Abstract
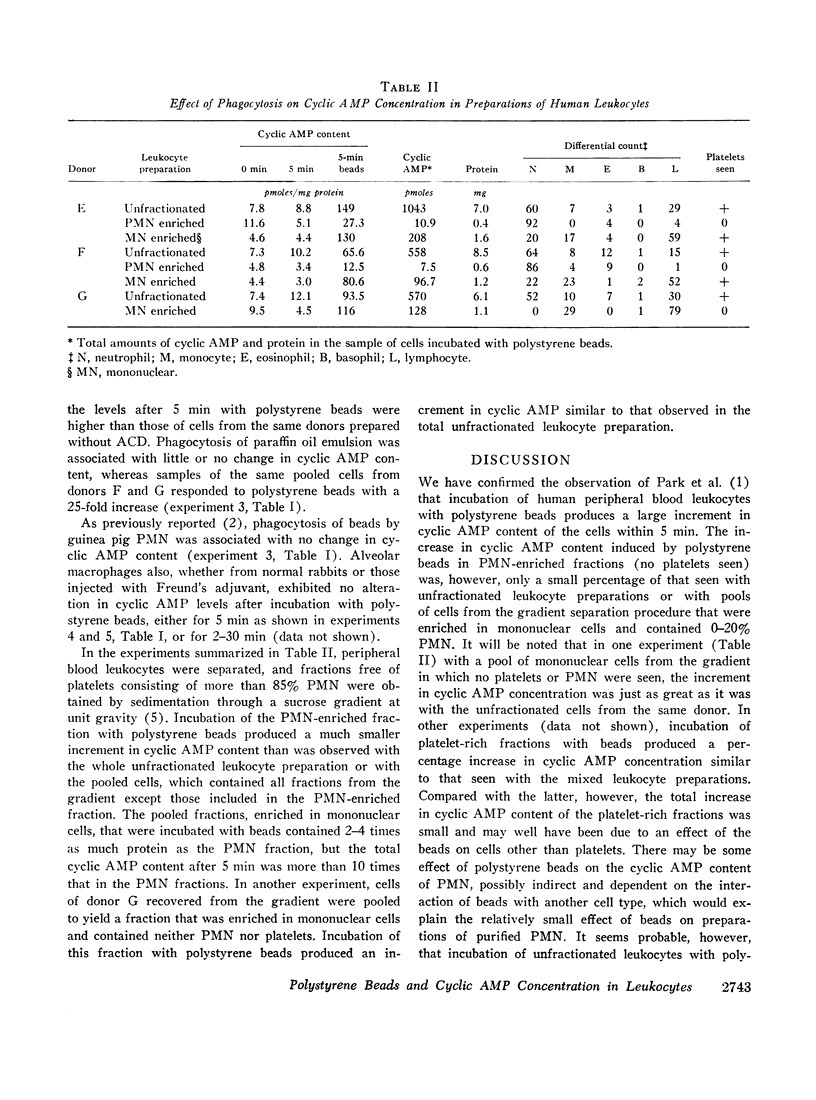
After incubation with polystyrene latex beads for 5 min. the cyclic 3′,5′-adenosine monophosphate (cyclic AMP) content of human peripheral blood leukocyte suspensions was increased severalfold. Preparations enriched in mononuclear cells and containing only 0-20% polymorphonuclear leukocytes (PMN) and no visible platelets exhibited a quantitatively similar response. Purified fractions of cells containing 85-90% PMN responded to polystyrene beads with a much smaller increase in cyclic AMP content. Phagocytosis of paraffin oil emulsion in the unfractionated mixed human leukocyte preparation was associated with little or no change in cyclic AMP levels. There was no change in cyclic AMP content of rabbit alveolar macrophages or guinea pig PMN during phagocytosis of polystyrene beads. All of these observations are consistent with the view that particle uptake per se does not increase cyclic AMP levels in phagocytic cells. It seems probable that the increase in cyclic AMP concentration that results when unfractionated human blood leukocytes are incubated with polystyrene beads occurs in cells other than PMN.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourne H. R., Lehrer R. I., Cline M. J., Melmon K. L. Cyclic 3',5'-adenosine monophosphate in the human lukocyte: synthesis, degradation, andeffects n neutrophil candidacidal activity. J Clin Invest. 1971 Apr;50(4):920–929. doi: 10.1172/JCI106564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker L. H., Evans W. H. Separation of granulocytes, monocytes, lymphocytes, erythrocytes, and platelets from human blood and relative tagging with diisopropylfluorophosphate (DFP). J Lab Clin Med. 1969 Jun;73(6):1036–1041. [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mosinger B., Vaughan M. The action of cyclic 3',5'-adenosine monophosphate on lipolysis in rat adipose tissue. Biochim Biophys Acta. 1967 Dec 5;144(3):569–582. doi: 10.1016/0005-2760(67)90046-x. [DOI] [PubMed] [Google Scholar]
- Rall T. W., Sattin A. Factors influencing the accumulation of cyclic AMP in brain tissue. Adv Biochem Psychopharmacol. 1970;3:113–133. [PubMed] [Google Scholar]
- Stossel T. P., Murad F., Mason R. J., Vaughan M. Regulation of glycogen metabolism in polymorphonuclear leukocytes. J Biol Chem. 1970 Nov 25;245(22):6228–6234. [PubMed] [Google Scholar]
- Stossel T. P., Pollard T. D., Mason R. J., Vaughan M. Isolation and properties of phagocytic vesicles from polymorphonuclear leukocytes. J Clin Invest. 1971 Aug;50(8):1745–1747. doi: 10.1172/JCI106664. [DOI] [PMC free article] [PubMed] [Google Scholar]


