Abstract
The development of cardiac hypertrophy in response to increased hemodynamic load and neurohormonal stress is initially a compensatory response that may eventually lead to ventricular dilation and heart failure. Regulator of G protein signaling 5 (Rgs5) is a negative regulator of G protein-mediated signaling by inactivating Gα(q) and Gα(i), which mediate actions of most known vasoconstrictors. Previous studies have demonstrated that Rgs5 expresses among various cell types within mature heart and showed high levels of Rgs5 mRNA in monkey and human heart tissue by Northern blot analysis. However, the critical role of Rgs5 on cardiac remodeling remains unclear. To specifically determine the role of Rgs5 in pathological cardiac remodeling, we used transgenic mice with cardiac-specific overexpression of human Rgs5 gene and Rgs5−/− mice. Our results demonstrated that the transgenic mice were resistant to cardiac hypertrophy and fibrosis through inhibition of MEK-ERK1/2 signaling, whereas the Rgs5−/− mice displayed the opposite phenotype in response to pressure overload. These studies indicate that Rgs5 protein is a crucial component of the signaling pathway involved in cardiac remodeling and heart failure.
Keywords: ERK1/2, RGS5, MEK1/2, signal transduction
The heart undergoes adaptive hypertrophic growth to augment cardiac output in response to a variety of pathological stimuli including hypertension, ischemia, pressure overload, and inherited gene mutations (1, 2). After a period of compensatory hypertrophy, the myocardium undergoes functional and histological deterioration in the setting of ongoing stress. Although much is known about the pathways that promote hypertrophic responses, mechanisms that antagonize these pathways have not been as clearly defined. The discovery and functional clarification of antihypertrophic targets are equally important for understanding the molecular mechanisms underlying cardiac hypertrophy.
Regulators of G-protein signaling (RGS) proteins are negative regulators of G protein-mediated signaling that act as GTPase accelerating proteins for heterotrimeric G proteins. One member of the RGS protein superfamily, RGS5, is expressed in vascular, cardiac, and skeletal muscle tissues (3). Indeed, it is a marker for angiogenic pericytes during neovascularization associated with skin wound healing and tumor angiogenesis (4, 5). RGS5 has been reported to inhibit several Gαi- and Gαq-mediated signaling pathways in cardiovascular tissues, including those acting via the cardiovascular signaling molecules angiotensin II (Ang II) and endothelin-1 (3, 6). Notably, ribozyme-mediated knockdown of RGS5 results in selective upreglation of Ang II-mediated activation of ERK1/2 compared with other RGS proteins (3).
Clinical work has shown that certain combinations of multiple SNPs in Rgs5 may confer risk for hypertension (6). Consistent with a role for RGS5 in blood pressure regulation, mean arterial blood pressures were lower in Rgs5-deficient mice compared with WT controls (7, 8). Cho et al. (7) reported that the hypotensive phenotype may be related to increased NO susceptibility combined with increased and ERK1/2 activation in vascular smooth muscle cells. In the heart, Rgs5 is known to be up-regulated in atria tissue of mice that overexpressed β2-adrenergic receptor, as well as in atria of rats that were chronically administered the β-adrenergic agonist isoproterenol (9). However, the role of Rgs5 as a regulator of cardiac hypertrophy and fibrosis has not previously been determined. In the present study, we show that the cardiac constitutive expression of human Rgs5 protects against cardiac hypertrophy and fibrosis by blocking MEK-ERK1/2 signaling, whereas Rgs5−/− mice displayed the opposite phenotype in response to pressure overload. Our studies with cardiac-specific transgenic Rgs5 mice and Rgs5−/− mice suggest that Rgs5 is a crucial modulator of cardiac remodeling and heart failure.
Results
Generation of Mice with Cardiac-Specific Overexpression of Human Rgs5.
To examine the function of endogenous Rgs5 in the mouse heart in vivo, transgenic mice with cardiac-specific overexpression of human Rgs5 (i.e., TG mice) were generated by using the α-myosin heavy chain promoter. We identified five transgenic founders by PCR analysis and established four independent lines. These lines were born in a normal Mendelian distribution. They also exhibited normal reproductive rate and sex distributions. The relative levels of Rgs5 protein expression in the different lines were line no. 21 > 3 > 28 > 7. Among four established lines of TG mice, line 3 was used for further experiments (Fig. S1A). We analyzed Rgs5 protein levels in various tissues by Western blot analysis using a human-specific anti-Rgs5 antibody. We found a robust expression of human Rgs5 protein in the heart, but did not detect it in other organs (Fig. S1B). To investigate whether Rgs5 expression is regulated by pressure overload, WT mice were subjected to aortic banding (AB) for different durations. Rgs5 protein expression was increased by 3.7-fold over basal levels in the second week of AB. However, Rgs5 expression in the LV was markedly decreased compared with basal levels after 8 wk of AB (Fig. S1 C and D). Thus, Rgs5 expression is regulated during LV remodeling induced by chronic pressure overload.
Effect of Rgs5 on Cardiac Hypertrophy.
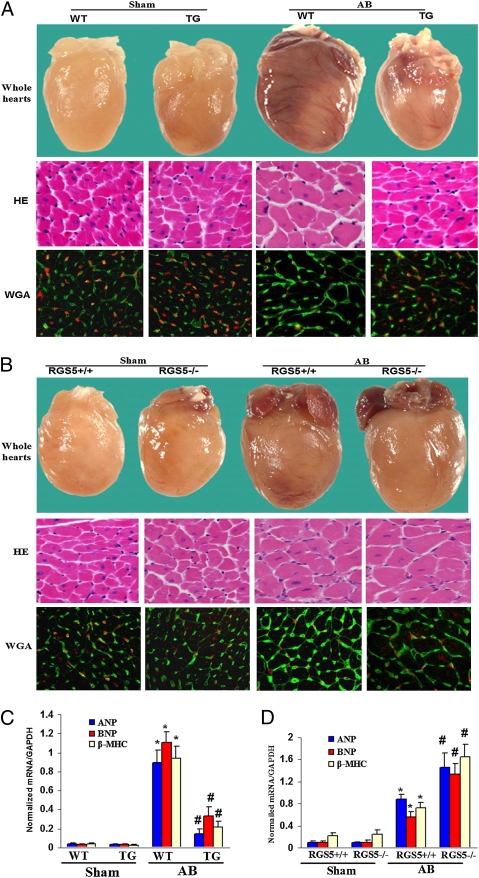
To determine whether cardiac overexpression of Rgs5 antagonized the hypertrophic response to pressure overload, WT littermates and TG mice were subjected to AB surgery or sham operation. TG mice showed significant attenuation of hypertrophy after 4 wk of AB compared with WT littermates, as measured by the ratios of heart weight/body weight (HW/BW), lung weight/body weight (LW/BW), and cardiomyocyte cross-sectional area (Table S1). No significant differences were observed in the sham-operated TG and WT mice. TG also inhibited cardiac dilation, wall thickness, and dysfunction, as evidenced by improvements in echocardiographic measurements (Table S1). Gross heart and HE staining further confirmed the inhibitory effect of Rgs5 on cardiac remodeling after AB (Fig. 1A). The induction of hypertrophic markers atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP), and β-MHC was also severely blunted in TG mice in response to AB (Fig. 1C). To further test the role of endogenous Rgs5 on cardiac hypertrophy, we applied AB to Rgs5−/− and Rgs5+/+ mice. After 4 wk, AB caused significant increases in HW/BW and CSA in both Rgs5−/− and Rgs5+/+ mice compared with sham operation. Interestingly, the percentage increases in HW/BW, LW/BW, and CSA were significantly greater in Rgs5−/− than in WT mice (Table S2). The results of echocardiographic measurements conducted at 4 weeks indicated that cardiac function was decreased in both Rgs5−/− and Rgs5+/+ mice (Table S2). Rgs5−/− mice hearts dilated after pressure overload, with end-diastolic and end-systolic dimensions increasing and fractional shortening decreasing more than in Rgs5+/+ mouse hearts (Table S2). These echocardiographic data were supported by morphologic analysis and more detailed invasive pressure–volume analysis (Table S2 and Fig. 1B). AB-induced changes in cardiac fetal genes ANP, BNP, and β-MHC were greater in Rgs5−/− than in Rgs5+/+ hearts (Fig. 1D). These results suggest that endogenous Rgs5 negatively regulates the extent of cardiac hypertrophy in response to pressure overload.
Fig. 1.
The effects of Rgs5 on cardiac hypertrophy. (A and B) Gross heart and WGA-FITC and HE staining of sham and AB mice at 4 wk after surgery in TG and WT mice (A) and in Rgs5−/− and Rgs5+/+ mice (B). Analysis of hypertrophic markers in TG and WT mice (C) and in Rgs5−/− and Rgs5+/+ mice (D). Total RNA was isolated from hearts of mice of the indicated groups, and expression of transcripts for ANP, BNP, and β-MHC induced by AB were determined by real-time PCR analysis. Data represent typical results of three to four different experiments as mean ± SEM (n = 4–11 mice per group). *P < 0.01 for WT/sham; #P < 0.01 for WT/AB after AB.
Effect of Rgs5 on MEK-ERK1/2 Signaling Pathway.
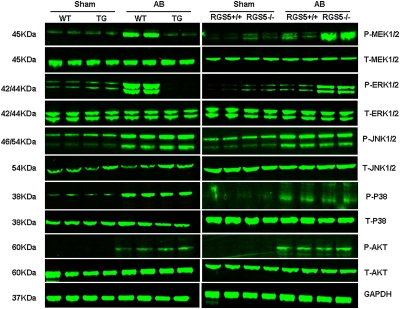
To explore the molecular mechanisms through which Rgs5 impairs the hypertrophic response, we examined the state of activation of MAPK in TG and WT hearts in response to pressure overload. We found that the phosphorylated levels of MEK1/2, ERK1/2, p38, and JNK1/2 were significantly increased in AB-infused WT hearts. However, the increased level of MEK1/2 and ERK1/2 was almost completely blocked in TG hearts, whereas p38 and JNK1/2 was similarly activated in the two groups (Fig. 2). Although AKT signaling plays a crucial role in the regulation of cardiac remodeling and apoptosis, we did not observe any differences in AKT activation between WT and TG mice (Fig. 2). Using Rgs5−/− and Rgs5+/+ mice, our further studies showed that AB significantly increased MEK and ERK1/2 phosphorylation in both Rgs5−/− and Rgs5+/+ mice. The increase of MEK-ERK1/2 signaling was more pronounced in Rgs5−/− than in Rgs5+/+ mice (Fig. 2). Collectively, these data suggest that constitutive expression of Rgs5 blunts the activation of MEK-ERK1/2 signaling, although it has no effect on p38, JNK, or AKT activation in hearts subjected to AB surgery. To further examine the role of Rgs5 on MEK-ERK1/2 signaling in the heart, we used Ad-Rgs5 to overexpress Rgs5 and Ad-shRgs5 to knock down Rgs5 protein expression (Fig. S2A) and exposed cultured neonatal rat cardiomyocytes to 1 μM Ang II infected with Ad-Rgs5 or Ad-shRgs5. Further studies showed that Ang II induced a significant phosphorylation of MEK and ERK1/2 that was almost completely blocked and sustained for all tested time points by overexpression of Rgs5 (Fig. S2B). More importantly, decreased Rgs5 levels by infection of Ad-shRgs5 resulted in pronounced activation of MEK and ERK1/2 in cardiac myocytes (Fig. S2B). Our findings suggest that Rgs5 inhibits MEK-ERK1/2 signaling in vitro and in vivo in response to hypertrophic stimuli. To examine whether MEK-ERK signaling has a causative role in Rgs5-mediated inhibition of cardiac hypertrophy, further in vitro experiments were performed. As expected, decreased Rgs5 levels led to pronounced hypertrophy induced by Ang II as assessed by ANP promoter activity and surface area measurements (Fig. S2 C and D). This response was strongly blunted by U0126, a MEK inhibitor that prevented ERK1/2 phosphorylation. These results suggest that Rgs5 inhibits cardiac hypertrophy through direct inhibition of MEK-ERK1/2 signaling in cardiac myocytes.
Fig. 2.
Effect of Rgs5 on MEK-ERK1/2 signaling pathway. Representative blots of ERK1/2, p38, and JNK, and AKT phosphorylation and their total protein expression at 4 wk after AB surgery in Rgs5 transgenic mice and WT mice or in Rgs5−/− and Rgs5+/+ mice.
Effect of Rgs5 on Fibrosis.
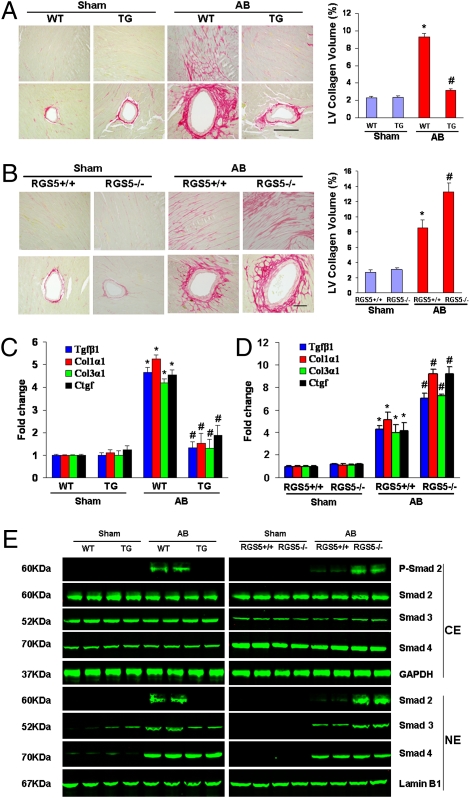
To determine the extent of fibrosis in the heart, paraffin-embedded slides were stained with picrosirius red (PSR). Marked perivascular and interstitial fibrosis were detected in the WT mice subjected to AB by PSR staining. The extent of cardiac fibrosis was remarkably reduced in TG mice (Fig. 3A). Subsequent analysis of mRNA expression levels of known mediators of fibrosis including Tgfβ1, procollagen, type I α1 (Col1α1), procollagen type III α1(Col3α1), and connective tissue growth factor (Ctgf), also demonstrated a blunted response in TG mice (Fig. 3C). Importantly, fibrosis was negligible in hearts from sham-operated Rgs5−/− and Rgs5+/+ mice. After AB, the fibrosis were present in Rgs5+/+ mice, but much more prominent in the Rgs5−/− mice (Fig. 3B). The expression of markers for fibrosis was also higher in Rgs5−/− than Rgs5+/+ mice (Fig. 3D). To confirm our in vivo data, we examined the effect of Rgs5 on collagen synthesis in isolated cardiac fibroblasts by [3H]-proline incorporation assays. Cells were serum-starved for 24 h in 0.5% FCS after infection with Ad-Rgs5 and Ad-shRgs5, and then treated with 15 ng/mL TGF-β1 for the indicated time. TGF-β1–stimulated [3H]-proline incorporation was attenuated by infection with Ad-Rgs5 and promoted by infection with Ad-shRgs5 (Fig. S3). To confirm the effects of Rgs5 on collagen synthesis, luciferase assay demonstrated an increase of promoter activity of CTGF with Rgs5 inhibition and the converse with Rgs5 overexpression (Fig. S3). To further elucidate the cellular mechanisms underlying the antifibrotic effects of Rgs5, we assessed the regulatory role of Rgs5 on Smad cascade activation. The increased level of Smad 2 phosphorylation and Smad2/3 nuclear translocation was attenuated in TG mice and promoted in Rgs5−/− mice in response to AB (Fig. 3E).
Fig. 3.
The effects of Rgs5 on fibrosis. (A and B) PSR staining on histological sections of the LV was performed on indicated mice 4 weeks after AB. (Scale bar: 10 μm.) Fibrotic areas from histological sections were quantified using an image analyzing system. (C and D) Real-time PCR analyses of Tgfβ1, Col1α1, Col3α1, and Ctgf were performed to determine mRNA expression levels in indicated mice. GAPDH was used as the sample loading control. Data represent typical results of three different experiments as mean± SEM (n = 4–6 mice/per group). *P < 0.01 for WT/sham. #P < 0.01 for WT/AB after AB. (E) Representative blots of Smad-2 phosphorylation and Smad-2/3/4 translocation from indicated groups 4 wk after AB in Rgs5 transgenic mice and WT mice or in Rgs5−/− and Rgs5+/+ mice (n = 4).
Inhibition of MEK-ERK1/2 Signaling Rescued Abnormalities in Rgs5−/− Mice.
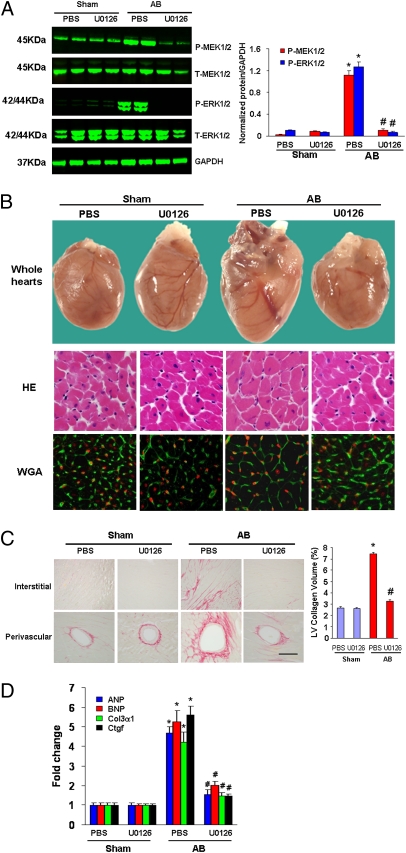
The aforementioned experimental results suggested that Rgs5 inhibits cardiac hypertrophy and fibrosis through blocking MEK-ERK1/2–dependent signaling pathways. To further confirm these findings, we evaluated whether the abnormalities in Rgs5−/− mice could be reversed through blocking MEK-ERK1/2 signaling using U0126 in vivo. We therefore treated Rgs5−/− mice with U0126 or PBS solution following AB. Western blot analysis showed that MEK and ERK1/2 phosphorylation levels were almost completely abrogated in our U0126-treated samples compared with the PBS solution–treated control mice (Fig. 4A). The results showed that U0126 treatment significantly reversed the deteriorative effects on cardiac dilation, wall thickness, and cardiac morphology, as well as fibrosis in response to 2 weeks of AB compared with PBS solution treatment in Rgs5−/− mice (Table S3 and Fig. 4 B and C). U0126 treatment also significantly reversed the increased mRNA levels of ANP, BNP, Ctgf, and Col1α1compared with those PBS-treated groups (Fig. 4D). These results suggest that inhibition of MEK-ERK1/2 signaling markedly reverses the deteriorative effects on cardiac hypertrophy and fibrosis caused by absent Rgs5 expression in the heart. These findings indicate that pharmacological inhibition of MEK-ERK1/2 signaling rescues abnormalities in Rgs5−/− mice in response to pressure overload.
Fig. 4.
Inhibition of MEK-ERK1/2 signaling rescued abnormalities in Rgs5−/− mice. (A) U0126 blocked MEK1/2 and ERK1/2 phosphorylation mediated by AB in Rgs5−/− mice. (B) Effects of U0126 on histological changes at 2 wk after surgery. (C) The effects of U0126 on fibrosis. Left, PSR staining; Right, statistical results of fibrotic areas. (D) The effects of U0126 on hypertrophic and fibrotic markers expression induced by AB in Rgs5−/− mice. mRNA was determined by real-time PCR analysis. Data represent typical results of three to five different experiments as mean ± SEM (n = 4–12 mice/per group). *P < 0.01 for PBS/sham values; #P < 0.01 for PBS/AB after AB.
Discussion
Previous studies have demonstrated that Rgs proteins play important role in the heart and vessels (3, 4). Rgs5 is a negative regulator of G protein-mediated signaling by inactivating Gα(q) and Gα(i) (3, 4). However, its function during cardiac hypertrophy and fibrosis was unclear. In the present study, we examined the role of Rgs5 in cardiac hypertrophy and fibrosis by using cardiac-specific Rgs5 transgenic mice and KO mice. The results demonstrated that elevated levels of Rgs5 protein expression in transgenic mice profoundly blunt hypertrophy, chamber dilation, and fibrosis via disruption of MEK-ERK1/2 signaling following chronic pressure overload. Conversely, loss of Rgs5 results in an exaggerated response of pathological cardiac remodeling and fibrosis. To our knowledge, this is the first report to demonstrate an important role of Rgs5 in the regulation of cardiac hypertrophy and fibrosis.
The mechanism by which Rgs5 mediates its antihypertrophic effects remains unclear. Cardiac hypertrophy is part of a compensatory response to mechanical loading and oxidative stresses that initially maintains cardiac output (1–3). With persistent stress, however, the compensatory hypertrophy can evolve into a decompensated state with profound changes in gene expression, contractile dysfunction, and extracellular remodeling. The signaling mechanism that mediates the critical transition from compensated hypertrophy to decompensated heart failure remains elusive. Recent studies demonstrated that MAPK signaling pathways play a key role in the progress of cardiac hypertrophy (9–11). The MAPK cascade consists of a sequence of successively acting kinases, including p38, JNKs, and ERKs, and is initiated in cardiac myocytes by stress stimuli (9–11). After it has been activated, p38, JNKs, and ERKs phosphorylate a wide array of intracellular targets, which include numerous transcription factors, resulting in the reprogramming of cardiac gene expression. The role that MEK1-ERK1/2 plays in the regulation of cardiac hypertrophy is an area of ongoing debate (9–11). It has been shown that ERK1/2 is activated in cultured neonatal rat cardiomyocytes by agonist stimulation (12, 13). Activation of MEK1 augmented cardiac hypertrophy in cultured cardiomyocytes whereas blocking MEK1 attenuated it. Similarly, use of the MEK1 inhibitor U0126 demonstrated that ERKs were required for the hypertrophic response induced by Ang II and ET-1 (12, 13). However, a number of additional studies have disputed the importance of MEK1-ERK1/2 in the regulation of cardiac hypertrophy, and one study has suggested that ERK activation prevents cardiac hypertrophy (9–11). To examine the molecular mechanisms involved in Rgs5’s ability to protect against cardiac hypertrophy, we examined the status of MAPKs signaling in our hypertrophic models. An important finding of this study is that MEK and ERK1/2 activation were blocked almost completely by cardiac expression of human Rgs5, whereas MEK and ERK1/2 phosphorylation levels were enhanced further by loss of Rgs5 expression in response to chronic pressure overload. However, the phosphorylation of p38, JNK1/2, and AKT was not affected by Rgs5. Therefore MEK-ERK1/2 signaling was a critical pathway through which Rgs5 influences cardiomyocyte growth. These findings are consistent with two recent studies that showed that Rgs5 expression blunts the activation of ERK1/2 in vascular smooth muscle cells (7, 14). We believe that inhibition of MEK-ERK1/2 signaling in the context of the adult heart under stress, such as pressure overload, may therefore provide a therapeutic strategy to regress cardiac hypertrophy. Consistent with this notion, blocking MEK-ERK1/2 signaling by U0126 rescued deteriorative cardiac dysfunction and dilation in Rgs5 mutant mice, indicating that the inhibitory effects of Rgs5 on cardiac hypertrophy are mediated through MEK-ERK1/2 signaling.
Pathological cardiac hypertrophy is accompanied by interstitial and perivascular fibrosis and approaches to limit collagen deposition in the heart have been limited to date (15–18). The present study revealed that Rgs5 blocks cardiac fibrosis in vivo and inhibits collagen synthesis in vitro. To our knowledge, our study is the first to report inhibition of fibrosis and TGF-β1–induced collagen synthesis in cardiac fibroblasts by Rgs5. In an attempt to elucidate the mechanisms underlying the inhibitory effect of Rgs5 on fibrosis, we analyzed key components of TGF-β1/Smad signal transduction. Blockade of this signaling pathway was predicted to blunt fibrosis (19). In line with these notions, our data suggest that Rgs5 abrogates Smad 2 phosphorylation and Smad 2/3 translocation in both cultured cardiac fibroblasts and hypertrophied hearts, thus inhibiting collagen synthesis and fibrosis. Recent studies indicate that TGF-β1/Smad signaling can be regulated by MEK-ERK1/2 signaling (9–11). We therefore examined the effects of MEK-ERK1/2 activation on fibrotic signaling and found that blocking MEK-ERK1/2 activation led to significant inhibition, whereas activation of MEK-ERK1/2 resulted in up-regulation of collagen synthesis and Smad 2/3 signaling. Furthermore, pharmacological inhibition of MEK-ERK1/2 signaling by U0126 markedly reversed the exaggerated fibrosis found in Rgs5−/− mice in vivo, indicating that Rgs5 attenuates fibrosis by blocking MEK-ERK1/2 signaling. In conclusion, the present study defines the role of Rgs5 in maintaining cardiac contractility and in reducing fibrosis in response to hypertrophic stimuli. The subcellular mechanism for the protective role of Rgs5 on the development of cardiac hypertrophy appears to be related to inhibition of the MEK-ERK1/2 signaling pathway. Our study provides insights into the mechanisms of cardiac hypertrophy and may have significant implications for the development of strategies for the treatment of cardiac hypertrophy through targeting of the Rgs5 signaling pathway.
Materials and Methods
Materials, Animals, and Animal Models.
Antibodies for the MAPK pathways were purchased from Cell Signaling Technology. The anti-Rgs5 antibody (reactive with mouse or human) was purchased from Abcam. The [3H]-leucine and [3H]-proline were purchased from Amersham. Other reagents were ordered from different company as described in SI Materials and Methods. All protocols were approved by the Animal Care and Use Committee of Renmin Hospital of Wuhan University. Human Rgs5 cDNA construct containing full-length human Rgs5 cDNA was cloned downstream of the cardiac MHC promoter. The detail for generation of cardiac specific transgenic mice of Rgs5 and animal models are described in SI Materials and Methods.
Blood Pressure, Echocardiography, and Histological Analysis.
A microtip catheter transducer (SPR-839; Millar Instruments) was inserted into the right carotid artery and advanced into the left ventricle. After stabilization for 15 min, the pressure signals and heart rate were recorded continuously with an ARIA pressure–volume conductance system coupled with a Powerlab/4SP A/D converter, stored, and displayed on a personal computer as described previously (18). Echocardiography was performed by Sonos 5500 ultrasound (Philips) with a 15-MHz linear array ultrasound transducer; details are provided in SI Materials and Methods. For histological analysis, hearts were excised, washed with saline solution, and placed in 10% formalin. Hearts were cut transversely close to the apex to visualize the left and right ventricles. Several sections of heart (4–5 μm thick) were prepared and stained with H&E for histopathology or PSR for collagen deposition and then visualized by light microscopy. For myocyte cross-sectional area, sections were stained for membranes with FITC-conjugated wheat germ agglutinin (WGA; Invitrogen) and for nuclei with DAPI. A single myocyte was measured with an image quantitative digital analysis system (NIH Image, version 1.6). The outline of 100 myocytes was traced in each group.
Recombinant Adenoviral Vectors, Cultured Neonatal Rat Cardiac Myocytes, and Fibroblasts.
We used replication-defective adenoviral vectors encoding for the entire coding region of Rgs5 gene (Open Biosystems) under the control of the cytomegalovirus promoter, and as a control, a similar adenoviral vector encoding for the GFP gene (AdEasy XL adenoviral Vector system; Strategene). We ordered three rat shRgs5 constructs from SuperArray (cat. no. KR42418G) and then generated three Ad-shRgs5 adenoviruses and selected one that led to a significant decrease in Rgs5 levels for further experiments. The details for generation of adenoviral, neonatal rat cardiac myocytes, and fibroblasts are provided in SI Materials and Methods.
Protein and Collagen Synthesis Assays and Surface Area.
Protein and collagen synthesis were assessed by [3H]-leucine and [3H]-proline incorporation as described previously (19, 20). For the surface areas, the cells were fixed with 3.7% formaldehyde in PBS solution, permeabilized in 0.1% Triton X-100 in PBS solution, and stained with α-actinin (Sigma) at a dilution of 1:100 by standard immunocytochemical techniques. Details are provided in SI Materials and Methods.
Reporter Assays, Real-Time RT-PCR, and Western Blotting.
The luciferase activity was assessed as described in SI Materials and Methods. Real-time PCR was used to detect the mRNA expression levels of hypertrophic and fibrotic markers. Quantification of Western blots was performed by Odyssey infrared imaging system (Li-Cor Biosciences) to detect protein expression. The secondary antibodies IRdye 800 antirabbit and IRdye 700 antimouse (Rockland) were used at 1:2,500 and 1:5,000, respectively, in Odyssey blocking for 1 h. The blots were scanned with the infrared Li-Cor scanner, allowing for simultaneous detection of two targets (anti- phospho and –total protein) in the same experiment. Details are provided in SI Materials and Methods.
Statistical Analysis.
Data are expressed as means ± SEM. Differences among groups were tested by two-way ANOVA followed by post hoc Tukey test. Comparisons between two groups were performed by unpaired Student t test. P < 0.05 was considered to be significantly different.
Supplementary Material
Acknowledgments
This research was supported by National Natural Science Foundation of China Grants 30900524, 30972954, and 30770733.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online atwww.pnas.org/lookup/suppl/doi:10.1073/pnas.1008397107/-/DCSupplemental.
References
- 1.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wencker D, et al. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manzur M, Ganss R. Regulator of G protein signaling 5: A new player in vascular remodeling. Trends Cardiovasc Med. 2009;19:26–30. doi: 10.1016/j.tcm.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Gu S, Cifelli C, Wang S, Heximer SP. RGS proteins: Identifying new GAPs in the understanding of blood pressure regulation and cardiovascular function. Clin Sci (Lond) 2009;116:391–399. doi: 10.1042/CS20080272. [DOI] [PubMed] [Google Scholar]
- 5.Hamzah J, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410–414. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- 6.Xiao B, Zhang Y, Niu WQ, Gao PJ, Zhu DL. Haplotype-based association of regulator of G-protein signaling 5 gene polymorphisms with essential hypertension and metabolic parameters. Clin Chem Lab Med. 2009;47:1483–1488. doi: 10.1515/CCLM.2009.344. [DOI] [PubMed] [Google Scholar]
- 7.Cho H, et al. Rgs5 targeting leads to chronic low blood pressure and a lean body habitus. Mol Cell Biol. 2008;28:2590–2597. doi: 10.1128/MCB.01889-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nisancioglu MH, et al. Generation and characterization of rgs5 mutant mice. Mol Cell Biol. 2008;28:2324–2331. doi: 10.1128/MCB.01252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jean-Baptiste G, et al. Beta adrenergic receptor-mediated atrial specific up-regulation of RGS5. Life Sci. 2005;76:1533–1545. doi: 10.1016/j.lfs.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Lorenz K, Schmitt JP, Vidal M, Lohse MJ. Cardiac hypertrophy: targeting Raf/MEK/ERK1/2-signaling. Int J Biochem Cell Biol. 2009;41:2351–2355. doi: 10.1016/j.biocel.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res. 2004;63:467–475. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Molkentin JD, Dorn GW., 2nd Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 13.Bueno OF, Molkentin JD. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ Res. 2002;91:776–781. doi: 10.1161/01.res.0000038488.38975.1a. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura T, et al. Mediating ERK 1/2 signaling rescues congenital heart defects in a mouse model of Noonan syndrome. J Clin Invest. 2007;117:2123–2132. doi: 10.1172/JCI30756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho H, Kozasa T, Bondjers C, Betsholtz C, Kehrl JH. Pericyte-specific expression of Rgs5: Implications for PDGF and EDG receptor signaling during vascular maturation. FASEB J. 2003;17:440–442. doi: 10.1096/fj.02-0340fje. [DOI] [PubMed] [Google Scholar]
- 16.Li HL, et al. Overexpression of myofibrillogenesis regulator-1 aggravates cardiac hypertrophy induced by angiotensin II in mice. Hypertension. 2007;49:1399–1408. doi: 10.1161/HYPERTENSIONAHA.106.085399. [DOI] [PubMed] [Google Scholar]
- 17.Li HL, et al. Targeted cardiac overexpression of A20 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circulation. 2007;115:1885–1894. doi: 10.1161/CIRCULATIONAHA.106.656835. [DOI] [PubMed] [Google Scholar]
- 18.Bian ZY, et al. LIM and cysteine-rich domains 1 regulates cardiac hypertrophy by targeting calcineurin/nuclear factor of activated T cells signaling. Hypertension. 2010;55:257–263. doi: 10.1161/HYPERTENSIONAHA.109.135665. [DOI] [PubMed] [Google Scholar]
- 19.Cai J, et al. Targeted expression of receptor-associated late transducer inhibits maladaptive hypertrophy via blocking epidermal growth factor receptor signaling. Hypertension. 2009;53:539–548. doi: 10.1161/HYPERTENSIONAHA.108.120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li HL, et al. Epigallocathechin-3 gallate inhibits cardiac hypertrophy through blocking reactive oxidative species-dependent and –independent signal pathways. Free Radic Biol Med. 2006;40:1756–1775. doi: 10.1016/j.freeradbiomed.2006.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.