Abstract
Chloroplast DNA (cpDNA) is under great photooxidative stress, yet its evolution is very conservative compared with nuclear or mitochondrial genomes. It can be expected that DNA repair mechanisms play important roles in cpDNA survival and evolution, but they are poorly understood. To gain insight into how the most severe form of DNA damage, a double-strand break (DSB), is repaired, we have developed an inducible system in Arabidopsis that employs a psbA intron endonuclease from Chlamydomonas, I-CreII, that is targeted to the chloroplast using the rbcS1 transit peptide. In Chlamydomonas, an I-CreII-induced DSB in psbA was repaired, in the absence of the intron, by homologous recombination between repeated sequences (20–60 bp) abundant in that genome; Arabidopsis cpDNA is very repeat poor, however. Phenotypically strong and weak transgenic lines were examined and shown to correlate with I-CreII expression levels. Southern blot hybridizations indicated a substantial loss of DNA at the psbA locus, but not cpDNA as a whole, in the strongly expressing line. PCR analysis identified deletions nested around the I-CreII cleavage site indicative of DSB repair using microhomology (6–12 bp perfect repeats, or 10–16 bp with mismatches) and no homology. These results provide evidence of alternative DSB repair pathways in the Arabidopsis chloroplast that resemble the nuclear, microhomology-mediated and nonhomologous end joining pathways, in terms of the homology requirement. Moreover, when taken together with the results from Chlamydomonas, the data suggest an evolutionary relationship may exist between the repeat structure of the genome and the organelle's ability to repair broken chromosomes.
Keywords: DNA repair, evolution, homing endonuclease, I-CreII, plastid DNA
Chloroplast DNA (cpDNA) is closely associated with the photosynthetic membranes that harvest radiant energy and strip electrons from water, producing molecular oxygen as a byproduct (1, 2). Highly reactive forms of oxygen are also generated, and despite the presence of detoxifying enzymes, photooxidative damage is a serious problem. Although there has been extensive study of the damage, protection, and repair of photosynthetic membranes, there has been little attention paid to the genetic consequences of photooxidative stress (3). This can be attributed, at least in part, to a grossly incomplete knowledge of how cpDNA is replicated and maintained throughout the life of a plant (3, 4).
However, despite the high-stress environment, the evolution of cpDNA is more conservative than nuclear or mitochondrial DNA (5). We can thus infer that DNA repair mechanisms play important roles in protecting cpDNA and are intimately involved in its evolution. And although some processes have been identified (6–11), the panoply of organelle repair mechanisms is not known, nor is it clear which are most important, or how they have coevolved with the genome. Although we expect that some cpDNA repair processes will reflect its prokaryotic ancestry, the consequences of eukaryotic cell integration and >1 billion years of evolution have surely produced some unique modifications to the repair machinery.
The most severe form of DNA damage is a double-strand break (DSB), which can result in gene loss, stalled DNA replication, and cell death (12). DSBs are caused by ionizing radiation, chemicals, oxidation, enzymes, and single-strand breaks during replication (12, 13). Mechanisms of DSB repair (diagrammed in Fig. S1) have been divided into two basic types, nonhomologous end joining (NHEJ) and homologous recombination (HR). NHEJ is the dominant nuclear response in animals and plants—it does not require homologous sequences, but is often mutagenic (14, 15). Repair by HR requires substantial homology, but the two pathways that use an intact chromosome to repair the broken one, double-strand break repair and synthesis-dependent strand annealing, are highly accurate. The third HR pathway, single-strand annealing (SSA), occurs between direct repeats >30 bp and results in deletions; in plants, it is favored over the other HR pathways (16). In recent years, microhomology-mediated end joining (MMEJ) has been recognized as a distinct type of DSB repair in eukaryotes. Only very short (2–14 bp) regions of homology are needed for this pathway, and it typically leaves deletions like SSA. It has also been distinguished genetically from the HR and NHEJ pathways and in mammalian cells acts as a backup to NHEJ (17–19).
Group I intron homing involves the repair of a DSB induced by the intron's rare-cutting endonuclease and results in gene conversion (7). Recently, we used a homing intron and its endonuclease (I-CreII) to examine DSB repair in the Chlamydomonas chloroplast—evidence of all three HR pathways was obtained, but not for NHEJ or MMEJ (20). In the absence of the intron, repair of the DSB was mostly by SSA between intergenic repeats of 20–60 bp that are abundant in that cp genome (21). Compared with Chlamydomonas, however, the cpDNA of land plants, especially Arabidopsis, is repeat poor (21, 22); e.g., REPuter (23) analysis of the 155-kb Arabidopsis genome revealed only 31 direct repeats >29 bp, and half of them map to the same small region of the genome (gb NC_000932). Thus, we were interested to see how this land plant chloroplast would handle a similar DSB, while also recognizing its potential for further study. To this end, an inducible system was developed based on I-CreII, which is targeted to the chloroplast with the transit peptide of rbcS1, and wherein, it cleaves the endogenous psbA gene (24–27).
Results
Generating Plants with an Inducible, Chloroplast-Targeted I-CreII.
The I-CreII endonuclease was expressed from the nucleus using chemical induction (24): the application of β-estradiol induces transcription of the Cre recombinase (via the XVE receptor-activator), which catalyzes a reaction between loxP sites, which places the G10-90 promoter upstream of the promoterless target gene, rbcS:I-CreII:GFP. This vector was chosen because unscheduled expression of the endonuclease was expected to be lethal. I-CreII was localized to the chloroplast with the N-terminal (amino acids 1–59) transit peptide of the rbcS1 gene (26, 27), and GFP was fused to the C terminus for possible fluorescence detection. It should be noted that the same construct, but without GFP, was also used, but because the initial results with each variant were comparable, we focused on rbcS:I-CreII:GFP.
Before creating transgenic plants, we verified that the rbcS:I-CreII:GFP construct had endonuclease activity using in vivo and in vitro assays. The in vivo assay was based on plasmid exclusion, wherein a target-site plasmid cannot be maintained in the same Escherichia coli cells as an active homing endonuclease plasmid, even if the plasmids have different selectable markers (28); the results with the I-CreII fusion constructs were included in Fig. S2). Although the fusion proteins were mostly insoluble when overexpressed in E. coli, there was sufficient soluble activity in crude extracts to detect specific cleavage of the pE4E5 substrate DNA in vitro (Fig. S3).
The rbcS:I-CreII:GFP plasmid and parental vector were introduced into Arabidopsis thaliana (Col-0 strain) using Agrobacterium-mediated transformation (29). Healthy kanamycin-resistant lines possessing the T-DNA insertion were obtained, and subsequently germinated on β-estradiol (and control medium) to identify those that inducibly expressed rbcS:I-CreII:GFP. The inducible phenotypes could be classified as either strong or weak, and an example of each is shown in Fig. 1B. Compared with the vector-only plants, the strong line, 243, had very poor shoot and leaf development, whereas the weaker line, 347, developed variegated leaves early in development, but then normally pigmented leaves thereafter. It should be said that the transgenic lines used in Fig. 1B, and in the remaining analyses, were homozygous for the T-DNA insertion, on the basis of segregation analysis. The phenotypes, however, are similar to those observed in the original hemizygotes, because of the dominant nature of the transgene.
Fig. 1.
I-CreII expression system and RT-PCR analysis of transgenic lines. (A) Diagram of pX6RIIIG before and after β-estradiol induction. PCR primers are also indicated. XVE, β-estradiol–response activator of CRE; KAN, kanamycin resistance; CRE, recombinase that acts on the loxP sites; rbcS:I-CreII:GFP, promoterless I-CreII fusion. (B) Transgenic plants germinated on induction medium for 2 wk. The Vector line was transformed with the parent plasmid and lines 243 and 347 with pX6RIIIG. (Scale bar, 3 mm.) (C) RT-PCR analysis of transgenic lines grown for 2 wk on control (−β-estradiol) and induction (+ β-estradiol) medium. The RT-PCR primers were for the I-CreII portion of rbcS:I-CreII:GFP (Top), and an actin (Act2) control gene. Size markers are indicated to the Right.
RT-PCR analysis indicated that both lines (243 and 347) displayed inducible expression of the I-CreII transgene, but the signal was reproducibly greater, relative to the control (actin) mRNA, in the stronger 243 line (Fig. 1C). No product was obtained with the Vector line, as expected. The RT-PCR analysis also indicated that there was some basal expression of rbcS:I-CreII:GFP in line 243 without β-estradiol, but those plants did not exhibit a variegated phenotype like 347. The level of GFP fluorescence was too low for microscopic detection in these induced lines, and in nearly all of the rbcS:I-CreII:GFP plants we examined, except for one that could not be sustained. The GFP fluorescence in that case was associated with plastids that lacked chlorophyll autofluorescence, indicative of severe damage (Fig. S4). Most likely, plants that had enough rbcS:I-CreII:GFP expression to allow visualization of sustained GFP fluorescence were not viable.
I-CreII–Induced Damage and Repair at the psbA Locus.
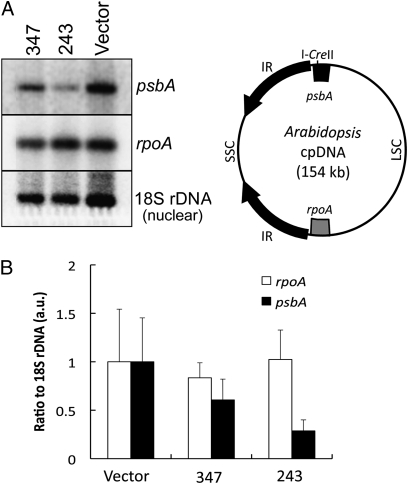
The recognition sequence for I-CreII is an ∼30-bp region in the highly conserved psbA gene (25), which is located in the large single-copy region, close to an inverted repeat (22) (Fig. 2A). Southern blot hybridizations of total DNA digested with restriction enzymes indicated only a slight loss of DNA at this locus in the 347 line, but a substantial (∼70%) reduction in line 243, relative to nuclear 18S rDNA (Fig. 2 and Fig. S5). Hybridizations with the rpoA gene, whose cpDNA location is far from psbA (Fig. 2A), indicated the reduction in psbA signal was not due to a general loss of cpDNA, because the rpoA/18S rDNA ratio was similar in all three plant lines. Thus, a preferential loss of psbA DNA had occurred in the stronger 243 line.
Fig. 2.
Hybridization analysis of transgenic plants expressing rbcS:I-CreII:GFP. Equal amounts of DNA from the rbcS:I-CreII:GFP (347 and 243) and vector-transformed (Vector) lines, grown on β-estradiol, were restricted, Southern blotted, and hybridized to the indicated genes. The blots were hybridized to each probe sequentially, after stripping of the previous probe. (A) Hybridization to SalI digests. The bands were 9.6 (psbA), 11.4 (rpoA), and 6 kb (18S), respectively. The full-length blot images are available as Fig. S5. The respective locations of the two plastid genes are indicated on the circular map. LSC, large single-copy region; SSC, small single-copy region; IR, inverted repeat. (B) Quantification of the ratios of the rpoA and psbA signals to 18S rDNA. The ratios are means (±SD, n = 3) obtained by hybridization to SalI, XhoI, and SalI + XhoI digests.
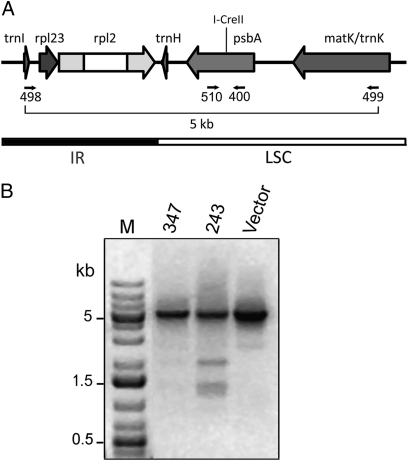
To precisely characterize the deletions (or other possible changes) in line 243, trial PCR reactions were performed with nested primers that flanked the I-CreII cleavage site and annealed ∼5, 2.5, and 0.25 kb, respectively, from that site. Only the primers that annealed ∼2.5 kb from the cleavage site were informative. They gave additional PCR products that were smaller than the 5-kb wild-type product, which was also correspondingly reduced in line 243 (Fig. 3). The smaller products were not seen with the Vector line. They were faintly visible (on the original gels) with DNA from line 347, but to facilitate further analysis only PCR products from line 243 were sequenced. It is worth mentioning that the sequence of a small product (∼0.4 kb) amplified from 243 with primers that annealed close to the cleavage site (400 and 510 in Fig. 3) was indistinguishable from wild type, making it unlikely that the large, 5-kb product in Fig. 3 contained many repair products with very short deletions or insertions.
Fig. 3.
PCR analysis at the psbA locus in plants expressing rbcS:I-CreII:GFP. Total DNA from the plants grown on β-estradiol (as in Fig. 2) was used for PCR with the indicated primers (498 and 499). (A) Map of the psbA region, with PCR primers and I-CreII cleavage site. MatK is the protein encoded within the trnK intron. (B) Agarose gel of the PCR products. Lane M contained size markers, and the ethidium-DNA fluorescence image was inverted.
The major induced PCR products, which were 1.9, 1.3, and 1.2 kb (Fig. 3), were excised from gels for direct sequencing and cloning. Also, the total PCR products were gel separated into three size fractions, which were cloned and sequenced. The results from all of this analysis are summarized in Table 1 and in Fig. 4, which provides a map of the paired deletion endpoints. First, it should be said that all of the mutant products had a deletion (no insertions were observed), which ranged in size from 3.1 to 4.8 kb. The fact that the paired endpoints for all these deletions map to either side of the I-CreII site suggests strongly that they are associated with a DSB. It is also apparent from the map (Fig. 4) that all of the deletions encompassed the trnH gene and included at least 50% of the psbA and rpl2 genes—in fact, psbA was completely deleted in most of them. These mutations, if accumulated to a sufficient level, would impact both plastid translation (trnH and rpl2) and photosynthesis (psbA), and could explain the poor shoot development in 243 (30).
Table 1.
Summary of microhomologous (microrepeats) and nonhomologous repair junctions
| Microrepeat* | PCR product (kb) | Deletion (kb) | Repeat size/mismatches | Repeat position† | Junction sequences‡ | No. of clones |
| 1§ | 1.2 | 3.8 | 12 | 152,604 | taggacAGAAATAAAGCAttgggt | — |
| taggacAGAAATAAAGCActtttg | 6 | |||||
| 1,953 | aataatAGAAATAAAGCActtttg | — | ||||
| 2 | 1.3 | 3.7 | 11 | 153,275 | tcactgATCCAATTTGAgtacct | — |
| tcactgATCCAATTTGAttctaa | 15 | |||||
| 2,485 | tttgctATCCAATTTGAttctaa | — | ||||
| 3 | 0.4 | 4.6 | 9 | 152,364 | aagtctATTGGAATTggctct | — |
| aagtctATTGGAATTttgcta | 1 | |||||
| 2,471 | gaagaaATTGGAATTttgcta | — | ||||
| 4 | 1.5 | 3.5 | 8 | 153,244 | aattagGTTTACGAcgaaac | — |
| aattagGTTTACGAgccaaa | 1 | |||||
| 2,244 | ttttgtGTTTACGAgccaaa | — | ||||
| 5 | 2.0 | 3.1 | 6 | 152,862 | cgtagaCAGTCAagtgaa | — |
| cgtagaCAGTCAtggtaa | 1 | |||||
| 1,438 | aaattgCAGTCAtggtaa | — | ||||
| 6§ | 1.3 | 3.7 | 16/2 | 153,165 | tataaaAATGGGAAATGCCCTAcctttg | — |
| tataaaAATGGGAAATGCCCTAatacat | 1 | |||||
| tataaaAATGGGA--TGCCCTAatacat | 3 | |||||
| 2,410 | tttactAATGGGA--TGCCCTAatacat | — | ||||
| 7 | 0.4 | 4.6 | 15/2 | 152,493 | tagggaAGAAAATCGATTTATggatgg | — |
| tagggaAGAAAATCGATTTATattgac | 0 | |||||
| tagggaAGATAATTGATTTATattgac | 2 | |||||
| 2,602 | atctttAGATAATTGATTTATattgac | — | ||||
| 8§ | 1.9 | 3.1 | 13/1 | 152,553 | tcggttATTGGGGAAAAATcaatat | — |
| tcggttATTGGGGAAAAATgcaatc | 1 | |||||
| tcggttATTGGGTAAAAATgcaatc | 6 | |||||
| 1,158 | tcccagATTGGGTAAAAATgcaatc | — | ||||
| 9 | 0.9 | 4.1 | 13/1 | 152,982 | gcgtctATACCGTAAAATAgatttt | — |
| gcgtctATACCGTAAAATAacattg | 0 | |||||
| gcgtctATACCGAAAAATAacattg | 7 | |||||
| 2,640 | gaaaccATACCGAAAAATAacattg | — | ||||
| 10 | 1.6 | 3.4 | 11/1 | 153,807 | gaaagcCGTATGCTTTGgaagaa | — |
| gaaagcCGTATGCTTTGttgcca | 1 | |||||
| gaaagcCGTATCCTTTGttgcca | 0 | |||||
| 2,704 | aagcggCGTATCCTTTGttgcca | — | ||||
| 11 | 1.5 | 3.5 | 11/1 | 153,184 | ggaaatGCCCTACCTTTgagtgc | — |
| ggaaatGCCCTACCTTTttaaaa | 2 | |||||
| ggaaatGCCCAACCTTTttaaaa | 0 | |||||
| 2,206 | accagaGCCCAACCTTTttaaaa | — | ||||
| 12 | 1.7 | 4.4 | 10/1 | 152,638 | tttggtGTCAAGGTAAtagcta | — |
| tttggtGTCAAGGTAAaatcct | 1 | |||||
| tttggtGTCATGGTAAaatcct | 0 | |||||
| 1,440 | attgcaGTCATGGTAAaatcct | — |
| Nonhomologous junction¶ | PCR product (kb) | Deletion (kb) | Position of junction nucleotide | Junction sequences | No. of clones |
| A | 0.6 | 4.4 | 152,686 | aaaggttaaaAgaatgggacc | — |
| aaaggttaaaAAttgccataa | 1 | ||||
| 2,655 | cgaaaaataacAttgccataa | — | |||
| B | 0.6 | 4.5 | 152,770 | tccacctcttAgaaagaaaag | — |
| tccacctcttAAtccttgagc | 1 | ||||
| 2,761 | tgtatgaaaggAtccttgagc | — | |||
| C | 0.2 | 4.8 | 152,446 | tcataacataTgaacagtaag | — |
| tcataacataTTccttgagca | 1 | ||||
| 2,762 | gtatgaaaggaTccttgagca | — | |||
| D | 0.2 | 4.8 | 152,277 | tccatggctgAatggttaaag | — |
| tccatggctgATtatattgac | 1 | ||||
| 2,614 | agataattgatTtatattgac | — |
*The repeats are the same as Fig. 4.
†The numbers are from the cpDNA sequence and refer to the first nucleotide of the repeat, which is in uppercase in the adjacent sequence column.
‡Potential (and observed) repair products are in italics. In some cases (i.e. repeats with mismatches) only one of the two possible repair products were found. The repeat nt, and the nonhomologous junction nt, are in uppercase, and the mismatched nt are underlined.
§Microrepeats associated with the prominent PCR products in Fig. 3.
¶The junctions correspond to those in Fig. 4.
Fig. 4.
Map of the microrepeats and nonhomologous DNA junctions involved in repair of the DSB. Microrepeats 1–12 and junctions A–D correspond to those in Table 1. The nucleotide numbering is from Arabidopsis cpDNA (gb NC_000932), and the hybridization probe was used in Fig. 2.
The junction sequences (Table 1) suggest that more than one process may be at work. Most of the repair events involved microhomology between the partners in the form of direct microrepeats—the perfect repeats were only 6–12 bp (microrepeats 1–5), whereas the imperfect repeats were 10–16 bp (microrepeats 6–12); the longer imperfect repeats (15–16 bp) had two mismatches, whereas the shorter ones had only one. It is noteworthy that microrepeats 1, 6, and 8 correspond to the dominant sequences obtained from the three major PCR products (lane 243 in Fig. 3). The fact that microrepeat 2 is highly represented in the sequenced clones (Table 1) probably reflects bias in the cloning process. The similarity of these repair junctions with those of the MMEJ pathway in the nucleus is striking (17, 18, 31).
We also detected several repair events that could not be explained by microhomology between the DNA partners (junctions A–D in Table 1), and hence may represent a form of nonhomologous repair, like NHEJ. This seems to be a quantitatively minor pathway under these conditions. However, the large sizes of those deletions could indicate an important backup role to the microhomology-mediated pathway.
Discussion
Transgenic homing endonucleases have been used extensively to study the repair of DSBs in nuclear chromosomes (16, 31, 32), but this reported usage for a cell organelle is unique. Transgenic restriction endonucleases have been used to damage mitochondrial DNA in animal cells (33), presumably because a homing enzyme target could not be introduced. Here, we used a relatively new homing endonuclease from a Chlamydomonas chloroplast intron (25) and retargeted it to the plastid. Because it cleaves the highly conserved psbA gene, I-CreII could likely be used on many other plants. Finally, using the steroid-inducible system (24) may also have been important, especially considering the severe growth impairment of the higher-expressing line, 243.
Unlike intron homing, which alters the target DNA, repair of this DSB by a similar HR pathway would restore the target, which cannot be distinguished from DNA that was never cleaved. Hence, we can only infer repair events that have changed the target. Given the deficit of repeated sequences >25 bp in Arabidopsis cpDNA, we were unsure how, or even if, the chromosome would become religated after the break. In the nucleus, repair with such limited or no homology occurs by pathways distinct from the HR pathways, and these processes were not known to occur in chloroplasts. However, these data clearly show that the organelle can repair a DSB using very limited homology, and with reasonable efficiency, based on the fact that there was little loss of cpDNA as a whole. Most of the repair events were mediated by microhomology between the partner DNAs, reminiscent of nuclear MMEJ (17–19). Several repair events, however, lacked any mediating homology, indicative of an NHEJ-like mechanism (12, 14). It is possible these latter DNAs are actually products of a microhomology-based machinery that can occasionally use nonhomologous DNA ends. Alternatively, the nonhomologous products are from a distinct pathway that, on the basis of deletion sizes, might back up the MMEJ-like pathway. Such a hierarchy would be the opposite of that in animal cells, where MMEJ backs up NHEJ (18).
An obvious question provoked by these experiments concerns the nature of the mediating proteins. The conserved Ku proteins, which are associated with NHEJ even in bacteria (34), have not been found in chloroplasts, including Arabidopsis (35, 36). Moreover, the substantial size of these deletions and those in Chlamydomonas are consistent with the absence of strong end-protecting proteins like Ku. On the other hand, the deletions are suggestive of roles for a 5′-to-3′ exonuclease, to resect the DNA and create 3′ tails, and for an endonuclease to remove the 3′ flaps (17). Presumably, one or more proteins would be needed to promote microannealing, but it is not clear what any of these proteins are in the chloroplast. Arabidopsis should be a promising system in which to identify them, however.
Previously, the lack of evidence for NHEJ repair in Chlamydomonas (20), or associated with an insertion element in tobacco (37), prompted the suggestion that the absence of this ability might account for the lack of horizontally acquired DNA in green plant chloroplasts. These results weaken that hypothesis, but stop short of negating it, because the NHEJ-like repair events were only a minor fraction of the total. It may also be relevant that we did not see any insertions of foreign (i.e., nuclear or mitochondrial) DNA in the break site; however, the laborious analysis of repair events limited the number that could be reasonably examined. Along this line, there are other factors that could limit horizontal transfer into cpDNA, e.g., the double-membrane envelope surrounding the chloroplast is a more formidable barrier than the nuclear envelope. Also, mitochondria have a peculiar tendency for fusion–subdivision cycles that might increase their exposure to foreign DNA (38). Finally, there are examples of horizontally acquired introns, and recently, whole genes in the cpDNA of unicellular green algae (39, 40). The equivalence of germ-line and vegetative cells in those organisms undoubtedly increases the probability of passing on horizontally acquired DNA.
The commonly held notion that cpDNA is circular rather than linear was challenged recently, on the basis of physical analysis of DNA from isolated plastids (41). The stabilization and replication of linear DNA typically requires special structured end sequences (e.g., telomeres), some of which are known from organelles (42, 43). However, such sequences have not been identified for a chloroplast chromosome. These results support the notion that stabilizing end structures would be needed to maintain linear cpDNA.
Repetitive DNA is an important feature of all genomes, and its proportion can vary considerably (44). The cpDNA of Chlamydomonas is relatively repeat rich (21), and the I-CreII–induced deletions described previously implicated perfect repeats of 15–62 bp, with a strong bias for repeats >30 bp (20). Despite the paucity of such repeats in Arabidopsis, the I-CreII–induced DSB was repaired efficiently, and the sizes of the accompanying deletions were similar to those obtained in Chlamydomonas. We suggest that evolution has endowed Arabidopsis, and probably other land plants, with the ability to repair DSBs without extensive homology, and that this ability may have impacted the evolution of cpDNA by reducing the need for sizable (>20 bp) repeats.
Materials and Methods
Plant Growth and Transformation.
A. thaliana ecotype Columbia (Col-0) and transgenic plants were grown at 23 °C under continuous light (150 μmol quanta m−2 × s−1) either axenically in Murashige-Skoog (MS) medium (45) or in soil. For induction, MS was supplemented with 1% sucrose and inducer plates received 10 μM β-estradiol (Sigma). Agrobacterium tumefaciens GV3101 (pMP90) was used for plant transformation by floral dip (29).
Plasmid Construction.
The N-terminal 59 aa of rbcS1a (AT1G67090) was amplified using oligos 485 and 486 (Table S1), which have XhoI and AfeI sites, from TAIR U13397 (26) obtained from the Arabidopsis Biological Resource Center (The Ohio State University). I-CreII was amplified from pI-CreII (25) using 488 and 489 (Table S1), which removed the stop codon and added AfeI and AvrII sites. GFP was amplified from pX6GFP (24) using oligos 490 and 491 (Table S1), which added AvrII and SpeI sites. The three products were cloned into the SmaI site of pUC18, creating pUC18-rbc, -ICre, and -GFP. GFP was excised with AvrII and cloned into AvrII-linearized pUC18-ICre, creating pUC18IIIG. The rbcS fragment was excised from pUC18-rbc with BamHI and AfeI and cloned into BamHI–AfeI digested pUC18IIIG, creating pUC18RIIIG. Its insert (rbcS:I-CreII:GFP) was completely sequenced, excised with XhoI and SpeI, and cloned into XhoI–SpeI cut pX6GFP; the final plasmid was pX6RIIIG.
RT-PCR.
Total RNA was isolated from 2-wk-old plantlets (∼50 mg fresh weight) using TRIzol (Invitrogen) (46) and reverse transcribed at 42 °C for 50 min (1 μg RNA in 25 μL) with SuperScript II (39). Aliquots (0.05 vol) were subjected to PCR with primers specific for I-CreII (488 and 489) and Act2 (535 and 536), and separated on 1% agarose gels for digital imaging.
DNA Analyses.
Total DNA was isolated as described (47). For the Southern blots, 3 μg DNA (per lane) was digested with SalI, XhoI, and SalI + XhoI overnight, resolved on long (20 cm) agarose (0.7%) gels, and transferred to cationic nylon membranes. The hybridization probes were obtained by PCR of wild-type Arabidopsis DNA, followed by random-primer labeling with α[32P]dCTP (7). The psbA and rpoA probes corresponded to nt 6–1,746 and 77,335–79,091, respectively, of cpDNA (gb NC_000932), and were synthesized with 524 and 525 and 522 and 523, respectively (Table S1). The 18S rDNA probe corresponded to nt 14,208,429–14,210,622 of chromosome 3 (gb NC_003074) and was amplified with 526 and 527 (Table S1).
For the PCR analysis, 200 ng of genomic DNA was subjected to 25–35 cycles of 94 °C (30 s), 60 °C (30 s), and 70 °C (5 min), using Taq polymerase (NEB) and primers 498 and 499 (Table S1), which amplify nt 152,241–2786 of cpDNA. The reactions were analyzed on 1% agarose, and the major products excised for direct sequencing and cloning into SmaI-linearized pUC18 or pGC-Blue (Lucigen). The PCR products were also gel separated into three fractions (<1 kb, 1–3 kb, and 3–5 kb) before cloning and sequencing. In parallel, genomic DNA was predigested with I-CreII (25) for 1 h at 37 °C to reduce wild-type target DNA and then used for PCR. PsbA primers 510 and 400 (Table S1), which amplify nt 611–947 of cpDNA, were also used for PCR, and the 337-bp product was directly sequenced.
Supplementary Material
Acknowledgments
We are grateful to W. Qiu (University of Texas-Austin, TX) for purified I-CreII, O. W. Odom for advice, and S. Oh and J. Kim for help with DNA analysis. N.-H. Chua (Rockefeller University) provided pX6GFP. This research was supported by Department of Energy Grant DE-FG03-02ER15352, Welch Grant F-1164, Texas-Advanced Research Program Grant 003658-0144-2007 (to D.L.H.), and National Science Foundation Grants IOS-0822811 and IOS-0849287 (to E.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004326107/-/DCSupplemental.
References
- 1.Rose RJ. The association of chloroplast DNA with photosynthetic membrane vesicles from spinach chloroplasts. J Cell Sci. 1979;36:169–183. doi: 10.1242/jcs.36.1.169. [DOI] [PubMed] [Google Scholar]
- 2.Sakai A, Takano H, Kuroiwa T. Organelle nuclei in higher plants: structure, composition, function, and evolution. Int Rev Cytol. 2004;238:59–118. doi: 10.1016/S0074-7696(04)38002-2. [DOI] [PubMed] [Google Scholar]
- 3.Guhamajumdar M, Sears BB. Chloroplast DNA base substitutions: An experimental assessment. Mol Genet Genomics. 2005;273:177–183. doi: 10.1007/s00438-005-1121-1. [DOI] [PubMed] [Google Scholar]
- 4.Maréchal A, et al. Whirly proteins maintain plastid genome stability in Arabidopsis. Proc Natl Acad Sci USA. 2009;106:14693–14698. doi: 10.1073/pnas.0901710106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korpelainen H. The evolutionary processes of mitochondrial and chloroplast genomes differ from those of nuclear genomes. Naturwissenschaften. 2004;91:505–518. doi: 10.1007/s00114-004-0571-3. [DOI] [PubMed] [Google Scholar]
- 6.Small GD, Greimann CS. Photoreactivation and dark repair of ultraviolet light-induced pyrimidine dimers in chloroplast DNA. Nucleic Acids Res. 1977;4:2893–2902. doi: 10.1093/nar/4.8.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dürrenberger F, Thompson AJ, Herrin DL, Rochaix J-D. Double strand break-induced recombination in Chlamydomonas reinhardtii chloroplasts. Nucleic Acids Res. 1996;24:3323–3331. doi: 10.1093/nar/24.17.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kmiec EB, Johnson C, May GD. Chloroplast lysates support directed mutagenesis via modified DNA and chimeric RNA/DNA oligonucleotides. Plant J. 2001;27:267–274. doi: 10.1046/j.1365-313x.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- 9.Khakhlova O, Bock R. Elimination of deleterious mutations in plastid genomes by gene conversion. Plant J. 2006;46:85–94. doi: 10.1111/j.1365-313X.2006.02673.x. [DOI] [PubMed] [Google Scholar]
- 10.Selby CP, Sancar A. A cryptochrome/photolyase class of enzymes with single-stranded DNA-specific photolyase activity. Proc Natl Acad Sci USA. 2006;103:17696–17700. doi: 10.1073/pnas.0607993103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutman BL, Niyogi KK. Evidence for base excision repair of oxidative DNA damage in chloroplasts of Arabidopsis thaliana. J Biol Chem. 2009;284:17006–17012. doi: 10.1074/jbc.M109.008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pâques F, Haber JE. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ragu S, et al. Oxygen metabolism and reactive oxygen species cause chromosomal rearrangements and cell death. Proc Natl Acad Sci USA. 2007;104:9747–9752. doi: 10.1073/pnas.0703192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wyman C, Kanaar R. DNA double-strand break repair: All's well that ends well. Annu Rev Genet. 2006;40:363–383. doi: 10.1146/annurev.genet.40.110405.090451. [DOI] [PubMed] [Google Scholar]
- 15.Bleuyard J-Y, Gallego ME, White CI. Recent advances in understanding of the DNA double-strand break repair machinery of plants. DNA Repair (Amst) 2006;5:1–12. doi: 10.1016/j.dnarep.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Puchta H. The repair of double-strand breaks in plants: Mechanisms and consequences for genome evolution. J Exp Bot. 2005;56:1–14. doi: 10.1093/jxb/eri025. [DOI] [PubMed] [Google Scholar]
- 17.Haber JE. Alternative endings. Proc Natl Acad Sci USA. 2008;105:405–406. doi: 10.1073/pnas.0711334105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennardo N, Cheng A, Huang N, Stark JM. Alternative-NHEJ is a mechanistically distinct pathway of mammalian chromosome break repair. PLoS Genet. 2008;4:e1000110. doi: 10.1371/journal.pgen.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heacock M, Spangler E, Riha K, Puizina J, Shippen DE. Molecular analysis of telomere fusions in Arabidopsis: Multiple pathways for chromosome end-joining. EMBO J. 2004;23:2304–2313. doi: 10.1038/sj.emboj.7600236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odom OW, Baek K-H, Dani RN, Herrin DL. Chlamydomonas chloroplasts can use short dispersed repeats and multiple pathways to repair a double-strand break in the genome. Plant J. 2008;53:842–853. doi: 10.1111/j.1365-313X.2007.03376.x. [DOI] [PubMed] [Google Scholar]
- 21.Maul JE, et al. The Chlamydomonas reinhardtii plastid chromosome: Islands of genes in a sea of repeats. Plant Cell. 2002;14:2659–2679. doi: 10.1105/tpc.006155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato S, Nakamura Y, Kaneko T, Asamizu E, Tabata S. Complete structure of the chloroplast genome of Arabidopsis thaliana. DNA Res. 1999;6:283–290. doi: 10.1093/dnares/6.5.283. [DOI] [PubMed] [Google Scholar]
- 23.Kurtz S, et al. REPuter: The manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001;29:4633–4642. doi: 10.1093/nar/29.22.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuo J, Niu QW, Møller SG, Chua N-H. Chemical-regulated, site-specific DNA excision in transgenic plants. Nat Biotechnol. 2001;19:157–161. doi: 10.1038/84428. [DOI] [PubMed] [Google Scholar]
- 25.Kim HH, Corina LE, Suh JK, Herrin DL. Expression, purification, and biochemical characterization of the intron-encoded endonuclease, I-CreII. Protein Expr Purif. 2005;44:162–172. doi: 10.1016/j.pep.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Krebbers E, Seurinck J, Herdies L, Cashmore AR, Timko MP. Four genes in two diverged subfamilies encode the ribulose-1,5-bisphosphate carboxylase small subunit polypeptides of Arabidopsis thaliana. Plant Mol Biol. 1998;11:745–759. doi: 10.1007/BF00019515. [DOI] [PubMed] [Google Scholar]
- 27.Lee DW, et al. Functional characterization of sequence motifs in the transit peptide of Arabidopsis small subunit of rubisco. Plant Physiol. 2006;140:466–483. doi: 10.1104/pp.105.074575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gruen M, Chang K, Serbanescu I, Liu DR. An in vivo selection system for homing endonuclease activity. Nucleic Acids Res. 2002;30:e29. doi: 10.1093/nar/30.7.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clough SJ, Bent AF. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 30.Ahlert D, Ruf S, Bock R. Plastid protein synthesis is required for plant development in tobacco. Proc Natl Acad Sci USA. 2003;100:15730–15735. doi: 10.1073/pnas.2533668100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glover L, McCulloch R, Horn D. Sequence homology and microhomology dominate chromosomal double-strand break repair in African trypanosomes. Nucleic Acids Res. 2008;36:2608–2618. doi: 10.1093/nar/gkn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez C, et al. Factors affecting double-strand break-induced homologous recombination in mammalian cells. Biotechniques. 2005;39:109–115. doi: 10.2144/05391GT01. [DOI] [PubMed] [Google Scholar]
- 33.Bacman SR, Williams SL, Moraes CT. Intra- and inter-molecular recombination of mitochondrial DNA after in vivo induction of multiple double-strand breaks. Nucleic Acids Res. 2009;37:4218–4226. doi: 10.1093/nar/gkp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowater R, Doherty AJ. Making ends meet: Repairing breaks in bacterial DNA by non-homologous end-joining. PLoS Genet. 2006;2:e8. doi: 10.1371/journal.pgen.0020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamura K, Adachi Y, Chiba K, Oguchi K, Takahashi H. Identification of Ku70 and Ku80 homologues in Arabidopsis thaliana: Evidence for a role in the repair of DNA double-strand breaks. Plant J. 2002;29:771–781. doi: 10.1046/j.1365-313x.2002.01258.x. [DOI] [PubMed] [Google Scholar]
- 36.West CE, et al. Disruption of the Arabidopsis AtKu80 gene demonstrates an essential role for AtKu80 protein in efficient repair of DNA double-strand breaks in vivo. Plant J. 2002;31:517–528. doi: 10.1046/j.1365-313x.2002.01370.x. [DOI] [PubMed] [Google Scholar]
- 37.Kohl S, Bock R. Transposition of a bacterial insertion sequence in chloroplasts. Plant J. 2009;58:423–436. doi: 10.1111/j.1365-313X.2009.03787.x. [DOI] [PubMed] [Google Scholar]
- 38.Ramonell KM, et al. Influence of atmospheric oxygen on leaf structure and starch deposition in Arabidopsis thaliana. Plant Cell Environ. 2001;24:419–428. doi: 10.1046/j.1365-3040.2001.00691.x. [DOI] [PubMed] [Google Scholar]
- 39.Odom OW, Shenkenberg DL, Garcia JA, Herrin DL. A horizontally acquired group II intron in the chloroplast psbA gene of a psychrophilic Chlamydomonas: in vitro self-splicing and genetic evidence for maturase activity. RNA. 2004;10:1097–1107. doi: 10.1261/rna.7140604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brouard J-S, Otis C, Lemieux C, Turmel M. Chloroplast DNA sequence of the green alga Oedogonium cardiacum (Chlorophyceae): Unique genome architecture, derived characters shared with the Chaetophorales and novel genes acquired through horizontal transfer. BMC Genomics. 2008;9:290. doi: 10.1186/1471-2164-9-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bendich AJ. Circular chloroplast chromosomes: The grand illusion. Plant Cell. 2004;16:1661–1666. doi: 10.1105/tpc.160771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vahrenholz C, Riemen G, Pratje E, Dujon B, Michaelis G. Mitochondrial DNA of Chlamydomonas reinhardtii: The structure of the ends of the linear 15.8-kb genome suggests mechanisms for DNA replication. Curr Genet. 1993;24:241–247. doi: 10.1007/BF00351798. [DOI] [PubMed] [Google Scholar]
- 43.La Claire JW, Wang J. Structural characterization of the terminal domains of linear plasmid-like DNA from the green alga Ernodesmis (Chlorophyta) J Phycol. 2004;40:1089–1097. [Google Scholar]
- 44.Shapiro JA, von Sternberg R. Why repetitive DNA is essential to genome function. Biol Rev Camb Philos Soc. 2005;80:227–250. doi: 10.1017/s1464793104006657. [DOI] [PubMed] [Google Scholar]
- 45.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant. 1962;15:473–497. [Google Scholar]
- 46.Chomczynski P, Mackey K. Modification of the TRIZOL reagent procedure for isolation of RNA from polysaccharide-and proteoglycan-rich sources. Biotechniques. 1995;19:942–945. [PubMed] [Google Scholar]
- 47.Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.