Abstract
Animals using sound for communication emit directional signals, focusing most acoustic energy in one direction. Echolocating bats are listening for soft echoes from insects. Therefore, a directional biosonar sound beam greatly increases detection probability in the forward direction and decreases off-axis echoes. However, high directionality has context-specific disadvantages: at close range the detection space will be vastly reduced, making a broad beam favorable. Hence, a flexible system would be very advantageous. We investigated whether bats can dynamically change directionality of their biosonar during aerial pursuit of insects. We trained five Myotis daubentonii and one Eptesicus serotinus to capture tethered mealworms and recorded their echolocation signals with a multimicrophone array. The results show that the bats broaden the echolocation beam drastically in the terminal phase of prey pursuit. M. daubentonii increased the half-amplitude angle from approximately 40° to approximately 90° horizontally and from approximately 45° to more than 90° vertically. The increase in beam width is achieved by lowering the frequency by roughly one octave from approximately 55 kHz to approximately 27.5 kHz. The E. serotinus showed beam broadening remarkably similar to that of M. daubentonii. Our results demonstrate dynamic control of beam width in both species. Hence, we propose directionality as an explanation for the frequency decrease observed in the buzz of aerial hawking vespertilionid bats. We predict that future studies will reveal dynamic control of beam width in a broad range of acoustically communicating animals.
Keywords: directionality, echolocation, buzz, frequency, perception
Acoustic communication plays a major role for conspecific and predator/prey interactions in many animals. Features of emitted sounds, such as time-frequency structure, intensity, and directionality, are central for communication range and direction. Flexibility in acoustic behavior allows for adaptive changes in sound signals to the constraints of a variety of contexts and purposes (1, 2). Directionality defines the angle of attention and is consequently a spatial filter for communication. Thus, directionality is as significant as other acoustic features, but as a result of the methodological challenge in measuring it, directionality has only very rarely been studied in the field (3 –6), and almost nothing is known about possible dynamic changes in directionality in response to behavioral tasks. Bats are ideal animals in which to study dynamic changes in directionality, as recording their high-intensity echolocation calls allow us to infer, from the bat's adaptive vocal changes to the changing context, which acoustic elements are important for perception through sound.
Echolocating bats can hunt and navigate without light, emitting short high-frequency sound pulses to determine the direction, distance, and features of objects in the environment from binaural cues, arrival time, amplitude, and spectrum of sonar reflections (7, 8). Bats modify echolocation call parameters such as duration, repetition rate, and intensity in response to obstacles and habitat (9, 10). In general, aerial insectivorous bats increase bandwidth and repetition rate and decrease duration of their calls as they close in on prey during a pursuit sequence (11). The biosonar beam is also directional (6, 12–14), which confers a number of advantages to the echolocating bat, i.e., inherent directional information, reduced clutter, and increased source level. Changes of sonar beam directionality during a pursuit have not been measured, but it is likely that a highly directional beam is most valuable at long range, where energy restrictions force the bats to emit a narrowly focused beam to achieve sufficient biosonar range. At close range, in contrast, a broader beam, providing “wide-angle view,” would be more beneficial. Therefore, a dynamic system capable of adjusting the directionality to a given context seems highly adaptive.
Vespertilionid bats emit their calls through the mouth. Because of their relatively simple facial features, the directionality of their sound emission can be modeled as a simple circular piston oscillating in an infinite baffle (15) as follows:
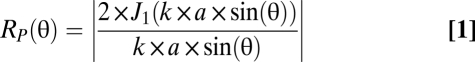 |
where Rp(θ) is the ratio between the on-axis pressure and the pressure at a given angle θ, J1 is a first-order Bessel function, k = 2π/λ, where λ is the wavelength, and a is the radius of the sound emitter. According to the model, directionality of bat echolocation calls increases with increasing frequency as well as with increasing size of the emitter. This means that bats can control the directionality of their echolocation calls by changing the frequency of their call or their emitter size (presumably the size of the open mouth) or through a combination of both these mechanisms.
In aerial insectivorous bats, the pursuit sequence falls into three main phases: search, approach, and terminal phase or buzz phase. The buzz is the phase right before the capture, in which pulse duration is short and pulse repetition rate very high. The buzz probably serves to provide frequent update of prey position data, but the specific significance—the acoustic characteristics of the buzz—is still not understood. However, the ubiquitous emission of buzzes in aerial insectivorous bats, the flexibility in duration, and the fact that bats may abort the pursuit sequence in the middle of the buzz in case of unsuccessful capture attempts all strongly indicate that the whole buzz is of high functional significance for prey capture (16 –18). In most vespertilionid bats, the buzz falls into two distinct parts, buzz I and buzz II, whereby buzz II is characterized by a lowering of the frequency of the echolocation pulse by almost an octave (11, 19). The frequency decrease has previously been speculated to represent a physical constraint of producing calls at the extremely high repetition rates (180–200 pulses/s) emitted during the final part of the pursuit sequence (20, 21). However, we hypothesize that the decrease in frequency is dictated by directionality and serves to provide the bat with a broader sonar beam to widen its field of “view” during the very last part of prey pursuit.
In this study, we used a multimicrophone array to measure changes in the horizontal and vertical directionality of the sound beam from five Myotis daubentonii as they approached and captured tethered mealworms in flight. Additionally, we present results of the horizontal emission pattern from one Eptesicus serotinus performing the same task.
Results
We recorded 293 trials from the five M. daubentonii and 67 from E. serotinus and selected four for each bat. We analyzed trials only in which the bats flew directly toward and pointed the beam at the center of the microphone cross and captured the mealworm without banking. Each trial resulted in around 12 approach calls and 14 buzz calls. Similar to previous findings for other bats under laboratory conditions, the bats in our study emitted search calls that were shorter in duration and of lower intensity compared with search signals emitted in the field; maximum duration for M. daubentonii was 3 ms (±0.35 ms) and that for E. serotinus was 3.4 ms (±0.15 ms), which corresponds to approach call durations reported for both species in the field (20, 22, 23). The terminal phases of the prey capture in the laboratory corresponded well with terminal phase recordings from the field. Both species consistently lowered the output frequency by approximately one octave in buzz II of the terminal phase. M. daubentonii decreased average lowest frequency from 39 kHz (±2.9) to 22 kHz (±1.9) and E. serotinus from 31 kHz (±1.0) to 16 kHz (±2.5). The average lowest frequency was measured as the low-frequency cutoff 10 dB from the peak frequency of the first harmonic.
For M. daubentonii, directionality was measured at 55 kHz through the entire hunting sequence to quantify the high-frequency directionality and at 27.5 kHz, one octave below, in the terminal phase to quantify the low-frequency directionality. A frequency of 55 kHz was chosen because it is approximately the peak frequency (54.4 kHz ±4.9) and is of high energy in the spectrum through the entire echolocation sequence. Similarly, for E. serotinus, directionality was measured at 35 kHz (peak, 35.6 ±1.8 kHz) for all calls and additionally at 17.5 kHz for the terminal buzz II calls (Fig. 1).
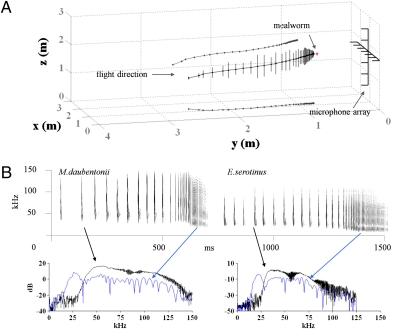
Fig. 1.
(A) A typical flight path for M. daubentonii during prey capture. The corresponding oscillogram for the trial is superimposed on the flight path. Lines with dots show the flight path projected on the x-z and x-y planes. (B) Spectrograms for two selected trials from M. daubentonii and E. serotinus, respectively. Lower: Spectra for a typical approach call (black) and a buzz II call (blue) for each species clearly illustrate how the frequency decreases in the terminal phase.
To estimate possible changes in emitter size (i.e., degree of mouth opening) from approach through buzz II, we fitted the piston model to the directionality measured for each call at the high frequency component through the entire pursuit sequence for M. daubentonii and E. serotinus and calculated the equivalent emitter size. The mouth opening radii were approximately 3.3 mm for M. daubentonii and approximately 5.9 mm for E. serotinus according to our estimates from the model (Table 1). Our analysis shows that emitter size (i.e., mouth opening) was constant throughout the entire pursuit. There was no significant gradual change in emitter size from beginning to end of either phase in either bat species (40 linear regressions; P > 0.05 for all). All estimated equivalent diameters stayed constant, except horizontal emitter size for M. daubentonii, for which our estimates indicated an increase from approach to terminal phase of approximately 0.2 mm (matched-pairs test, P < 0.05), i.e., a change so minor that it has no consequence for beam width.
Table 1.
Mean ± SD equivalent piston radius of the emission pattern measured for the five M. daubentonii and E. serotinus
|
M. daubentonii |
||||||
| Pattern | 1 | 2 | 3 | 4 | 5 | E. serotinus |
| Approach high-frequency | n = 56 | n = 49 | n = 44 | n = 36 | n = 48 | n = 55 |
| Horizontal (mm) | 3.3 ± 0.3 | 3.3 ± 0.1 | 3.4 ± 0.2 | 3.2 ± 0.1 | 3.3 ± 0.2 | 5.7 ± 0.7 |
| Vertical (mm) | 3.1 ± 0.1 | 3.2 ± 0.1 | 3.0 ± 0.2 | 3.0 ± 0.2 | 3.0 ± 0.2 | — |
| Terminal high-frequency | n = 53 | n = 67 | n = 50 | n = 48 | n = 69 | n = 72 |
| Horizontal (mm) | 3.6 ± 0.2 | 3.4 ± 0.2 | 3.6 ± 0.1 | 3.6 ± 0.2 | 3.5 ± 0.1 | 6.0 ± 0.4 |
| Vertical (mm) | 3.1 ± 0.1 | 3.2 ± 0.2 | 3.0 ± 0.2 | 3.1 ± 0.1 | 3.1 ± 0.2 | — |
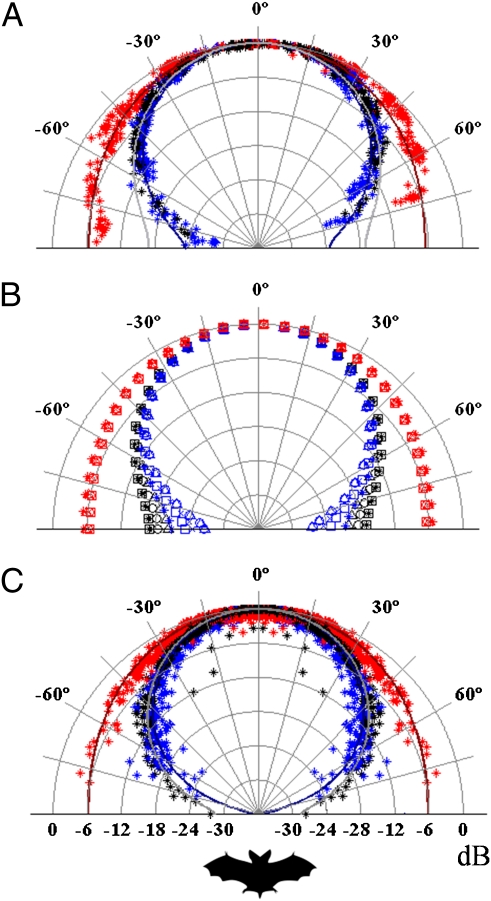
Fig. 2 shows the horizontal beam pattern for M. daubentonii and E. serotinus in the approach and terminal phases. Fig. 2 illustrates how the bats add a much broader component to their sonar beam during the terminal phase by lowering the frequency. The half amplitude angle, i.e., the off-axis angle at which sound pressure has decreased by 6 dB relative to the on-axis (0°) pressure, was approximately 40° at 55 kHz and approximately 90° at 27.5 kHz for M. daubentonii, and approximately 36° at 35 kHz and approximately 80° at 17.5 kHz for E. serotinus. We estimated beam aim and beam shape for each call for two reasons: (i) to ensure that the bat's emitter size was constant and (ii) to determine at what angle each of the 12 microphones recorded the call to allow us to pool all data. The half amplitude angles reported here are estimated from the entire data set (Fig. 1 B and C). Thus, we have no variance in half amplitude angle. However, the SD of the estimated emitter size (Table 1) is a good measure of the (slight) variation in the half amplitude angle from call to call, as half amplitude angle follows the emitter size.
Fig. 2.
(A) Horizontal directionality for one M. daubentonii (number 5) measured at 55 kHz during the approach (black marks) and terminal phase (blue marks) and at 27.5 kHz in the terminal phase (red marks). The fitted emission patterns of a piston at 55 kHz both for the approach (light gray line) and the terminal phase (blue line) and at 27.5 kHz for the terminal phase (red line) are plotted for comparison. (B) Fitted horizontal directionality for the five M. daubentonii (□, *, x, o, and Δ) measured at 55 kHz during the approach (black) and terminal phase (blue) and at 27.5 kHz (red) in the terminal phase. (C) Horizontal directionality for E. serotinus measured at 35 kHz during the approach (asterisk) and terminal phase (asterisk) and at 17.5 kHz in the terminal phase (asterisk).The fitted emission patterns of a piston at 35 kHz both for approach (light gray line) and terminal phase (blue line) and at 17.5 kHz for the terminal phase (red line) are superimposed on the data points. For easier interpretation of the E. serotinus date, all points are mirrored around the 0° line. 0° represents the estimated beam aim, and all recorded pressure values are normalized to the highest value for each call.
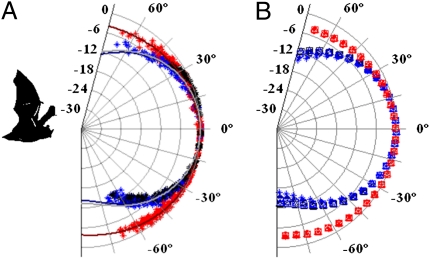
The beam also widened in the vertical direction during buzz II. Vertical beam patterns for M. daubentonii at 55 kHz and 27.5 kHz are plotted in Fig. 3 (approximately 45° half amplitude angle at 55 kHz, >90° at 27.5 kHz). The vertical beam pattern is plotted relative to the measured beam direction of the 55 kHz component and shows that the lower-frequency component of the terminal phase is pointed downward relative to the high-frequency component. On average for all bats, the low-frequency component was aimed 15.2° below the high-frequency component (±2.3°). The extraordinary resemblance of the directionality from all five M. daubentonii both vertically and horizontally demonstrates how similar directionality is across individuals (Table 1).
Fig. 3.
(A) Vertical emission patterns for one M. daubentonii (number 5) measured at 55 kHz during the approach (black marks) and terminal phase (blue marks) and at 27.5 kHz during the terminal phase (red marks).The fitted emission patterns of a piston at 55 kHz both for the approach (light gray line) and the terminal phase (blue line) and at 27.5 kHz (red line) are plotted for comparison. (B) Fitted horizontal directionality for the five M. daubentonii (□, *, x, o, and Δ) measured at 55 kHz during the approach (black) and terminal phase (blue) and at 27.5 kHz (red) in the terminal phase. The 27.5-kHz pattern is plotted 15° below the 55-kHz pattern to comply with the measured difference in aim for the two components. For all plots, 0° represents the estimated beam aim at 55 kHz, and all recorded pressure values are normalized to the highest value for each call.
We estimated the beam aim accuracy at 55 kHz for M. daubentonii (i.e., the direction in which the bat emitted most energy at 55 kHz). The results show that the bats lock onto the mealworm with very high accuracy, on average 0.2° (±2.5°) horizontally and 1.1° (±2.7°) vertically, i.e., the bats point the axis of their sonar beam precisely on the mealworm both horizontally and vertically. The beam is bilaterally symmetrical across frequencies. Thus, beam aim measured at 55 kHz is representative for horizontal beam aim at all frequencies in the entire sweep.
Discussion
The present study shows that the bats broaden their echolocation beam dramatically in the terminal phase by lowering the output frequency by almost an octave compared with the approach phase. Our results also show that M. daubentonii and the single E. serotinus keep a constant emitter size or opening of the mouth during the entire pursuit sequence. The individual beam shapes of M. daubentonii were very similar. Finally, the results show that the beam aim is asymmetrical across frequencies in the vertical plane. The main axis of the low-frequency component is, on average, pointing 15° below the high-frequency component. This is in accordance with Hartley and Suthers (13), who showed that the main axis of the beam moves downward with decreasing frequency for Eptesicus fuscus.
For any emitter, the beam gets broader the lower the frequency is (24). For M. daubentonii, we chose to measure directionality at the peak power frequencies (55 kHz and 27.5 kHz), but there is energy in the echolocation calls also below these frequencies. The minimum frequencies of the spectrum bandwidth (−10 dB) are 39 kHz and 22 kHz, respectively. This indicates that the signal contains a slightly broader component both in the approach and the buzz than the values we report here. For E. serotinus, the low-frequency cutoff is more steep (Fig. 3) and thus contains very little energy below the peak power frequency at which we measured; hence our results represent the maximum directionality available to the bat during both phases. However, the dramatic broadening from approach to terminal phase caused by the lowering of frequency is unaffected no matter whether we compare at the peak power frequency or the lowest frequency of each phase.
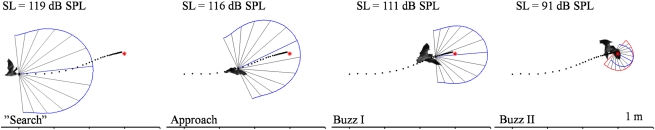
The broadening of the echolocation beam in the final part of the pursuit is advantageous to bats that hunt prey, which can escape by making unpredictable evasive maneuvers. Many insects like noctuid moths and field crickets have ears sensitive to ultrasound, and when they detect echolocating bats, they make erratic escape behaviors in response to the ultrasonic bat calls (25). Bats emit a narrow echolocation beam (12 –14), and in the wild the beam is even more directional than in the laboratory. M. daubentonii emitted calls with half amplitude value at 55 kHz of 40° in the laboratory versus only 20° in the field (6). With such a high degree of directionality all the way through the pursuit, combined with a decrease in emitted output intensity, the “field of view” would gradually decrease, which would enable the prey to escape the bat's echolocation beam by a relatively small movement in space. By broadening the beam, the bats increase their field of view, thus enabling them to keep track of prey trying to escape capture. Fig. 4 illustrates how the bat's field of view, at the higher frequencies (blue trace) used during the approach, gradually decreases as the output level is reduced. In contrast, the red curve shows a dramatic increase in “peripheral vision” in the final part of pursuit, which the bat obtains by decreasing the frequency. The figure focuses on the directionality of the outgoing sonar beam, but for the bat, directionality of hearing and the dynamic decrease in hearing sensitivity when the bat closes in on the prey (10) also play important roles for sensing through sonar. Both effects on the receiving side would make the detection space continuously smaller as the bat closes the distance to its prey. In future studies it is likely that technological improvements will make it possible to test the value of the broadened beam by determining when escaping prey is “illuminated” by sound in real encounters between bats and hearing insects.
Fig. 4.
The estimated vertical detection area for M. daubentonii during a hunting sequence. The area outlined for each of the four selected calls indicates the area in which a bat can detect a mealworm (estimated target strength, −40 dB at 10 cm). The estimate is based on source levels measured during the experiment, and assuming a hearing threshold of 20 dB. The plots are based on the directionality measured at 55 kHz (approach, blue) and 27.5 kHz (buzz II, red), clearly illustrating how the bat changes from “telephoto lens” to “wide angle” view by lowering the frequency.
Approach calls have most energy at approximately 55 kHz, which is the reason we chose to determine beam aim at this frequency for M. daubentonii. Through all sequences, the beam aim (at 55 kHz) was directly on the worm (average, 0.2 ± 2.5° horizontally and 1.1 ± 2.7° vertically). This extremely high accuracy correlates well with the 3° horizontal accuracy reported for big brown bats fixating their beam on the target during the last 300 ms of pursuit (26), and underlines how the aim of the echolocation beam in bats resembles closely the gaze of visually orienting animals (27). Because the bats direct their beam on the mealworm with such high accuracy, it seems reasonable to assume that the beam aim of the 55 kHz component is the focus of the bat's attention. This is not to be confused with the field of view, which is the area ahead of the bat ensonified sufficiently to produce audible echoes. Compared with visually orienting animals, the focus of the beam corresponds to the focus of visual attention, i.e., what is foveated, whereas the entire filed ensonified sufficiently to generate returning echoes corresponds to the entire field of view including peripheral vision. Thus, when the bat shifts to lower frequencies in the buzz, it is not shifting its attention, but broadening the field of view, much like the change from a telephoto lens at great distances to a wide-angle lens for close-ups.
The downward orientation of the low-frequency component in buzz II may be an adaptation to the escape response of flying prey with bat-detecting ears (i.e., power dives and passive falls) or to most bats’ preferred mode of capture using the interfemoral membrane. In case of M. daubentonii, their preferred hunting strategy, trawling over water surfaces, may also add adaptive value to a downward shift. The distantly related Macrophyllum macrophyllum (Phyllostomidae) trawls for insects in a manner similar to M. daubentonii. M. macrophylum emits echolocation calls through the nostrils, and is characterized by its nose leaf, a fleshy structure around the nostrils. M. macrophylum bends down the nose leaf in the final phase of the pursuit, presumably to point the sound beam downward (28), which may be a morphological parallel to M. daubentonii’s downward acoustic view by the low frequencies in the final phase.
Although our results from E. serotinus must be interpreted with caution in view of the very low sample size and a lack of vertical measurements, it is striking how similar the dynamic changes in the horizontal beam pattern of M. daubentonii and E. serotinus are. Hence, this indicates that vespertilionid bats in general obtain a broadening of the beam by lowering the frequency during buzz II, i.e., they keep the emitter size constant while lowering the frequency enough to achieve the required increase in directionality. The lowering of frequency is typical for vespertilionid bats hunting aerial insects. Several species of molossid bats are also reported to lower the frequency of their echolocation calls in the final part of pursuit although not as prominently as the vespertilionids [e.g., Tadarida brasiliensis, Molossus molossus, Molossops temminckii (29 –31)]. Mystacina tuberculata (Mystacinidae) lowers the frequency by shifting energy from the second to the first harmonic during the buzz (32). Hence, broadening the echolocation beam in the final part of prey pursuit may be a common feature of adaptive value for aerial hawking bats.
Several theories have been proposed concerning the function of the buzz II. One is simply a physiological constraint, i.e., that bats cannot produce calls at such high repetition rates and still maintain high frequencies (20, 21). This theory seems unlikely as multiple species of bats produce equally high repetition rates without the concomitant decrease in frequency [e.g., Craseonycteris thonglongyai and Balantiopteryx plicata (33, 34)]. It has also been suggested that, by decreasing the first harmonic by an octave, the bat can retain the frequency range of the sweep—now of the second harmonic—without unrealistic physiological demands for muscle speed, because the corresponding range of the first harmonic is much smaller (33). However, we believe it much more likely that the frequency decrease performed by aerial hawking vespertilionid bats is to achieve a wider detection angle at this stage in the pursuit. Aerial hawking bats not producing the buzz II may use different means to broaden the beam when in close proximity to prey, i.e., they reduce their emitter size (closing the mouth). The frequency decrease could be an indication of a more sophisticated signal design in vespertilionids and molossids. The echolocation signals emitted by those two families are hypothesized to be more recently evolved (<20 Mya) compared with short multiharmonic signals emitted by for instance the Emballonuridae, an ancient lineage that has existed for more than 45 million years (35, 36). Thus, the broadening of the echolocation beam observed could be a derived trait in these two sister groups that may in part explain their recent and massive species radiation such that together vespertilionids and molossids constitute almost half of all extant bat species (approximately 500 species).
The present study shows that M. daubentonii can dynamically control the directionality of its echolocation beam in response to situational changes. Our preliminary results from E. serotinus indicate that this may be a general phenomenon for vespertilionid bats. As mouth emitters, they have two basic methods available for controlling directionality: by altering the size of the emitter, i.e., the size of the open mouth; and/or by changing the frequency of their calls. Presumably nasal-emitters like rhinolophids or phyllostomids have less flexibility as it is hard to imagine dynamic control over emitter size, which must be dictated by the nostrils and the distance between them (15, 37). However, the elaborate nose leaves found in many of these bats could function in modifying the beam shape by reflection, thus indicating high importance of directionality for sound signals.
The other group of echolocating mammals, the toothed whales (Odontoceti), also seem capable of modifying their echolocation beam pattern. For false killer whales (Pseudorca crassidens) and Risso dolphins (Grampus griseus), there is a correlation between directionality, intensity, and frequency (38); and bottlenose dolphins (Tursiops truncatus) change the beam width and direction in response to the perceptual problem presented to them (39). Although it has not been shown that toothed whales can modify their beam rapidly and dynamically in response to click-echo feedback—as bats may be able to—the fact that they do adjust directionality in different situations corroborates our theory that directionality of the outgoing sound pulse is essential to the function of the sonar. Also, data from nonecholocating animals point to the importance of flexible directionality control. Red-winged blackbirds vary the directionality of their song to suit a given context emitting omnidirectional alert signals to warn multiple conspecifics but directional signals during courtship to reduce eavesdropping (3, 4). Thus, we conclude that directionality and directional flexibility are important features of acoustic signals in general, not only of biosonar signals. We predict that future studies of acoustic communication will corroborate the significance of directionality for all animals using acoustic signals.
Materials and Methods
We trained six bats to capture tethered mealworms in a laboratory setting: five M. daubentonii (average weight, 8 g) and one E. serotinus (weight, 24 g). All recordings were performed in a 7 × 4.8 × 2.4-m flight room at the University of Southern Denmark in June 2007 and March to April 2010.
The research in the present study adhered to the Association for the Study of Animal Behaviour/Animal Behaviour Society Guidelines for the Use of Animals in Research (published on the Animal Behaviour Web site), the legal requirements of Denmark (where the work was carried out), and all institutional guidelines.
Recordings.
The echolocation calls emitted by M. daubentonii were recorded with an array of 12 quarter-inch 40 BF G.R.A.S. microphones (grids removed) amplified 40 dB by 12 AA G.R.A.S. amplifiers with 13.5 kHz build-in high-pass filters and sampled at 350 kHz by a 12-channel A/D converter (Avisoft 1216 ultrasound gate). The microphones were arranged as a cross with seven microphones placed on a horizontal line 40 cm apart (approximately 150 cm above the ground) and six microphones on a vertical line (also 40 cm apart), with two placed above the center microphone and three below it (Fig. 1). For E. serotinus, we recorded the echolocation calls with a seven-microphone array, with six on a horizontal line and one placed above the third microphone from the left. To get enough off-axis recordings even when the bat was far away, the distance between the microphones was adjusted according to the distance from bat to array, such that we used 70-cm microphone spacing for approach calls and 35 cm for buzz call recordings. For E. serotinus, the microphones and amplifiers were the same as for M. daubentonii, but for A/D conversion we used two 512 Wavebooks (sampling rate, 250 kHz/channel; IOtech) synchronized by external triggering. A single mealworm was suspended in front of the array at level with the horizontal microphones, approximately 75 cm from the array (100 cm from the large array for E. serotinus).
We recorded 1.5-s files, 1 s before trigger and 0.5 s after trigger. Triggering occurred at approximately the time of capture. Additionally, we videotaped each trial using a MotionPro X-4 high-speed video camera (Redlake) or a Sony digital night-shot video camera. We used video recordings to ensure that the bats were not banking during the capture attempt, which would change the plane in which the beam pattern was recorded. Microphones were calibrated daily using a sound calibrator (type 4231; Brühl & Kjaer).
Positioning and Beam Aim.
The bats positions were estimated at each sound emission using the time of arrival differences on the microphones (6). We filtered the recordings using third-octave band-pass filters centered on the approximate peak frequency of the approach call for each species and of the low component of the terminal phase calls, i.e., 55 kHz and 27.5 kHz for M. daubentonii and 35 kHz and 17.5 kHz for E. serotinus. The rms pressure of each recorded signal was calculated for each third-octave band-pass filter and the rms pressures were compensated for spherical spreading loss [20×log10(distance/10 cm)], atmospheric attenuation (40) and angle of incidence on the microphones (41).
We determined the beam aim, the direction the bat was aiming its beam by computing the horizontal and vertical angle from the bat's position at the time of the call to each recording microphone. We then fitted the compensated rms pressure as a function of the recorded angle to a second-order polynomial by least squares in the horizontal and vertical plane and the peak of the polynomial was used as a proxy for the beam direction. We did this for the high- and low-frequency bands separately. For the recordings of M. daubentonii, the estimated beam aim had to be within 10° of the center microphone for a call to be included in the analysis. For E. serotinus, it had to be within the outer two microphones.
We estimated detection space (Fig. 4) from the emitted intensities, the directional patterns, an assumed detection threshold of 20 dB for the bat, and a target-strength for the prey of −40 dB at 10 cm (42).
Acknowledgments
We thank John Ratcliffe and Simon Boel Pedersen for valuable comments on the manuscript. This study was funded by the Oticon Foundation, the Danish Council for Natural Sciences (FNU), Wissenschaftskolleg zu Berlin, Carlsberg, and the European Commission via the Seventh Framework Programme project ChiRoPing, Information Society Technologies Contract 215370.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.McGregor PK, Otter KA, Peake TM. Communication networks: receiver and signaller perspectives. In: Espmark Y, Amundsen T, Rosenqvist G, editors. Animal Signals: Signalling and Signal Design in Animal Communication. Trondheim, Norway: Tapir Academic Press; 2000. pp. 329–340. [Google Scholar]
- 2.Arch VS, Narins PM. “Silent” signals: Selective forces acting on ultrasonic communication systems in terrestrial vertebrates. Anim Behav. 2008;76:1423–1428. doi: 10.1016/j.anbehav.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patricelli GL, Dantzker MS, Bradbury JW. Differences in acoustic directionality among vocalizations of the male red-winged blackbird (Agelaius pheoniceus) are related to function in communication. Behav Ecol Sociobiol. 2007;61:1099–1110. [Google Scholar]
- 4.Patricelli GL, Dantzker MS, Bradbury JW. Acoustic directionality of red-winged blackbird (Agelaius phoeniceus) song relates to amplitude and singing behaviours. Anim Behav. 2008;76:1389–1401. [Google Scholar]
- 5.Rasmussen MH, Wahlberg M, Miller LA. Estimated transmission beam pattern of clicks recorded from free-ranging white-beaked dolphins (Lagenorhynchus albirostris) J Acoust Soc Am. 2004;116:1826–1831. doi: 10.1121/1.1775274. [DOI] [PubMed] [Google Scholar]
- 6.Surlykke A, Boel Pedersen S, Jakobsen L. Echolocating bats emit a highly directional sonar sound beam in the field. Proc Biol Sci. 2009;276:853–860. doi: 10.1098/rspb.2008.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neuweiler G. Auditory adaptations for prey capture in echolocating bats. Physiol Rev. 1990;70:615–641. doi: 10.1152/physrev.1990.70.3.615. [DOI] [PubMed] [Google Scholar]
- 8.Schnitzler H-U, Moss CF, Denzinger A. From spatial orientation to food acquisition in echolocating bats. Trends Ecol Evol. 2003;18:386–394. [Google Scholar]
- 9.Griffin DR, Webster FA, Michael CR. The echolocation of flying insects by bats. Anim Behav. 1960;3:141–154. [Google Scholar]
- 10.Hartley DJ. Stabilization of perceived echo amplitudes in echolocating bats. I. Echo detection and automatic gain control in the big brown bat, Eptesicus fuscus, and the fishing bat, Noctilio leporinus . J Acoust Soc Am. 1992;91:1120–1132. doi: 10.1121/1.402639. [DOI] [PubMed] [Google Scholar]
- 11.Griffin DR. Listening in the Dark. 2nd Ed. New Haven, CT: Yale Univ Press; 1958. [Google Scholar]
- 12.Shimozawa T, Suga N, Hendler P, Schuetze S. Directional sensitivity of echolocation system in bats producing frequency-modulated signals. J Exp Biol. 1974;60:53–69. doi: 10.1242/jeb.60.1.53. [DOI] [PubMed] [Google Scholar]
- 13.Hartley DJ, Suthers RA. The sound emission pattern of the echolocating bat, Eptesicus fuscus . J Acoust Soc Am. 1989;85:1348–1351. doi: 10.1121/1.395684. [DOI] [PubMed] [Google Scholar]
- 14.Henze D, O'Neill WE. The emission pattern of vocalizations and directionality of the sonar system in the echolocating bat, Pteronotus parnelli . J Acoust Soc Am. 1991;89:2430–2434. doi: 10.1121/1.400975. [DOI] [PubMed] [Google Scholar]
- 15.Strother GK, Mogus M. Acoustical beam patterns for bats: Some theoretical considerations. J Acoust Soc Am. 1970;48(suppl 2) doi: 10.1121/1.1912304. [DOI] [PubMed] [Google Scholar]
- 16.Schnitzler HU, Kalko EKV, Miller LA, Surlykke A. The echolocation and hunting behavior of the bat, Pipistrellus kuhli. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1987;161:267–274. doi: 10.1007/BF00615246. [DOI] [PubMed] [Google Scholar]
- 17.Moss CF, Bohn K, Gilkenson H, Surlykke A. Active listening for spatial orientation in a complex auditory scene. PLoS Biol. 2006;4:e79. doi: 10.1371/journal.pbio.0040079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Britton AR, Jones G. Echolocation behaviour and prey-capture success in foraging bats: Laboratory and field experiments on Myotis daubentonii. J Exp Biol. 1999;202:1793–1801. doi: 10.1242/jeb.202.13.1793. [DOI] [PubMed] [Google Scholar]
- 19.Schnitzler HU, Kalko EKV. Echolocation by insect-eating bats. Bioscience. 2001;51:557–569. [Google Scholar]
- 20.Kalko EKV, Schnitzler HU. The echolocation and hunting behavior of Daubenton's bat, Myotis daubentoni . Behav Ecol Sociobiol. 1989;24:225–238. [Google Scholar]
- 21.Faure PA, Barclay RMR. Substrate-gleaning versus aerial-hawking: Plasticity in the foraging and echolocation behaviour of the long-eared bat, Myotis evotis . J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1994;174:651–660. doi: 10.1007/BF00217386. [DOI] [PubMed] [Google Scholar]
- 22.Miller LA, Degn HJ. The acoustic behavior of four species of vespertilionid bats studied in the field. J Comp Physiol. 1981;142:67–74. [Google Scholar]
- 23.Jensen ME, Miller LA. Echolocation signals of the bat Eptesicus serotinus recorded using a vertical microphone array: Effect of flight altitude on searching signals. Behav Ecol Sociobiol. 1999;47:60–69. [Google Scholar]
- 24.Mogensen F, Møhl B. Sound radiation patterns in the frequency domain of cries from a Vespertilionid bat. J Comp Physiol. 1979;134:165–171. [Google Scholar]
- 25.Miller LA, Surlykke A. How some insects detect and avoid being eaten by bats: Tactics and countertactics of prey and predator. Bioscience. 2001;51:570–581. [Google Scholar]
- 26.Ghose K, Moss CF. The sonar beam pattern of a flying bat as it tracks tethered insects. J Acoust Soc Am. 2003;114:1120–1131. doi: 10.1121/1.1589754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Surlykke A, Ghose K, Moss CF. Acoustic scanning of natural scenes by echolocation in the big brown bat, Eptesicus fuscus . J Exp Biol. 2009;212:1011–1020. doi: 10.1242/jeb.024620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinbeer M, Kalko EKV. Ecological niche and phylogeny: The highly complex echolocation behavior of the trawling long-legged bat, Macrophyllum macrophyllum . Behav Ecol Sociobiol. 2007;61:1337–1348. [Google Scholar]
- 29.Simmons JA, Fenton MB, O'Farrell MJ. Echolocation and pursuit of prey by bats. Science. 1979;203:16–21. doi: 10.1126/science.758674. [DOI] [PubMed] [Google Scholar]
- 30.Mora EC, Macías S, Vater M, Coro F, Kössl M. Specializations for aerial hawking in the echolocation system of Molossus molossus (Molossidae, Chiroptera) J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004;190:561–574. doi: 10.1007/s00359-004-0519-2. [DOI] [PubMed] [Google Scholar]
- 31.Guillen-Servent A, Ibanez I. Unusual echolocation behavior in a small molossid bat, Molossops temminckii, that forages near background clutter. Behav Ecol Sociobiol. 2007;61:1599–1613. [Google Scholar]
- 32.Jones G, Webb PI, Sedgeley JA, O'Donnell CFJ. Mysterious Mystacina: How the New Zealand short-tailed bat (Mystacina tuberculata) locates insect prey. J Exp Biol. 2003;206:4209–4216. doi: 10.1242/jeb.00678. [DOI] [PubMed] [Google Scholar]
- 33.Surlykke A, et al. Echolocation in two very small bats from Thailand: Craseonycteris thonglongyai and Myotis siligorensis . Behav Ecol Sociobiol. 1993;33:1–12. [Google Scholar]
- 34.Ibáñez C, Juste J, López-Wilchis R, Albuja VL, Núñez-Garduño A. Echolocation of three species of sac-winged bats (Balantiopteryx) J Mammal. 2002;83:1049–1057. [Google Scholar]
- 35.Schnitzler H-U, Kalko EKV, Denzinger A. Evolution of echolocation in bats. In: Thomas J, Moss CF, Vater M, editors. Echolocation in Bats and Dolphins. Chicago: Univ of Chicago Press; 2004. pp. 331–339. [Google Scholar]
- 36.Jones G, Teeling EC. The evolution of echolocation in bats. Trends Ecol Evol. 2006;21:149–156. doi: 10.1016/j.tree.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Hartley DJ, Suthers RA. The sound emission pattern and the acoustical role of the noseleaf in the echolocating bat, Carollia perspicillata . J Acoust Soc Am. 1987;82:1892–1900. doi: 10.1121/1.395684. [DOI] [PubMed] [Google Scholar]
- 38.Madsen PT, Kerr I, Payne R. Echolocation clicks of two free-ranging, oceanic delphinids with different food preferences: False killer whales Pseudorca crassidens and Risso's dolphins Grampus griseus . J Exp Biol. 2004;207:1811–1823. doi: 10.1242/jeb.00966. [DOI] [PubMed] [Google Scholar]
- 39.Moore PW, Dankiewicz LA, Houser DS. Beamwidth control and angular target detection in an echolocating bottlenose dolphin (Tursiops truncatus) J Acoust Soc Am. 2008;124:3324–3332. doi: 10.1121/1.2980453. [DOI] [PubMed] [Google Scholar]
- 40.American National Standards Institute . American National Standard. Method for the Calculation of the Absorption of Sound by the Atmosphere ANSI S1.26-1978, American Institute of Physics (for Acoustical Society of America) New York: American National Standards Institute; 1978. [Google Scholar]
- 41.Brüel & Kjær . Condenser Microphones and Microphone Preamplifiers for Acoustic Measurements: Data Handbook. Nærum, Denmark: Brüel & Kjær; 1982. [Google Scholar]
- 42.Surlykke A, Filskov M, Fullard JH, Forrest E. Auditory relationships to size in noctuid moths: Bigger is better. Naturwissenshaften. 1999;86:238–241. [Google Scholar]