Abstract
Coffee farms are often embedded within a mosaic of agriculture and forest fragments in the world's most biologically diverse tropical regions. Although shade coffee farms can potentially support native pollinator communities, the degree to which these pollinators facilitate gene flow for native trees is unknown. We examined the role of native bees as vectors of gene flow for a reproductively specialized native tree, Miconia affinis, in a shade coffee and remnant forest landscape mosaic. We demonstrate extensive cross-habitat gene flow by native bees, with pollination events spanning more than 1,800 m. Pollen was carried twice as far within shade coffee habitat as in nearby forest, and trees growing within shade coffee farms received pollen from a far greater number of sires than trees within remnant forest. The study shows that shade coffee habitats support specialized native pollinators that enhance the fecundity and genetic diversity of remnant native trees.
Keywords: agriculture, pollination, tropical ecology, gene flow, fragmentation
An estimated 13 million ha of tropical forest are destroyed every year (1) by the expansion of crops, pasture, and logging (1, 2). In deforested tropical regions, one of the most widely cultivated and economically valuable crops is coffee. Often grown adjacent to remnant forest patches, coffee covers over 11 million ha of land within many of the world's most biodiverse regions (3, 4). Intensively farmed “sun” coffee supports little native vegetation and can create an inhospitable matrix that isolates plant and animal populations living within forest fragments (3, 5). Alternatively, coffee grown under a canopy of overstory trees in the traditional “shade-grown style” can preserve ecological processes and provide farmers with ecosystem services (4, 5). For example, shade coffee farms serve as a habitat for migratory birds and nonmigratory bats, both of which provide farmers with pest control (6–8). Tropical animals living within shade coffee farms may also facilitate seed dispersal for native trees, thus providing opportunities for the maintenance of shade trees and future reforestation (9).
However, pollination mutualisms may be particularly vulnerable to habitat alteration, however, and many tropical tree species are self-incompatible and dependent on animal pollinators for reproduction and gene flow (reviewed in 10, 11–14). Although some agricultural landscapes provide suitable habitat for pollinators (15–17), many agricultural practices negatively impact native pollinator communities (18–20). Agricultural landscapes can also have high densities of exotic honey bees (Apis mellifera scutellata), which may not provide pollinator services for reproductively specialized native plants (21, reviewed in ref. 22). Exotic honey bees can maintain gene flow in altered habitats for plants with unspecialized flowers and abundant nectar and/or pollen, even if a subset of the native pollinators is lost (reviewed in ref. 12; e.g., refs. 23, 24). However, loss of the native pollinators of reproductively specialized plants can lead to reduced genetic diversity and inbreeding depression (25–27). Global declines in native pollinator populations (e.g., 28) and increased agricultural intensification magnify the threat of pollinator limitation for native plants in human altered landscapes (29–31).
In this study, we present a genetic analysis of pollen dispersal across the ubiquitous tropical shade coffee landscape. We used a combination of field experiments and seed parentage analyses to examine the capacity of the native bee community to maintain gene flow processes for tropical trees and bridge populations in shade coffee and remnant forest habitats. Specifically, we examine pollen dispersal processes for Miconia affinis, a neotropical tree that exhibits the “buzz-pollination” syndrome, in which tubular anthers require sonication by native bees for pollen release (32). Because exotic honey bees cannot sonicate, buzz-pollinated plants provide a unique opportunity to examine the impact of habitat change on pollination and gene flow by native bees.
Results
Pollinators and Plant Reproduction.
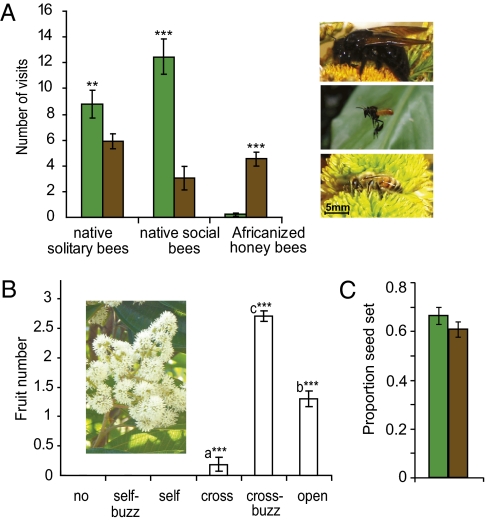
We found a greater number of native solitary and social bees visiting M. affinis in forest habitat than in shade coffee habitat (t test: F28,29 = 8.65, P = 0.004 and F28,29 = 3.88, P = 0.003, respectively), whereas nonpollinating Africanized honey bees were more frequent visitors of M. affinis in shade coffee habitat (F28,29 = 5.51, P < 0.001; Fig. 1A). Africanized honey bees conducted 1% of the visits in forest habitat but 26% of the visits in shade coffee habitat. The remaining visitors were native bees, 98% of which were buzz-pollinating species. Buzz-pollinating native solitary bees, such as the large-bodied carpenter bees (Xylocopa spp.), made up a greater proportion of visits in shade coffee habitat (43% of visits), whereas the smaller bodied buzz-pollinating social bees (Scaptotrigona and Trigona spp.) made up a greater proportion of visits in forest habitat (58% of visits) (t test: F28,29 = 9.918, P = 0.003). Controlled pollination experiments revealed that M. affinis is self-incompatible and yields a significantly higher fruit set with cross-buzz pollination than with any other treatment type (adjusted R2 = 0.131, P < 0.001; Fig. 1B). Ambient seed set, measured as the proportion of ovules that set viable seed without experimental manipulation (SI Text), did not differ between habitat types (P = 0.151; Fig. 1C).
Fig. 1.
(A) Visitation to M. affinis by buzz-pollinating native bees, buzz-pollinating social bees, and non–buzz-pollinating Africanized honey bees in forest (green) and shade coffee (brown) habitats based on pollinator observations (n = 59 trees). Photographs (Top to Bottom) of the native solitary bee (Xylocopa tabaniformis), native social bee (Trigona fulviventris), and Africanized honey bee (A. mellifera scutellata). (B) Photograph of M. affinis inflorescence and fruit set results for six pollination treatments: no pollination (no), self-pollinated flowers (self), self–buzz-pollinated flowers (self-buzz), cross-pollinated flowers (cross), cross–buzz-pollinated flowers (cross-buzz), and open pollinated flowers (open) (n = 30). (C) Seed set from fruits collected for seed arrays (n = 60). Error bars represent SE. **P < 0.01; ***P < 0.001.
Paternity Analyses.
Paternity was inferred for 329 seeds (68% of seeds analyzed), with an average probability of exclusion of 0.996. The multilocus outcrossing rate was 100% in both shade coffee (tm = 0.99 ± 0.06) and forest (tm = 1.01 ± 0.08) habitats, consistent with hand pollination experiments. Biparental inbreeding (tm − ts) was negligible in shade coffee (0.00 ± 0.04) and forest (0.06 ± 0.11) habitats. The number of unique pollen donors per seed tree was markedly higher in shade coffee habitat (0.946 ± 0.012) than in forest habitat (0.814 ± 0.030). The effective number of pollen donors per seed family (Nep, TWOGENER; ref. 33) was nearly two times higher in shade coffee habitat (Nep = 10.6) than in forest habitat (Nep = 5.7), indicating higher genetic diversity of seeds produced in shade coffee habitats.
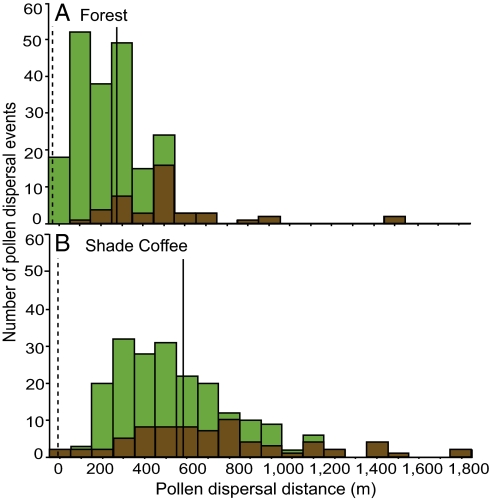
Across the landscape, pollen dispersal ranged from 0.10 to 1,890 m. Cross-habitat gene flow accounted for 43% of all pollen dispersal events (Fig. 2). Mean pollen dispersal distance based on paternity analyses was 589 ± 90.6 m (n = 189) in shade coffee habitat and 261 ± 52.2 m (n = 224) in forest habitat, indicating more than 2-fold longer pollen dispersal in the former (P = 0.0005). Mean pollen dispersal distances based on the TWOGENER analysis yielded similar values: 803.6 m in shade coffee habitat and 101.5 m in forest habitat. The exponential power function best fit the pollen dispersal data in both forest and shade coffee habitats (coffee: a = 401.8, b = 0.094, error = 0.095; forest: a = 47.35, b = 0.057, error = 0.223; explanation of parameters provided in SI Text) (Fig. 3), and a fat-tailed distribution could be inferred from the low value of the curve-shaped parameter (b < 1) (34).
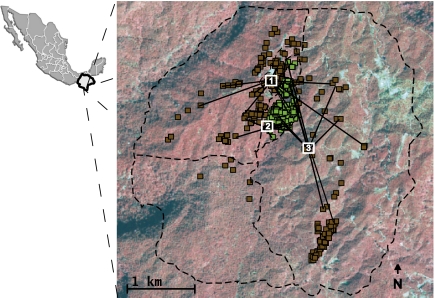
Fig. 2.
(A) Study region in Chiapas, Mexico, showing mapped M. affinis individuals in forest (green squares) and coffee (brown squares) habitats within the 1,200-ha agroecosystem (dashed lines). Solid black lines radiating from three focal trees (numbered 1, 2, and 3) connect to 10 pollen sources, as revealed by paternity analysis.
Fig. 3.
Pollen dispersal patterns for M. affinis seed trees in forest (A) and shade coffee (B) habitats binned in 100-m distance categories with the proportion of pollen derived from forest (green) and shade coffee (brown) habitats indicated. Dotted vertical lines represent mean nearest flowering neighbor distances in each habitat, and solid lines represent mean pollen dispersal distances in each habitat.
Mean pollen dispersal distance greatly exceeded nearest neighbor distances (nnds) in both forest (nnd = 9.24 ± 2.46 m) and shade coffee (nnd = 28.8 ± 9.11 m) habitats (Z = 0.83, P < 0.0001 and Z = 6.34, P < 0.0001, respectively) (Fig. 3). Pollen dispersal distances were significantly correlated with habitat type (P = 0.005) but not significantly associated with seed tree size (P = 0.267) or nnd (P = 0.520) (linear mixed effects model, full-model adjusted R2 = 0.545). Forest trees sired 65.1% of seeds sampled in a shade coffee habitat, whereas shade coffee trees sired 32.0% of seeds sampled in a forest habitat. Given the overall mean pollen dispersal distance (419 m), the proportion of seeds with forest sires within the shade coffee habitat was not significantly different from expected (66% expected) (Wilcoxon signed rank test: Z = −0.784, P = 0.433), but the proportion of seeds with shade coffee sires produced in a forest habitat was greater than expected (14.6% expected) (Wilcoxon signed rank test: Z = −2.43, P = 0.014).
Conclusions
Although native trees within shade coffee farms have received attention for supporting migratory birds, it was not known if native pollinators could link these trees with populations in nearby forest. In this study, we show that shade coffee farms support native bee communities, which facilitate the gene flow of native trees across the landscape mosaic. Paternity analysis revealed high outcrossing rates in M. affinis, consistent with self-incompatibility and negligible levels of biparental inbreeding. Distinct differences in breeding structure were observed, however, within shade coffee and forest habitats. The estimated number of pollen donors per seed array was twice as high in a shade coffee habitat as in a forest habitat, despite the higher density of potential sires in the forest. Additionally, M. affinis trees in shade coffee habitat sired an unexpectedly high proportion of seeds collected from forest trees, suggesting that in shade coffee systems, native trees and their pollinators can play a disproportionate role in mediating gene flow across the mosaic landscape.
Previous studies have shown that tropical trees in low-population densities (e.g., logged forests) tend to have lower outcrossing rates than trees in high-population densities (e.g., uncut forests) (reviewed in 14, 35), suggesting that self-pollination or pollen limitation (i.e., reduced fecundity) may be more prevalent in agricultural habitats in which trees are more scattered. We observed equally high seed sets in both habitats, despite the 7-fold higher population density of M. affinis in the remnant forest. Pollen dispersal curves in both habitats exhibited a fat-tailed distribution, indicating that even isolated trees received pollen from a relatively large number of pollen donors (i.e., 36). Past gene flow studies have focused on partly self-compatible species with unspecialized flowers (23, 37–43) and have occasionally found that isolated trees in agricultural landscapes receive pollen from a large number of distant sires (23, 38). However, Africanized honey bees may have been the primary vectors of gene flow in these studies (23, 24). Our focus on a buzz-pollinated tree allowed us to exclude Africanized honey bees and highlights the role of native bees as both pollinators and vectors of gene flow in the shade coffee landscape mosaic. The results should apply to other buzz-pollinated plants, which represent ≈8% of the world's flowering plant species (32), as well as to other native plants whose limited pollen and nectar rewards may not attract exotic honey bees.
Native bees dispersed M. affinis pollen significantly further than predicted by nnds in both shade coffee and forest habitats. The breakdown of nearest neighbor mating was strongest in the shade coffee habitat, where mean pollen dispersal distances were more than double those found in the forest habitat, despite similarly sized tree clusters in both habitats (Fig. 3). Deviations from nearest neighbor mating have been documented in breeding structure studies for trees in undisturbed tropical forests (44) and in human modified tropical landscapes (23, 38), and they are most often attributed to asynchronous flowering, pollen carryover, and changes in pollinator flight behavior within more open habitats (reviewed in 14). M. affinis trees flower synchronously, and although intertree distances are longer in the shade coffee habitat, our analyses revealed that pollen dispersal distance was significantly influenced by habitat but not by degree of isolation. Seed set and pollen dispersal distance were also unaffected by tree size, indicating that larger trees did not participate in a disproportionately greater number of pollination or long-distance pollen dispersal events. Instead, our results suggest that differences in breeding structure most likely derive from changes in the pollinator community and pollinator foraging behavior, both of which are influenced by habitat (reviewed in 45, 46).
Small-bodied social bees dominated the buzz-pollinating bee community in the forest habitat, whereas large-bodied solitary bees were the most common buzz pollinators in the shade coffee habitat. This is consistent with other surveys of tropical habitats with differing levels of agricultural intensification (16). The broader foraging range of larger bodied bees (47) may partly explain the longer distances of pollen dispersal in the shade coffee habitat. Although pollinator visitation rates for M. affinis trees were lower in the shade coffee habitat, there was no difference in fecundity between the two habitat types. High levels of seed set, despite self-incompatibility, provide additional evidence for substantial intertree pollinator movement within the shade coffee landscape mosaic. Other studies have found that native pollinators can persist in agricultural landscapes and provide ecosystem services to crops (15, 17). This study documents native bee-mediated gene flow across an active agricultural landscape. The study also highlights a system in which the negative impacts of agriculture on native bee richness and abundance did not reduce the ecosystem function provided by outcross pollination.
In summary, this study shows that traditional shade coffee farms can maintain native insect communities that mediate outcross pollination in reproductively specialized native plants. Furthermore, we document some of the longest precisely recorded pollination distances by native tropical bees (14). Unlike past studies in which exotic honey bees have been the primary source of extensive gene flow across human-altered tropical habitats, we show that native bees link shade coffee and forest fragments and provide essential pollination services for native tropical trees. Native bee communities within shade coffee farms therefore not only ensure against the loss of introduced honey bees (15) and increase coffee yields (47) but maintain the reproduction and genetic diversity of native trees.
Materials and Methods
The study was conducted in the highlands of the Soconusco, a coffee-growing region located in southern Chiapas, Mexico. In the Soconusco, coffee is cultivated in the traditional style, under a canopy of overstory trees (5, 48). Typical of Central American coffee-growing regions, the landscape studied is dominated by shade coffee, with forest representing less than 10% of the land cover (49). The 1,200-ha study site encompasses an uncut forest fragment and three shade coffee farms, each relatively consistent in vegetation management style. Canopy trees within the study site include nitrogen-fixing legumes (Inga spp.), nonnative fruit trees (Citrus sinensis and Mangifera indica), and a diverse spectrum of native trees (mean of 157.21 overstory trees per ha−1 and 14.67 tree species per ha−1). Since the establishment of the shade coffee farms, land managers have allowed for the colonization of a few native understory trees because of their service in reducing soil erosion (9). One such species is M. affinis D.C. (Melastomatacea), a small understory tree (3–6 m) that is broadly distributed in the neotropics, ranging from Mexico to Brazil (50). M. affinis is a synchronously flowering species that blooms in Chiapas for ≈3 d at the onset of the first rains in mid-March. Fruits develop over a 3- to 4-mo period and are dispersed by a variety of birds and bats (9, 51).
Following exhaustive searches in 2006–2008, 445 M. affinis adult (reproductive) trees were identified across the landscape (306 in the forest habitat and 139 in the coffee habitat). Tree diameter at breast height and degree of spatial isolation at three different spatial scales were measured per tree. The scales included (i) distance to the nearest neighbor, (ii) average distance to the nearest 20 neighbors, and (iii) average distance to all trees in the landscape. For the mating system study, 30 adult M. affinis trees (15 in the forest habitat and 15 in the coffee habitat) were selected, whereas 59 trees (29 in the forest habitat and 30 in the coffee habitat) were selected for the pollinator observation study. Pollinator observation consisted of a 30-min observation period per tree (59 observation periods in total), during which four fully blooming inflorescences were monitored during the period of peak insect visitation activity (9:00–15:00). Flower-visiting insects were identified, and buzz-pollinating ability (based on observation of sonication and pollen release) was recorded for each visit.
To measure seed set and to collect seed arrays for paternity analyses, 20 fruits were randomly sampled from 60 randomly chosen adult M. affinis trees. For the paternity analyses, 24 of these trees were chosen (12 in the coffee habitat and 12 in the forest habitat) and one seed was randomly selected from each fruit, yielding an array of 20 seeds for each tree. The seeds were soaked for 48 h in sterile water before DNA extraction. DNA was extracted from both adult leaf tissue and seed tissue using the DNeasy Plant kit (Qiagen) and then cleaned using the Geneclean kit (Qbiogene). All trees and seeds were genotyped at eight microsatellite loci (9, 52). Paternity analysis was used to infer pollen dispersal distances using the program CERVUS 3.0 (53). Paternity was assigned only to seeds with a confidence criterion of >0.95, resulting in a simple exclusion probability of >0.996. We used a maximum-likelihood method (54) to calculate multilocus outcrossing rates for populations in each habitat. Because direct paternity analysis does not account for unidentified sires, we also used the indirect TWOGENER analysis (33), as implemented in the software package POLDISP (55), to measure differentiation in seed tree pollen allele pools (Φft) and the Nep [Nep = (2 Фft)−1] in each habitat (56). Details on the study site, mating system study, and paternity analyses are provided in SI Materials and Methods.
A Student's t test was used to compare insect visit frequency between habitat types for each of three groups: (i) native solitary bees, (ii) native social bees, and (iii) Africanized honey bees. Fruit set was calculated as the number of flowers to set fruit divided by the total number of flowers in each treatment. A linear mixed effects model was used to examine the effects of (i) habitat, (ii) tree size, and (iii) degree of spatial isolation on seed set and pollen dispersal distance, with maternal tree as a random factor. The three spatial isolation scales were examined independently for each model, although only the results for the “distance to nearest neighbor” scale are reported because this scale was the most predictive. Pollen dispersal distances calculated from the direct paternity analysis were compared with nnds using a Kolmogorov–Smirnov Z test. All counts were square root-transformed, all proportions were arcsine square root-transformed before analyses (57), and all significance values were Bonferroni-corrected. All statistical analyses were conducted with the R software environment (R Development Core Team; http://www.r-project.org). For outcrossing rates and biparental inbreeding, the ± values represent SDs, whereas all other ± values represent SEs.
Supplementary Material
Acknowledgments
We thank J. Vandermeer, I. Perfecto, B. Rathcke, B. Ferguson, and S. Pereira for help in the development of this project; G. Dominguez-Martinez for his help in scouting trees; and the farmers of Nueva Alemania for permission to conduct this study on their land. All experiments were in compliance with current laws governing biodiversity protection in Mexico. This project was supported by the Helen Olson Brower Fellowship at the University of Michigan and National Science Foundation Awards DEB 0908661 and DEB 043665.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002490107/-/DCSupplemental.
References
- 1.Food and Agriculture Organization of the United Nations . 2005 Global Forest Resources Assessment. Rome, Italy: United Nations Food and Agriculture Organization; 2005. [Google Scholar]
- 2.Butler RA, Laurance WF. New strategies for conserving tropical forests. Trends Ecol Evol. 2008;23:469–472. doi: 10.1016/j.tree.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Donald PF. Biodiversity impacts of some agricultural commodity production systems. Conserv Biol. 2004;18:17–37. [Google Scholar]
- 4.Hardner J, Rice R. Rethinking green consumerism. Sci Am. 2002;286:88–95. doi: 10.1038/scientificamerican0502-88. [DOI] [PubMed] [Google Scholar]
- 5.Perfecto I, Rice RA, Greenberg R, VanderVoort ME. Shade coffee: A disappearing refuge for biodiversity. Bioscience. 1996;46:598–608. [Google Scholar]
- 6.Williams-Guillén K, Perfecto I, Vandermeer J. Bats limit insects in a neotropical agroforestry system. Science. 2008;320:70. doi: 10.1126/science.1152944. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg R, et al. The impact of avian insectivory on arthropods and leaf damage in some Guatemalan coffee plantations. Ecology. 2000;81:1750–1755. [Google Scholar]
- 8.Van Bael SA, et al. Birds as predators in tropical agroforestry systems. Ecology. 2008;89:928–934. doi: 10.1890/06-1976.1. [DOI] [PubMed] [Google Scholar]
- 9.Jha S, Dick CW. Shade coffee farms promote genetic diversity of native trees. Curr Biol. 2008;18:R1126–R1128. doi: 10.1016/j.cub.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Bawa KS. Mating systems, genetic differentiation and speciation in tropical rain-forest plants. Biotropica. 1992;24:250–255. [Google Scholar]
- 11.Bawa KS, Hadley M. Reproductive Ecology of Tropical Forest Plants. Carnforth: United Nations Educational, Scientific, and Cultural Organization, Paris, and Parthenon Press; 1990. [Google Scholar]
- 12.Aguilar R, Ashworth L, Galetto L, Aizen MA. Plant reproductive susceptibility to habitat fragmentation: Review and synthesis through a meta-analysis. Ecol Lett. 2006;9:968–980. doi: 10.1111/j.1461-0248.2006.00927.x. [DOI] [PubMed] [Google Scholar]
- 13.Ashworth L, Aguilar R, Galetto L, Aizen MA. Why do pollination generalist and specialist plant species show similar reproductive susceptibility to habitat fragmentation? J Ecol. 2004;92:717–719. [Google Scholar]
- 14.Dick CW, Jones FA, Hardy OJ, Petit RJ. Spatial scales of seed and pollen-mediated gene flow in tropical forest trees. Tropical Plant Biology. 2008;1:20–33. [Google Scholar]
- 15.Winfree R, Williams NM, Dushoff J, Kremen C. Native bees provide insurance against ongoing honey bee losses. Ecol Lett. 2007;10:1105–1113. doi: 10.1111/j.1461-0248.2007.01110.x. [DOI] [PubMed] [Google Scholar]
- 16.Tylianakis JM, Klein AM, Tscharntke T. Spatiotemporal variation in the diversity of hymenoptera across a tropical habitat gradient. Ecology. 2005;86:3296–3302. [Google Scholar]
- 17.Klein AM, Steffan-Dewenter I, Buchori D, Tscharntke T. Effects of land-use intensity in tropical agroforestry systems on coffee flower-visiting and trap-nesting bees and wasps. Conserv Biol. 2002;16:1003–1014. [Google Scholar]
- 18.Allen-Wardell G, et al. The potential consequences of pollinator declines on the conservation of biodiversity and stability of food crop yields. Conserv Biol. 1998;12:8–17. [Google Scholar]
- 19.Kearns CA, Inouye DW, Waser NM. Endangered mutualisms: The conservation of plant-pollinator interactions. Annu Rev Ecol Syst. 1998;29:83–112. [Google Scholar]
- 20.Steffan-Dewenter I, Potts SG, Packer L. Pollinator diversity and crop pollination services are at risk. Trends Ecol Evol. 2005;20:651–652. doi: 10.1016/j.tree.2005.09.004. author reply 652–653. [DOI] [PubMed] [Google Scholar]
- 21.Aizen MA, Feinsinger P. Habitat fragmentation, native insect pollinators, and feral honeybees in Argentine chaco serrano. Ecol Appl. 1994;4:378–392. [Google Scholar]
- 22.Butz-Huryn V. Ecological impacts of introduced honey bees. Q Rev Biol. 1997;72:275–297. [Google Scholar]
- 23.Dick CW. Genetic rescue of remnant tropical trees by an alien pollinator. Proc R Soc London Ser B. 2001;268:2391–2396. doi: 10.1098/rspb.2001.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dick CW, Etchelecu G, Austerlitz F. Pollen dispersal of tropical trees (Dinizia excelsa: Fabaceae) by native insects and African honeybees in pristine and fragmented Amazonian rainforest. Mol Ecol. 2003;12:753–764. doi: 10.1046/j.1365-294x.2003.01760.x. [DOI] [PubMed] [Google Scholar]
- 25.Loveless MD, Hamrick JL. Ecological determinants of genetic structure in plant populations. Annu Rev Ecol Syst. 1984;15:65–95. [Google Scholar]
- 26.Aguilar R, Quesada M, Ashworth L, Herrerias-Diego Y, Lobo J. Genetic consequences of habitat fragmentation in plant populations: Susceptible signals in plant traits and methodological approaches. Mol Ecol. 2008;17:5177–5188. doi: 10.1111/j.1365-294X.2008.03971.x. [DOI] [PubMed] [Google Scholar]
- 27.Levin DA, Kerster HW. Gene flow in seed plants. Evol Biol. 1974;7:139–218. [Google Scholar]
- 28.Biesmeijer JC, et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science. 2006;313:351–354. doi: 10.1126/science.1127863. [DOI] [PubMed] [Google Scholar]
- 29.Kremen C, Williams NM, Thorp RW. Crop pollination from native bees at risk from agricultural intensification. Proc Natl Acad Sci USA. 2002;99:16812–16816. doi: 10.1073/pnas.262413599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ricketts TH, et al. Landscape effects on crop pollination services: Are there general patterns? Ecol Lett. 2008;11:499–515. doi: 10.1111/j.1461-0248.2008.01157.x. and erratum (2008) 11:1121. [DOI] [PubMed] [Google Scholar]
- 31.Klein AM, et al. Importance of pollinators in changing landscapes for world crops. Proc R Soc London Ser B. 2007;274:303–313. doi: 10.1098/rspb.2006.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buchmann S. Buzz pollination in angiosperms. In: Jones CER, editor. Little Handbook of Experimental Pollination Biology. New York: Van Nostrand; 1983. pp. 73–113. [Google Scholar]
- 33.Smouse PE, Dyer RJ, Westfall RD, Sork VL. Two-generation analysis of pollen flow across a landscape. I. Male gamete heterogeneity among females. Evolution. 2001;55:260–271. doi: 10.1111/j.0014-3820.2001.tb01291.x. [DOI] [PubMed] [Google Scholar]
- 34.Clark JS. Why trees migrate so fast: Confronting theory with dispersal biology and the paleorecord. Am Nat. 1998;152:204–224. doi: 10.1086/286162. [DOI] [PubMed] [Google Scholar]
- 35.Mimura M, Barbour RC, Potts BM, Vaillancourt RE, Watanabe KN. Comparison of contemporary mating patterns in continuous and fragmented Eucalyptus globulus native forests. Mol Ecol. 2009;18:4180–4192. doi: 10.1111/j.1365-294X.2009.04350.x. [DOI] [PubMed] [Google Scholar]
- 36.Klein EK, Lavigne C, Gouyon PH. Mixing of propagules from discrete sources at long distance: Comparing a dispersal tail to an exponential. BMC Biol. 2006;6 doi: 10.1186/1472-6785-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nason JD, Hamrick JL. Reproductive and genetic consequences of forest fragmentation: Two case studies of neotropical canopy trees. J Hered. 1997;88:264–276. [Google Scholar]
- 38.Chase MR, Moller C, Kesseli R, Bawa KS. Distant gene flow in tropical trees. Nature. 1996;383:398–399. [Google Scholar]
- 39.Sampson JF, Byrne M. Outcrossing between an agroforestry plantation and remnant native populations of Eucalyptus loxophleba. Mol Ecol. 2008;17:2769–2781. doi: 10.1111/j.1365-294X.2008.03779.x. [DOI] [PubMed] [Google Scholar]
- 40.Bacles CFE, Burczyk J, Lowe AJ, Ennos RA. Historical and contemporary mating patterns in remnant populations of the forest tree Fraxinus excelsior L. Evolution. 2005;59:979–990. [PubMed] [Google Scholar]
- 41.Byrne M, Elliott CP, Yates CJ, Coates DJ. Maintenance of high pollen dispersal in Eucalyptus wandoo, a dominant tree of the fragmented agricultural region in Western Australia. Conserv Genet. 2008;9:97–105. [Google Scholar]
- 42.Born C, et al. Small-scale spatial genetic structure in the Central African rainforest tree species Aucoumea klaineana: A stepwise approach to infer the impact of limited gene dispersal, population history and habitat fragmentation. Mol Ecol. 2008;17:2041–2050. doi: 10.1111/j.1365-294X.2007.03685.x. [DOI] [PubMed] [Google Scholar]
- 43.Kamm U, et al. Frequent long-distance gene flow in a rare temperate forest tree (Sorbus domestica) at the landscape scale. Heredity. 2009;103:476–482. doi: 10.1038/hdy.2009.70. [DOI] [PubMed] [Google Scholar]
- 44.Stacy EA, et al. Pollen dispersal in low-density populations of three neotropical tree species. Am Nat. 1996;148:275–298. [Google Scholar]
- 45.Tscharntke T, Brandl R. Plant-insect interactions in fragmented landscapes. Annu Rev Entomol. 2004;49:405–430. doi: 10.1146/annurev.ento.49.061802.123339. [DOI] [PubMed] [Google Scholar]
- 46.Ghazoul J. Pollen and seed dispersal among dispersed plants. Biol Rev Camb Philos Soc. 2005;80:413–443. doi: 10.1017/s1464793105006731. [DOI] [PubMed] [Google Scholar]
- 47.Klein AM, Steffan-Dewenter I, Tscharntke T. Fruit set of highland coffee increases with the diversity of pollinating bees. Proc R Soc London Ser B. 2003;270:955–961. doi: 10.1098/rspb.2002.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moguel P, Toledo VM. Biodiversity conservation in traditional coffee systems of Mexico. Conserv Biol. 1999;13:11–21. [Google Scholar]
- 49.Philpott SM, Lin B, Jha S, Brines SJ. A multi-scale assessment of hurricane impacts on agricultural landscapes based on land use and topographic features. Agric Ecosyst Environ. 2008;128:12–20. [Google Scholar]
- 50.Wurdack J. Flora of Ecuador. Stockholm, Sweden: Swedish Natural Science Research Council Editorial Service; 1980. [Google Scholar]
- 51.Luck GW, Daily GC. Tropical countryside bird assemblages: Richness, composition, and foraging differ by landscape context. Ecol Appl. 2003;13:235–247. [Google Scholar]
- 52.Jha S, Dick CW. Isolation and characterization of nine microsatellite loci for the tropical understory tree Miconia affinis Wurdack (Melastomataceae) Mol Ecol Resour. 2009;9:344–345. doi: 10.1111/j.1755-0998.2008.02428.x. [DOI] [PubMed] [Google Scholar]
- 53.Marshall TC, Slate J, Kruuk LEB, Pemberton JM. Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol. 1998;7:639–655. doi: 10.1046/j.1365-294x.1998.00374.x. [DOI] [PubMed] [Google Scholar]
- 54.Ritland K. Extensions of models for the estimation of mating systems using n independent loci. Heredity. 2002;88:221–228. doi: 10.1038/sj.hdy.6800029. [DOI] [PubMed] [Google Scholar]
- 55.Robledo-Arnuncio JJ, Austerlitz F, Smouse PE. POLDISP: A software package for indirect estimation of contemporary pollen dispersal. Mol Ecol Notes. 2007;7:763–766. [Google Scholar]
- 56.Smouse PE, Sork VL. Measuring pollen flow in forest trees: An exposition of alternative approaches. For Ecol Manage. 2004;197:21–38. [Google Scholar]
- 57.Sokal RR, Rohlf FJ. Biometry: The Principles and Practice of Statistics in Biological Research. 3rd Ed. New York: Freeman; 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.