Abstract
The Myc protein and proteins that participate in mitosis represent attractive targets for cancer therapy. However, their potential is presently compromised by the threat of side effects and by a lack of pharmacological inhibitors of Myc. Here we report that a circumscribed exposure to the aurora kinase inhibitor, VX-680, selectively kills cells that overexpress Myc. This synthetic lethal interaction is attributable to inhibition of aurora-B kinase, with consequent disabling of the chromosomal passenger protein complex (CPPC) and ensuing DNA replication in the absence of cell division; executed by sequential apoptosis and autophagy; not reliant on the tumor suppressor protein p53; and effective against mouse models for B-cell and T-cell lymphomas initiated by transgenes of MYC. Our findings cast light on how inhibitors of aurora-B kinase may kill tumor cells, implicate Myc in the induction of a lethal form of autophagy, indicate that expression of Myc be a useful biomarker for sensitivity of tumor cells to inhibition of the CPPC, dramatize the virtue of bimodal killing by a single therapeutic agent, and suggest a therapeutic strategy for killing tumor cells that overexpress Myc while sparing normal cells.
Keywords: apoptosis, aurora kinase, autophagy synthetic lethality, targeted therapy, chromosomal passenger protein complex
The MYC proto-oncogene encodes a bHLHzip-type transcription factor (Myc) that is a key regulator of cell growth, proliferation, and survival (1). Overexpression of Myc transforms various cells in vitro, causes tumors in mice, and ranks among the more common tumorigenic anomalies found in a wide variety of human malignancies (1, 2). Although these features make Myc a potential target for cancer therapy, practicable inhibition of the protein with pharmaceuticals has yet to be achieved. An alternative approach is to exploit synthetic lethal interactions between overexpression of Myc and the therapeutic induction of other cellular aberrations that do not kill normal cells (3–5).
VX-680 is a potent and reversible small-molecule inhibitor of the aurora family of mitotic kinases (6). Among the targets for VX-680 is aurora-B kinase/AURK B, the catalytic subunit of the chromosomal passenger protein complex (CPPC), which also contains the proteins survivin/BIRC5, INCENP, and Borealin (7). The complex is involved in multiple facets of cell division, including the spindle checkpoint, chromosome segregation, and cytokinesis (7). Here we report a synthetic lethal interaction between VX-680 and Myc overexpression. This effect can be achieved by any means that inhibits or depletes the aurora-B kinase or otherwise disables the CPPC. The synthetic lethality is mediated mainly by a combination of apoptosis and lethal autophagy. It kills cells that overexpress Myc in vitro while sparing normal cells, and it has therapeutic efficacy when tested in mice bearing tumors elicited by a transgene of MYC. We suggest that overexpression of Myc might represent a biomarker for identifying tumors that are likely to respond to treatment with inhibitors of aurora-B kinase at a favorable therapeutic index. Moreover, the bimodal nature of cell killing may provide a deterrent to the emergence of drug resistance.
Results
Synthetic Lethal Interaction Between MYC and VX-680.
To elucidate the cellular response to VX-680, we examined the effect of the drug on human retinal pigment epithelial cells that expressed either the neomycin resistance gene (RPE-NEO) or NEO plus an ectopic allele of human MYC (RPE-MYC) (Fig. 1A and Fig. S1). Over the course of 7–9 d, continuous application of VX-680 (300 nM) killed the entire population of RPE-MYC cells. Moreover, the killing proceeded to completion even if VX-680 was withdrawn after 3 d (Fig. 1A and Fig. S1B).
Fig. 1.
VX-680 elicits apoptosis and autophagy in cells that overexpress Myc. (A) Cell killing by VX-680. RPE-NEO cells (□ and ■) and RPE-MYC cells (○ and ●) were treated with 300 nM VX-680 for 3 d and then either transferred into drug-free medium (○ and □) or maintained in fresh medium containing VX-680 (● and ■). Cells were harvested at daily intervals and then assayed for viability by the trypan blue exclusion assay. The data are expressed as mean ± SD for three independent experiments. The arrow indicates the point at which VX-680 treatment was terminated. (B) Reversible inhibition of aurora-B kinase by VX-680. RPE-MYC cells were treated for 3 d with 300 nM VX-680 and then transferred into drug-free medium. Cells were fixed either before (i and iii) or 3 h after (ii and iv) the transfer and stained for DNA in blue (iii and iv) and phosphorylation of histone H3 at Ser-10 in green (i and ii), a surrogate assay for the activity of aurora-B kinase. (C) Activation of caspase-9. RPE-MYC cells were treated for 3 d with either 0.03% DMSO or 300 nM VX-680, fixed, and stained for DNA (blue) and active caspase-9 (red). (D) Ultrastructural analysis of cell morphology. RPE-MYC cells were treated with 300 nM VX-680 for 3 d and then transferred into drug-free medium. Representative electron micrographs of RPE-MYC cells either before (a) or 7 d after (b–d) administration of VX-680 are shown. M, mitochondria; N, nucleus. White arrows denote vacuoles containing electron-dense material in b, multimembrane vesicles in c, and localized cytoplasmic degeneration in d. Black arrowheads in c and d point to double-membrane vesicles sequestering a portion of cytoplasm.
Withdrawal of the drug was accompanied by prompt reactivation of aurora-B in the cells (Fig. 1B). In contrast, RPE-NEO cells were not killed by VX-680. Instead, the cells failed to proliferate in the presence of VX-680, but doubled in number within 6 d after withdrawal of the drug (Fig. 1A and Fig. S1B). A similar arrest of proliferation without death in response to VX-680 was observed with other rodent and human cells that do not overexpress Myc (Table S1).
Initial Lethal Response to VX-680 Is Apoptotic Death.
Exposure to VX-680 for 3 d killed ∼30% of RPE-MYC cells (Fig. 1A and Fig. S1A). By the end of the 3-d treatment, virtually all of the surviving cells had become multinucleated and polyploid, and had entered a new phase of killing (see below). Thus, we divided the killing by VX-680 into two phases, defined as “early” (death within 3 d) and “delayed” (all subsequent deaths). VX-680 caused both early and late death of RPE-MYC cells and other cells that overexpressed ectopic Myc, including mouse embryo fibroblasts (MEF-MYC), rat embryo fibroblasts (Rat1A-MYC), and human kidney epithelial cells (HA1E-MYC) (Table S1).
Early cell death was associated with activation of the canonical form of apoptosis in RPE-MYC, Rat1A-MYC, and HA1E-MYC cells (Fig. 1C, Fig. S2A, and Table S1). The fraction of cells affected by apoptosis could account for at least two-thirds of the early cell deaths (Fig. S2A). Apoptosis first became apparent by day 2 of exposure to VX-680, reached a peak at day 3, and faded with the inception of the delayed phase of death (Fig. S2B). We do not know why apoptosis was limited to a minority of the cell population, but this limit was seen in mouse, rat, and human cells (Table S1).
We demonstrated that apoptosis was responsible for the concurrent cell death by two means. First, we examined the proapoptotic protein Bim, which is known to mediate apoptosis in the presence of excess Myc (8). Bim was vigorously expressed in RPE-MYC cells but was undetectable in RPE-NEO cells (Fig. S2C). Partial depletion of Bim with RNAi (Fig. S2C) provided substantial protection against the early death elicited by VX-680 (Fig. S2D). Second, we found that the pancaspase inhibitor z-VAD-fmk effected a two-thirds reduction in early cell death (Fig. S2E).
To further characterize the selectivity of early cell killing by VX-680, we surveyed a variety of genes that have been implicated in tumorigenesis. To date, we have observed synthetic lethality with MYCN, the E6 oncogene of human papillomavirus 16, the E1A gene of adenovirus, and NOTCH1-IC, which encodes a constitutively active version of the Notch protein (Fig. S3A). MYCN is closely related to MYC and has been implicated in a synthetic lethal interaction with inhibition of the CDK2 kinase (9). In accordance with previous reports (10–12), E6, E1A, and NOTCH1-IC all induced vigorous expression of Myc in the RPE-NEO test cells (Fig. S3B), which provides a credible explanation for their synthetic lethal interaction with VX-680. Deficiencies in the tumor suppressor proteins p53 and Rb were not synthetic lethal with VX-680 (Fig. S3C).
Myc Overexpression Permits Continuation of DNA Replication During Arrested Cytokinesis.
VX-680 arrests cells late in cytokinesis (6), which normally would lead to arrest of DNA replication (13–15). However, during a 3-d treatment with VX-680, RPE-MYC cells continued DNA replication (Fig. 2A) and mitosis (Fig. 2B), steadily increased their numbers of centrosomes and chromosomes (Fig. 2 C and D), and became enlarged, multinucleated, and polyploid (Fig. 2 E and F). By day 10, no cells remained, however (Fig. 1A and Fig. S1B). In contrast, the same treatment caused RPE-NEO cells to arrest DNA synthesis and mitosis (Fig. 2 A and B), but not before ∼70% of the cells had become tetraploid (Fig. 2F), presumably due to inhibition of the spindle checkpoint by aurora-B kinase (14). DNA synthesis was renewed when VX-680 was removed from RPE-NEO cells after 3 d (Fig. 2A), in accordance with the revival of cellular proliferation (Fig. 1A and Fig. S1B). By day 10, ∼75% of the cells were actively synthesizing DNA (Fig. 2A).
Fig. 2.
VX-680 elicits polyploidy in cells that overexpress Myc. (A) The effect of VX-680 on DNA synthesis. RPE-NEO cells (□) and RPE-MYC cells (■) were treated with 300 nM VX-680 for 3 d and then transferred into drug-free medium. BrdU incorporation assays were performed at the indicated times after initiation of VX-680 treatment. Each column, here and in B, represents the average of three independent experiments, and each experiment was done in triplicate. Error bars represent 1 SD. #Cells below the limit of detection. (B) The effect of VX-680 on the mitotic index. RPE-NEO cells (□) and RPE-MYC cells (■) were treated with 300 nM VX-680 for 0 or 3 d, fixed, and stained for DNA. The mitotic index was expressed as the percentage of cells in mitosis. (C) VX-680 elicits abnormalities in centrosome number, centromere number, and the mitotic spindle. RPE-MYC was treated with 300 nM VX-680 for 3 d and then stained for centrosomes with anti-pericentrin antibodies in green (a, an untreated RPE-MYC cell; b, a treated RPE-MYC cell), centromeres with the CREST human autoserum in red (c), microtubules with anti–α-tubulin antibodies in red (d), and DNA with DAPI in blue (a–d). A representative cell is shown in each panel. (Scale bar: 5 μm.) (D) The effect of VX-680 on centrosome number. The percentage of mitotic RPE-MYC cells with the indicated number of centrosomes (numbered squares) was determined after various durations of treatment with 300 nM VX-680. A representative experiment is shown. (E) The effect of VX-680 on cell morphology. RPE-MYC cells were treated with either 0.03% DMSO (a and c) or 300 nM VX-680 (b and d) for 3 d and then photographed. Three-fold magnifications of a field of RPE-NEO cells and a single multinucleated RPE-MYC cell are shown in c and d, respectively. (Scale bar: 20 μm.) (F) Polyploidy elicited by VX-680 in cells that overexpress Myc. RPE-NEO cells and RPE-MYC cells were treated with either DMSO or 300 nM VX-680 for the indicated days, and DNA content was analyzed by FACS after staining DNA with 10 μg/mL of propidium iodide. (G) Overexpression of Myc prevents induction of the p53-dependent G1 checkpoint pathway. RPE-NEO cells and RPE-MYC cells were treated for the indicated time periods with 300 nM VX-680, and expression of the indicated proteins was assessed by Western blot analysis. Equal loading was confirmed by staining of total proteins on the transfer membrane with Ponceau S.
The arrest of DNA synthesis elicited by disablement of the CPPC in normal cells is mediated by the p53-p21 pathway (13–15). Accordingly, the amounts of p53 and p21 increased in RPE-NEO cells treated with VX-680 (Fig. 2G). In contrast, the constitutive expression of p21 in RPE-MYC cells was greatly diminished compared with that in RPE-NEO cells (Fig. 2G). Moreover, although treatment with VX-680 elicited a modest induction of p53 in RPE-MYC cells, it produced no notable change in the expression of p21 (Fig. 2G). It appears that activation of the p53-p21 pathway was blocked when Myc was overexpressed, as described previously (16), accounting for the failure of VX-680 to arrest DNA synthesis.
The cellular response to cytotoxic agents also is typically mediated by the p53-p21 pathway (17), and a deficiency in this pathway is a common means through which tumor cells become resistant to cytotoxic drugs (18). In contrast, such a deficiency did not create resistance to the synthetic lethal interaction between VX-680 and Myc in either model cell lines (Table S1) or human tumor cell lines (Fig. S4). We conclude that the synthetic lethal interaction between VX-680 and Myc does not require the p53-p21 pathway—a valuable property, given the frequency at which that pathway is deficient in human tumors (18).
Myc Overexpression Elicits Autophagy in Response to VX-680.
Apoptosis in RPE-MYC cells was not readily detectable during delayed death. Instead, the cells displayed features of autophagy (Fig. 1D and Fig. S5 A–F). This phenotype first became apparent at 3 d after initiation of treatment with VX-680 (Fig. S5 A, B, D, and F), reached a maximum by 5 d, and eventually affected at least 80% of the cells (Fig. S5 B and D), even though the drug had been withdrawn after 3 d. RPE-NEO cells did not respond to VX-680 in this manner (Fig. S5 A, B, and D–F).
We suspected that the interaction between VX-680 and Myc might induce the expression of genes responsible for autophagy. We focused on ATG6 and ATG9, because these genes are involved in initiating the genesis of autophagosomes (19). We found that treatment with VX-680 augmented the expression of both ATG6 and ATG9 in RPE-MYC cells, but not in RPE-NEO cells (Fig. S5A). Induction of the ATG genes became evident within 2 d after administration of VX-680, when autophagy was still at constitutive levels, and reached a maximum within 5 d, congruent with the peak of autophagy (Fig. S5 A, B, D, and F). We conclude that Myc and VX-680 induce ATG6 and ATG9 in a collaborative manner, and that this induction is probably integral to the augmentation of autophagy by the synthetic lethal interaction.
Autophagy Is a Major Effector of Delayed Death.
The congruence of autophagy with delayed death raised the possibility that autophagy was the agency of delayed death. We addressed this possibility in two ways. First, we used RNAi to deplete cells of either Atg5 or Atg7 protein (Fig. 3A), two essential components of the canonical autophagy pathway (19). These depletions greatly suppressed autophagy (Fig. 3B) and provided substantial, but incomplete, protection against the delayed death of RPE-MYC cells in response to VX-680 (Fig. 3C). In contrast, depletion of the Atg proteins provided no protection against the early, apoptotic form of death (Fig. 3D). Second, we used mouse embryo fibroblasts established from atg5+/+ and atg5−/− mice (20). Ectopic overexpression of Myc rendered both the WT and null cells susceptible to early death elicited by VX-680 (Fig. 3E). In contrast, the absence of atg5 provided >2-fold greater protection against delayed death (Fig. 3F).
Fig. 3.
The autophagy genes ATG5 and ATG7 contribute to delayed cell death elicited by VX-680. (A) Depletion of Atg5 and Atg7 by their cognate RNAi. RPE-MYC cells were infected with either pLKO.1-puro control lentiviral particles or lentiviral particles encoding shRNA against either ATG5 or ATG7, selected with 0.5 μg/mL of puromycin for 2 d, and subjected to Western blot analysis for Atg7 and Atg5, which are found mainly as Atg5–Atg12 conjugates. Actin was used as a loading control. (B) Suppression of autophagy by depletion of either Atg5 or Atg7. RPE-MYC cells were stably transfected with Cherry-LC3, infected with the indicated RNAi viruses, treated with 300 nM VX-680 for 3 d, and then transferred into drug-free medium. Quantification of autophagy with Cherry-LC3 was performed 2 d after withdrawal of VX-680. Cells that had more than 40 Cherry-LC3 puncta per cell were scored as positive. Each column, here and in ensuing panels, represents the average of three independent experiments, and each experiment was done in triplicate. Error bars represent SD. (C and D) Depletion of autophagy proteins suppresses delayed cell death, but not early death. RPE-MYC cells were infected with the indicated RNAi lentiviruses, treated with 300 nM VX-680 for 3 d, and then transferred into drug-free medium. Cells were harvested at 0, 3, and 6 d after initiation of VX-680 administration and assayed for viability by the trypan blue exclusion assay. (C) Delayed death was calculated using the following formula: percentage of dead cells = [(live cell number at day 3 − live cell number at day 6)/(live cell number at day 3)] × 100. (D) Early death was expressed as the percentage of dead cells at day 3. (E and F) Absence of atg5 protects cells against delayed death, but not against early death. atg5+/+ and atg5−/− MEF cells were infected with either a control virus (pMig) or a Myc-expressing virus (pMigMyc). The infected cells were then treated with DMSO or 300 nM VX-680 for 3 d and transferred into drug-free medium. Cells were harvested at 0, 3, and 6 d after initiation of treatment with VX-680 and assayed for viability by the trypan blue exclusion assay. Early death (E) and delayed death (F) were calculated as described for C and D. #Cells below the limit of detection. In the experiment represented by the hatched column, 100 μM z-VAD-fmk was included in the medium during the delayed cell death assay.
We conclude that a substantial portion of the delayed cell death was executed by autophagy. However, neither depletion of Atg proteins by RNAi nor a genetic deficiency in atg5 provided complete protection against delayed death. The residual death was not likely the product of apoptosis, because it could not be blocked by z-VAD-fmk (Fig. 3F). A possible explanation is the action of a noncanonical pathway to autophagy that is independent of Atg5 and Atg7 (21).
Synthetic Lethal Interaction Between VX-680 and Myc Overexpression Is Due to Disablement of the CPPC.
The pharmacological effects of VX-680 are not limited to aurora kinases (22). We used two approaches to examine whether the killing of cells by VX-680 was due to inhibition of aurora-B kinase and consequent disablement of the CPPC. First, we exploited another inhibitor of aurora kinases, AZD1152 (23), which affects aurora-B and -C, but not aurora-A. AZD1152 replicated the early and delayed killing of RPE-MYC cells described above for VX-680 (Fig. 4A). Second, we demonstrated that depletion of aurora-B, survivin, and INCENP with RNAi (Fig. 4B) elicited apoptosis (Fig. 4C), autophagy (Fig. 4D), and delayed death that eliminated virtually all of the remaining cells (Fig. 4E). In contrast, RPE-NEO cells recovered from each of the depletions (Fig. 4F) as the effect of RNAi decayed over time. We conclude that both the early and delayed cell death elicited by VX-680 can be attributed to disablement of the CPPC by inhibition of aurora-B kinase, and that any other form of such disablement likely would also display a synthetic lethal interaction with overexpression of Myc.
Fig. 4.
Synthetic lethal interaction between disablement of the CPPC and overexpression of Myc. (A) Cell killing by AZD1152. RPE-NEO cells (□ and ■) and RPE-MYC cells (○ and ●) were treated with 50 nM AZD1152 for 3 d and then either transferred into drug-free medium (○ and □) or maintained in fresh medium containing VX-680 (● and ■). Cells were harvested at the indicated intervals and then assayed for viability by the trypan blue exclusion assay. The data are expressed as mean ± SD for three independent experiments. The arrow indicates the time at which VX-680 treatment was terminated. (B) Depletion of the chromosomal passenger proteins survivin, aurora-B, and INCENP by RNAi. Extracts from RPE-MYC cells treated with RNAi against luciferase, INCENP, surviving, or aurora-B were analyzed by immunoblotting for expression of survivin, aurora-B, and INCENP at 4 d after transfection with RNAi. (C) Early apoptosis elicited by depletion of chromosomal passenger proteins in cells that overexpress Myc. Apoptosis was assessed by DAPI staining of DNA at 4 d after transfection of RPE-NEO cells (□) and RPE-MYC cells (■) with RNAi against the indicated genes. Cells with fragmented nuclei were scored as positive for apoptosis. Each column, here and in D, represents the average of three independent experiments, and each experiment was done in triplicate. Error bars represent 1 SD. (D) Autophagy elicited by depletion of chromosomal passenger proteins in cells that overexpress Myc. Autophagy was assessed by immunostaining for LC3 at 7 d after transfection of RPE-NEO cells (□) and RPE-MYC cells (■) with RNAi against the indicated genes. Cells that had more than 40 LC3 puncta per cell were scored as positive. (E and F) Delayed cell death elicited by depletion of the chromosomal passenger proteins. RPE-MYC cells (E) and RPE-NEO cells (F) were replated at 5% and 20% confluence, respectively, at 4 d after transfection with RNAi against indicated genes. The depletions by RNAi were comparable to those illustrated in B. Cells were photographed after being fixed and stained with crystal violet at 4 d (E) or 7 d (F) after replating.
DNA Synthesis Is Required for the Synthetic Lethal Interaction Between Myc and VX-680.
It seemed possible to us that illicit DNA synthesis was the trigger for the synthetic lethality in RPE-MYC cells treated with VX-680. We tested this possibility by either growing RPE-MYC cells to confluence or treating these cells with aphidicolin before exposing them to VX-680. Both procedures arrested DNA synthesis (Fig. S6 A and B) and substantially protected the cells from both early and delayed killing by VX-680 (Fig. S6 C–F). The elevated expression of Myc in RPE-MYC cells was not affected by either confluence or aphidicolin (Fig. S6G). We conclude that sustained DNA synthesis is essential for the synthetic lethal interaction between Myc and VX-680, perhaps by virtue of the ensuing polyploidy or DNA damage (Discussion).
Therapeutic Efficacy of VX-680 Against Myc-Driven Models of Lymphoma.
Our studies in vitro indicated that VX-680 might be useful in the treatment of tumors that overexpress Myc. We explored this possibility with mouse models that develop either B-cell (24) or T-cell (25) lymphomas in response to Myc overexpression. The B-cell tumors are driven by a constitutively active transgene of MYC (24), whereas the transgene responsible for the T-cell tumors can be repressed by doxycycline (25). We used pulse therapy to exploit the fact that the synthetic lethal interaction continues to kill cells after withdrawal of VX-680, whereas at least some fraction of normal cells may recover from the effects of the drug.
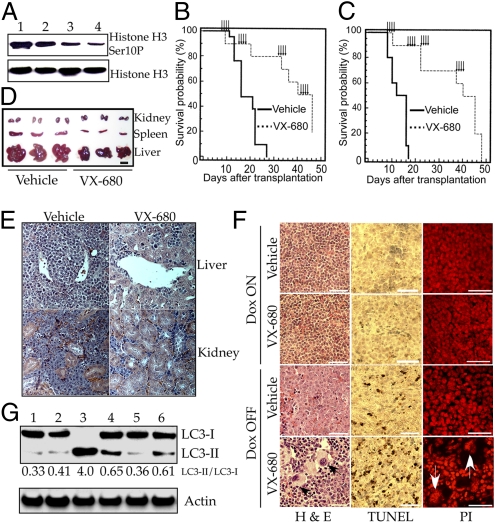
Treatment with VX-680 was initiated at 7 d after cells from primary tumors were transplanted to naive isogenic mice, by which point enlargement of the liver and spleen was inevitably apparent. Intermittent pulses of treatment were continued until more than half of the animals were deceased (Fig. 5 B and C). The result was an ∼3-fold increase in survival. Treatment of primary T-cell tumors led to a prompt and major reduction in phosphorylation of histone H3 at Ser-10 at a dose that proved effective against both B-cell and T-cell tumors (75 mg/kg body weight) (Fig. 5A), demonstrating that the drug inhibited aurora-B kinase in vivo, consistent with the inhibition being responsible for the tumor response.
Fig. 5.
VX-680 extends the survival of mice with lymphomas elicited by Myc. (A) Inhibition in vivo of aurora-B kinase in tumor cells. Tet-o-MYC/Eμ-SR-tTA transgenic mice maintained on a doxycycline-free diet were treated with 0 mg/kg (lane 1), 45 mg/kg (lane 2), 65 mg/kg (lane 3), and 75 mg/kg (lane 4) of VX-680 via i.p. injection when tumors became evident. Higher doses were precluded by limited solubility of VX-680. Single-cell suspensions were prepared from spleen at 6 h after injection and analyzed for histone H3 and its phosphorylation at Ser-10 by Western blot analysis. (B) Survival of mice bearing T-cell lymphomas. Isogenic naïve mice were transplanted with T-cell lymphoma cells (1 million cells per recipient) from a single Tet-o-MYC/Eμ-SR-tTA transgenic mouse. One week later, the recipients were treated with either vehicle (n = 20) or 75 mg/kg of VX-680 (n = 10) by daily i.p. injection at the times indicated by arrows (see SI Materials and Methods for details). Survival is displayed as a Kaplan-Meier plot. Survival curves for vehicle treatment and VX-680 treatment were compared using the log-rank test; P = 0.0001. (C) Survival of mice bearing B-cell lymphomas. Isogenic naïve mice underwent transplantation with B-cell lymphoma cells (50,000 per recipient) from a single Eμ-MYC mouse and treated as described in B. Survival curves for vehicle treatment and VX-680 treatment were compared using the log-rank test; P = 0.0001. (D and E) Tumor suppression by VX-680. Isogenic naïve mice underwent transplantation with T-cell lymphomas (1 million cells per animal) and treated with either vehicle or 75 mg/kg of VX-680 for 4 d by daily i.p. injection that was initiated 1 wk after transplantation. Six days after the final injection, the indicated internal organs from animals were harvested for photography (D) and H&E staining of fixed sections (E). (F) VX-680 elicits apoptosis and polyploidy in MYC-driven lymphomas in vivo. Tet-o-MYC/Eμ-tTA transgenic mice maintained on a doxycycline-free diet were treated with five daily i.p. injections of 75 mg/kg of VX-680 or vehicle when tumors became evident (Dox OFF). One day after the final injection, spleens were removed and fixed with 4% paraformaldehyde. Spleens for controls were obtained from Tet-o-MYC/Eu-tTA mice that had been kept tumor-free by the administration of doxycycline in the diet (Dox ON). Sections of spleens were analyzed by H&E staining, TUNEL assay, and propidium iodide staining. Black and white arrows indicate polyploid cells. (G) Induction of autophagy by VX-680 in vivo. Six Tet-o-MYC/Eμ-tTA transgenic mice were treated with vehicle (lanes 1 and 2) or five daily i.p. injections of 75 mg/kg of VX-680 (lanes 3–6) when tumors became evident. Single-cell suspensions were prepared from spleen at 24 h after the last injection and assessed by Western blot analysis with antibodies against LC3 and actin. The numbers indicate the ratio between LC3-II and LC3-I.
A single 4-d treatment of animals bearing the transplanted T-cell lymphoma shrank the spleen, liver and kidney (Fig. 5D and Table S2), and greatly reduced the number of infiltrating tumor cells in nonlymphoid organs such as liver and kidney (Fig. 5E). A single 5-d treatment of animals bearing a primary T-cell lymphoma increased the amount of apoptosis in tumors 4- to 5-fold (Fig. 5F and Fig. S7A) and the number of polyploid cells by 8-fold (Fig. 5F and Fig. S7B), and elicited autophagy in tumor tissue (Fig. 5G).
Mice subjected to the intermittent treatment described in Fig. 5B gained weight (Fig. S7C) and demonstrated little if any change in peripheral blood cells (Fig S7D). In contrast, daily treatment for 16 d caused a modicum of weight loss and a 7-fold reduction in neutrophils, in accordance with a previous report (6). In addition, a 5-d treatment with VX-680 had no appreciable effect on weight, histology, apoptosis, or ploidy in the spleens of normal mice (Fig. 5F and Fig. S7 A and B). We conclude that a synthetic lethal interaction between VX-680 and Myc overexpression can be elicited in vivo with consequent therapeutic efficacy, and that the therapeutic regimen that we used was well tolerated.
Discussion
We have found that a circumscribed exposure to the aurora kinase inhibitor VX-680 selectively kills cells that overexpress Myc. The synthetic lethal interaction is demonstrable in experimental cell lines that overexpress ectopic MYC, human tumor cell lines that overexpress intrinsic MYC or MYCN, and murine tumors initiated by transgenes of MYC. The killing results from prompt apoptosis and delayed autophagy, is not compromised by withdrawal of the drug after 3 d of treatment, and has demonstrable therapeutic efficacy in animal models. We were able to demonstrate that aurora-B kinase is the crucial target for VX-680 in both the early and the delayed killing, and that the lethal response can be attributed to disablement of the CPPC.
Functions upstream of Myc in cell signaling pathways might display similar synthetic lethality. NOTCH1-IC, HPV-E6, and adenovirus E1A provide examples, because they can induce the expression of Myc and display synthetic lethality with VX-680 in RPE-NEO cells (Fig. S3A). It should be worthwhile to pursue other such examples, in the hope of amplifying the number of therapeutic targets that might display synthetic lethality with inhibitors of the CPPC.
Molecular Path to Synthetic Lethality.
It is possible to postulate a sequence of events that gives rise to the bimodal synthetic lethal interaction between Myc and disablement of the CPPC. The sequence begins when cytokinesis is arrested by failure of the CPPC. In the presence of excess Myc, the G1/S checkpoint is compromised and illicit DNA synthesis ensues, leading to polyploidy in the absence of cytokinesis. Inhibition of aurora-B causes the spindle checkpoint to fail, resulting in multinucleation. Myc overexpression may contribute to this mayhem in any of several ways, including direct promotion of DNA replication (26) and circumvention of both the G1/S and G2/M checkpoints (16, 27, 28). The end result is an unleashing of the proapoptotic activity of Myc (29) and induction of ATG genes by a demonstrable (albeit yet unexplained) collaboration between Myc and VX-680. Thus, the cells that survive the wave of apoptotic killing are destined to die later, due mainly to lethal autophagy.
The illicit DNA synthesis that occurs during the synthetic lethal interaction might well give rise to DNA damage (30, 31). The cytostatic and apoptotic responses to DNA damage are typically mediated by p53 (17) and overexpression of Myc channels that respond to apoptosis (16). But the synthetic lethal interaction does not require p53, so DNA damage may not be the trigger for the interaction. Instead, the polyploidy resulting from illicit DNA synthesis might be responsible (32).
There has been uncertainty about the role of p53 in the cellular response to VX-680 and other inhibitors of aurora-B kinase (33, 34). The uncertainty may arise in part from unappreciated variables. For example, a previous study found that VX-680 induces apoptosis preferentially in cells with defective p53 function (34), whereas in our experience, deficiencies in p53 were not synthetic lethal with VX-680 (Table S1 and Fig. S3C). At least two factors could account for this discrepancy. First, the previous study used HPV-E6 to inactivate p53 in cells with the wild-type allele of TP53, and we found that the E6 gene itself has a synthetic lethal interaction with VX-680, presumably mediated by induction of Myc. Second, the p53-null cells that responded to VX-680 in that study are known to overexpress intrinsic Myc.
Synthetic Lethal Approaches to Cancer Therapy.
Previous reports have described synthetic lethal interactions between Myc and various external agents; examples include activation of the DR5 death receptor pathway with the TRAIL ligand (3), inhibition of cyclin-dependent kinase CDK1 (4), and the antimalarial drug chloroquine (5). In addition, inhibition of CDK2 is synthetically lethal with overexpression of MycN (9). The synthetic lethal effect described here has several advantageous features, however.
First, cytotoxic therapies typically elicit apoptosis. The bimodal nature of the synthetic lethality that we have described may permit the treatment of cancer cells that are defective in apoptosis, a common circumstance that can confer resistance to therapy (18). For example, we have encountered a number of human tumor cell lines that overexpress either MYC or MYCN and are defective in apoptosis, yet were killed by the delayed response to VX-680 (Table S3). Second, the strategy described here targets proteins expressed only in dividing cells, and it might have minimal toxicity for nondividing cells. Finally, the cytotoxic effect of VX-680 on proliferating cells might be minimalized by administering the agent in circumscribed pulses, which can kill tumor cells that overexpress Myc. It is notable that normal mice appeared to tolerate pulsed treatment with VX-680 well.
The Myc-selective killing of cells by chloroquine involved both apoptosis and autophagy (5), but in a manner different from what we have described here. Treatment with chloroquine induced large-scale autophagy but impaired its terminal steps, resulting in induction of p53-dependent apoptosis. Cell death was not impaired by a genetic deficiency of atg7, and an unexplained form of killing occurred when apoptosis was blocked. Chloroquine appeared to be effective as a preventive agent in a mouse model for B-cell lymphoma, but demonstrated no efficacy as a therapeutic.
We conclude that overexpression of Myc and genes that activate MYC are potential biomarkers for sensitivity of tumor cells to VX-680 or any other agent that compromises the CPPC. There may well be genes that are capable of a similar synthetic interaction without the intervention of Myc, but we have yet to uncover an example of such genes. The synthetic lethal interaction between Myc and VX-680 may create a favorable therapeutic index, and may be valuable as a biomarker in the design of both future clinical trials and therapeutic regimens. Preclinical studies of combination therapy exploiting two or more of the several synthetic lethal interactions with Myc that have now been defined also merit consideration.
Note Added in Proof.
Subsequent to review of the manuscript and final submission to the editor, an online publication reported that inhibition of aurora-B kinase selectively killed cells that overexpress Myc and displayed efficacy in a preclinical model for B-cell lymphoma (Blood, doi 10.1182/blood-2009-11-251074). The bimodal nature of the killing reported here was not described.
Materials and Methods
See SI Materials and Methods for details on all materials and procedures, including (i) cell lines, transfections, and retroviral transductions; (ii) assays for cellular proliferation, DNA synthesis, cell cycle, apoptosis, and autophagy; (iii) chemical and immunologic reagents; (iv) imaging procedures; (v) mouse strains, tumorigenesis, and treatment protocols; (vi) analysis of cellular RNA and proteins; (vii) the use of RNAi; and (viii) statistical analyses.
Supplementary Material
Acknowledgments
We thank Gerard Evan, David Morgan, and Scott Kogan for assistance with the manuscript; Jay Debnath (University of California, San Francisco) for the Cherry-LC3 construct; Robert Weinberg (Massachusetts Institute of Technology) for HA1E cells; Noboru Mizushima (Tokyo Medical and Dental University) for the atg5−/− MEF cells; and Tyler Jacks (Massachusetts Institute of Technology) for the Trp53−/− MEF cells. We also thank Ivy Hsieh and Juan Engel for excellent support with transmission electron microscopy and Jane Gordon for confocal laser microscopy. This research was supported by the Susan G. Komen Breast Cancer Research Fund (D.Y.), a Howard Hughes Medical Institute Physician-Scientist Fellowship and National Institutes of Health Grant K08 CA104032 (to A.G.), National Cancer Institute Grant K01 CA115681 (to S.K.), and funds from the G.W. Hooper Research Foundation (to J.M.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008366107/-/DCSupplemental.
References
- 1.Dang CV. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell Biol. 1999;19:1–11. doi: 10.1128/mcb.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nesbit CE, Tersak JM, Prochownik EV. MYC oncogenes and human neoplastic disease. Oncogene. 1999;18:3004–3016. doi: 10.1038/sj.onc.1202746. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, et al. Synthetic lethal targeting of MYC by activation of the DR5 death receptor pathway. Cancer Cell. 2004;5:501–512. doi: 10.1016/s1535-6108(04)00113-8. [DOI] [PubMed] [Google Scholar]
- 4.Goga A, Yang D, Tward AD, Morgan DO, Bishop JM. Inhibition of CDK1 as a potential therapy for tumors over-expressing MYC. Nat Med. 2007;13:820–827. doi: 10.1038/nm1606. [DOI] [PubMed] [Google Scholar]
- 5.Maclean KH, Dorsey FC, Cleveland JL, Kastan MB. Targeting lysosomal degradation induces p53-dependent cell death and prevents cancer in mouse models of lymphomagenesis. J Clin Invest. 2008;118:79–88. doi: 10.1172/JCI33700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrington EA, et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10:262–267. doi: 10.1038/nm1003. [DOI] [PubMed] [Google Scholar]
- 7.Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: Conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- 8.Hemann MT, et al. Evasion of the p53 tumour surveillance network by tumour-derived MYC mutants. Nature. 2005;436:807–811. doi: 10.1038/nature03845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molenaar JJ, et al. Inactivation of CDK2 is synthetically lethal to MYCN over-expressing cancer cells. Proc Natl Acad Sci USA. 2009;106:12968–12973. doi: 10.1073/pnas.0901418106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weng AP, et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 2006;20:2096–2109. doi: 10.1101/gad.1450406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tworkowski KA, et al. Adenovirus E1A targets p400 to induce the cellular oncoprotein Myc. Proc Natl Acad Sci USA. 2008;105:6103–6108. doi: 10.1073/pnas.0802095105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinoshita T, et al. Transactivation of prothymosin alpha and c-myc promoters by human papillomavirus type 16 E6 protein. Virology. 1997;232:53–61. doi: 10.1006/viro.1997.8536. [DOI] [PubMed] [Google Scholar]
- 13.Beltrami E, Plescia J, Wilkinson JC, Duckett CS, Altieri DC. Acute ablation of survivin uncovers p53-dependent mitotic checkpoint functions and control of mitochondrial apoptosis. J Biol Chem. 2004;279:2077–2084. doi: 10.1074/jbc.M309479200. [DOI] [PubMed] [Google Scholar]
- 14.Ditchfield C, et al. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161:267–280. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang D, Welm A, Bishop JM. Cell division and cell survival in the absence of survivin. Proc Natl Acad Sci USA. 2004;101:15100–15105. doi: 10.1073/pnas.0406665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seoane J, Le HV, Massagué J. Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature. 2002;419:729–734. doi: 10.1038/nature01119. [DOI] [PubMed] [Google Scholar]
- 17.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 18.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: A link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 19.Xie Z, Klionsky DJ. Autophagosome formation: Core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 20.Kuma A, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 21.Nishida Y, et al. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461:654–658. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
- 22.Carter TA, et al. Inhibition of drug-resistant mutants of ABL, KIT, and EGF receptor kinases. Proc Natl Acad Sci USA. 2005;102:11011–11016. doi: 10.1073/pnas.0504952102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mortlock AA, et al. Discovery, synthesis, and in vivo activity of a new class of pyrazoloquinazolines as selective inhibitors of aurora B kinase. J Med Chem. 2007;50:2213–2224. doi: 10.1021/jm061335f. [DOI] [PubMed] [Google Scholar]
- 24.Adams JM, et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- 25.Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol Cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- 26.Dominguez-Sola D, et al. Non-transcriptional control of DNA replication by c-Myc. Nature. 2007;448:445–451. doi: 10.1038/nature05953. [DOI] [PubMed] [Google Scholar]
- 27.Sheen JH, Dickson RB. Overexpression of c-Myc alters G(1)/S arrest following ionizing radiation. Mol Cell Biol. 2002;22:1819–1833. doi: 10.1128/MCB.22.6.1819-1833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Q, Dang CV. c-Myc overexpression uncouples DNA replication from mitosis. Mol Cell Biol. 1999;19:5339–5351. doi: 10.1128/mcb.19.8.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelengaris S, Khan M, Evan G. c-MYC: More than just a matter of life and death. Nat Rev Cancer. 2002;2:764–776. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]
- 30.Felsher DW, Bishop JM. Transient excess of MYC activity can elicit genomic instability and tumorigenesis. Proc Natl Acad Sci USA. 1999;96:3940–3944. doi: 10.1073/pnas.96.7.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vafa O, et al. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: A mechanism for oncogene-induced genetic instability. Mol Cell. 2002;9:1031–1044. doi: 10.1016/s1097-2765(02)00520-8. [DOI] [PubMed] [Google Scholar]
- 32.Ganem NJ, Pellman D. Limiting the proliferation of polyploid cells. Cell. 2007;131:437–440. doi: 10.1016/j.cell.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 33.Li M, et al. Aurora kinase inhibitor ZM447439 induces apoptosis via mitochondrial pathways. Biochem Pharmacol. 2010;79:122–129. doi: 10.1016/j.bcp.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Gizatullin , et al. The Aurora kinase inhibitor VX-680 induces endoreduplication and apoptosis preferentially in cells with compromised p53-dependent postmitotic checkpoint function. Cancer Res. 2006;66:7668–7677. doi: 10.1158/0008-5472.CAN-05-3353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.