Abstract
Slow and persistent synaptic inhibition is mediated by metabotropic GABAB receptors (GABABRs). GABABRs are responsible for the modulation of neurotransmitter release from presynaptic terminals and for hyperpolarization at postsynaptic sites. Postsynaptic GABABRs are predominantly found on dendritic spines, adjacent to excitatory synapses, but the control of their plasma membrane availability is still controversial. Here, we explore the role of glutamate receptor activation in regulating the function and surface availability of GABABRs in central neurons. We demonstrate that prolonged activation of NMDA receptors (NMDA-Rs) leads to endocytosis, a diversion from a recycling route, and subsequent lysosomal degradation of GABABRs. These sorting events are paralleled by a reduction in GABABR-dependent activation of inwardly rectifying K+ channel currents. Postendocytic sorting is critically dependent on phosphorylation of serine 783 (S783) within the GABABR2 subunit, an established substrate of AMP-dependent protein kinase (AMPK). NMDA-R activation leads to a rapid increase in phosphorylation of S783, followed by a slower dephosphorylation, which results from the activity of AMPK and protein phosphatase 2A, respectively. Agonist activation of GABABRs counters the effects of NMDA. Thus, NMDA-R activation alters the phosphorylation state of S783 and acts as a molecular switch to decrease the abundance of GABABRs at the neuronal plasma membrane. Such a mechanism may be of significance during synaptic plasticity or pathological conditions, such as ischemia or epilepsy, which lead to prolonged activation of glutamate receptors.
Keywords: endocytic, recycling, excitation-inhibition, glutamate
The availability of neurotransmitter receptors, a major determinant of synaptic efficacy, is regulated by coordinated mechanisms of intracellular trafficking that deliver newly synthesized receptors to the plasma membrane and remove them for storage, recycling, or degradation (1). The molecular mechanisms controlling the availability of GABAB receptors (GABABRs), which are central players in the modulation of excitatory and inhibitory synaptic activity, are unclear.
GABABRs mediate slow and prolonged inhibitory synaptic signals (2, 3). Consistent with these roles, modifications in the function of GABABRs are implicated in epilepsy, anxiety, stress, sleep disorders, nociception, depression, cognition, and addictive mechanisms to drugs of abuse (3–7). GABABRs are members of the G protein-coupled receptor (GPCR) superfamily and are obligatory heteromers composed of two related subunits, namely GABABR1 and GABABR2 (3, 8). GABABR1 binds agonist with high affinity, whereas GABABR2 mediates coupling to Gαi (9, 10). GABABRs are located in GABA-ergic and glutamatergic pre- and postsynaptic terminals, but their distribution does not coincide with the active zone, postsynaptic density, or inhibitory postsynaptic specializations. Rather, they are perisynaptic receptors activated by GABA spillover (3, 11). Stimulation of GABABRs decreases the levels of cAMP, inhibits neurotransmitter release from presynaptic terminals, and hyperpolarizes postsynaptic neurons (2).
The availability and function of GPCRs are typically controlled by mechanisms such as agonist-induced desensitization and internalization, followed by recycling or degradation (12, 13). In the case of GABABRs, the role that agonist-dependent desensitization plays in controlling signaling and cell surface availability remains controversial (14), but phosphorylation by GPCR-dependent kinases and arrestin binding have been excluded (15, 16). On the contrary, constitutive endocytosis in neurons has been reported consistently (16–22). Electron microscopy studies have demonstrated that GABABRs are enriched in the vicinity of glutamatergic synapses (23–27), but the role of glutamate receptors in controlling the efficacy of GABABR signaling remains largely unexplored.
Here, we investigate the molecular mechanisms underlying the control of GABABR cell surface availability and effector coupling by glutamate receptor activity. Our study demonstrates that glutamate, acting through NMDA receptors (NMDA-Rs), activates AMP-dependent protein kinase (AMPK) and protein phosphatase 2A (PP2A), resulting in transient changes to the phosphorylation state of the GABABR2 subunit on serine 783 (S783). Phosphorylation changes alter the fate of endocytosed GABABRs, which divert from a recycling to a lysosomal degradation route, attenuating GABABR function. Our observations provide a unique mechanism to regulate the functional availability of GABABRs via a nonconventional extracellular signal, uncovering an added interplay between excitation and inhibition in central neurons.
Results
Activation of NMDA-Rs Triggers GABABR Endocytosis.
We measured the steady-state abundance of endogenous GABABRs by surface biotinylation. Glutamate produced a biphasic response in the cell surface levels of GABABRs. Short exposure to glutamate (1–5 min) increased the abundance of both GABABR1 and GABABR2 subunits at the plasma membrane, whereas longer treatments (10–30 min) triggered a pronounced and steady decrease of surface receptors (Fig. 1A). Neurons were treated with NMDA to determine directly whether activation of NMDA-Rs was sufficient to promote receptor disappearance. Activation of NMDA-Rs mimicked the effect of glutamate but triggered a faster disappearance of GABABRs (Fig. 1B). A23187, a calcium ionophore that potentiates the response to NMDA, also markedly reduced surface GABABRs (Fig. S1). To evaluate if glutamate affects functional GABABRs under physiological conditions, we used whole-cell patch-clamp recording of hippocampal neurons and applied a 15-s pulse of 1 mM glutamate to reproduce synaptic concentrations of the excitatory transmitter. Glutamate caused a significant reduction in the baclofen-induced GABABR-dependent activation of inwardly rectifying K+ currents (Fig. 1 C and D). This reduction was relatively rapid (onset within 2 min after glutamate application) and was maintained for a further 7 min before slowly recovering in amplitude after 10 min (Fig. 1D). MK801 (100 nM), a selective NMDA-R antagonist, abolished the regulatory effect of glutamate, and baclofen-activated currents remained at control levels (Figs. 1 C and D). Glutamate acted directly via NMDA-Rs to modulate the GABABR-activated current, because the application of 1 mM glutamate to a cell line expressing GABABR1a and GABABR2, with inwardly rectifying K+ channels Kir3.1 and Kir3.2, did not affect their activity following the addition of 10 μM GABA (Control IK = 100%; +1 mM glutamate = 95.3 ± 7%; n = 6). Taken together, these observations suggest that activation of NMDA-Rs plays a significant role in the plasticity of cell surface GABABRs.
Fig. 1.
(A) Cortical neurons were left untreated or exposed to 20 μM glutamate for 0–30 min. Biotinylated (surface) and total proteins were visualized by immunoblotting with GABABR2 (R2) and GABABR1 (R1) antibodies. (Lower) Immunoblots for each condition were analyzed by densitometry. **P < 0.01. (B) Surface expression of GABABR2 was evaluated as above after exposure to 20 μM NMDA for 0–10 min. (Lower) Immunoblots were quantified as above. *P < 0.05; **P < 0.01. (C) Whole-cell recordings from cultured hippocampal neurons with 10 μM baclofen-activated K+ currents before (Left) and after (Right) glutamate receptor activation (1 mM, 15 s) in the absence (Upper) and presence (Lower) of 100 nM MK801 at 7, 10, 13, and 19 min. Baclofen responses were obtained in a high K+ Krebs’ solution supplemented with AP-5 (20 μM), CNQX (10 μM), TTX (500 nM), and Bicuculline (25 μM). (D) Baclofen-activated currents normalized to the first response at t = 0 min were recorded as control responses (■) and responses interposed with glutamate (○) or glutamate and MK801 applications (▲). All points represent the mean ± SEM (n = 5–12 cells; *P < 0.05). (E) Cortical neurons were left untreated or exposed to 20 μM glutamate, 20 μM PAO, or 20 μM glutamate plus 20 μM PAO for 30 min. Biotinylated (surface) and total proteins were visualized by immunoblotting with GABABR2 and GABABR1 antibodies. (F) Hippocampal neurons were transfected with MYC-GABABR1a and HA-GABABR2 and processed for immunoendocytosis with MYC antibodies. Cells were left untreated (Upper) or exposed to 20 μM glutamate for 30 min (Lower). Surface receptors were detected with MYC- and Texas Red-conjugated secondary antibodies (red channel). Internalized receptors were detected using FITC-conjugated secondary antibodies (green channel). (Right) Merged images are shown. (Scale bar: 5 μm.)
To determine whether the disappearance of surface GABABRs was the result of internalization, we first examined whether phenyl arsine oxide (PAO) affected their removal. PAO is a widely used inhibitor of clathrin-mediated endocytosis (28, 29) that completely blocks the endocytosis of GABABRs (Fig. S2). Surface levels of GABABRs were reduced by glutamate, an effect prevented by PAO (Fig. 1E). Because no differences were observed for GABABR1 and GABABR2, both subunits were suitable to explore the effect of glutamate. We used an immunoendocytosis assay to visualize directly the effect of NMDA-R activation on GABABRs by following the GABABR1 subunit. GABABRs were efficiently detected at the neuronal plasma membrane and concentrated in intracellular structures after constitutive internalization. These structures accumulated throughout the cell body and frequently displayed perinuclear localization (Fig. 1F Upper, arrowhead). In contrast, after glutamate stimulation, the majority of internalized GABABRs remained spatially restricted in the proximity of the plasma membrane (Fig. 1F Lower, arrowhead). Combined, these findings provide direct evidence that activation of NMDA-Rs decreases GABABR function by triggering their internalization and altering their intracellular distribution after endocytosis.
Activation of NMDA-Rs Diverts GABABRs from a Recycling Route.
After internalization, GABABRs recycle to the plasma membrane, a mechanism that is accelerated by receptor agonist (21, 22). To determine whether glutamate stimulation interferes with recycling, we combined immunoendocytosis with detection of Rab11, a marker for recycling endosomes (30). After endocytosis, GABABRs colocalized significantly with Rab11 (Fig. 2A Upper, arrowhead). By contrast, after glutamate stimulation, internalized GABABRs were restricted to the proximity of the plasma membrane, dissociated from Rab11-positive compartments (Fig. 2A Lower, arrowhead).
Fig. 2.
(A) Hippocampal neurons were transfected with MYC-GABABR1a and HA-GABABR2 and processed for immunoendocytosis with MYC antibodies. Cells were left untreated (Upper) or exposed to 20 μM glutamate for 30 min (Lower). Surface receptors were detected with MYC- and cyanine-conjugated secondary antibodies (blue channel). Internalized receptors were detected using Texas Red-conjugated secondary antibodies (red channel). Rab11-GFP was visualized to compare distribution (green channel). (Right) Merged images show internalized GABABRs and Rab11. (Scale bar: 5 μm.) (B) Same as above for neurons treated with glutamate under control conditions (Upper) or after incubation with lysosomal inhibitors (200 nM bafilomycin and 100 μM leupeptin) (Lower). (C) Cortical neurons were left untreated or exposed to either 20 μM glutamate, 50 μM baclofen plus 20 μM glutamate, or 50 μM baclofen for 30 min. Biotinylated (surface) proteins were visualized by immunoblotting with GABABR2 antibodies. (Right) Immunoblots for each condition were analyzed by densitometry. Ctrl, control; ns, nonsignificant. **P < 0.01.
To determine whether postglutamate internalized GABABRs were targeted for degradation, immunoendocytosis was performed after pretreating the neurons with lysosomal blockers. The redistribution of internalized GABABRs by glutamate was markedly inhibited following lysosomal blockade. Thus, in the presence of lysosomal inhibitors, GABABRs did not divert from recycling endosomes after glutamate exposure and still concentrated in Rab11-positive perinuclear compartments (Fig. 2B Lower, arrowheads). These results indicate that lysosomal blockade forces internalized GABABRs to continue along the recycling pathway.
Next, based on the observations that GABABR activation induces postsynaptic hyperpolarization and accelerates recycling, we hypothesized that baclofen might counteract the NMDA-R–dependent degradation signal. To test this prediction, we measured the abundance of cell surface GABABRs after coincident stimulation of glutamate and baclofen for 30 min. Under such conditions, baclofen significantly reduced the disappearance of GABABRs after glutamate stimulation (Fig. 2C). Together, these observations demonstrate that glutamate receptor activation alters the endocytic route of GABABRs and produces a diversion from a recycling route to a degradation route. Furthermore, they indicate that activation of GABABRs is sufficient to evade the NMDA-R–triggered degradation switch partially, which is possibly attributable to reduced activation of NMDA-Rs at hyperpolarized potentials.
GABABR Sorting After NMDA-R Activation Is Controlled by AMPK Activation and Phosphorylation of S783 in GABABR2.
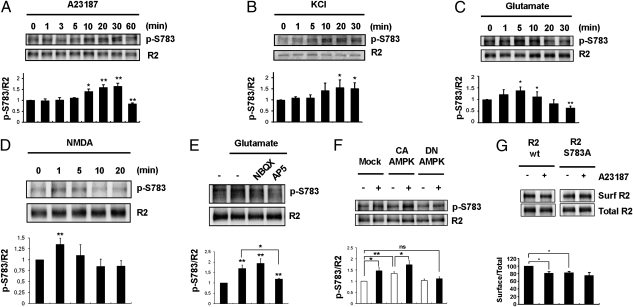
Multiple phosphorylation sites regulate the function and surface stability of GABABRs (17, 31). To explore whether phosphorylation plays a role in the glutamate-mediated removal of GABABRs, we examined known phosphoacceptor sites. Phosphorylation of S783 in GABABR2 was significantly stimulated by augmented intracellular Ca2+ levels and by KCl-induced depolarization, two biochemical models of increased neuronal activity (Fig. 3 A and B). Glutamate also enhanced phosphorylation of S783 (Fig. 3C). To determine if NMDA-Rs were involved, phosphorylation of S783 was examined after treatment with specific glutamate receptor agonists and antagonists. Phosphorylation was rapidly stimulated by NMDA and robustly inhibited by AP5, a selective NMDA-R antagonist (Fig. 3 D and E). Importantly, the time course of S783 phosphorylation triggered by NMDA-R activation paralleled the trafficking behavior of cell surface GABABRs, showing an early rise in phosphorylation followed by steady dephosphorylation after 5 min of treatment.
Fig. 3.
(A) Cortical neurons were treated with 2 μM A23187 for 0–60 min. Total cell lysates were prepared and visualized by immunoblotting with anti-S783 phosphospecific antibodies or GABABR2 antibodies. Immunoblots for each condition were analyzed by densitometry and plotted with the abscissa directly below the corresponding lane (Lower). *P < 0.05; **P < 0.01. Using the same conditions as above, neurons were treated with 30 mM KCl for 0–30 min (B), 20 μM glutamate for 0–30 min (C), 20 μM NMDA for 0–20 min (D), and 20 μM glutamate in the absence and presence of AP-5 (20 μM) or NBQX (10 μM) (E). (F) Cortical neurons were left untreated (−) or exposed to 20 μM glutamate for 30 min (+) in the absence or presence of a constitutively active (CA-AMPK) or dominant negative AMPK (DN-AMPK). Total cell lysates were prepared and visualized by immunoblotting with anti-S783 phosphospecific antibodies or GABABR2 antibodies. Immunoblots for each condition were analyzed by densitometry and plotted with the abscissa directly below the corresponding lane (Lower). ns, not significant. (G) HEK293 cells were transfected with GABABR1 and WT GABABR2 or GABABR2-S783A. Cells were left untreated or exposed to 2 μM A23187 for 30 min. Biotinylated (surface) and total proteins were visualized by immunoblotting with GABABR2 antibodies and analyzed by densitometry as above.
Next, we investigated the signaling mechanisms involved in the phosphorylation of S783 after NMDA-R activation. Ca2+ release from intracellular stores and Ca2+ influx were necessary to increase S783 phosphorylation (Fig. S3A). This led us to examine the role of AMPK, a metabolic sensor activated by low ATP/AMP ratios and high Ca2+ levels (32), which is responsible for phosphorylating S783 (31). Phosphorylation of S783 was stimulated by a constitutive form of AMPK in the absence or presence of glutamate and was blocked by a dominant negative AMPK (Fig. 3F). In agreement with these findings, phosphorylation of S783 was prevented by STO-609, a selective inhibitor of the upstream kinase CaM KK (Fig. S3B).
To demonstrate that neuronal activity and NMDA-R activation stimulate AMPK, we measured phosphorylation of threonine 172 (T172) in AMPK as an indicator of kinase activity. In accordance with the data presented above, AMPK was activated by A23187, by KCl, by glutamate, and by NMDA (Fig. S4). Thus, NMDA-Rs activate AMPK, resulting in phosphorylation of GABABR2 at S783.
The significant changes in GABABR2 phosphorylation led us to investigate whether S783 regulates receptor removal. HEK293 cells were transfected with WT GABABR1 and GABABR2 subunits or with GABABR1 and GABABR2 containing a serine-to-alanine substitution at position 783 (S783A). Ca2+ influx was used as a stimulus in these cells lacking NMDA-Rs. A23187 promoted the disappearance of recombinant receptors from the plasma membrane, but removal was significantly attenuated in receptors containing the S783A mutated subunit (Fig. 3G). Cell surface levels of heterodimers containing S783A were lower than those of WT, suggesting that dephosphorylation favors degradation, thus reducing the availability of plasma membrane GABABRs.
We used immunoendocytosis to visualize directly the fate of internalized receptors containing mutated phosphorylation sites. The majority of WT receptors accumulated throughout the cell body after constitutive endocytosis and glutamate produced a marked redistribution of the internalized receptors (Fig. 4A). In contrast, internalized receptors containing S783A were spatially restricted to the proximity of the plasma membrane even before glutamate treatment, mimicking the endocytic pattern of postglutamate WT receptors (Fig. 4A, arrowheads). Additionally, glutamate did not affect the pattern of internalized receptors containing S783A (Fig. 4A Lower). These data imply that transient changes in S783 phosphorylation regulate the postendocytic sorting of GABABRs after NMDA-R activation.
Fig. 4.
(A) Hippocampal neurons were transfected with MYC-GABABR1a and WT HA-GABABR2 or FLAG-GABABR2-S783A and processed for immunoendocytosis with MYC antibodies. Surface receptors were detected with MYC- and cyanine-conjugated secondary antibodies (blue channel). Internalized receptors were detected using Texas Red-conjugated secondary antibodies (red channel). Rab11-GFP was visualized to compare distribution (green channel). Merged images showing internalized GABABRs and Rab11 are shown. Neurons were analyzed under control conditions (Upper) or after treatment with 20 μM glutamate for 30 min (Lower). (Scale bar: 5 μm.) (B) Cortical neurons were left untreated or exposed to 100 nM OA for 0–60 min. Biotinylated (surface) and total proteins were visualized by immunoblotting with GABABR2 antibodies. Immunoblots for each condition were analyzed by densitometry and plotted with the abscissa directly below the corresponding lane (Lower). (C) HEK293 cells were transfected with MYC-GABABR1b-α-Bgt and GABABR2. Cells were first incubated with unlabeled α-Bgt and then with Rd-α-Bgt in the absence (○) or presence of 100 nM OA (●). Newly inserted receptors were quantified by flow cytometry. (D) Cortical neurons were treated with 100 nM OA for 0–60 min. Total cell lysates were prepared and visualized by immunoblotting with anti-S783 phosphospecific antibodies or anti-T172 phosphospecific antibodies. Immunoblots for each condition were analyzed by densitometry (Lower). *P < 0.05; **P < 0.01; ***P < 0.001. (E) Cortical neurons were left untreated or exposed to 20 μM glutamate in the absence or presence of 100 nM OA. Biotinylated (surface) and total proteins were visualized by immunoblotting with GABABR2 antibodies. Immunoblots for each condition were analyzed by densitometry and plotted with the abscissa directly below the corresponding lane (Lower). (F) Cortical neurons were exposed to 20 μM glutamate for 30 min in the absence or presence of 100 nM OA, 500 nM OA, or 20 μM cyclosporin A (CyA). Total cell lysates were prepared and visualized by immunoblotting with anti-S783 phosphospecific antibodies, GABABR2 antibodies, anti-T172 phosphospecific antibodies, or AMPK antibodies. (Right) Immunoblots for each condition were analyzed by densitometry.
Because S783A is a dephosphomimetic substitution, these results suggest that dephosphorylation of S783 is involved in diverting from the recycling route. Thus, we explored the role of dephosphorylation on the surface availability of GABABRs. Surface levels of GABABRs were significantly enhanced by okadaic acid (OA) at a concentration that efficiently inhibits PP2A but not PP1 (Fig. 4B). This effect was not caused by increased GABABR exocytosis, because OA treatment had no effect on the insertion of de novo synthesized receptors (Fig. 4C). Consistent with these data, phosphorylation of S783 in GABABR2 and T172 in AMPK was stimulated by OA (Fig. 4D). More importantly, reduction of GABABRs surface levels required PP2A activity (Fig. 4E). In addition, dephosphorylation of GABABRs after 30 min of glutamate treatment was blocked by OA and not by cyclosporin A, consistent with the role of PP2A in receptor dephosphorylation (Fig. 4F). In agreement with these observations, PP2A associated with GABABR1 in cortical neurons (Fig. S5). These observations indicate that PP2A-mediated dephosphorylation is necessary for reduction of cell surface GABABRs.
Discussion
Dynamic Balance Between Recycling and Degradation Controls the Abundance of GABABRs Through a Phosphorylation Switch.
Our observations are consistent with an integrated intracellular trafficking model in which activation of NMDA-Rs and GABABRs controls the balance between endocytosis, recycling, and degradation of GABABRs in neurons (Fig. S6). Briefly, GABABRs internalize and recycle in a constitutive manner. Activation of NMDA-Rs raises intracellular Ca2+, activating AMPK and promoting a transient increase in cell surface GABABRs through S783 phosphorylation. Prolonged NMDA-R stimulation triggers the endocytosis of GABABRs (by a mechanism not uncovered in this study) and the activation of PP2A. PP2A favors dephosphorylation of S783 in GABABR2 and diversion of the endocytosed pool from recycling to degradation. Combined, these events downstream of NMDA-Rs effectively decrease the availability of cell surface receptors. GABABR activation overcomes the glutamate effect, possibly by hyperpolarizing the cell membrane, thereby preventing persistent depolarization and favoring recycling over degradation. However, concurrent GABABR activation can also attenuate the Ca2+ signal induced by NMDA-R activation without affecting postsynaptic currents. This effect proceeds via GABABR down-regulation of adenylate cyclase activity and consequent attenuation of cAMP-dependent protein kinase activity, which reduces the NMDA-R–induced Ca2+ signal (33).
We refer to the postendocytic sorting model as the glutamate switch model. Overall, it is similar to the control of AMPA receptor availability by activation of different glutamate receptors, which alter their endocytic fate (34). The implications of the model for the functional localization of GABABRs are not clear. For example, it is not known whether GABABR exocytosis occurs at dedicated sites or randomly throughout the neuron and whether phosphorylation by AMPK or dephosphorylation by PP2A serves as a tag to redirect endocytosed populations from recycling endosomes to specific domains, such as synapses or perisynaptic sites.
Mechanisms Controlling Recycling and Degradation of GABABRs.
The involvement of lysosomes in GABABR degradation has been suggested before (16, 20, 21). In addition, the connection between degradative and recycling routes is strongly supported by the fact that blocking recycling of GABABRs with monensin produced an increase in lysosomal degradation (21). These data are in agreement with our switch model. At the molecular level, a di-leucine motif in the C-terminal domain of GABABR1 may act as a lysosomal degradation signal because its removal results in accumulation of GABABR1 at the plasma membrane (35). This idea is consistent with di-leucine motifs playing multiple functions as export, endocytosis, recycling, or lysosomal targeting signals for GPCRs (36), but their role in GABABR degradation has not been directly demonstrated.
A23187 and KCl produced slower GABABR phosphorylation than direct activation of NMDA-Rs. One explanation for this discrepancy is that the physical proximity of NMDA-Rs and GABABRs in many synaptic regions favors local and rapid signaling between receptors. Alternatively, activation of phosphatases may be faster or more efficient after stimulation of NMDA-Rs, which are known to initiate a variety of second-messenger signaling cascades (37). Importantly, the time course of S783 phosphorylation triggered by NMDA-Rs paralleled the behavior of cell surface availability of GABABRs, showing an early rise followed by a steady decline.
The specific postendocytic sorting consequence of NMDA-R activity and S783 phosphorylation suggests that different phosphorylation sites regulate distinct aspects of the biosynthetic pathway of GABABRs, a postulate that has been convincingly demonstrated for other neurotransmitter receptors (38, 39).
Glutamate, Hyperexcitability, and Neuronal Damage.
High-resolution microscopy in the visual cortex, cerebellum, thalamus, and hippocampus has shown that GABABRs are enriched in the vicinity of excitatory synapses, supporting the participation of glutamate receptor activation in the regulation of GABABR function (23–27). In agreement with this, we have recently shown that glutamate produces a rapid disappearance of GABABR1 and GABABR2 from the plasma membrane of primary neurons (22). Additionally, activation of NMDA-Rs causes a pronounced decrease in the total levels of GABABR2 in organotypic slices of rat hippocampus (40). Thus, reduction in the abundance of GABABRs has been associated with NMDA-induced excitotoxicity, suggesting that hyperexcitability may include the elimination of an inhibitory component. Activation of kainate receptors also produces a decline in GABABR levels after 24 h in adult mice, possibly contributing to temporal lobe epilepsy (41). Our present results demonstrate that GABABR function is attenuated by brief pulses of glutamate, consequently decreasing inwardly rectifying K+ currents and leading to reduced postsynaptic hyperpolarization. This attenuation is blocked by MK801-implicating NMDA-Rs in the regulation of functional cell surface GABABRs. Our data also indicate that persistent NMDA-R activity initiates a signaling cascade, including AMPK and PP2A, which effectively reduces the availability of GABABRs, possibly favoring hyperexcitability and neuronal damage. These observations are in agreement with those published earlier, which showed that increased phosphorylation of S783 reduces the attenuation of GABABR-mediated responses during desensitization by promoting cell surface stability (31). They also agree with the initial increase in phosphorylation of S783 during transient ischemic injury (31). Additionally, they provide an integrated molecular explanation for the neuroprotective effect of GABABR activity on NMDA-induced cell death (40).
Removal of GABABRs from pre- or postsynaptic terminals after glutamate receptor activation may be a general mechanism involved in hyperexcitability in diverse physiological scenarios. For example, the interplay between glutamate and GABABR activity has been reported to modulate oscillatory activity in the auditory cortex (42). Additionally, glutamate-mediated removal of GABABRs may be involved in the modulation of LTP by GABABRs (43). Finally, it may operate in glutamatergic terminals of large and small fibers in the dorsal horn of the spinal cord, promoting the hyperactivation of nociceptive pathways. Recently, enhanced excitatory synaptic activity has been involved in the removal of GABAARs from synaptic sites in a Ca2+ and calcineurin-dependent manner (44). Thus, it is tempting to speculate that Ca2+ signals originating from NMDA-Rs produce a transient reduction in GABA-ergic inhibition affecting ionotropic and metabotropic receptors.
Experimental Procedures
Chemicals and Plasmids.
Glutamate, baclofen, GABA, PAO, leupeptin, bafilomycin, and OA were purchased from Sigma. NMDA, CNQX, and D-AP5 were purchased from Tocris Bioscience. Fluorescently conjugated α-bungarotoxin (α-Bgt) was purchased from Invitrogen. The constructs containing MYC-GABABR1, HA-GABABR2, FLAG-GABABR2-S783A, and FLAG-GABABR2 in pRK5 have been described previously and contain their epitope tags on the extracellular N-terminal domains (17, 31). Myc-GABABR1b-α-Bgt was generated by inserting a 13-aa α-Bgt tag into a restriction site contained in the N terminus of GABABR1b. EGFP-WT-Rab11 was kindly provided by F. Bronfman (Pontificia Universidad Católica de Chile, Santiago, Chile).
Antibodies.
GABABR1 and GABABR2 antibodies have been described previously (17, 31). Anti-phospho S783-GABABR2 (p-S783) has been described (31). Anti-phospho T172-AMPK (p-T172) was purchased from Cell Signaling Technology. Actin, β-tubulin, anti-FLAG, and anti-MYC antibodies were purchased from Sigma. HA antibodies were purchased from Roche. The secondary anti-mouse and anti-rabbit antibodies conjugated to Texas Red, FITC, cyanine, and HRP were purchased from Jackson Immuno Research Laboratories.
Animals, Primary Neurons, and Transfections.
Adult pregnant female Sprague–Dawley rats were purchased from the Central Animal Facility at Universidad Católica de Chile and killed by means of asphyxia in a CO2 chamber, according to the National Academy of Sciences’ guidelines for the care and use of laboratory animals. Primary cultures of cortical and hippocampal neurons were obtained from embryonic day 18 rats (45) and used at 5 and 14 days in vitro (div), respectively. Hippocampal neurons were transfected by calcium phosphate, as reported elsewhere (46).
Biotinylation, GST Pull-Downs, Immunoprecipitations, and Phosphorylation.
Biotinylation of cortical neurons was conducted as reported elsewhere (16). Briefly, biotinylated proteins were precipitated with Neutravidin beads (Pierce Protein Research Products, Thermo Scientific), and samples were separated by SDS/PAGE. Surface and total proteins were visualized by immunoblotting. Actin or tubulin was used as an internal control (Fig. S7). GST pull-downs, immunoprecipitations, and detection of phosphorylated subunits were performed as described earlier and visualized by immunoblotting and chemiluminescence (17, 31). Immunoblots were analyzed by densitometry. Graphical representations correspond to the mean ± SEM of at least three independent experiments. Significance was evaluated using Student's t test (ns, nonsignificant; *P < 0.05; **P < 0.01; ***P < 0.001).
Immunofluorescence, Immunoendocytosis, and Image Processing.
Immunofluorescence, immunoendocytosis, and image processing, including colocalization, were conducted in hippocampal neurons as described elsewhere (16, 22, 47).
Patch-Clamp Recording and Analysis of Ligand-Activated Membrane Currents.
Recordings were conducted on 14-div hippocampal neurons as described previously (31).
Flow Cytometry.
HEK293 cells transfected with GABABR1b-α-Bgt and HA-GABABR2 were incubated with unlabeled α-Bgt for 60 min at 37 °C and subsequently with rhodamine-conjugated α-Bgt for increasing periods of time. Cells were subject to flow cytometry, as described elsewhere (48).
Supplementary Material
Acknowledgments
M.T. is recipient of a National Scientist Development Award from the American Heart Association. K.J.V. was funded by Comisión Nacional de Investigación Científica y Tecnológica (CONICYT). A.C. and S.J.M. were supported by National Institutes of Health-Fogarty International Research Collaboration Award (FIRCA) Grant R03-NS065725. A.C. was supported by Fondo Nacional de Desarrollo Científico y Tecnológico (Fondecyt) Grant 1100137 and Iniciativa Científico Milenio (ICM) Grant P07-048-F. S.J.M. supported by National Institute of Neurological Disorders and Stroke Grants NS047478, NS048045, NS051195, NS056359, and NS054900. T.G.S. was supported by the Medical Research Council, United Kingdom.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000853107/-/DCSupplemental.
References
- 1.Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 2.Mott DD, Lewis DV. The pharmacology and function of central GABAB receptors. Int Rev Neurobiol. 1994;36:97–223. doi: 10.1016/s0074-7742(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 3.Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- 4.Cousins MS, Roberts DC, de Wit H. GABA(B) receptor agonists for the treatment of drug addiction: A review of recent findings. Drug Alcohol Depend. 2002;65:209–220. doi: 10.1016/s0376-8716(01)00163-6. [DOI] [PubMed] [Google Scholar]
- 5.Schuler V, et al. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)) Neuron. 2001;31:47–58. doi: 10.1016/s0896-6273(01)00345-2. [DOI] [PubMed] [Google Scholar]
- 6.Gassmann M, et al. Redistribution of GABAB(1) protein and atypical GABAB responses in GABAB(2)-deficient mice. J Neurosci. 2004;24:6086–6097. doi: 10.1523/JNEUROSCI.5635-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thuault SJ, et al. The GABA(B2) subunit is critical for the trafficking and function of native GABA(B) receptors. Biochem Pharmacol. 2004;68:1655–1666. doi: 10.1016/j.bcp.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 8.Kaupmann K, et al. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 9.Robbins MJ, et al. GABA(B2) is essential for g-protein coupling of the GABA(B) receptor heterodimer. J Neurosci. 2001;21:8043–8052. doi: 10.1523/JNEUROSCI.21-20-08043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duthey B, et al. A single subunit (GB2) is required for G-protein activation by the heterodimeric GABA(B) receptor. J Biol Chem. 2002;277:3236–3241. doi: 10.1074/jbc.M108900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scanziani M. GABA spillover activates postsynaptic GABA(B) receptors to control rhythmic hippocampal activity. Neuron. 2000;25:673–681. doi: 10.1016/s0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 12.von Zastrow M. Mechanisms regulating membrane trafficking of G protein-coupled receptors in the endocytic pathway. Life Sci. 2003;74:217–224. doi: 10.1016/j.lfs.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Tan CM, Brady AE, Nickols HH, Wang Q, Limbird LE. Membrane trafficking of G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2004;44:559–609. doi: 10.1146/annurev.pharmtox.44.101802.121558. [DOI] [PubMed] [Google Scholar]
- 14.Couve A, Calver AR, Fairfax B, Moss SJ, Pangalos MN. Unravelling the unusual signalling properties of the GABA(B) receptor. Biochem Pharmacol. 2004;68:1527–1536. doi: 10.1016/j.bcp.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 15.Perroy J, Adam L, Qanbar R, Chénier S, Bouvier M. Phosphorylation-independent desensitization of GABA(B) receptor by GRK4. EMBO J. 2003;22:3816–3824. doi: 10.1093/emboj/cdg383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fairfax BP, et al. Phosphorylation and chronic agonist treatment atypically modulate GABAB receptor cell surface stability. J Biol Chem. 2004;279:12565–12573. doi: 10.1074/jbc.M311389200. [DOI] [PubMed] [Google Scholar]
- 17.Couve A, et al. Cyclic AMP-dependent protein kinase phosphorylation facilitates GABA(B) receptor-effector coupling. Nat Neurosci. 2002;5:415–424. doi: 10.1038/nn833. [DOI] [PubMed] [Google Scholar]
- 18.Wilkins ME, Li X, Smart TG. Tracking cell surface GABAB receptors using an alpha-bungarotoxin tag. J Biol Chem. 2008;283:34745–34752. doi: 10.1074/jbc.M803197200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramoino P, et al. Endocytosis of GABAB receptors modulates membrane excitability in the single-celled organism Paramecium. J Cell Sci. 2006;119:2056–2064. doi: 10.1242/jcs.02931. [DOI] [PubMed] [Google Scholar]
- 20.Grampp T, Sauter K, Markovic B, Benke D. Gamma-aminobutyric acid type B receptors are constitutively internalized via the clathrin-dependent pathway and targeted to lysosomes for degradation. J Biol Chem. 2007;282:24157–24165. doi: 10.1074/jbc.M702626200. [DOI] [PubMed] [Google Scholar]
- 21.Grampp T, Notz V, Broll I, Fischer N, Benke D. Constitutive, agonist-accelerated, recycling and lysosomal degradation of GABA(B) receptors in cortical neurons. Mol Cell Neurosci. 2008;39:628–637. doi: 10.1016/j.mcn.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Vargas KJ, et al. The availability of surface GABA B receptors is independent of gamma-aminobutyric acid but controlled by glutamate in central neurons. J Biol Chem. 2008;283:24641–24648. doi: 10.1074/jbc.M802419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulik A, et al. Subcellular localization of metabotropic GABA(B) receptor subunits GABA(B1a/b) and GABA(B2) in the rat hippocampus. J Neurosci. 2003;23:11026–11035. doi: 10.1523/JNEUROSCI.23-35-11026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fritschy JM, et al. GABAB-receptor splice variants GB1a and GB1b in rat brain: Developmental regulation, cellular distribution and extrasynaptic localization. Eur J Neurosci. 1999;11:761–768. doi: 10.1046/j.1460-9568.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- 25.Gonchar Y, Pang L, Malitschek B, Bettler B, Burkhalter A. Subcellular localization of GABA(B) receptor subunits in rat visual cortex. J Comp Neurol. 2001;431:182–197. doi: 10.1002/1096-9861(20010305)431:2<182::aid-cne1064>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 26.Kulik A, et al. Distinct localization of GABA(B) receptors relative to synaptic sites in the rat cerebellum and ventrobasal thalamus. Eur J Neurosci. 2002;15:291–307. doi: 10.1046/j.0953-816x.2001.01855.x. [DOI] [PubMed] [Google Scholar]
- 27.Luján R, Shigemoto R. Localization of metabotropic GABA receptor subunits GABAB1 and GABAB2 relative to synaptic sites in the rat developing cerebellum. Eur J Neurosci. 2006;23:1479–1490. doi: 10.1111/j.1460-9568.2006.04669.x. [DOI] [PubMed] [Google Scholar]
- 28.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell. 2004;15:176–188. doi: 10.1091/mbc.E03-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yumoto R, et al. Clathrin-mediated endocytosis of FITC-albumin in alveolar type II epithelial cell line RLE-6TN. Am J Physiol Lung Cell Mol Physiol. 2006;290:L946–L955. doi: 10.1152/ajplung.00173.2005. [DOI] [PubMed] [Google Scholar]
- 30.Jones MC, Caswell PT, Norman JC. Endocytic recycling pathways: Emerging regulators of cell migration. Curr Opin Cell Biol. 2006;18:549–557. doi: 10.1016/j.ceb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Kuramoto N, et al. Phospho-dependent functional modulation of GABA(B) receptors by the metabolic sensor AMP-dependent protein kinase. Neuron. 2007;53:233–247. doi: 10.1016/j.neuron.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carling D. The AMP-activated protein kinase cascade—A unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Chalifoux JR, Carter AG. GABAB receptors modulate NMDA receptor calcium signals in dendritic spines. Neuron. 2010;66:101–113. doi: 10.1016/j.neuron.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- 35.Restituito S, et al. Multiple motifs regulate the trafficking of GABA(B) receptors at distinct checkpoints within the secretory pathway. Mol Cell Neurosci. 2005;28:747–756. doi: 10.1016/j.mcn.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Dong C, Filipeanu CM, Duvernay MT, Wu G. Regulation of G protein-coupled receptor export trafficking. Biochim Biophys Acta. 2007;1768:853–870. doi: 10.1016/j.bbamem.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sattler R, Tymianski M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol Neurobiol. 2001;24:107–129. doi: 10.1385/MN:24:1-3:107. [DOI] [PubMed] [Google Scholar]
- 38.Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moss SJ, Smart TG. Constructing inhibitory synapses. Nat Rev Neurosci. 2001;2:240–250. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- 40.Cimarosti H, Kantamneni S, Henley JM. Ischaemia differentially regulates GABAB receptor subunits in organotypic hippocampal slice cultures. Neuropharmacology. 2009;56:1088–1096. doi: 10.1016/j.neuropharm.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Straessle A, Loup F, Arabadzisz D, Ohning GV, Fritschy JM. Rapid and long-term alterations of hippocampal GABAB receptors in a mouse model of temporal lobe epilepsy. Eur J Neurosci. 2003;18:2213–2226. doi: 10.1046/j.1460-9568.2003.02964.x. [DOI] [PubMed] [Google Scholar]
- 42.Oswald AM, Doiron B, Rinzel J, Reyes AD. Spatial profile and differential recruitment of GABAB modulate oscillatory activity in auditory cortex. J Neurosci. 2009;29:10321–10334. doi: 10.1523/JNEUROSCI.1703-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davies CH, Starkey SJ, Pozza MF, Collingridge GL. GABA autoreceptors regulate the induction of LTP. Nature. 1991;349:609–611. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- 44.Bannai H, et al. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron. 2009;62:670–682. doi: 10.1016/j.neuron.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 45.Goslin K, Banker G. Rat hippocampal neurons in low-density cultures. In: Banker G, Goslin K, editors. Culturing Nerve Cells. Cambridge, MA: MIT; 1991. pp. 251–281. [Google Scholar]
- 46.Jiang M, Chen G. High Ca2+-phosphate transfection efficiency in low-density neuronal cultures. Nat Protoc. 2006;1:695–700. doi: 10.1038/nprot.2006.86. [DOI] [PubMed] [Google Scholar]
- 47.Ramírez OA, et al. Dendritic assembly of heteromeric γ-aminobutyric acid type B receptor subunits in hippocampal neurons. J Biol Chem. 2009;284:13077–13085. doi: 10.1074/jbc.M900575200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riquelme E, Carreño LJ, González PA, Kalergis AM. The duration of TCR/pMHC interactions regulates CTL effector function and tumor-killing capacity. Eur J Immunol. 2009;39:2259–2269. doi: 10.1002/eji.200939341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.