Ret51 and slows down its ubiquitin-dependent degradation.
Fig. 5.
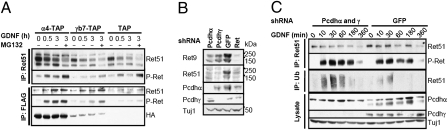
Pcdhs are required for stabilization of activated Ret. (A) Differentiated CAD cells stably expressing Pcdhα4-TAP (α4-TAP), Pcdhγb7-TAP (γb7-TAP), or TAP vector control (TAP) were left untreated or treated with GDNF/GFRα1 for 0.5 or 3 h. Anti-Ret51 and anti-FLAG (TAP) immunoprecipitates (IPs) were blotted with anti-Ret51 and anti-P-Ret. The blots were reprobed with anti-Ret51 antibody. The anti-FLAG (TAP) immunoprecipitation blot was reprobed with anti-HA antibody. (B) CAD cells infected with anti-Pcdhα, anti-Pcdhγ, anti-GFP, or anti-Ret lentiviral shRNA were differentiated. Total cell lysate was blotted for Ret9, Ret51, Pcdhα, Pcdhγ, and Tuj1. (C) Sympathetic neurons were infected with lentivirus encoding shRNA plasmids targeting Pcdhα and Pcdhγ or GFP. Neurons were cultured for an additional 7 d and stimulated with GDNF for various times. Anti-Ret51 IPs were blotted with anti-Ret51 and anti-P-Ret. Antiubiquitin IPs were blotted with anti-Ret51. Total cell lysate was blotted with anti-P-Ret, anti-Ret51, Pcdhα, Pcdhγ, and Tuj1 antibodies.
We next investigated whether reducing Pcdh levels affects Ret stability. We find that shRNA-mediated double-knockdown of Pcdhα and Pcdhγ protein in CAD cells and sympathetic neuron cultures leads to a reduction in the total levels of Ret51 protein and an increase in the levels of ubiquitinated Ret51 (Fig. 5 B and C and quantification in Fig. S5C). In addition, pretreatment of CAD cells with MG132 increases the level of phosphorylated Ret51 but not the total levels of Ret51 (Fig. 5A). shRNA-mediated knockdown of Pcdhα or Pcdhγ alone also reduces steady-state levels of both Ret9 and Ret51. We conclude that Pcdhα and Pcdhγ associate with and stabilize both Ret isoforms (Fig. 5B). Taken together, our data suggest that binding of phosphorylated Ret to Pcdhα and/or Pcdhγ increases stability and delays turnover of Ret.
Discussion
The data presented here provide evidence for a signaling function of Pcdhs. Affinity purification studies in CAD cells reveal that Pcdhα4-TAP interacts with different Pcdhα, Pcdhβ, and Pcdhγ isoforms as well as with a variety of cell adhesion and signaling molecules (Fig. S1 A and B). Using CAD cells and primary cell culture, we show that the tyrosine kinase Ret binds to and regulates Pcdhα and Pcdhγ tyrosine phosphorylation and protein levels (Figs. 1 B–D, 3, and 4 A–C). We also found that Pcdhs stabilize activated Ret in CAD cells (Fig. 5 A and B) and sympathetic neurons (Fig. 5C). Thus, Pcdhs and Ret appear to stabilize each other and are components of a membrane complex that can induce efficient signaling (Fig. 6). In addition, Pcdhα4-TAP associates with tyrosine phosphatases and tyrosine kinases other than Ret, and Pcdhγ undergoes Ret-independent tyrosine phosphorylation in glial cell cultures (Fig. 4D). Thus, Ret-independent pathways may contribute to Pcdh tyrosine phosphorylation. The tyrosine kinase PYK2, which has recently been identified as a Pcdhγ interactor by a yeast two-hybrid screen, likely does not phosphorylate Pcdhs, because Pcdhα and Pcdhγ bind preferentially to the inactive form of PYK2 and suppress its kinase activity. In addition, Pcdhα and Pcdhγ are not phosphorylated by PYK2 in an in vitro kinase assay (17).
Fig. 6.
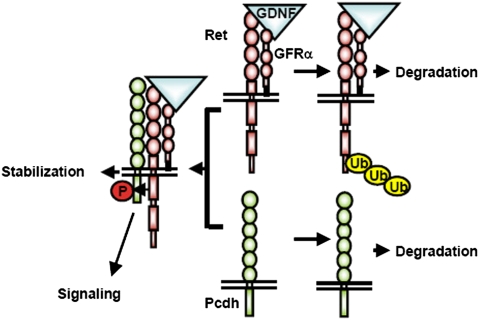
Model of Pcdh and Ret interaction and stabilization in CAD and sympathetic neurons. Activation of Ret with GDNF/GFRα1 leads to ubiquitination and rapid degradation. Pcdhs not bound to Ret also undergo degradation. Activated Ret bound to Pcdh is stabilized. Pcdhs bound to activated Ret are stabilized and phosphorylated and might initiate downstream signaling.
Pcdhs and Ret localize to growth cones and synapses of neurons and have been implicated in axonal pathfinding and neuronal survival (1, 3, 29, 30). In addition, Pcdhs and Ret localize to endosomes and the cell surface (24, 31), and we detected Pcdhα4-TAP and Pcdhγ7-TAP colocalization in a punctate pattern along processes in CAD cells (Fig. 1D). Pcdh and Ret may form stable intercellular complexes by binding in trans to Pcdhs, Ret, or other membrane proteins on neighboring cells. Formation of such a complex could alter Pcdh and Ret phosphorylation and endocytosis and induce downstream signaling. Tyrosine phosphorylation of Pcdhs by Ret may create binding sites for downstream effectors. Notably, we identified several proteins that copurify with Pcdhα4-TAP and are involved in neurite outgrowth and axonal pathfinding, such as stathmin-2, stathmin-3, and LAR (19, 20). Furthermore, in yeast two-hybrid screens, Pcdhα was reported to bind to the cytoskeletal proteins neurofilament M and fascin and Pcdhγb1 was found to bind to stathmin-2 (reviewed in 16). Cellular events induced by specific binding of Pcdhs in trans to other Pcdhs, Ret, and/or other tyrosine kinases could contribute to neurite outgrowth and the stabilization of interneuronal connections. Pcdh binding-induced signaling could be part of a mechanism for establishing synaptic connections or confirming synaptic identity. Depending on the specific set of protein interactions on the cell surface, signaling could induce neuronal survival or the maturation of a functional synapse. Identification and analysis of factors downstream of Pcdh and Ret are necessary to examine which specific role the Pcdh-Ret interaction may play.
Using a series of Pcdhα4-TAP deletion mutants, we determined that Pcdhα4 can bind to Ret through its extracellular and/or transmembrane domain and that the Pcdhα4 cytoplasmic domain alone is not sufficient to interact with Ret (Fig. S2B). Interestingly, both Ret and Pcdhs have cadherin-like extracellular domains. Thus, it is possible that the Pcdh-Ret interaction is mediated by these conserved sequences in a ligand- or differentiation-dependent manner. Ret binding to Pcdhs through the extracellular and transmembrane domains could bring the intracellular domains in close proximity and allow phosphorylation of the Pcdh intracellular domain by the Ret intracellular kinase domain. Mutagenesis studies suggest that Tyr-750 and Tyr-763 in Pcdhα4 are the most likely Ret-dependent phosphorylation sites (Fig. S2 C and D). Our data do not allow us to distinguish between direct phosphorylation of the Pcdhs by Ret and Pcdh phosphorylation by a Ret-dependent kinase. However, we note that knocking down c-Src, the major tyrosine kinase downstream of Ret, did not affect Pcdh tyrosine phosphorylation, making it more likely that Ret itself phosphorylates Pcdhs (Fig. S3B). The elevated levels of the Ret ligand persephin, the Ret coreceptor GFRα1, and Ret itself in differentiated CAD cells might be responsible for the Ret-dependent phosphorylation of Pcdhα4-TAP and Pcdhγ7-TAP in the absence of exogenously added GDNF and GFRα1 (Figs. S3 and S6 A and B). It has been reported recently that persephin can bind to GFRα1 and activate Ret (32). Recently, Pcdhγ was shown to associate with other Pcdh isoforms and to form a ≈1,000-kDa complex with several proteins of the postsynaptic density in mouse brain (8). Other than the Pcdhs, the Pcdhγ-binding partners identified in that report differ from the candidates in our study. Presumably, this is because Pcdhγ rather than Pcdhα was used in the initial purification and the candidates were purified from brain lysate rather than CAD cells. Ret was likely not identified because expression levels of Ret are low in the brain (33).
In summary, we show that Pcdhα and/or Pcdhγ forms a functional complex with Ret and that Pcdh proteins and Ret interact to regulate tyrosine phosphorylation and stability. Ret-dependent tyrosine phosphorylation of Pcdhs in response to the GDNF may be only one of many interactions capable of triggering the phosphorylation and activation of Pcdh-dependent signaling.
Materials and Methods
Reagents.
Pcdhα4-TAP full-length; Pcdhα4-TAP truncation mutants; and Pcdhγb7-TAP, DDR2-GFP, metadherin-GFP, CD98-GFP, RPTPα-GFP, Ret9-GFP, and Ret51-GFP were generated as described in SI Materials and Methods. The following chemical reagents were used at the following final concentrations: 10 μg/mL MG132 (Z-Leu-Leu-Leu-al; Sigma) in methanol, 10 mM N-ethylmaleimide (Sigma) in lysis buffer, and 20 μM PP2 in DMSO (Calbiochem).
Antibodies.
Antibodies are described in SI Materials and Methods.
Cell Culture.
CAD cells were cultured in DMEM (Gibco) containing 10% (vol/vol) Fetalclone III Serum (HyClone). Differentiation was induced by withdrawing serum for 48 h unless stated differently. Transfections of CAD cell lines were carried out with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Stably transfected CAD cell lines were produced by Lipofectamine 2000 transfection of the TAP-tagged plasmids and selection with G418 (Gibco). siRNA oligonucleotides and shRNA constructs and their usage are described in SI Materials and Methods.
Western Blot and Immunoprecipitation.
Cells were induced with mouse recombinant GDNF (R&D Systems) and recombinant GDNF receptor (GFR)α1/Fc fusion (R&D Systems) as indicated. Cell lysis, Western blotting, and immunoprecipitation were performed following standard protocols, as described in SI Materials and Methods.
MNs, Glia, and SCG Cultures.
ES cell lines were derived from mice transgenic for Hb9::GFP (Jackson Labs; stock number 005029) and differentiated into MNs as described by Di Giorgio et al. (27) (details provided in SI Materials and Methods). Glia were cultured as previously described by Di Giorgio et al. (27) (details provided in SI Materials and Methods). Rat SCG neurons were cultured as previously described (34) (details provided in SI Materials and Methods).
Immunostaining.
CAD cells were grown on coverslips coated with poly-d-lysine and laminin (BD Biosciences), which were fixed and permeabilized in methanol. Where appropriate, anti-EGFP coupled to Alexa 488 (A21311; Invitrogen) or anti-HA (HA.11; Covance) was added overnight. Fluorescently coupled secondary antibodies were obtained from Jackson ImmunoResearch. Coverslips were mounted in Vectashield with DAPI (Vector Laboratories). Images were collected with a Zeiss LSM 510 Meta confocal microscope.
Supplementary Material
Acknowledgments
We thank Jacinthe Gingras, Monica Carrasco, Nick Atwater, Hilary Bowden, and Emiko Morimoto for expert technical assistance; Weisheng Victor Chen, Kevin Monahan, and Polina Kehayova for critical reading of the manuscript; and Junqiang Ye for helpful discussions. This work was supported by National Institutes of Health Grant R01NS043915 (to T.M.), The Vermont Genetics Network through National Institutes of Health Grant P20 RR16462 from the IDeA Networks of Biomedical Research Excellence (INBRE) of the National Center for Research Resources (NCRR) (to B.A.B.), and Grant NS051238 and an Irma T. Hirschl Research Award (to G.R.P.).
Footnotes
The authors declare no conflict of interest.
See Commentary on page 13565.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007182107/-/DCSupplemental.
References
- 1.Kohmura N, et al. Diversity revealed by a novel family of cadherins expressed in neurons at a synaptic complex. Neuron. 1998;20:1137–1151. doi: 10.1016/s0896-6273(00)80495-x. [DOI] [PubMed] [Google Scholar]
- 2.Wu Q, Maniatis T. A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell. 1999;97:779–790. doi: 10.1016/s0092-8674(00)80789-8. [DOI] [PubMed] [Google Scholar]
- 3.Phillips GR, et al. Gamma-protocadherins are targeted to subsets of synapses and intracellular organelles in neurons. J Neurosci. 2003;23:5096–5104. doi: 10.1523/JNEUROSCI.23-12-05096.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morishita H, Murata Y, Esumi S, Hamada S, Yagi T. CNR/Pcdhalpha family in subplate neurons, and developing cortical connectivity. NeuroReport. 2004;15:2595–2599. doi: 10.1097/00001756-200412030-00007. [DOI] [PubMed] [Google Scholar]
- 5.Tasic B, et al. Promoter choice determines splice site selection in protocadherin alpha and gamma pre-mRNA splicing. Mol Cell. 2002;10:21–33. doi: 10.1016/s1097-2765(02)00578-6. [DOI] [PubMed] [Google Scholar]
- 6.Esumi S, et al. Monoallelic yet combinatorial expression of variable exons of the protocadherin-alpha gene cluster in single neurons. Nat Genet. 2005;37:171–176. doi: 10.1038/ng1500. [DOI] [PubMed] [Google Scholar]
- 7.Morishita H, Yagi T. Protocadherin family: Diversity, structure, and function. Curr Opin Cell Biol. 2007;19:584–592. doi: 10.1016/j.ceb.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Han MH, Lin C, Meng S, Wang X. Proteomic analysis reveals overlapping functions of clustered protocadherins. Mol Cell Proteomics. 2009;9:71–83. doi: 10.1074/mcp.M900343-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernández-Monreal M, Kang S, Phillips GR. Gamma-protocadherin homophilic interaction and intracellular trafficking is controlled by the cytoplasmic domain in neurons. Mol Cell Neurosci. 2009;40:344–353. doi: 10.1016/j.mcn.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc Natl Acad Sci USA. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasegawa S, et al. The protocadherin-alpha family is involved in axonal coalescence of olfactory sensory neurons into glomeruli of the olfactory bulb in mouse. Mol Cell Neurosci. 2008;38:66–79. doi: 10.1016/j.mcn.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Katori S, et al. Protocadherin-alpha family is required for serotonergic projections to appropriately innervate target brain areas. J Neurosci. 2009;29:9137–9147. doi: 10.1523/JNEUROSCI.5478-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, et al. Gamma protocadherins are required for survival of spinal interneurons. Neuron. 2002;36:843–854. doi: 10.1016/s0896-6273(02)01090-5. [DOI] [PubMed] [Google Scholar]
- 14.Weiner JA, Wang X, Tapia JC, Sanes JR. Gamma protocadherins are required for synaptic development in the spinal cord. Proc Natl Acad Sci USA. 2005;102:8–14. doi: 10.1073/pnas.0407931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lefebvre JL, Zhang Y, Meister M, Wang X, Sanes JR. gamma-Protocadherins regulate neuronal survival but are dispensable for circuit formation in retina. Development. 2008;135:4141–4151. doi: 10.1242/dev.027912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yagi T. Clustered protocadherin family. Dev Growth Differ. 2008;50(Suppl 1):S131–S140. doi: 10.1111/j.1440-169X.2008.00991.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, et al. alpha- and gamma-Protocadherins negatively regulate PYK2. J Biol Chem. 2009;284:2880–2890. doi: 10.1074/jbc.M807417200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi Y, Wang JK, McMillian M, Chikaraishi DM. Characterization of a CNS cell line, CAD, in which morphological differentiation is initiated by serum deprivation. J Neurosci. 1997;17:1217–1225. doi: 10.1523/JNEUROSCI.17-04-01217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grenningloh G, Soehrman S, Bondallaz P, Ruchti E, Cadas H. Role of the microtubule destabilizing proteins SCG10 and stathmin in neuronal growth. J Neurobiol. 2004;58:60–69. doi: 10.1002/neu.10279. [DOI] [PubMed] [Google Scholar]
- 20.Chagnon MJ, Uetani N, Tremblay ML. Functional significance of the LAR receptor protein tyrosine phosphatase family in development and diseases. Biochem Cell Biol. 2004;82:664–675. doi: 10.1139/o04-120. [DOI] [PubMed] [Google Scholar]
- 21.Enomoto H. Regulation of neural development by glial cell line-derived neurotrophic factor family ligands. Anat Sci Int. 2005;80:42–52. doi: 10.1111/j.1447-073x.2005.00099.x. [DOI] [PubMed] [Google Scholar]
- 22.Carroll P, et al. Juxtaposition of CNR protocadherins and reelin expression in the developing spinal cord. Mol Cell Neurosci. 2001;17:611–623. doi: 10.1006/mcne.2001.0966. [DOI] [PubMed] [Google Scholar]
- 23.Anders J, Kjar S, Ibáñez CF. Molecular modeling of the extracellular domain of the RET receptor tyrosine kinase reveals multiple cadherin-like domains and a calcium-binding site. J Biol Chem. 2001;276:35808–35817. doi: 10.1074/jbc.M104968200. [DOI] [PubMed] [Google Scholar]
- 24.Richardson DS, Lai AZ, Mulligan LM. RET ligand-induced internalization and its consequences for downstream signaling. Oncogene. 2006;25:3206–3211. doi: 10.1038/sj.onc.1209349. [DOI] [PubMed] [Google Scholar]
- 25.Carlomagno F, et al. Efficient inhibition of RET/papillary thyroid carcinoma oncogenic kinases by 4-amino-5-(4-chloro-phenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) J Clin Endocrinol Metab. 2003;88:1897–1902. doi: 10.1210/jc.2002-021278. [DOI] [PubMed] [Google Scholar]
- 26.Garrett AM, Weiner JA. Control of CNS synapse development by gamma-protocadherin-mediated astrocyte-neuron contact. J Neurosci. 2009;29:11723–11731. doi: 10.1523/JNEUROSCI.2818-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierchala BA, Tsui CC, Milbrandt J, Johnson EM. NGF augments the autophosphorylation of Ret via inhibition of ubiquitin-dependent degradation. J Neurochem. 2007;100:1169–1176. doi: 10.1111/j.1471-4159.2006.04292.x. [DOI] [PubMed] [Google Scholar]
- 29.Blank M, Triana-Baltzer GB, Richards CS, Berg DK. Alpha-protocadherins are presynaptic and axonal in nicotinic pathways. Mol Cell Neurosci. 2004;26:530–543. doi: 10.1016/j.mcn.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Airaksinen MS, Saarma M. The GDNF family: Signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 31.Murata Y, Hamada S, Morishita H, Mutoh T, Yagi T. Interaction with protocadherin-gamma regulates the cell surface expression of protocadherin-alpha. J Biol Chem. 2004;279:49508–49516. doi: 10.1074/jbc.M408771200. [DOI] [PubMed] [Google Scholar]
- 32.Sidorova YA, et al. Persephin signaling through GFRalpha1: The potential for the treatment of Parkinson’s disease. Mol Cell Neurosci. 2010;44:223–232. doi: 10.1016/j.mcn.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Lee KY, Samy ET, Sham MH, Tam PK, Lui VC. 3′ Splicing variants of ret receptor tyrosine kinase are differentially expressed in mouse embryos and in adult mice. Biochim Biophys Acta. 2003;1627:26–38. doi: 10.1016/s0167-4781(03)00068-x. [DOI] [PubMed] [Google Scholar]
- 34.McFarlane S, Cooper E. Postnatal development of voltage-gated K currents on rat sympathetic neurons. J Neurophysiol. 1992;67:1291–1300. doi: 10.1152/jn.1992.67.5.1291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.