Abstract
Neurite outgrowth represents a critical stage in the correct development of neuronal circuitries, and is dependent on the complex regulation of actin filament and microtubule dynamics by intrinsic as well as extrinsic signals. Previous studies have implicated the tumor suppressor factor, p53, in the regulation of axonal outgrowth through a nontranscriptional effect involving local regulation of the Rho kinase signaling pathway that controls these dynamics. In the present study, we first showed that semaphorin 3A-induced growth cone collapse in cultured hippocampal neurons was associated with the partial truncation of phosphorylated p53, and that both effects were prevented by calpain inhibition with either m-calpain–specific siRNA or inhibitors. We further determined that semaphorin 3A-mediated calpain activation and growth cone collapse were associated with m-calpain phosphorylation and prevented by inhibition of MAPK, ERK, or p38. In vitro studies confirmed that p53 and especially phosphorylated p53 were partially truncated by calpain. Thus, our results indicate that semaphorin 3A-mediated growth cone collapse is mediated in part by m-calpain activation, possibly through MAPK-mediated phosphorylation, and the resulting truncation of phosphorylated p53, leading to Rho kinase activation and cytoskeletal reorganization. They provide a pathway by which extrinsic signals regulate axonal growth through activation of m-calpain and p53 truncation.
Keywords: ERK, p38 MAPK, phosphorylation, Rho kinase, hippocampus
Normal CNS development depends on appropriate neuronal migration and establishment of organized structures with correct circuits. Neurite outgrowth, especially axonal outgrowth, is driven in part by highly motile growth cones that respond to intrinsic factors and extrinsic cues. Growth cone motility depends on a dynamic actin network, with polymerization occurring at the leading edge, depolymerization in the central region, and interactions with myosin pulling on actin filaments (1, 2). Besides actin filaments, microtubule dynamics are also critical for neurite outgrowth (3, 4). Numerous proteins have been demonstrated to regulate actin filaments and microtubules, including the Rho family GTPases (4–8). Emerging evidence has also implicated calpains in axonal growth regulation; calpains are a family of neutral calcium-dependent proteases involved in a wide range of cellular functions (9). It has recently become widely acknowledged that calpain, by partially truncating a variety of cytoskeletal proteins, plays a critical role in the regulation of shape and motility in numerous cell types (see refs. 10 and 11 for reviews). A recent study indicates that m-calpain functions as a molecular switch in CHO cells to control cell spreading and retraction (12). Interestingly, integrin activation results in calpain activation and, depending on the state of phosphorylation of the integrin cytoplasmic domain, leads to inhibition of RhoA and cell spreading or activation of RhoA and cell retraction. Recent studies have more specifically implicated calpain in growth cone regulation. Robles et al. (13) proposed that calcium transients in filipodia activate calpain, resulting in inhibition of axonal growth through Src inhibition. A variant of this idea has been recently developed (14). It has been further proposed that the combined activation of calpain and Rho kinase (ROCK) signaling is required to produce growth cone collapse (15). However, the links between calpain activation and growth cone collapse were not elucidated.
Recent evidence has also implicated the tumor suppressor protein p53 in cell migration and neurite outgrowth regulation. Nerve growth factor–induced neurite growth in PC12 cells and in cultured cortical neurons depends on the presence of functional p53 (16, 17), possibly through increased expression of coronin 1b (a filamentous actin-binding protein) and the small GTPase Rab13 (18). We recently showed that phosphorylated p53 (p-p53) was abundantly present in axons and growth cones and that p53 suppression by inhibitors or siRNA-induced rapid growth cone collapse (19). Furthermore, in a mouse model of Niemann-Pick type C (NPC), a neurodegenerative disease caused by mutations in the cholesterol transport proteins NPC1 and NPC2, axon development was impaired during early postnatal development, and this impairment was associated with a significant decrease in axonal p-p53 levels (20). Treatment of NPC-deficient mice with a ROCK inhibitor not only increased p-p53 expression but also improved axonal development (20). Previous in vitro studies indicated that calpain could partially truncate p53 (21, 22), thus providing a potential link between calpain and p53 in axonal growth regulation. In the present study we first showed that growth cone collapse triggered by the repulsive cue, semaphorin 3A, was associated with partial truncation of p-p53, and both effects were significantly reduced by calpain inhibition with inhibitors or treatment with siRNAs against m- but not μ-calpain. We further showed that semaphorin 3A–induced calpain activation depended on activation of ERK and p38 MAPK. Thus, our results identified a signaling pathway in semaphorin 3A–induced axon repulsion, which consists of activation of MAPK, calpain phosphorylation/activation, p-p53 truncation, and growth cone collapse.
Results
Calpain Inhibition Suppressed Semaphorin 3A-Induced Growth Cone Collapse and p-p53 Degradation.
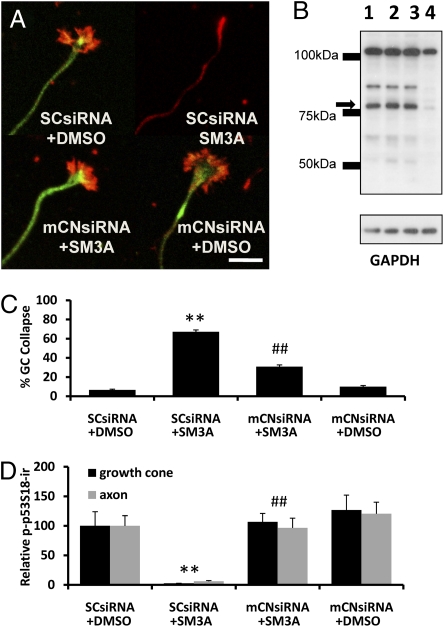
Two sets of experiments were used to test whether calpain was involved in semaphorin 3A-induced growth cone collapse in cultured hippocampal neurons: suppression of calpain expression with siRNAs against μ-calpain or m-calpain and inhibition of calpain activation by specific inhibitors. For siRNA experiments, hippocampal neurons at 3 d in vitro (DIV) were incubated with siRNAs specific for μ- or m-calpain or with scrambled control siRNA followed by 5 min semaphorin 3A (1 nM) exposure 24 h later. They were then fixed and processed for immunostaining with anti–phospho-p53S18 (equivalent to human S15; Fig. 1A, green) or anti-E6AP antibodies [Fig. 1A, red; E6AP is an E3 ligase encoded by UBE3a gene and our recent results indicate that it is highly expressed in growth cones (19)]. Semaphorin 3A treatment induced rapid growth cone collapse in the majority of neurons and a marked reduction in immunoreactivity of p53 phosphorylated at Ser18 (hereafter referred to as p-p53S18) in axons and growth cones (Fig. 1A). Immunoblotting results indicated that m-calpain levels were markedly reduced by siRNAs against m- but not μ-calpain (Fig. 1B). Down-regulation of m-calpain from axons and growth cones was confirmed by immunohistochemical staining (Fig. S1). Image analysis revealed that down-regulation of m-calpain, but not μ-calpain (Fig. S2), significantly prevented semaphorin 3A-induced growth cone collapse (Fig. 1 A and C) and reduction in p-p53S18-ir in axons and growth cones (Fig. 1 A and D).
Fig. 1.
Effect of siRNA against m-calpain on semaphorin 3A–induced growth cone collapse and decrease in p-p53S18. (A) Cultured hippocampal neurons were transfected on DIV 3 with siRNA against m-calpain (mCNsiRNA) or scrambled siRNA (SCsiRNA) and treated on DIV 4 with DMSO or semaphorin 3A (SM3A) for 5 min before being fixed and processed for immunostaining with anti-p-p53 (green) and anti-E6AP (red) antibodies as described in Materials and Methods. Results are representatives of three or four culture dishes from three independent experiments. (Scale bar, 20 μm.) (B) Immunoblotting analysis of m-calpain in cultured cortical neurons. Cultured cortical neurons were transfected on DIV 3 with or without siRNA (lane 1), scrambled siRNA (lane 2), μ-calpain siRNA (lane 3), or m-calpain siRNA (lane 4) and processed on DIV 4 for immunoblotting with anti–m-calpain (arrow indicates native m-calpain, Mr approximately 80 kDa). GAPDH was used as loading control. (C) Percentage of collapsed growth cones (GC) (defined as described in Materials and Methods) in hippocampal neurons in experiments shown in Fig. 1A (n = 100 growth cones from three independent experiments; **P < 0.001 vs. DMSO-treated and ##P < 0.01 vs. SM3A-treated). (D) Levels of p-p53S18 immunoreactivity (p-p53S18-ir) in axons and growth cones of hippocampal neurons in experiments shown in Fig. 1A (data are means ± SEM percent of DMSO-treated neurons; n = 25–30 growth cones; **P < 0.01 vs. DMSO group and ##P < 0.01 vs. SM3A-treated neurons).
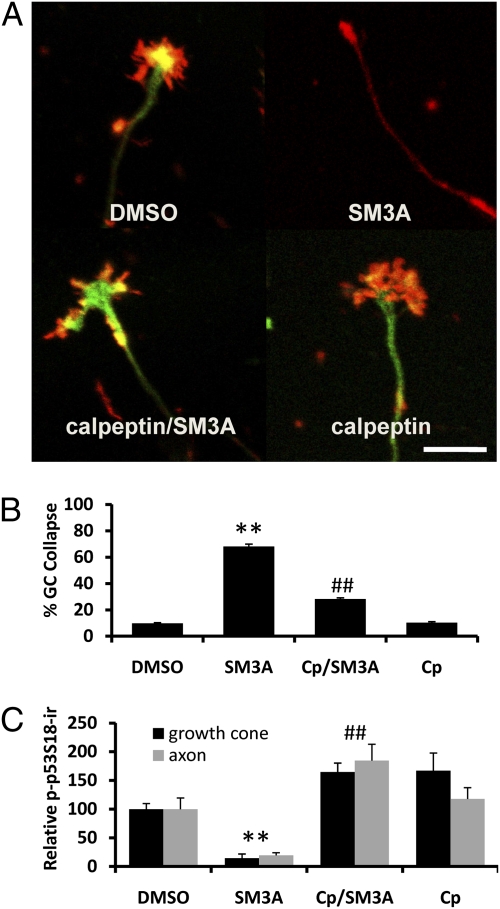
For experiments with calpain inhibitors, hippocampal neurons at DIV 4 were preincubated with calpain inhibitor III (5 μM) or calpeptin (10 μM) for 3 h followed by incubation with semaphorin 3A (1 nM) for 5 min. Both inhibitors significantly prevented semaphorin 3A-induced growth cone collapse (Fig. 2 A and B and Fig. S3), and reduction in p-p53S18-ir in axons and growth cones (Fig. 2 A and C). Immunohistochemical analyses provided evidence that both μ- and m-calpain were present in axons and growth cones; interestingly, semaphorin 3A-induced growth cone collapse was associated with a decrease in μ-calpain, but not m-calpain, immunoreactivity in growth cones (Fig. S4).
Fig. 2.
Calpain inhibition reduces semaphorin 3A-induced growth cone collapse. (A) Cultured hippocampal neurons were treated on DIV4 with DMSO or 1 nM semaphorin 3A (SM3A) in the presence or absence of calpeptin (10 μM pretreatment for 3 h) and processed for immunostaining with anti-p-p53 (green) and anti-E6AP (red) antibodies. (Scale bar, 20 μm.) (B) Percentage of collapsed growth cones (GC) in hippocampal neurons in experiments shown in Fig. 2A (**P < 0.01 vs. DMSO-treated and ##P < 0.01 vs. SM3A-treated neurons; n = 100 growth cones from three independent experiments). Cp, calpeptin. (C) Levels of p-p53S18 immunoreactivity (p-p53S18-ir) in axons and growth cones of DIV 4 hippocampal neurons in experiments shown in Fig. 2A (n = 25–30 growth cones from three independent experiments; **P < 0.01 vs. DMSO-treated and ##P < 0.01 vs. SM3A-treated neurons).
Calpain-Mediated Truncation of p-p53.
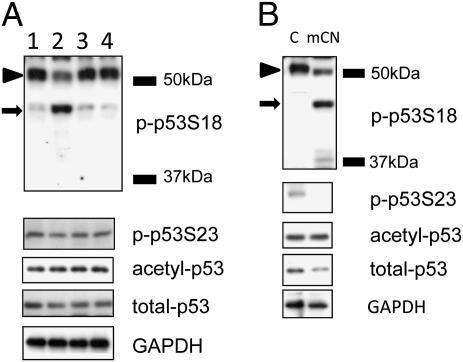
Calpain involvement in semaphorin 3A-induced decrease in p-p53S18 levels was further studied by immunoblotting analysis. Cortical neurons were first incubated with calpeptin for 3 h followed by treatment with 1 nM semaphorin 3A for 5 min. Immunoblotting results showed that semaphorin 3A treatment induced the appearance of a p-p53S18-ir band (arrow, Fig. 3A) that migrated slightly faster than the native p-p53S18-ir band (arrowhead, Fig. 3A) with an apparent Mr of approximately 48 kDa; formation of this band was significantly decreased in the presence of the calpain inhibitor calpeptin (Fig. 3A). Semaphorin 3A treatment did not alter levels of acetylated p53 (acetyl-p53; K382, equivalent to K379 in human) and only slightly reduced levels of total p53 (nonmodified) and p53 phosphorylated at Ser23 (p-p53S23; equivalent to S20 in human). Decrease in both total-p53 and p-p53S23 was also reduced by pretreatment with calpeptin (Fig. 3A).
Fig. 3.
Semaphorin 3A-induced p53 truncation is mediated by calpain. (A) Immunoblotting analysis of various isoforms of p53 proteins in cultured cortical neurons. Cortical neurons were treated on DIV 4 with DMSO (vehicle, lane 1), 1 nM semaphorin 3A (lane 2), semaphorin 3A in the presence of and following pretreatment with 10 μM calpeptin for 3 h (lane 3), or calpeptin alone (lane 4), and processed for immunoblotting as described in Materials and Methods. Representative images of immunoblots probed with anti–phospho-p53Ser18 (p-p53S18), anti–p-p53Ser23, anti–acetyl-p53, and anti–total (nonmodified) p53 antibodies. Arrowhead indicates the native p-p53S18 band; arrow indicates p-p53S18 breakdown product. GAPDH was used as loading control. (B) In vitro analysis of m-calpain–mediated p53 truncation. Whole homogenates were prepared from DIV 4 cortical neuronal cultures and incubated in the absence (C) or presence (mCN) of purified m-calpain (0.5 U/mL) with 1.0 mM CaCl2 at 37 °C for 15 min. Reaction was stopped by adding 2× SDS sample buffers and p53 truncation was analyzed by immunoblotting. Shown are representative images of immunoblots probed with anti-p-p53S18, anti–p53S23, anti–acetyl-p53, and anti–total-p53 antibodies. GAPDH was used as loading control. Arrow indicates p-p53S18 breakdown product.
We next studied calpain-mediated truncation of p53 by incubating whole homogenates prepared from cortical neurons at DIV 4 with purified m-calpain and calcium followed by immunoblotting analysis. Incubation with m-calpain (0.5 U/mL) for 15 min induced the disappearance of the native p-p53S18 band and the appearance of a p-p53S18-ir band with an apparent Mr of approximately 48 kDa, which was similar to that induced by semaphorin 3A treatment (arrow, Fig. 3B). Incubation with exogenous m-calpain also resulted in the degradation of p-p53S23 and total p53, but did not change levels of acetylated p53 (Fig. 3B).
Semaphorin 3A-Induced Growth Cone Collapse and p53 Truncation Is Associated with Activation of ERK and p38 MAPK.
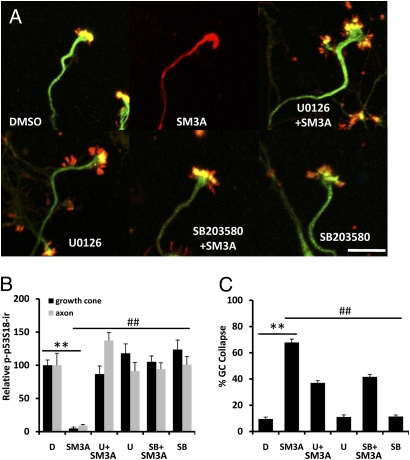
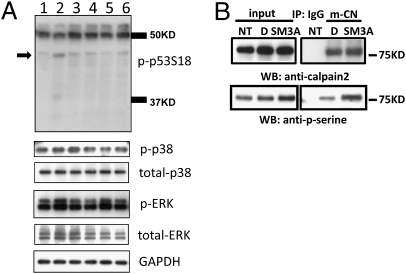
Recent studies in fibroblasts demonstrated that calpain activation by EGF was mediated by ERK phosphorylation of m-calpain at Ser50 (23). We recently discovered that both EGF and BDNF activated m-calpain in neurons as a result of MAPK-mediated serine phosphorylation (24, 25). In addition, stimulation of adenylate cyclase or protein kinase A (PKA) inhibited calpain activation by phosphorylating calpain at serine/threonine 369–370 (26). As semaphorin 3A has been shown to activate MAPK (27), we used an inhibitor of MAPK kinase, U0126, which is widely used to suppress ERK activity, to determine whether this pathway was involved in semaphorin 3A–induced growth cone collapse. We recently reported that p38 MAPK was involved in p53 truncation and growth cone collapse induced by cholesterol perturbation (20); therefore the potential role of p38 in semaphorin 3A–induced growth cone collapse was also tested by using the p38 MAPK inhibitor SB203580. The MAP kinase inhibitors U0126 (500 nM) or SB203580 (400 nM) were applied 3 h before semaphorin 3A treatment (1 nM, 5 min); both inhibitors significantly reduced growth cone collapse (Fig. 4 A and C) and prevented the decrease in p-p53S18-ir in axons and growth cones (Fig. 4 A and B). As neither inhibitor completely blocked semaphorin 3A-induced growth cone collapse, we tested whether the two inhibitors had additive effects. Results from two experiments indicated that, in the presence of both inhibitors, semaphorin 3A-induced growth cone collapse was further reduced to approximately 23% (compared with 37% and 41% as shown in Fig. 4C). Immunoblotting results indicated that semaphorin 3A treatment induced a rapid increase in levels of activated/phosphorylated ERK (at Thr202/Tyr204) and p38 (at Thr180/Tyr182; hereafter referred to as p-ERK and p-p38, respectively; Fig. 5A). Semaphorin 3A-induced p-ERK increase was blocked by U0126 (Fig. S5) but not by SB203580, whereas that increase in p-p38 was blocked by SB203580 but not by U0126 (Fig. 5A). Levels of nonphosphorylated ERK (total-ERK) and p38 MAPK (total-p38) were not significantly altered by preincubation with the inhibitors or semaphorin 3A treatment (Fig. 5A).
Fig. 4.
Semaphorin 3A–induced growth cone collapse and p53 degradation are blocked by ERK and p38 inhibition. Cultured hippocampal neurons were treated on DIV 4 with DMSO or semaphorin 3A (SM3A) in the presence or absence of U0126 or SB203580 pretreatment and processed for immunostaining with anti–p-p53 (green) and anti-E6AP (red) antibodies. (A) Representative images. (Scale bar, 20 μm.) (B) Quantitative results of p-p53S18 levels in axons and growth cones of hippocampal neurons shown in Fig. 4A (**P < 0.01 vs. DMSO-treated and ##P < 0.01 vs. semaphorin 3A–treated; n = 25–40 growth cones from three individual experiments). (C) Growth cone (GC) collapse rate in hippocampal neurons shown in Fig. 4A (**P < 0.01 vs. DMSO-treated and ##P < 0.01 vs. semaphorin 3A–treated; n = 100 growth cones from three individual experiments).
Fig. 5.
ERK and p38 MAPK activation is involved in p-p53 truncation and calpain activation. (A) Immunoblotting analysis of various proteins in cultured cortical neurons. Cortical neurons were treated on DIV4 with DMSO (lane 1), semaphorin 3A (lane 2), semaphorin 3A following a 3-h pretreatment with ERK inhibitor U0126 (lane 3), U0126 treatment alone (lane 4), semaphorin following a 3-h pretreatment with p38 inhibitor SB203580 (lane 5), or SB203580 treatment alone (lane 6), and processed for immunoblotting. Representative images of immunoblots probed with anti–p-p53S18, anti–phospho-p38 MAPK (p-p38), anti–total-p38, anti–phospho-ERK (p-ERK), and anti–total-ERK antibodies. Arrow indicates p-p53S18 breakdown products. GAPDH was used as loading control. (B) Immunoprecipitation and immunoblotting analysis of m-calpain phosphorylation. Cultured cortical neurons were treated with (D) or without DMSO (NT, no treatment) or with semaphorin 3A (SM3A) and processed for immunoprecipitation with anti–m-calpain (m-CN) or control (IgG) antibodies as described in Materials and Methods. Immunoblots were then probed with anti–m-calpain or anti–phospho-serine antibodies. Input: whole lysates from the same samples before immunoprecipitation was performed. Note that phosphorylation of m-calpain is markedly enhanced by semaphorin 3A treatment.
Immunoprecipitation experiments were performed to examine whether semaphorin 3A-induced m-calpain activation was mediated by serine phosphorylation of the protease, as previously reported (24). Cortical neurons treated at DIV 4 with DMSO or semaphorin 3A as described in the previous sections were lysed in immunoprecipitation buffer and proteins were immunoprecipitated with anti–m-calpain antibodies. Immunoprecipitates were then analyzed by immunoblotting using anti–phospho-serine and anti–m-calpain antibodies. Semaphorin 3A treatment increased serine phosphorylation of immunoprecipitated m-calpain (Fig. 5B), in good agreement with our previous studies (24).
Discussion
The present study identified a pathway linking a negative regulator of axonal growth, semaphorin 3A, to growth cone collapse, which consists of activation of ERK and p38 MAPK kinases, phosphorylation and activation of m-calpain, and calpain-mediated truncation of p-p53S18. We are also postulating that the final step in growth cone collapse is a result of actin filament contraction (7, 28) via the activation of the RhoA–ROCK pathway, as we previously reported (19, 20). Semaphorin 3A-induced rapid growth cone collapse in cultured hippocampal neurons was associated with marked decrease in p-p53S18 levels in axons and growth cones and with the formation of a lower molecular weight fragment; both effects were significantly reduced by two calpain inhibitors. Although the p-p53S18-ir breakdown product was still detected by the antibody in immunoblots, immunohistochemical staining showed a marked decrease in p-p53S18-ir in axons and growth cones. The discrepancy may result from the ability of the antibody to recognize the denatured (as in immunoblots) but not the nondenatured breakdown products (as in immunohistochemical studies). Experiments with specific siRNAs indicated that m-calpain, but not μ-calpain, was responsible for both growth cone collapse and p-p53S18 truncation. The involvement of calpain in p53 truncation was further confirmed by results indicating that calpain treatment of homogenates from cortical neurons produced a similar breakdown product of p-p53S18 as semaphorin 3A treatment. We further showed that activation of ERK and p38 MAPK was involved in semaphorin 3A–induced p53 truncation and growth cone collapse, possibly through phosphorylation of m-calpain, as we recently reported (24, 25). Previous studies have implicated MAPK in m-calpain activation in nonneuronal cells (29) and downstream from the EGF-ErbB1 pathway (23). Furthermore, it was shown that m-calpain was phosphorylated at serine 50 and was activated independently of calcium. Our results indicated that semaphorin 3A treatment resulted in activation/phosphorylation of ERK/MAPK, and that inhibiting ERK prevented semaphorin 3A-induced p-p53 truncation. These results are consistent with the hypothesis that semaphorin 3A-induced p-p53 truncation and growth cone collapse involves the activation of ERK/MAPK, and the resulting phosphorylation/activation of m-calpain. However, we cannot exclude the participation of p38 MAPK in this process, as p38 MAPK inhibition also reduced both p-p53 truncation and growth cone collapse. It is also noteworthy that inhibitors of calpain, ERK, and p38 MAPK, while completely preventing semaphorin 3A–induced p53 truncation, produced only a partial blockade of growth cone collapse. As the combination of inhibitors of both ERK and p38 MAPK resulted in an additive effect on growth cone collapse, it is conceivable that additional downstream pathway(s) distinct from p53 degradation may be involved in growth cone collapse. These possibilities will be tested in future studies.
It is now well documented that calpain, by truncating a variety of proteins, plays a critical role in the regulation of shape and motility in numerous cell types (see refs. 10 and 11 for reviews). To this list of substrates and functions, p53 and calpain-mediated truncation of its phosphorylated form needs to be added as playing a major role in regulating axonal growth and growth cone growth. We previously showed that p-p53 played a critical role in growth cone regulation through a dual effect: (i) p-p53 interferes with ROCK signaling by inhibiting RhoA synthesis, and (ii) p-p53 interferes with ROCK signaling by directly binding to ROCK (20). We therefore postulate that calpain-mediated truncation of p-p53 eliminates this dual action of p-p53, which increases ROCK activity and in turn induces actin filament contraction and growth cone collapse (7, 28).
Previous in vitro studies have shown that calpain cleavage of mouse p53 generates two major truncated products with apparent Mrs of 41 and 33 kDa (22). Interestingly, mutations affecting serine 15 did not affect calpain-mediated p53 truncation, although the effect of phosphorylation at this residue has not been tested to our knowledge. Deletion of aa 13–19 rendered p53 completely resistant to calpain cleavage and the conformation mutant p53 recognized by PAb240 is also resistant to calpain-mediated truncation, although, again, there is no information for the phosphorylated variant of this mutant conformation (22). Current studies are directed at identifying the calpain truncation site(s) in p53 and p-p53 and whether this truncation is affected by phosphorylation at different sites.
Our results demonstrate the existence of a unique mechanism linking extrinsic and intrinsic signals to the regulation of axonal outgrowth. Calpain, and in particular m-calpain, is ideally suited to integrate these various signals, as a result of its dual regulation by MAPK- and PKA-mediated phosphorylation, and, by truncating various proteins participating in the regulation of actin dynamics, to provide a critical switch for axonal outgrowth or retraction. In particular, the role of cAMP in axonal growth has been widely documented (30, 31). It is therefore tempting to propose that cAMP, by activating PKA and inhibiting m-calpain, protects p-p53 from degradation, thereby promoting axonal outgrowth. Interestingly, a role for calpain in adult axonal regeneration/degeneration has also been discussed (32), suggesting that the function of calpain in regulation of axonal growth extends beyond the developmental period.
Materials and Methods
Animals.
Time-pregnant BALB/C mice were purchased from Charles River, and kept under temperature- and humidity-controlled conditions with a 12-h light: 12-h dark cycle and food ad libitum before being used. The use of animals followed roles and regulations of the Office of Laboratory Animal Welfare of the National Institutes of Health and was approved by the Institutional Animal Care and Use Committee of Western University of Health Sciences.
Chemicals and Antibodies.
Purified m-calpain, calpeptin (N-benzyloxycarbonyl-L-leucylnorleucinal, a calpain inhibitor), SB203580 (a p38 kinase inhibitor), and U0126 (an ERK inhibitor) were purchased from EMD Chemicals. Control rabbit serum, Tau1 antibodies, and anti-E6AP and anti–phospho-serine antibodies were from Sigma. Semaphorin 3A/Fc was from R&D Systems. Anti–total p53 antibodies were from Santa Cruz Biotechnology. Anti-GAPDH antibody was from Millipore. Anti–phospho-p53 (Ser23), anti–phospho-p53 (Ser18), anti–μ-calpain, anti–m-calpain, anti–acetyl-p53 (Lys382), anti–phospho-ERK (Thr202/Tyr204), anti-ERK, anti–phospho-p38 MAPK (Thr180/Tyr182), and anti-p38 MAPK antibodies were from Cell Signaling Technology. Alexa 488–conjugated anti-rabbit and Alexa 594–conjugated anti-mouse antibodies were from Invitrogen.
Neuronal Cultures.
Cortical and hippocampal neurons were prepared from E18 BALB/C mouse embryos and cultured in NeuroBasal (Gibco) with 10% BSA, 2% B27, and 1% glutamine for 3 to 4 d before being used.
Small Interfering RNA and Transfection.
Transfection with siRNAs for μ-calpain, m-calpain, or scrambled control siRNA was performed as previously described (24). Briefly, neurons were incubated with DMEM (HyClone) with the addition (per 900 μL) of 2 μL of 50 μM siRNA (siRNA final concentration, 100 nM), 48 μL of 0.25 M CaCl2, and 50 μL of BES [pH 7.1; N-N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid] for 3 h. Cultured neurons were then changed to fresh culture medium and cultured for 18 to 24 h before being processed for immunoblot experiments or immunostaining analysis. The following siRNA sequences were used: μ-calpain, TACCTCTGTTCAATTGCTCTA; m-calpain, GCGGTCAGATACCTTCATCAA (Qiagen).
Drug Treatment.
Hippocampal and cortical neurons were treated at DIV 4 with the following inhibitors for 3 h: calpeptin (10 μM), calpain inhibitor III (5 μM), SB203580 (400 nM), and U0126 (500 nM), followed by semaphorin 3A/Fc (1 nM) for 5 min.
Immunofluorescence and Image Analysis.
Hippocampal neurons were fixed with 4% paraformaldehyde in phosphate buffer (pH 7.4) for 15 min. After washing with 1× PBS solution, cells were permeabilized with 0.05% Triton X-100 in 1× PBS solution for 15 min and incubated with blocking buffer (3% BSA, 0.02% Triton X-100 in 1× PBS solution) for 15 min before being probed with primary antibodies. The following primary antibodies were used: Tau-1 (1:1,000), anti-E6AP (1:1,000), anti–phospho-p53S18 (1:250), anti–μ-calpain (1:250), anti–m-calpain (1:500), and anti–phospho-ERK (1:250). All primary antibodies were diluted in blocking buffer and incubated at 4 °C for 18 h. After six washes (6 × 10 min) with 1× PBS solution at room temperature, cells were incubated with the secondary antibodies Alexa 488–anti-rabbit (1:500) or Alexa 594-anti-mouse (1:500); both antibodies were diluted in blocking buffer and incubated at room temperature for 1 h. Cells were then washed with 1× PBS solution (6 × 10 min) and sealed with mounting medium (Vectashield; Vector Laboratories) containing DAPI to stain nuclei. Immunofluorescent signal was detected with a confocal microscope (TE 2000U with D-Eclipse C1 system; Nikon).
Quantification of Growth Cone Morphology and p-p53S18 Immunoreactivity.
Confocal images were taken with a 60× objective. Approximately 20 to 30 images were randomly selected from each culture dish (20 mm in diameter); at least four to six dishes from three to six independent culture preparations were used for each experimental group. Within an experiment, cultures used for different experimental groups and designed for comparison were stained simultaneously and imaged with the same acquisition parameters. Quantification was done blindly by multiple researchers. Growth cones with less than one filopodium were considered collapsed. ImageJ software was used to quantify immunoreactivity intensity of p-p53S18 in axons and growth cones; the “total integrated density” was used instead of “average intensity.” Briefly, individual growth cones were outlined manually and the total integrated density was measured using ImageJ software. For quantification of immunoreactivity in axons, a 50-μm fragment of axons from the neck of growth cones toward the cell body was selected and integrated density was measured. Results were expressed as means ± SEM, and P values were determined by one-way ANOVA followed by post hoc analysis; P values less than 0.05 were considered statistically significant.
Immunoprecipitation and Immunoblotting Procedures.
For immunoprecipitation, cultured cortical neurons were lysed in a lysis buffer [0.05 M Tris base, 0.9% NaCl, pH 7.6, and 0.5% Triton X-100 plus protease inhibitors mixture (1:100; EMD Biosciences) and phosphatase inhibitor mixtures (1:500; Sigma)]. Lysates were centrifuged at 16,000 × g for 30 min at 4 °C. Supernatants were then cleared with a mixture of protein A/G–agarose beads (each 50%) for 1 h at 4 °C, and after a brief spin, pellets were discarded. A small portion of supernatants was used as input. The reminder of the supernatant was immunoprecipitated overnight with control IgG or m-calpain antibodies. Immunoprecipitates were captured by incubation with protein A/G–agarose beads for 3 h at 4 °C. After several washes, the beads were resuspended in 2× SDS sample buffer (4% SDS, 100 mM Tris-HCl, pH 6.8, 10% β-mercaptoethanol, 20% glycerol and 0.2% bromophenol blue) and boiled for 10 min. The resulting proteins were separated by SDS/PAGE and transferred to PVDF membranes for immunoblotting according to previously described protocols (19).
In Vitro m-Calpain–Mediated p53 Cleavage Assay.
Purified m-calpain (0.5 U/mL; Calbiochem) and CaCl2 (1.0 mM; Sigma) were added to whole homogenate lysates prepared from DIV 4 cortical neurons as described in the previous section except with the omission of the protease inhibitor mixture; samples were then incubated at 37 °C for 15 min. Incubation was terminated by adding 100 μM EDTA, EGTA (EMD Chemicals), and 2× SDS sample buffer. Aliquots of proteins (20 μg) were then separated by SDS/PAGE and transferred to PVDF membranes for immunoblotting.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS062167 (to M.B.) and by funds from Western University of Health Sciences (to X.B.). X.B. was also supported by funds from the Daljit and Elaine Sarkaria Chair.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008652107/-/DCSupplemental.
References
- 1.Diefenbach TJ, et al. Myosin 1c and myosin IIB serve opposing roles in lamellipodial dynamics of the neuronal growth cone. J Cell Biol. 2002;158:1207–1217. doi: 10.1083/jcb.200202028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowery LA, Van Vactor D. The trip of the tip: Understanding the growth cone machinery. Nat Rev Mol Cell Biol. 2009;10:332–343. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaefer AW, et al. Coordination of actin filament and microtubule dynamics during neurite outgrowth. Dev Cell. 2008;15:146–162. doi: 10.1016/j.devcel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witte H, Neukirchen D, Bradke F. Microtubule stabilization specifies initial neuronal polarization. J Cell Biol. 2008;180:619–632. doi: 10.1083/jcb.200707042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giniger E. How do Rho family GTPases direct axon growth and guidance? A proposal relating signaling pathways to growth cone mechanics. Differentiation. 2002;70:385–396. doi: 10.1046/j.1432-0436.2002.700801.x. [DOI] [PubMed] [Google Scholar]
- 6.Pak CW, Flynn KC, Bamburg JR. Actin-binding proteins take the reins in growth cones. Nat Rev Neurosci. 2008;9:136–147. doi: 10.1038/nrn2236. [DOI] [PubMed] [Google Scholar]
- 7.Gallo G. RhoA-kinase coordinates F-actin organization and myosin II activity during semaphorin-3A-induced axon retraction. J Cell Sci. 2006;119:3413–3423. doi: 10.1242/jcs.03084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rex CS, et al. Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. J Cell Biol. 2009;186:85–97. doi: 10.1083/jcb.200901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 10.Carragher NO, Frame MC. Calpain: a role in cell transformation and migration. Int J Biochem Cell Biol. 2002;34:1539–1543. doi: 10.1016/s1357-2725(02)00069-9. [DOI] [PubMed] [Google Scholar]
- 11.Perrin BJ, Huttenlocher A. Calpain. Int J Biochem Cell Biol. 2002;34:722–725. doi: 10.1016/s1357-2725(02)00009-2. [DOI] [PubMed] [Google Scholar]
- 12.Flevaris P, et al. A molecular switch that controls cell spreading and retraction. J Cell Biol. 2007;179:553–565. doi: 10.1083/jcb.200703185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robles E, Huttenlocher A, Gomez TM. Filopodial calcium transients regulate growth cone motility and guidance through local activation of calpain. Neuron. 2003;38:597–609. doi: 10.1016/s0896-6273(03)00260-5. [DOI] [PubMed] [Google Scholar]
- 14.Mingorance-Le Meur A, O'Connor TP. Neurite consolidation is an active process requiring constant repression of protrusive activity. EMBO J. 2009;28:248–260. doi: 10.1038/emboj.2008.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.To KC, Church J, O'Connor TP. Growth cone collapse stimulated by both calpain- and Rho-mediated pathways. Neuroscience. 2008;153:645–653. doi: 10.1016/j.neuroscience.2008.02.043. [DOI] [PubMed] [Google Scholar]
- 16.Fábián Z, Vecsernyés M, Pap M, Szeberényi J. The effects of a mutant p53 protein on the proliferation and differentiation of PC12 rat phaeochromocytoma cells. J Cell Biochem. 2006;99:1431–1441. doi: 10.1002/jcb.21019. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Yan W, Chen X. p53 is required for nerve growth factor-mediated differentiation of PC12 cells via regulation of TrkA levels. Cell Death Differ. 2006;13:2118–2128. doi: 10.1038/sj.cdd.4401972. [DOI] [PubMed] [Google Scholar]
- 18.Di Giovanni S, et al. The tumor suppressor protein p53 is required for neurite outgrowth and axon regeneration. EMBO J. 2006;25:4084–4096. doi: 10.1038/sj.emboj.7601292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin Q, et al. A novel function for p53: regulation of growth cone motility through interaction with Rho kinase. J Neurosci. 2009;29:5183–5192. doi: 10.1523/JNEUROSCI.0420-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin Q, Liao G, Baudry M, Bi X. Cholesterol perturbation in mice results in p53 degradation and axonal pathology through p38 MAPK and Mdm2 activation. PLoS ONE. 2010;5:e9999. doi: 10.1371/journal.pone.0009999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubbutat MH, Vousden KH. Proteolytic cleavage of human p53 by calpain: A potential regulator of protein stability. Mol Cell Biol. 1997;17:460–468. doi: 10.1128/mcb.17.1.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pariat M, et al. Proteolysis by calpains: A possible contribution to degradation of p53. Mol Cell Biol. 1997;17:2806–2815. doi: 10.1128/mcb.17.5.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glading A, et al. Epidermal growth factor activates m-calpain (calpain II), at least in part, by extracellular signal-regulated kinase-mediated phosphorylation. Mol Cell Biol. 2004;24:2499–2512. doi: 10.1128/MCB.24.6.2499-2512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zadran S, et al. Brain-derived neurotrophic factor and epidermal growth factor activate neuronal m-calpain via mitogen-activated protein kinase-dependent phosphorylation. J Neurosci. 2010;30:1086–1095. doi: 10.1523/JNEUROSCI.5120-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zadran S, et al. 17-Beta-estradiol increases neuronal excitability through MAP kinase-induced calpain activation. Proc Natl Acad Sci USA. 2009;106:21936–21941. doi: 10.1073/pnas.0912558106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiraha H, Glading A, Chou J, Jia Z, Wells A. Activation of m-calpain (calpain II) by epidermal growth factor is limited by protein kinase A phosphorylation of m-calpain. Mol Cell Biol. 2002;22:2716–2727. doi: 10.1128/MCB.22.8.2716-2727.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bechara A, et al. FAK-MAPK-dependent adhesion disassembly downstream of L1 contributes to semaphorin3A-induced collapse. EMBO J. 2008;27:1549–1562. doi: 10.1038/emboj.2008.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang XF, Schaefer AW, Burnette DT, Schoonderwoert VT, Forscher P. Rho-dependent contractile responses in the neuronal growth cone are independent of classical peripheral retrograde actin flow. Neuron. 2003;40:931–944. doi: 10.1016/s0896-6273(03)00754-2. [DOI] [PubMed] [Google Scholar]
- 29.Niapour M, Berger S. Flow cytometric measurement of calpain activity in living cells. Cytometry A. 2007;71:475–485. doi: 10.1002/cyto.a.20399. [DOI] [PubMed] [Google Scholar]
- 30.Lohof AM, Quillan M, Dan Y, Poo MM. Asymmetric modulation of cytosolic cAMP activity induces growth cone turning. J Neurosci. 1992;12:1253–1261. doi: 10.1523/JNEUROSCI.12-04-01253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ming GL, et al. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997;19:1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 32.Hou ST, Jiang SX, Smith RA. Permissive and repulsive cues and signalling pathways of axonal outgrowth and regeneration. Int Rev Cell Mol Biol. 2008;267:125–181. doi: 10.1016/S1937-6448(08)00603-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.