Abstract
Previous reports suggested that culture as 3D aggregates or as spheroids can increase the therapeutic potential of the adult stem/progenitor cells referred to as mesenchymal stem cells or multipotent mesenchymal stromal cells (MSCs). Here we used a hanging drop protocol to prepare human MSCs (hMSCs) as spheroids that maximally expressed TNFα stimulated gene/protein 6 (TSG-6), the antiinflammatory protein that was expressed at high levels by hMSCs trapped in the lung after i.v. infusion and that largely explained the beneficial effects of hMSCs in mice with myocardial infarcts. The properties of spheroid hMSCs were found to depend critically on the culture conditions. Under optimal conditions for expression of TSG-6, the hMSCs also expressed high levels of stanniocalcin-1, a protein with both antiinflammatory and antiapoptotic properties. In addition, they expressed high levels of three anticancer proteins: IL-24, TNFα-related apoptosis inducing ligand, and CD82. The spheroid hMSCs were more effective than hMSCs from adherent monolayer cultures in suppressing inflammatory responses in a coculture system with LPS-activated macrophages and in a mouse model for peritonitis. In addition, the spheroid hMSCs were about one-fourth the volume of hMSCs from adherent cultures. Apparently as a result, larger numbers of the cells trafficked through the lung after i.v. infusion and were recovered in spleen, liver, kidney, and heart. The data suggest that spheroid hMSCs may be more effective than hMSCs from adherent cultures in therapies for diseases characterized by sterile tissue injury and unresolved inflammation and for some cancers that are sensitive to antiinflammatory agents.
Keywords: inflammation, MSC, stanniocalcin-1, spheroid, TNFα stimulated gene/protein 6
There has been considerable interest in the therapeutic potentials of the cells from bone marrow referred to initially as colony forming units-fibroblastic, then as marrow stromal cells, subsequently as mesechymal stem cells, and most recently as multipotent mesenchymal stromal cells (MSCs) (1–6). The cells are relatively easy to isolate from human donors or patients, expand rapidly for 30 or more population doublings in culture, and differentiate into several cellular phenotypes in vitro and in vivo. These and related properties prompted testing the therapeutic potential of the cells in animal models and in clinical trials for a large number of diseases (see www.clinicaltrials.gov). The initial assumption in exploring the therapeutic benefits of MSCs was that they might engraft and differentiate to replace injured cells. Engraftment and differentiation was observed in rapidly grown embryos, with extreme tissue injury, or after local administrations of large concentrations of the cells. Frequently, however, therapeutic benefits were observed without evidence of engraftment. Instead, the cells enhanced tissue repair or limited tissue destruction by paracrine secretions or cell-to-cell contacts that modulated inflammatory or immune reactions (3, 6–8). The potential paracrine effects of the cells was suggested by the observations that the cells in culture secrete a large number of cytokines (9, 10). Recent reports, however, have demonstrated that MSCs are activated by cross-talk with injured cells to express high levels of a large number of additional genes (9, 11–15).
We previously observed (14) that i.v.-infused human MSCs (hMSCs) improved cardiac function and decreased scarring in a mouse model of myocardial infarction in part because the cells that were trapped in the lung as microemboli were activated to secrete the antiinflammatory protein TNFα stimulated gene/protein 6 (TSG-6) (16). The TSG-6 decreased the inflammatory reactions in the heart and thereby limited deterioration of the cardiac tissue. However, the hMSCs did not express TSG-6 until 12–24 h after they created microemboli in lungs and until about half the hMSCs had undergone destruction through apoptosis and necrosis. We also observed that standard cultures of hMSCs did not express TSG-6 but were activated to express the protein if incubated for 24 h or longer with the inflammatory cytokine TNFα (14).
The observations suggested that appropriate manipulation of hMSCs in culture before in vivo administration might enhance their therapeutic benefits by eliminating the lag period for activation on the cells by signals from injured tissues.
Recently there has been a series of publications on aggregation of MSCs either as a procedure for enhancing chrondrogenic differentiation of the cells (17–19) or to increase their therapeutic potential (20–23). Because aggregated hMSCs were detected in the pulmonary microemboli observed after i.v. infusion of the cells (14, 24), we tested the hypothesis that aggregation of hMSCs in culture may provide an effective procedure to preactivate the cells to express TSG-6, and thereby, enhance their antiinflammatory effects through a reduction in the lag period for expression of the gene in vivo.
Results
Aggregation of hMSCs in Hanging Drops into Spheroids.
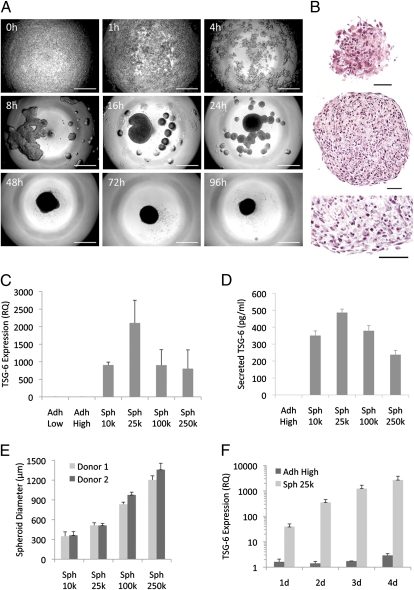
To aggregate hMSCs, we used a hanging drop protocol. Time-lapse microscopy demonstrated that hMSCs cultured in hanging drops first formed a loose network and then numerous small aggregates that gradually coalesced into a single central spheroid along the lower surface of the drop (Fig. 1A). Once assembled, the spheroid did not increase in size but progressively compacted between 48 and 96 h. H&E staining of sections revealed the spheroids were solid throughout with small round cells evenly distributed and embedded in matrix (Fig. 1B). The surface of the spheroid had a layer of epithelium-like cells that were more elongated and flatter. As expected, the sizes of the spheroids were dependent on the number of hMSCs suspended in the hanging drops (Fig. 1E). hMSC spheroids of all sizes expressed and secreted very high levels of the antiinflammatory molecule TSG-6 compared with either low or high density monolayer cultures, but spheroids of 25,000 cells (Sph 25k) showed the highest expression and secretion of TSG-6 (Fig. 1 C and D). Moreover, TSG-6 expression increased in a time dependent manner with spheroids of 25,000 hMSCs and was consistently much higher than in standard cultures of adherent hMSCs (Fig. 1F).
Fig. 1.
The expression of TSG-6 was increased as hMSCs aggregated into spheroids in hanging drops. (A) Phase contrast microscopy showing the time course of the aggregation of 25,000 hMSCs into a spheroid in a hanging drop. (Scale bar, 500 μm.) (B) H&E staining of hMSC spheroid sections from 3-d hanging drop cultures. Surface (Top), and center (Middle and Bottom) of a spheroid. (Scale bar, 50 μm.) (C) Real-time RT PCR measurements of TSG-6 expression in hMSCs shown as relative to Adh Low sample (n = 3). (D) ELISA measurements of TSG-6 secretion over 24 h from hMSCs grown for 3 d at high density or as hanging drops at different cell densities (n = 4). (E) Sizes of spheroids generated by hMSCs from two donors grown in hanging drops for 3 d. Sizes were measured from captured images of transferred spheroids (n = 7–13). (F) Real-time RT PCR measurements of TSG-6 expression in hMSCs grown at high density or in hanging drops at 25,000 cells/drop for 1–4 d shown as relative to hMSCs grown at low density (n = 3). Values are mean ± SD. Abbreviations: RQ, relative quantity; Adh Low, hMSCs plated at 100 cells/cm2 for 7–8 d until about 70% confluent; Adh High, hMSCs harvested from same Adh Low cultures, plated at 5,000 cells/cm2 and incubated for 3 d; Sph 10k-250k, hMSCs harvested from same Adh Low cultures and incubated for 3 d in hanging drops at 10,000-250,000 cells/drop.
Viability of hMSCs in Spheroids.
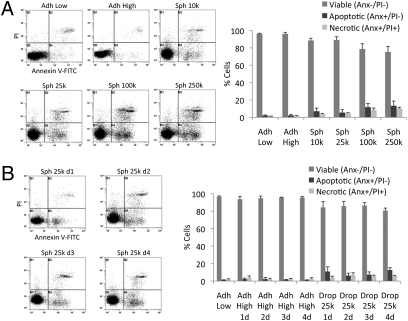
Because hMSCs in spheroids may have less access to nutrients, it was of interest to establish whether the cells remained viable. In 3-d cultures of spheroids of 10,000 or 25,000 hMSCs, almost 90% of the harvested cells were viable as assayed by propidium iodide (PI) uptake and labeling with annexin V-FITC (Fig. 2A). The number of apoptotic or necrotic cells was greater in spheroids prepared with 100,000 or 250,000 hMSCs (Fig. 2A). Also, the number of apoptotic or necrotic cells increased slightly when the incubation period was extended from 3 d to 4 d (Fig. 2B).
Fig. 2.
Viability of hMSCs in spheroids. (A and B) Viability of hMSCs as determined by flow cytometry measuring PI uptake and annexin V-FITC labeling. Spheroids were dissociated with trypsin/EDTA. Representative log fluorescent dot plots and summary of the data are shown. Values are mean ± SD (n = 3). Abbreviations: As in Fig. 1 with 1d to 4d indicating days of incubation.
Analysis of Spheroid hMSC Size in Vitro and Relative Tissue Distribution After i.v. Infusion.
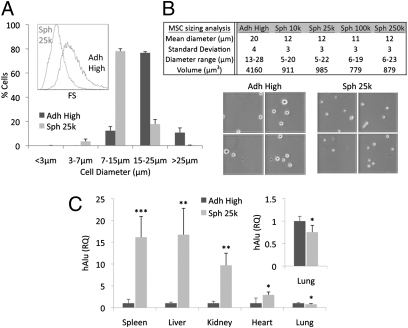
As suggested by histological sections (Fig. 1B), hMSCs in spheroids appeared smaller than hMSCs from standard monolayer cultures. The cells released from spheroids by trypsinization were nearly half the diameter and approximately one-fourth the volume of hMSCs derived from adherent monolayers as shown by flow cytometry (Fig. 3A and Fig. S1) and microscopy (Fig. 3B).
Fig. 3.
Size analysis and i.v. infusion of spheroid hMSCs. (A) Assays of cell size by flow cytometry (n = 3). hMSC sizes were estimated from forward scatter (FS) (Inset) properties of the viable population (calcein AM+/7AAD−) relative to beads with known diameters (3, 7, 15, and 25 μm). (B) Cell size assayed by microscopy. (C) Relative tissue distribution of i.v. infused hMSCs. NOD/scid mice were infused i.v. with 106 monolayer or spheroid derived hMSCs. After 15 min, tissues were harvested for genomic DNA and tissue distribution of hMSCs was determined with real-time PCR for human Alu and GAPDH (n = 4–5) and shown as relative to Adh High sample. *P < 0.05, **P < 0.01, and ***P < 0.001. Values are mean ± SD. Abbreviations: as in Fig. 1.
To test if the smaller size of the hMSCs dissociated from spheroids would allow the cells to traffic through the lung microvasculature and therefore distribute more efficiently into other tissues, both monolayer and spheroid hMSCs were injected i.v. into the tail vein of NOD/scid mice. Real-time PCR for human Alu sequences in the lungs collected 15 min after hMSC infusion suggested that the number of trapped cells decreased by about 25% with spheroid-derived hMSCs compared with monolayer hMSCs, At the same time, a larger fraction of infused spheroid hMSCs were recovered in the liver, spleen, kidney, and heart (Fig. 3C).
hMSCs Dissociated from Spheroids Retain the Properties of Adherent hMSCs.
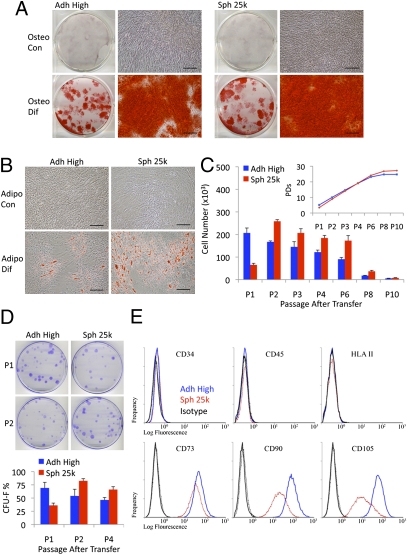
hMSCs dissociated from spheroids retained the ability to differentiate into mineralizing cells and adipocytes (Fig. 4 A and B). The dissociated cells expanded more slowly during an initial passage and then more rapidly than adherent hMSCs through four passages before reaching senescence at about the same number of population doublings (Fig. 4C and Fig. S2A). In addition, the dissociated cells readily generated colonies (CFUs) when plated at clonal densities (Fig. 4D and Fig. S2B). Consistent with the data on rates of propagation (Fig. 4C), the number of CFUs from spheroid cells was initially less than the number of CFUs from adherent cultures but was greater in later passages (Fig. 4D and Fig. S2B). The surface epitopes of the hMSCS dissociated from spheroids were similar to the surface epitopes of hMSCs from adherent monolayers when dissociated under the same conditions with trypsin (10 min at 37 °C): the dissociated cells were negative for hematopoietic markers, and they were slightly less positive for CD73, CD90, and CD105, apparently because of the smaller size of the cells (Fig. 4E, Figs. S3–S6, and Table S1).
Fig. 4.
Spheroid hMSCs retain the properties of hMSCs from adherent cultures. (A) Differentiation of hMSCs in osteogenic medium (Osteo Dif) and control medium (Osteo Con). Cultures were stained with Alizarin Red after 14 d. (Scale bar, 200 μm.) (B) Differentiation of hMSCs in adipogenic medium (Adipo Dif) and control medium (Adipo Con). Cultures were stained with Oil Red O after 14 d. (Scale bar, 200 μm.) (C) Growth of hMSCs (donor 2) as monolayers from high density and hanging drop cultures plated at low density (5,500 cells/plate) and passaged every 7 d (n = 4). Cumulative population doublings (PDs) after each passage are shown (Inset). (D) CFU-F assays of hMSCs (donor 2) plated at 83 cells/plate and incubated for 14 d (n = 4). Representative plates at passage 1 and passage 2 after transfer. Values are mean ± SD. (E) Flow cytometry of surface protein expression on hMSCs. Abbreviations: as in Fig. 1 with P1 to P10 indicating passage number.
Transcriptome Changes in the Spheroid hMSCs.
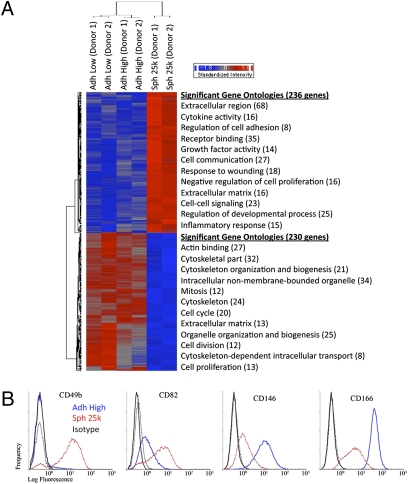
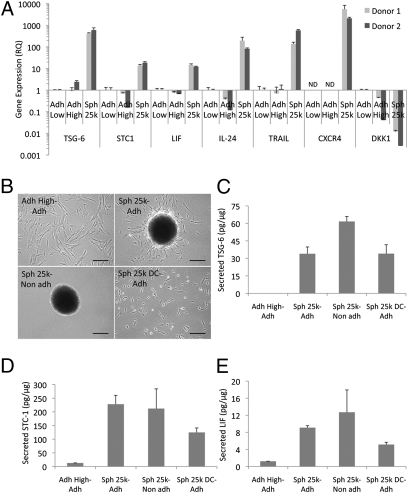
Surveys with microarray assays demonstrated that 236 genes were up-regulated and 230 genes were down-regulated in a comparison of spheroid cells with hMSCs from adherent monolayers (Fig. 5A and Table S2). There were increases in genes with ontologies for extracellular region, regulation of cell adhesion, receptor binding, cell communication, extracellular matrix, and negative regulation of cell proliferation (Fig. 5A). Also, there were parallel decreases in genes with ontologies for cytoskeleton organization and biogenesis, mitosis, cell cycle, and extracellular matrix (Fig. 5A). Of special interest was the increase in genes with ontologies for response to wounding and inflammatory response (Fig. 5A). Real time RT PCR assays (Fig. 6A) demonstrated marked increases in the expression of TSG-6; stanniocalcin-1 (STC-1), an antiinflammatory/antiapoptotic protein; leukemia inhibitory factor (LIF), a cytokine for growth and development; IL-24, a tumor suppressor protein; TNFα-related apoptosis inducing ligand (TRAIL), a protein with selectivity for killing certain cancer cells; and CXC chemokine receptor 4 (CXCR4), a receptor involved in MSC homing. As expected from its stimulatory effect of MSC proliferation (25), there was decreased expression of dickkopf 1 (DKK1), an inhibitor of Wnt signaling (Fig. 6A).
Fig. 5.
Microarray assays of hMSCs from two donors. (A) Hierarchical clustering of differentially expressed genes. Genes that were either up- (236 genes) or down-regulated (230 genes) in spheroids (Sph 25k) at least twofold compared with their adherent culture counterparts (Adh Low and Adh High), were used in hierarchical clustering. The most significant Gene Ontology terms for up-regulated genes (red) and down-regulated genes (blue) are shown next to the heat map. (B) Flow cytometry of differentially expressed surface epitopes on hMSCs. Abbreviations: as in Fig. 1.
Fig. 6.
Spheroid hMSCs express high levels of antiinflammatory and antitumorigenic molecules. (A) Real-time RT PCR measurements for antiinflammatory genes (TSG-6, STC-1, and LIF), antitumorigenic genes (IL-24 and TRAIL), gene for an MSC homing receptor (CXCR4), and gene for the Wnt signaling inhibitor (DKK1) for two donors. Values are mean RQ ± 95% confidence interval from triplicate assays compared with Adh Low sample. (B) Images of high density monolayer (Adh High), spheroids (Sph 25k), and spheroid derived hMSCs (Sph 25k DC) 24 h after transfer onto adherent (Adh) or nonadherent (Non adh) surfaces. Cultures were in six-well plates containing 1.5 mL CCM and either 200,000 hMSCs from high density cultures, eight spheroids, or 200,000 hMSCs dissociated from spheroids. After 24 h, medium was recovered for ELISAs and cells lyzed for protein assays. (Scale bar, 200 μm.) TSG-6 (C), STC-1 (D), and LIF (E) ELISAs on medium, normalized to total cellular protein. Values are mean ± SD (n = 3). Abbreviations: as in Fig. 1 with ND indicating not detectable and Sph 25k DC-Adh indicating hMSCs dissociated from Sph 25k and plated on cell adherent surfaces.
Changes in Cell Surface Protein Expression and Cell Cycle Distribution in hMSC Spheroids.
Assays by flow cytometry demonstrated decreased expression of podocalyxin-like protein (PODXL), an anticell-adhesion protein; and α4-integrin (CD49d), an integrin subunit associated with lymphocyte homing (Figs. S3–S6). There was partial down-regulation of the melanoma cell adhesion molecule (MCAM or CD146) that is used as a marker for endothelial cells and pericytes, and of ALCAM (CD166), a cell adhesion molecule (Fig. 5B). At the same time, there was increased expression of an integrin subunit for cell adhesion (α2-integrin of CD49b), and a protein associated with suppression of metastases (CD82) (Fig. 5B). As expected from microarray results, assays by flow cytometry also demonstrated a decrease of spheroid hMSCs in S-phase compared with monolayer hMSCs (Fig. S7 A–D).
Spheroid hMSCs Secrete Antiinflammatory Proteins.
Spheroids of hMSCs plated on adherent culture surfaces gradually generated spindle-shaped cells that migrated away from the spheroids (Fig. 6B). No migration was seen with spheroids plated on nonadherent surfaces (Fig. 6B). ELISAs demonstrated that hMSCs either in spheroids or dissociated from spheroids continued to secrete TSG-6, STC-1, and LIF when plated on culture dishes for 24 h (Fig. 6 C–E). The levels of all three factors were much higher than with adherent monolayer hMSCs. About the same levels of STC-1 and LIF were observed in spheroids cultured directly either on adherent or nonadherent plates, but spheroids cultured on nonadherent dishes secreted more TSG-6 (Fig. 6 C–E). The levels of TSG-6, STC-1, and LIF decreased when the hMSCs were dissociated from spheroids and cultured on adherent plates but the levels remained much higher than with adherent monolayers (Fig. 6 C–E).
Spheroid hMSCs Decrease Activation of Macrophages in Vitro and Inflammation in Vivo.
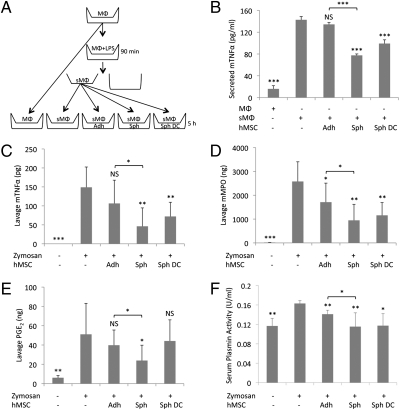
The increased secretion of antiinflammatory molecules TSG-6 and STC-1 by the spheroid hMSCs suggested that the cells would be more effective than adherent monolayer cultures of hMSCs in reducing inflammatory responses. To test this prediction, mouse macrophages were preactivated with LPS in the upper chamber of a transwell, followed by a transfer of the chamber to a test well (Fig. 7A). Under the conditions of the experiment, the presence in the test well of hMSCs from adherent monolayers had no significant effect on the expression or secretion of TNFα by the stimulated macrophages (Fig. 7B and Fig. S8A). In contrast, TNFα expression and secretion was decreased significantly by the presence in the test well of intact spheroids or hMSCs dissociated from spheroids (Fig. 7B and Fig. S8A). The results indicated therefore that the spheroid derived hMSCs secreted more effective antiinflammatory factors.
Fig. 7.
hMSC spheroids exhibit enhanced antiinflammatory effects in vitro and in vivo. (A) Schematic of the mouse macrophage (mMΦ) assay. mMΦs were seeded in the upper chamber of a transwell, stimulated with LPS for 90 min, the LPS was removed, and the chamber transferred to a six-well dish plated with monolayer (Adh), spheroid (Sph), or spheroid-derived hMSCs (Sph DC) at the same cell density. MΦ:hMSC (2:1). After 5 h, medium was collected for ELISAs. (B) ELISA for mTNFα in medium from cocultures (n = 3). (C–F) Antiinflammatory activity of hMSCs in a mouse model of peritonitis. C57BL/6 mice were injected i.p. with zymosan to induce inflammation. After 15 min, the mice were injected i.p. with 1.5 × 106 monolayer hMSCs, 60 spheroids, or 1.5 × 106 spheroid derived cells. After 6 h, peritoneal lavage was collected and mTNFα (C), mMPO (D), and PGE2 (E) levels were determined with ELISAs. Total amounts of the specific molecules in the lavage are shown (n = 4–8). After 24 h, blood was collected and plasmin activity was measured from serum (n = 3–6). Values are mean ± SD. Not significant (NS) P ≥ 0.05, *P < 0.05, **P < 0.01, and ***P < 0.001. Abbreviations: as in Figs. 1 and 6.
To test the effects of spheroid hMSCs on inflammation in vivo, a mouse model of zymosan-induced peritonitis was used (26). Six hours after i.p. administration of monolayer, spheroid, or spheroid derived hMSCs, inflammatory exudates were collected and used in estimating the level of inflammation. hMSC spheroids significantly decreased the protein content of the lavage fluid (Fig. S8B) and the volume (Fig. S8C), neutrophil activity, as assayed by secreted myeloperoxidase (MPO) (Fig. 7D), and levels of the proinflammatory molecules TNFα (Fig. 7C), IL-1β (Fig. S8D), CXCL2/MIP-2 (Fig. S8E), and PGE2 (Fig. 7E). In addition, serum levels of plasmin activity, an inflammation associated protease that is inhibited by TSG-6 (16), were decreased significantly by hMSC spheroids (Fig. 7F). Serum plasmin activity was reduced approximately to the levels of noninflammatory control animals 24 h after spheroid injection (Fig. 7F). Spheroid-derived hMSCs also substantially decreased levels of the inflammatory markers assayed, although to a lesser extent than intact spheroids (Fig. 7 C–F and Fig. S8 B–E). Moreover, hMSC spheroids were significantly more effective than adherent monolayer hMSC in suppressing inflammation (Fig. 7 C–F and Fig. S8B).
Discussion
Classically hMSCs were isolated and expanded as adherent monolayer cultures, but it was soon recognized that centrifugation of the cells to form micropellets or large aggregates greatly enhanced their chondrogenic differentiation that slowly occurred over several weeks (18, 27). However, several recent publications demonstrated that culture of MSCs in 3D or as spheroids for shorter periods of time improved their therapeutic potential by increased expression of genes such as CXCR4 to promote adhesion to endothelial cells or of IL-24 that has tumor suppressing properties (20, 22, 23). The experiments presented here were designed to prepare hMSCs as spheroids that maximally expressed TSG-6, the antiinflammatory protein that produced beneficial effects in mice with myocardial infarcts because it was expressed at high levels after i.v.-infused hMSCs were trapped in the lung (14).
The results demonstrated that the properties of hMSCs cultured as spheroids depend critically on the experimental conditions. In hanging drops, the cells first formed a network and then most of the cells coalesced into a single spheroid. Optimal levels of TSG-6 expression were observed with spheroids approximately 500 μm in diameter and incubated for 3 d. Expression levels remained high but were lower in larger spheroids, and more of the cells became apoptotic or necrotic in the larger spheroids. Also, more of the cells became apoptotic or necrotic with longer times of incubation. The cells in spheroids retained most of the surface epitopes of hMSCs from adherent cultures. Also, hMSCs dissociated from spheroids retained the potential to differentiate into mineralizing cells and adipocytes. They also expanded at a similar rate as hMSCs from adherent monolayer cultures after a delay through one passage. In addition, spheroid-dissociated hMSCs remained highly clonogenic.
As was observed previously with large hMSC spheroids (28) and hMSCs in 3D culture (22), surveys with mRNA/cDNA microarrays demonstrated marked differences in the transcriptomes compared with hMSCs from adherent cultures. Quantitative assays confirmed some of the important differences. As expected, there was a marked decrease in the anticell-adhesion protein PODXL (24) and a decrease in cell cycling. Of special note was that several of the differences had important implications for the potential therapeutic uses of hMSCs. There were higher levels of expression of the antiinflammatory protein TSG-6 than previously observed by preincubation of hMSCs with TNFα (14). Also, there was a high level of expression of STC-1, a protein with both antiinflammatory and antiapoptotic effects (13, 29). The high levels of expression of both TSG-6 and STC-1 were maintained for at least 1 d after the cells were dissociated from the spheroids. Therefore the results suggested that both spheroids and spheroid derived hMSCs may be more effective than hMSCs from adherent cultures in modulating inflammatory reactions. The suggestion was confirmed by the demonstration that the spheroids and spheroid derived hMSCs were more effective in suppressing TNFα production by LPS stimulated macrophages in culture. In addition, they were more effective in suppressing inflammation in an in vivo model for zymosan induced peritonitis. Also of special interest was that the spheroid hMSCs expressed high levels of transcripts for the tumor suppressor protein IL-24, an observation made previously with 3D cultures of hMSCs prepared using spinner flasks and a rotating wall vessel bioreactor (22). In addition, the spheroid hMSCs prepared under the conditions optimized to express TSG-6 also expressed high levels of transcripts for TRAIL that is selective for killing certain cancer cells (30, 31) and for CD82 that suppresses some metastases (32). Therefore, spheroids and spheroid derived hMSCs may be particularly effective as an adjunct therapy for some types of cancers, particularly for therapy of cancers sensitive to antiinflammatory agents such as aspirin or steroids (33). A further advantage of the spheroid hMSCs was that they were less than one-fourth the volume of hMSCs from adherent cultures. Therefore a significantly smaller number was trapped in the lung after i.v. infusion and thus larger numbers were found in many tissues (14, 24).
The molecular forces that increase expression of antiinflammatory and antitumorigenic genes in hMSCs assembled into spheroids are intriguing but unclear. Cells in spheroids are in close association with each other and probably signal cues to each other much easier than in monolayer cultures, where only a very small part of the cell can touch another cell and secreted molecules must be present in high amounts to ensure communication. The changes in the hMSCs as they form spheroids are probably the result of the nonadherent culture conditions, high degree of confluency, nutrient deprivation, air-liquid interface, and “microgravity” of hanging drops. More detailed studies of each of these and other possible factors must be conducted to have a better understanding of the changes hMSCs accrue when they aggregate into spheroids.
The results presented here indicated that hMSCs can be activated nonchemically in hanging drops to secrete substantial quantities of potent antiinflammatory proteins and express antitumorigenic molecules. Therefore spheroid hMSCs may have advantages for many therapeutic applications. In addition, hMSCs dissociated from spheroids provide extremely small activated cells that could have major advantages for i.v. administration.
Materials and Methods
More detailed methods are presented in the SI Materials and Methods.
hMSC Cell Culture.
Frozen vials of passage 1 hMSCs from bone marrow were obtained from the Center for the Preparation and Distribution of Adult Stem Cells (http://medicine.tamhsc.edu/irm/msc-distribution.html). After 24-h recovery, hMSCs were seeded at low density (100 cells/cm2), and incubated in complete culture medium (CCM) containing 17% FBS for 7–8 d until approximately 70% confluent. hMSCs were passed under the same conditions through no more than three passages before being used for assays.
Spheroid Generation and Dissociation.
hMSCs were plated in hanging drops in 35 μL of CCM containing 10,000–250,000 cells/drop for up to 4 d. To obtain spheroid derived cells, spheroids were incubated with trypsin/EDTA for 5–30 min (depending on the size of the spheroid) while pipetting every 2–3 min.
Intravenous Infusion of hMSCs and Alu PCR.
Male NOD/scid mice were infused with 106 monolayer or spheroid derived hMSCs i.v. followed by collection of tissues 15 min later. Genomic DNA was isolated and used to determine the relative quantity of human DNA in each tissue with real-time PCR for human Alu and GAPDH and mouse GAPDH (14, 24, 34).
Mouse Model of Peritonitis and Measurements of Inflammation.
To induce inflammation in male C57BL/6J mice, zymosan solution was administered i.p., followed by i.p. injection of either 1.5 × 106 monolayer hMSCs, 1.5 × 106 spheroid derived cells, or 60 spheroids 15 min later. After 6 h, inflammatory exudates were collected by peritoneal lavage and the cell-free supernatant was used to measure total protein, neutrophil activity (secreted mMPO), and levels of the proinflammatory molecules mTNFα, mIL-1β, mCXCL2/MIP-2, and PGE2. Twenty-four hours after cell injection, blood was collected from the right ventricle and the mouse plasmin activity was measured from the serum.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health Grants P01HL075161, R01HL073755, and P40RR17447.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008117107/-/DCSupplemental.
References
- 1.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–274. [PubMed] [Google Scholar]
- 2.Owen M, Friedenstein AJ. Stromal stem cells: Marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 3.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 4.Eaves CJ, et al. Molecular analysis of primitive hematopoietic cell proliferation control mechanisms. Ann N Y Acad Sci. 1991;628:298–306. doi: 10.1111/j.1749-6632.1991.tb17260.x. [DOI] [PubMed] [Google Scholar]
- 5.Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 6.Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): Controversies, myths, and changing paradigms. Mol Ther. 2009;17:939–946. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 8.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 9.Ren G, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184:2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 11.Gunn WG, et al. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: A potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24:986–991. doi: 10.1634/stemcells.2005-0220. [DOI] [PubMed] [Google Scholar]
- 12.Ohtaki H, et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci USA. 2008;105:14638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Block GJ, et al. Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1. Stem Cells. 2009;27:670–681. doi: 10.1002/stem.20080742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee RH, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romieu-Mourez R, et al. Cytokine modulation of TLR expression and activation in mesenchymal stromal cells leads to a proinflammatory phenotype. J Immunol. 2009;182:7963–7973. doi: 10.4049/jimmunol.0803864. [DOI] [PubMed] [Google Scholar]
- 16.Wisniewski HG, Vilcek J. Cytokine-induced gene expression at the crossroads of innate immunity, inflammation and fertility: TSG-6 and PTX3/TSG-14. Cytokine Growth Factor Rev. 2004;15:129–146. doi: 10.1016/j.cytogfr.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Steck E, et al. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23:403–411. doi: 10.1634/stemcells.2004-0107. [DOI] [PubMed] [Google Scholar]
- 18.Arufe MC, De la Fuente A, Fuentes-Boquete I, De Toro FJ, Blanco FJ. Differentiation of synovial CD-105(+) human mesenchymal stem cells into chondrocyte-like cells through spheroid formation. J Cell Biochem. 2009;108:145–155. doi: 10.1002/jcb.22238. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, et al. 3D spheroid culture system on micropatterned substrates for improved differentiation efficiency of multipotent mesenchymal stem cells. Biomaterials. 2009;30:2705–2715. doi: 10.1016/j.biomaterials.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 20.Potapova IA, Brink PR, Cohen IS, Doronin SV. Culturing of human mesenchymal stem cells as three-dimensional aggregates induces functional expression of CXCR4 that regulates adhesion to endothelial cells. J Biol Chem. 2008;283:13100–13107. doi: 10.1074/jbc.M800184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qihao Z, Xigu C, Guanghui C, Weiwei Z. Spheroid formation and differentiation into hepatocyte-like cells of rat mesenchymal stem cell induced by co-culture with liver cells. DNA Cell Biol. 2007;26:497–503. doi: 10.1089/dna.2006.0562. [DOI] [PubMed] [Google Scholar]
- 22.Frith JE, Thomson B, Genever P. Tissue Eng Part C Methods. 2009. Dynamic Three-Dimensional Culture Methods Enhance Mesenchymal Stem Cell Properties and Increase Therapeutic Potential. -Not available-, ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Wang CC, et al. Spherically symmetric mesenchymal stromal cell bodies inherent with endogenous extracellular matrices for cellular cardiomyoplasty. Stem Cells. 2009;27:724–732. doi: 10.1634/stemcells.2008-0944. [DOI] [PubMed] [Google Scholar]
- 24.Lee RH, et al. The CD34-like protein PODXL and alpha6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood. 2009;113:816–826. doi: 10.1182/blood-2007-12-128702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gregory CA, Singh H, Perry AS, Prockop DJ. The Wnt signaling inhibitor dickkopf-1 is required for reentry into the cell cycle of human adult stem cells from bone marrow. J Biol Chem. 2003;278:28067–28078. doi: 10.1074/jbc.M300373200. [DOI] [PubMed] [Google Scholar]
- 26.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 28.Potapova IA, et al. Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells. 2007;25:1761–1768. doi: 10.1634/stemcells.2007-0022. [DOI] [PubMed] [Google Scholar]
- 29.Huang L, et al. Anti-inflammatory and renal protective actions of stanniocalcin-1 in a model of anti-glomerular basement membrane glomerulonephritis. Am J Pathol. 2009;174:1368–1378. doi: 10.2353/ajpath.2009.080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahmood Z, Shukla Y. Death receptors: Targets for cancer therapy. Exp Cell Res. 2010;316:887–899. doi: 10.1016/j.yexcr.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Mellier G, Huang S, Shenoy K, Pervaiz S. TRAILing death in cancer. Mol Aspects Med. 2010;31:93–112. doi: 10.1016/j.mam.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Smith SC, Theodorescu D. Learning therapeutic lessons from metastasis suppressor proteins. Nat Rev Cancer. 2009;9:253–264. doi: 10.1038/nrc2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McBride C, Gaupp D, Phinney DG. Quantifying levels of transplanted murine and human mesenchymal stem cells in vivo by real-time PCR. Cytotherapy. 2003;5:7–18. doi: 10.1080/14653240310000038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.