Abstract
Heterotrimeric GTP-binding proteins (G proteins) transmit extracellular stimuli perceived by G protein-coupled receptors (GPCRs) to intracellular signaling cascades. Hundreds of GPCRs exist in humans and are the targets of a large percentage of the pharmaceutical drugs used today. Because G proteins are regulated by GPCRs, small molecules that directly modulate G proteins have the potential to become therapeutic agents. However, strategies to develop modulators have been hampered by a lack of structural knowledge of targeting sites for specific modulator binding. Here we present the mechanism of action of the cyclic depsipeptide YM-254890, which is a recently discovered Gq-selective inhibitor. YM-254890 specifically inhibits the GDP/GTP exchange reaction of α subunit of Gq protein (Gαq) by inhibiting the GDP release from Gαq. X-ray crystal structure analysis of the Gαqβγ–YM-254890 complex shows that YM-254890 binds the hydrophobic cleft between two interdomain linkers connecting the GTPase and helical domains of the Gαq. The binding stabilizes an inactive GDP-bound form through direct interactions with switch I and impairs the linker flexibility. Our studies provide a novel targeting site for the development of small molecules that selectively inhibit each Gα subunit and an insight into the molecular mechanism of G protein activation.
Keywords: G protein, crystal structure, specific inhibitor
Heterotrimeric guanine nucleotide-binding proteins (G proteins) serve as critical relays that transmit extracellular stimuli perceived by G protein-coupled receptors (GPCRs) at the cell surface to intracellular signaling cascades (1–3). Heterotrimeric G proteins comprise three subunits, α, β, and γ. The Gα subunit conserves the Ras-like GTPase domain that is essential for hydrolyzing GTP to GDP. In an in vivo resting state, the Gα subunit usually exists in the GDP-bound state and forms a trimer with the β and γ subunits. A ligand-activated GPCR acts as a guanine nucleotide exchange factor (GEF) that stimulates exchange of bound GDP for GTP on the Gα subunit. GTP binding alters three flexible regions, switches I, II, and III, of Gα, leading to Gβγ dissociation and the subsequent binding of Gα and Gβγ to downstream effectors. Signal termination is achieved by GTP hydrolysis, resulting in the reformation of an inactive heterotrimer.
GPCR-mediated signal transduction controls a wide variety of organ functions through multiple signaling pathways (3). The 15 human genes encoding Gα subunits are categorized into four subfamilies: Gαs, GαI, Gαq, and Gα12. The multiplicity of receptor-G protein interactions makes it difficult to analyze the intracellular signaling network. The most straightforward approach is to apply a specific inhibitor or activator for one type of G protein. However, until now, availability of small molecules has been limited to a subset of Gα subunits. Strategies to develop small molecule inhibitors have been hampered by a lack of structural information about the inhibitor binding site on the molecular surface.
To identify such an inhibitor binding site, we focused our studies on the cyclic depsipeptide YM-254890, which was isolated from Chromobacterium sp. QS3666 as an inhibitor of ADP-induced platelet aggregation (4). YM-254890 strongly inhibits intracellular calcium ion mobilization and serum response element (SRE)-mediated transcription stimulated by several receptors coupled to Gq, but not those coupled to Gi, Gs, or G15 (5). YM-254890 also exhibited antithrombotic and thrombolytic effects in an electrically induced carotid artery thrombosis model in rats (6). YM-254890 is the first and only compound that specifically inhibits Gq signaling. Accordingly, it is an invaluable tool for analyzing G protein activation and Gq-mediated cell responses. However, the details of the inhibitory mechanism of YM-254890 remain to be revealed.
In this study, we carried out the biochemical characterization of YM-254890 using purified G proteins in vitro and determined the X-ray crystal structure of the heterotrimeric Gq protein complexed with YM-254890. Our results revealed a unique inhibitory mechanism by which YM-254890 selectively blocks GDP release from the Gq protein.
Results
Specific Inhibition of Gq Family Members by YM-254890.
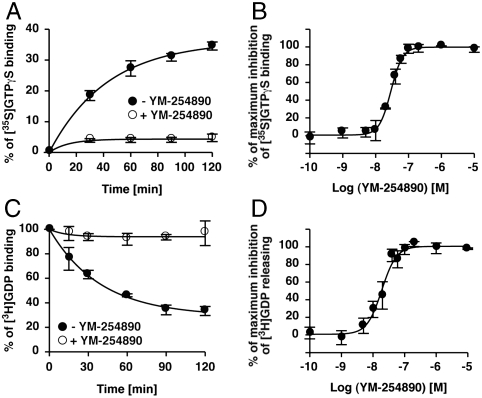
Previously, it was reported that YM-254890 blocks agonist-induced GTPγS binding to Gq/11 in crude cell membranes (5). This finding suggests the possibility that YM-254890 directly binds to Gαq and inhibits the GDP/GTP exchange. To test this, we first performed an in vitro [35S]GTPγS binding assay using purified Gαq protein. YM-254890 blocked spontaneous GTPγS binding to Gαq in a concentration-dependent manner, suggesting a stoichiometric interaction between Gαq and YM-254890 (Fig. 1 A and B). On the other hand, YM-254890 had no effect on the spontaneous GTPγS binding to Gαs, Gαi1, Gαo, and Gα13 (Fig. S1). GTP binding to GDP-bound Gα consists of two reactions: dissociation of bound GDP and binding of GTP to nucleotide-free Gα. The dissociation of GDP is a rate-limiting step in the GDP/GTP exchange reaction. We investigated the effect of YM-254890 on GDP dissociation from Gαq. [3H]GDP was preloaded on Gαq, and [3H]GDP released from Gαq was analyzed in the presence or absence of YM-254890. YM-254890 blocked GDP dissociation from Gαq in a concentration-dependent manner (Fig. 1 C and D), indicating that YM-254890 acts as a Gαq-specific guanine nucleotide dissociation inhibitor (GDI).
Fig. 1.
Inhibitory effects of YM-254890. (A) Inhibition of GTPγS binding of Gαq. Purified Gαq (100 nM) preincubated without (filled circles) or with (open circles) 10 μM YM-254890 was assayed in the presence of 300 mM (NH4)2SO4. (B) Dose-dependent inhibition of GTPγS binding to Gαq. YM-254890-preincubated Gαq was incubated with GTPγS for 120 min. (C) Inhibition of GDP dissociation from Gαq. [3H]GDP dissociation from Gαq (50 nM) was monitored without (filled circles) or with (open circles) 10 μM YM-254890 in the presence of 500 μM unlabeled GDP. (D) Dose-dependent inhibition of GDP dissociation from Gαq (120 min). Each value represents the mean ± S.D. from three independent experiments.
Aluminum fluoride (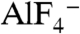 ) is known to activate Gα proteins by mimicking the γ-phosphate of GTP in the presence of GDP (7, 8). Thus, the addition of
) is known to activate Gα proteins by mimicking the γ-phosphate of GTP in the presence of GDP (7, 8). Thus, the addition of 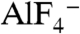 induces conformational changes in Gα proteins from the GDP-bound inactive form to the
induces conformational changes in Gα proteins from the GDP-bound inactive form to the 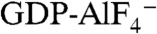 -bound active state (9, 10). YM-254890 inhibited the
-bound active state (9, 10). YM-254890 inhibited the 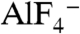 -induced conformational change in Gαq and Gα14 but not Gα16 (Fig. S2). In Fig. S2, we used ectopically expressed Gα proteins in lysates from mammalian cells, because both Gα14 and Gα16 proteins were difficult to purify. The results of a previous study (5) and those of our studies indicate that YM-254890 selectively inhibits Gαq, Gα11, and Gα14 among mammalian Gα members.
-induced conformational change in Gαq and Gα14 but not Gα16 (Fig. S2). In Fig. S2, we used ectopically expressed Gα proteins in lysates from mammalian cells, because both Gα14 and Gα16 proteins were difficult to purify. The results of a previous study (5) and those of our studies indicate that YM-254890 selectively inhibits Gαq, Gα11, and Gα14 among mammalian Gα members.
To examine whether YM-254890 inhibits Gαq bound to Gβγ, we prepared heterotrimer His-Gαi/qβ1γ2C68S (henceforth, referred to as His-Gαi/qβγ), which contains His-Gαi/q, Gβ1, and nonprenylated His-Gγ2C68S. Gαi/q is a soluble and functional Gαq chimera in which the wild-type N-terminal helix was replaced with that of Gαi1 (11). We compared the YM-254890 sensitivity of monomeric His-Gαi/q and heterotrimeric His-Gαi/qβγ by using the [35S]GTPγS binding assay. YM-254890 inhibited the GTPγS binding to His-Gαi/qβγ as well as Gβγ-free His-Gαi/q (Fig. S3).
Overall Structure of Heterotrimeric G Protein Complexed with YM-254890.
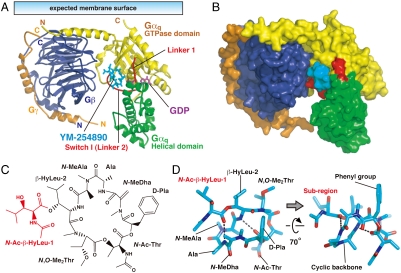
To clarify the molecular basis of the YM-254890 action, the crystal structure of Gαi/qβγ bound to YM-254890 was determined at a 2.9 Å resolution (Table S1). The structure reveals the heterotrimer in which Gγ2-bound Gβ1 forms extensive contacts with Gαi/q, which consists of the N-terminal helix as well as the GTPase and helical domains (Fig. 2A). The major α/β interface covers the N-terminal helix and switch II from the GTPase domain, but not the helical domain, of Gαi/q. GDP binds to a cleft between the GTPase and helical domains without contacting Gβγ. These structural characteristics are basically similar to the other reported G protein heterotrimer structures (12, 13). YM-254890 docks into the cleft between Linker 1 and Switch I (Linker 2), which connect the GTPase and helical domains. To our knowledge, this cleft has never been described as a critical contact site with other molecules, including Gα effectors (11, 14), Gβγ (12, 13), GoLoco (15), GPCRs (16), or GPCR-mimic peptides (17). A structural comparison of our YM-254890-bound Gαi/qβγ with the inhibitor-free heterotrimers containing Gαi1 or Gαt/i1 (12, 13) suggested a slightly different configuration of the helical domain against the GTPase domain (Fig. S4A). Although a crystal packing effect can not be excluded, this rotation of the helical domain relative to the GTPase domain may represent a basic property of Gαq rather than a conformation induced by the binding of YM-254890. The configuration of the two domains of YM-254890-bound Gαi/q appears to overlap with the active form of Gαi/q (11, 14) (Fig. S4B). It is known from the structures of GDP-bound and GTP-bound Gαt that the orientation of the helical domain is unaffected by nucleotide exchange; the structures are quite similar except for the three switch regions. Furthermore, there is precedence for a difference of the configuration of the two domains among Gα subfamilies in the structures reported for Gαi1 and Gαt (9). Switches I–III of Gαi/q preserved the canonical GDP-bound conformation (Fig. S4C).
Fig. 2.
Overall structure of the Gαi/qβγ heterotrimer in complex with YM-254890. (A) The heterotrimer is viewed with the expected orientation at the plasma membrane. Gαi/q consists of the GTPase (yellow) and the helical (green) domains connected by two linker regions (red), Linker 1 and Switch I (Linker 2). Gβ and Gγ are blue and orange, respectively. GDP (purple) and YM-254890 (cyan) are shown as stick models. (B) Surface representation of the heterotrimer as shown in (A). (C) Chemical structure of YM-254890. YM-254890 consists of a cyclic backbone (black) and an additional subregion (red). (D) Structure model of YM-254890 bound to the heterotrimer. Oxygen and nitrogen are shown in red and blue, respectively. The hydrogen bonds are shown as dashed lines.
YM-254890 consists of a cyclic backbone and an additional subregion (red region in Fig. 2C) with seven amide bonds. YM-254890 in our complex may exhibit a combination of cis- or trans-amide bond conformations identical to the major conformer suggested by a previous NMR analysis in solution (18) and display an overall conformation of a folded V-shape, which is in part stabilized by intramolecular hydrogen bonds (Fig. 2D and Fig. S5). The compact form of YM-254890 has 1047 Å2 of the total solvent accessibility area, of which 52% (543 Å2) is masked by direct contact with Gαi/q (Fig. 2B).
Binding Mode of YM-254890 to G Protein.
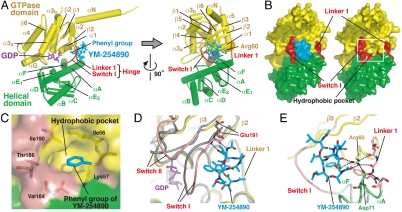
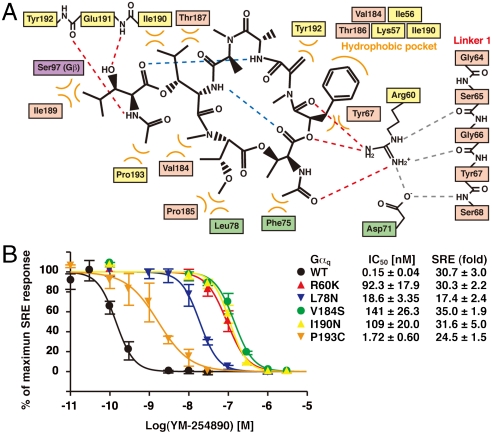
The interface between YM-254890 and Gαi/q comprises the α1 helix and β2 strand from the GTPase and the αA helix from the helical domains in addition to the two interdomain linkers: Linker 1, connecting the α1 and αA helices, and Switch I (Linker 2), connecting the αF helix and the β2 strand (Fig. 3A). The inhibitor–protein interactions are summarized in Fig. 4A. Thirteen residues of the binding cleft participate in nonpolar may contacts with YM-254890. At the bottom of the cleft, the aromatic phenyl group of YM-254890 D-Pla docks into a small hydrophobic pocket (Fig. 4A) and should make intimate contacts with residues from Switch I (Val184 and Thr186) and the GTPase domain (Ile56, Lys57, and Ile190) (Fig. 3 B and C). To validate the contribution of these resides to the binding affinity, we generated Gαq-point mutants and measured the YM-254890 sensitivity of each Gαq mutant. Using the trypsin protection assay, we first confirmed that each Gαq mutant has the capacity to undergo a proper conformation change (Fig. S6). The sensitivity of YM-254890 was evaluated by the SRE-reporter activity induced by the coexpression of the Gαq mutant and the M1 muscarinic acetylcholine receptor. Each substitution of Val184 or Ile190 to a hydrophilic residue resulted in a marked reduction of YM-254890 sensitivity (940- and 730-fold, respectively) (Fig. 4B). Two nonpolar residues (Phe75 and Leu78) from the helical domain (αA helix) also contacted YM-254890. The mutation of Leu78 exhibited reduction of sensitivity of two orders of magnitude.
Fig. 3.
Specific binding of YM-254890 to Gαi/q. (A) Side and front views of the YM-254890-binding cleft on Gαi/q in our complex structure. The key residue Arg60 on the α1 helix is shown in a stick model. The color codes are the same as those in Fig. 2A. (B) Surface representation of the front view of Gαi/q with (Left) and without (Right) YM-254890 to show the pocket between two linkers at the interdomain region. (C) Close-up view of the hydrophobic pocket [white square area in (B)] that accommodates the phenyl group of YM-254890 (cyan). Residues that form this pocket are depicted as stick models and labeled. The two linkers are pink. (D) Switch I-YM-254890 interactions. The inactive GDP-bound form of Gαi/q (yellow) in our complex is superimposed on the active form of Gαq (gray) bound to 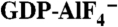 and GRK2 (11). Glu191 in Switch I is shown as a stick model. The hydrogen bonds between Switch I backbone and the subregion of YM-254890 are shown as dashed lines. The Gαi/q switches (pink) are shifted from those of the active form. (E) The Arg-Asp pair in Linker 1–YM-254890 interactions. Arg60, Asp71, and Linker 1 residues are shown as stick models. The hydrogen bonds involving the Arg-Asp pair are shown as dashed lines.
and GRK2 (11). Glu191 in Switch I is shown as a stick model. The hydrogen bonds between Switch I backbone and the subregion of YM-254890 are shown as dashed lines. The Gαi/q switches (pink) are shifted from those of the active form. (E) The Arg-Asp pair in Linker 1–YM-254890 interactions. Arg60, Asp71, and Linker 1 residues are shown as stick models. The hydrogen bonds involving the Arg-Asp pair are shown as dashed lines.
Fig. 4.
Summary of the YM-254890–Gαi/q interactions and mutation studies. (A) Schematic representation of the inhibitor-protein interactions. Intramolecular (blue) and intermolecular (red) hydrogen bonds of YM-254890 are shown as dashed lines. Hydrogen bonds between Arg60 and Linker 1 are shown as gray dashed lines. Hydrophobic interactions are indicated as curved orange lines. (B) Mutational analysis of Gαq residues that appear to directly interact with YM-254890. YM-254890 sensitivity of each Gαq mutant was evaluated by SRE activation. The calculated IC50 values of YM-254890 for each Gαq mutant and SRE activity of each Gαq mutant are shown. Each value represents the mean ± S.D. from three independent experiments performed in duplicate. Mutant protein samples were confirmed to retain proper conformations by the trypsin protection assay (Fig. S6).
YM-254890 may fit its nonpolar side chains of both the subregion and the cyclic peptide to Switch I (Val182-Tyr192), and form two hydrogen bonds to Switch I backbone (Fig. 3D). These intimate interactions cannot be retained in the GTP-bound form, whose Switch I is shifted approximately 2 Å away from the YM-254890-bound cleft (19, 20). In sharp contrast to Switch I (Linker 2), Linker 1 lacks most direct contacts with YM-254890. Instead, the salt bridge between Arg60 from the GTPase domain (α1 helix) and Asp71 from the helical domain (αA helix), the Arg-Asp pair, would mediate the Linker 1-inhibitor interactions by forming multiple hydrogen bonds (Fig. 3E and Fig. S7). The terminal guanidium group of Arg60 would be essential for these interactions and contribute to the stabilization of the V-shaped ring of the inhibitor. Mutation of Arg60 with a lysine residue resulted in approximately 620-fold less sensitivity to YM-254890 (Fig. 4B).
Discussion
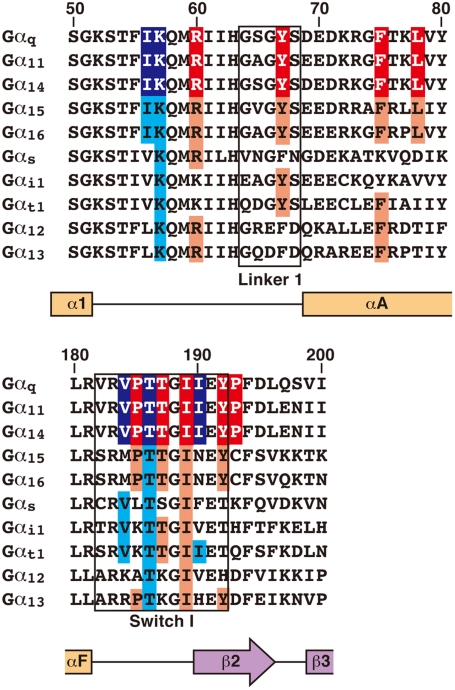
YM-254890 exhibits exquisite substrate specificity for Gαq, Gα11, and Gα14. Our sequence alignment reveals that the Gα residues directly interacting with the inhibitor are completely conserved in all YM-254890-sensitive Gαq, Gα11, and Gα14 but not in other Gα members (Fig. 5). In particular, Gα15 and Gα16 conserve all the key residues except Switch I. Our Switch I mutations, I190N and P193C, which replace each Gαq residue with the corresponding residue in Gα15 and Gα16, respectively, reduce inhibitor sensitivity (Fig. 4B). Thus, we speculate that, in principle, Switch I would ensure the specificity. Other Gα members display sequence diversity in regions other than Switch I. Such an example is Gαi1, which possesses lysine instead of the key residue Arg60. The R60K mutation severely reduces the activity, as already described (Fig. 4B).
Fig. 5.
Sequence alignment of G protein α subunits around the YM-254890 binding site. Linker 1 and Switch I regions are outlined in black. The predicted secondary structure is represented by orange squares and a purple arrow for the α helices and the β strand, respectively. The residue numbering is based on Gαq. The residues that appear to directly interact with YM-254890 are shown on a red background. In particular, the residues that form the hydrophobic pocket, which would bind to the phenyl group of YM-254890, are marked with a blue background. Conserved residues at these positions in YM-254890-insensitive Gα are shown on a light-red or light-blue background. These residues are completely conserved only in YM-254890-sensitive Gαq, Gα11, and Gα14.
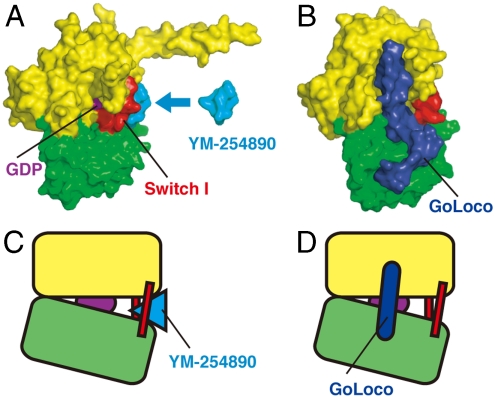
YM-254890-mediated stabilization of both interdomain linkers, Linker 1 and Switch I (Linker 2), directly accounts for the repression of the GDP release from Gαq (Fig. 1 C and D) and, thereby, the repression of the exchange with GTP (Fig. 1 A and B). A large body of experimental evidence (16, 21–25) supports the importance of the rearrangement of the GTPase and helical domains of Gα to create the necessary route for GDP dissociation. In particular, two flexible interdomain linkers appear to act together as a hinge during the opening of the helical domain away from the GTPase domain. A site-directed spin-labeling study showed that a ligand-activated GPCR triggers the conformational change in Switch I (21). A previous mutation study also indicated that the flexibility of interdomain linkers controls the GDP release rate of Gα (25). In our structure model, YM-254890 forms a cone-like structure with its phenyl group at the head and was stuck deeply into the cleft between both interdomain linkers. As described above, YM-254890 should stabilize Switch I in the inactive GDP-bound conformation through direct interaction (Fig. 3D). The Linker 1–YM-254890 interaction with multiple hydrogen bonds would also contribute to the stabilization of interdomain linkers (Fig. 3E and Fig. S7). Thus, YM-254890 may repress GDP release by docking into the cleft to reduce a hinge motion of interdomain linkers that are necessary for the rearrangement of the GTPase and helical domains (Fig. 6 A and C). In contrast, it has been shown that the GoLoco motif, which is known to exert GDI activity against Gi subfamily members, acts as a “clamp” by contacting both the GTPase and helical domains to restrict the domain rearrangement (Fig. 6 B and D) (15, 26).
Fig. 6.
Comparison of the structural basis for the inhibition of GDP release by YM-254890 and the GoLoco peptide. (A) Surface representation of the Gαi/q–YM-254890 complex (PDB ID: 3AH8). (B) Surface representation of the Gαi1–GoLoco peptide complex (PDB ID: 1KJY). The GoLoco peptide is shown in blue. (C) Schematic representation of (A). YM-254890 directly inhibits a hinge motion of the helical domain away from the GTPase domain. (D) Schematic representation of (B). The GoLoco peptide may act as a Gα clamp that restricts the movement of the two domains (14, 25).
The unique binding mode of YM-254890 to Gq is reminiscent of that of the “interfacial inhibitor,” which is an alternative to a competitive inhibitor in the development of innovative therapeutics (27, 28). The interfacial inhibitor binds to two macromolecules (protein–protein or protein–nucleic acid) and forms a dead-end complex. Brefeldin A is a typical interfacial inhibitor that stabilizes the abortive complex of Arf and Arf-GEF, inhibiting Arf activation by its GEF (29). In our current model, YM-254890 binds to a cleft between the GTPase and helical domains within one molecule and restricts the conformational rearrangement of these domains. We propose that YM-254890 should be categorized as a unique type of inhibitor, which fixes a domain–domain interface of one macromolecule by binding to hinge regions connecting two intramolecular domains. In some signal-transduction proteins, the interdomain rearrangement is important for the regulation of their molecular functions. The molecular basis of YM-254890 action provides the possibility that a pocket formed by hinge regions becomes a unique site for inhibitor binding.
More recently, constitutive activation of the heterotrimeric G protein α subunit with somatic mutations has been found in uveal melanoma and blue naevi (30). YM-254890 suppresses the oncogenic mutant-induced transcriptional activation (5), suggesting a potential YM-254890 application in cancer in addition to several other diseases. The nucleotide exchange reaction of some α subunits, Gαq, Gαi1, and Gαo, is promoted by Ric-8A (31). Interestingly, YM-254890 also inhibits the Ric-8A-mediated exchange reaction of Gαq (32), which is consistent with the notion that the inhibitor directly binds to Gαq. Cholera (33), pertussis (34), and Pasturella multocida (35) toxins are well known modulators of G protein activity. As a Gq-specific inhibitor, YM-254890 should be a useful addition to the tools available to analyze diverse G protein signaling pathways. A close look at the previously reported Gα structures shows that each α subunit preserves the interdomain cleft, which is similar to that of our structure but displays unique surface shapes and properties. This observation suggests that YM-254890 derivatives or YM-254890-mimic compounds could be developed for the specific inhibition of each Gα.
Materials and Methods
Protein Expression and Purification.
Baculoviruses encoding His-Gαi/q and His-Gγ2C68S were generated using the Bac-to-Bac baculovirus expression system (Invitrogen). Baculoviruses encoding Gαq, Gα13, Gβ1, and His-Gγ2 were kindly provided by Tohru Kozasa (University of Illinois at Chicago). Purification of His-Gαi/q and Gαi/qβ1γ2C68S from baculovirus-infected High Five cells was performed as previously described (11), with the addition of an anion-exchange chromatography step before a final gel-filtration step. For crystallization, Gαi/qβ1γ2C68S was finally concentrated to 40 mg/mL using Centricon YM-30. Purification of Gαq, Gαi1, and Gα13 from baculovirus-infected Sf9 cells was performed as previously described (36). His-Gαo was purified from BL21(DE3) cells using Ni-NTA agarose (QIAGEN). Gαs was purified from BL21(DE3) cells as previously described (37).
Crystallization.
Gαi/qβγ (375 μM, 28 mg/mL) mixed with 2 mM YM-254890 in a buffer containing 20 mM Hepes-NaOH pH 8.0, 100 mM NaCl, 1 mM MgCl2, 2 mM DTT, 0.4 mM GDP, and 4% (v/v) DMSO was used for crystallization. Crystallization screening was carried out by the vapor-diffusion method at 20 °C using commercial screening kits (Nextal Biotechnologies). The protein was mixed in a 1∶1 ratio with the reservoir solution. Crystallization conditions were subsequently optimized by the sparse matrix method. The prismatic crystal of the Gαi/qβγ–YM-254890 complex was obtained under conditions that included 7% PEG 4,000, 70 mM acetate-NaOH pH 5.1, and 30% (v/v) glycerol.
GTPγS Binding Assay.
GTPγS binding of the purified Gα was measured using a filter-binding method. The spontaneous GTPγS binding of Gαq and Gαi1 was promoted in the presence of (NH4)2SO4, as previously described (38). Briefly, purified Gα (100 nM) was preincubated with YM-254890 for 3 min at 20 °C in assay buffer A (50 mM Hepes-NaOH pH 7.5, 1 mM EDTA, 1 mM DTT, 0.9 mM MgSO4, and 0.05% Genapol C-100). Reactions were started by the addition of 10 μM [35S]GTPγS (10,000 cpm/pmol) and 300 mM (NH4)2SO4. The GTPγS binding of Gαs and Gαo was performed in assay buffer B (20 mM Hepes-NaOH pH 8.0, 100 mM NaCl, 1 mM EDTA, 1 mM DTT, 10 mM MgSO4, and 0.05% Lubrol-PX) (31). The GTPγS binding of Gα13 was performed in assay buffer C (50 mM Hepes-NaOH pH 7.6, 1 mM EDTA, 1 mM DTT, 0.5 mM MgSO4, and 0.05% Lubrol-PX) (39). Reactions of Gαq and Gαs were carried out at 20 °C, whereas those of Gαi1, Gαo, and Gα13 were performed at 25 °C. The reactions were stopped by the addition of an ice-cold stop buffer (20 mM Tris-HCl pH 7.7, 100 mM NaCl, 2 mM MgSO4, 0.05% Genapol C-100 or Lubrol-PX, and 1 mM GTP), and the mixtures were filtered through nitrocellulose membranes. The membranes were washed twice with an ice-cold wash buffer (20 mM Tris-HCl pH 7.7, 100 mM NaCl, and 2 mM MgSO4) and air-dried.
GDP Dissociation Assay.
Gαq (100 nM) was incubated with 2 μM [3H]GDP (10,000 cpm/pmol) for 18 h at 20 °C in assay buffer A with 50 mM (NH4)2SO4. Approximately 25% of Gαq incorporated the radiolabeled nucleotide. The reaction mixtures were then mixed with the same volume of an assay buffer containing an excess of unlabeled GDP (1 mM), DMSO or YM-254890, and 750 mM (NH4)2SO4 to monitor the dissociation of [3H]GDP. The reactions were stopped by the addition of an ice-cold wash buffer, and the mixtures were filtered.
Luciferase Assay of the SRE-Mediated Transcription.
293T cells were seeded on a 48-well plate and transfected using Lipofectamine 2000 (Invitrogen) with pCMV5-Gαq or its mutant plasmids (50 - 70 ng/well), pCMV5-M1 muscarinic acetylcholine receptor (10 ng/well), pSRE-Luc (50 ng/well), pEF-RLuc (0.5 ng/well), and pCMV5 up to a total of 300 ng/well of plasmid DNA. YM-254890 was added 20 min after transfection. At 18 h posttransfection, the cells were harvested and subjected to the Dual-Luciferase Reporter Assay System (Promega) using a multilabel plate reader (PerkinElmer). Expression levels of Gαq and its mutants were confirmed by immunoblotting. Firefly luciferase activity derived from pSRE-Luc was normalized to the Renilla luciferase activity derived from pEF-RLuc.
For more information, see SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank T. Kozasa (University of Illinois at Chicago) for the gift of the baculoviruses encoding Gαq, Gα13, Gβ1, and His-Gγ2; T. Kawano (University of Illinois at Chicago) for technical advice about the purification of the Gαi/qβγ heterotrimer; Y. Sugawara (Osaka University) for helpful discussions; M. Orita (Astellas Pharma) for the docking simulation of Gαqβγ-YM-254890; N. Shimizu, M. Kawamoto, and M. Yamamoto at SPring-8 for their assistance with the synchrotron experiments; J. Tsukamoto [Nara Institute of Science and Technology (NAIST)] for mass spectrometry; Y. Kaziro (Kyoto University) and M. Linder (Cornell University) for reviewing the manuscript; and all of the members of our laboratories at NAIST for helpful discussions. This work was supported by a Ministry of Education, Culture, Sports, Science, and Technology (MEXT) Grant-in-Aid for Scientific Research on Priority Areas (17079006 and 19036013).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 3AH8).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003553107/-/DCSupplemental.
References
- 1.Gilman AG. G proteins: Transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 2.Kaziro Y, Itoh H, Kozasa T, Nakafuku M, Satoh T. Structure and function of signal-transducing GTP-binding proteins. Annu Rev Biochem. 1991;60:349–400. doi: 10.1146/annurev.bi.60.070191.002025. [DOI] [PubMed] [Google Scholar]
- 3.Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 4.Taniguchi M, et al. YM-254890, a novel platelet aggregation inhibitor produced by chromobacterium sp. QS3666. J Antibiot. 2003;56:358–363. doi: 10.7164/antibiotics.56.358. [DOI] [PubMed] [Google Scholar]
- 5.Takasaki J, et al. A novel Galphaq/11-selective inhibitor. J Biol Chem. 2004;279:47438–47445. doi: 10.1074/jbc.M408846200. [DOI] [PubMed] [Google Scholar]
- 6.Kawasaki T, et al. Antithrombotic and thrombolytic efficacy of YM-254890, a Gq/11 inhibitor, in a rat model of arterial thrombosis. Thromb Haemost. 2003;90:406–413. doi: 10.1160/TH03-02-0115. [DOI] [PubMed] [Google Scholar]
- 7.Sternweis PC, Gilman AG. Aluminum: A requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc Natl Acad Sci USA. 1982;79:4888–4891. doi: 10.1073/pnas.79.16.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bigay J, Deterre P, Pfister C, Chabre M. Fluoroaluminates activate transducin-GDP by mimicking the gamma-phosphate of GTP in its binding site. FEBS Lett. 1985;191:181–185. doi: 10.1016/0014-5793(85)80004-1. [DOI] [PubMed] [Google Scholar]
- 9.Coleman DE, et al. Structures of active conformations of Gi alpha 1 and the mechanism of GTP hydrolysis. Science. 1994;265:1405–1412. doi: 10.1126/science.8073283. [DOI] [PubMed] [Google Scholar]
-
10.Sondek J, Lambright DG, Noel JP, Hamm HE, Sigler PB. GTPase mechanism of G proteins from the 1.7- Å crystal structure of transducin α-GDP
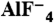 . Nature. 1994;372:276–279. doi: 10.1038/372276a0. [DOI] [PubMed] [Google Scholar]
. Nature. 1994;372:276–279. doi: 10.1038/372276a0. [DOI] [PubMed] [Google Scholar] - 11.Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJ. Snapshot of activated G proteins at the membrane:The Galphaq-GRK2-Gbetagamma complex. Science. 2005;310:1686–1690. doi: 10.1126/science.1118890. [DOI] [PubMed] [Google Scholar]
- 12.Wall MA, et al. The structure of the G protein heterotrimer Gi alpha 1 beta 1 gamma 2. Cell. 1995;83:1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 13.Lambright DG, et al. The 2.0 Å crystal structure of a heterotrimeric G protein. Nature. 1996;379:311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- 14.Lutz S, et al. Structure of Galphaq-p63RhoGEF-RhoA complex reveals a pathway for the activation of RhoA by GPCRs. Science. 2007;318:1923–1927. doi: 10.1126/science.1147554. [DOI] [PubMed] [Google Scholar]
- 15.Kimple RJ, Kimple ME, Betts L, Sondek J, Siderovski DP. Structural determinants for GoLoco-induced inhibition of nucleotide release by Galpha subunits. Nature. 2002;416:878–881. doi: 10.1038/416878a. [DOI] [PubMed] [Google Scholar]
- 16.Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9:60–71. doi: 10.1038/nrm2299. [DOI] [PubMed] [Google Scholar]
- 17.Johnston CA, Siderovski DP. Structural basis for nucleotide exchange on G alpha i subunits and receptor coupling specificity. Proc Natl Acad Sci USA. 2007;104:2001–2006. doi: 10.1073/pnas.0608599104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Taniguchi M, et al. YM-254890 analogues, novel cyclic depsipeptides with Galpha(q/11) inhibitory activity from chromobacterium sp. QS3666. Bioorg Med Chem. 2004;12:3125–3133. doi: 10.1016/j.bmc.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Lambright DG, Noel JP, Hamm HE, Sigler PB. Structural determinants for activation of the alpha-subunit of a heterotrimeric G protein. Nature. 1994;369:621–628. doi: 10.1038/369621a0. [DOI] [PubMed] [Google Scholar]
- 20.Mixon MB, et al. Tertiary and quaternary structural changes in Gi alpha 1 induced by GTP hydrolysis. Science. 1995;270:954–960. doi: 10.1126/science.270.5238.954. [DOI] [PubMed] [Google Scholar]
- 21.Oldham WM, Van Eps N, Preininger AM, Hubbell WL, Hamm HE. Mapping allosteric connections from the receptor to the nucleotide-binding pocket of heterotrimeric G proteins. Proc Natl Acad Sci USA. 2007;104:7927–7932. doi: 10.1073/pnas.0702623104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Remmers AE, Engel C, Liu M, Neubig RR. Interdomain interactions regulate GDP release from heterotrimeric G proteins. Biochemistry. 1999;38:13795–13800. doi: 10.1021/bi990887f. [DOI] [PubMed] [Google Scholar]
- 23.Gales C, et al. Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat Struct Mol Biol. 2006;13:778–786. doi: 10.1038/nsmb1134. [DOI] [PubMed] [Google Scholar]
- 24.Ceruso MA, Periole X, Weinstein H. Molecular dynamics simulations of transducin:Interdomain and front to back communication in activation and nucleotide exchange. J Mol Biol. 2004;338:469–481. doi: 10.1016/j.jmb.2004.02.064. [DOI] [PubMed] [Google Scholar]
- 25.Majumdar S, Ramachandran S, Cerione RA. Perturbing the linker regions of the alpha-subunit of transducin: A new class of constitutively active GTP-binding proteins. J Biol Chem. 2004;279:40137–40145. doi: 10.1074/jbc.M405420200. [DOI] [PubMed] [Google Scholar]
- 26.Kimple RJ, Willard FS, Siderovski DP. The GoLoco motif: Heralding a new tango between G protein signaling and cell division. Mol Interv. 2002;2:88–100. doi: 10.1124/mi.2.2.88. [DOI] [PubMed] [Google Scholar]
- 27.Arkin MR, Wells JA. Small-molecule inhibitors of protein-protein interactions: Progressing towards the dream. Nat Rev Drug Discov. 2004;3:301–317. doi: 10.1038/nrd1343. [DOI] [PubMed] [Google Scholar]
- 28.Pommier Y, Cherfils J. Interfacial inhibition of macromolecular interactions: Nature’s paradigm for drug discovery. Trends Pharmacol Sci. 2005;26:138–145. doi: 10.1016/j.tips.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Renault L, Guibert B, Cherfils J. Structural snapshots of the mechanism and inhibition of a guanine nucleotide exchange factor. Nature. 2003;426:525–530. doi: 10.1038/nature02197. [DOI] [PubMed] [Google Scholar]
- 30.Van Raamsdonk CD, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tall GG, Krumins AM, Gilman AG. Mammalian ric-8A (synembryn) is a heterotrimeric Galpha protein guanine nucleotide exchange factor. J Biol Chem. 2003;278:8356–8362. doi: 10.1074/jbc.M211862200. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura A, et al. Ric-8A potentiates Gq-mediated signal transduction by acting downstream of G protein-coupled receptor in intact cells. Genes Cells. 2006;11:487–498. doi: 10.1111/j.1365-2443.2006.00959.x. [DOI] [PubMed] [Google Scholar]
- 33.Cassel D, Pfeuffer T. Mechanism of cholera toxin action: Covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci USA. 1978;75:2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katada T, Ui M. Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP-ribosylation of a membrane protein. Proc Natl Acad Sci USA. 1982;79:3129–3133. doi: 10.1073/pnas.79.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson BA, Ho M. Pasteurella multocida toxin as a tool for studying Gq signal transduction. Rev Physiol Biochem Pharmacol. 2004;152:93–109. doi: 10.1007/s10254-004-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozasa T. Purification of G protein subunits from Sf9 insect cells using hexahistidine-tagged alpha and beta gamma subunits. Methods Mol Biol. 2004;237:21–38. doi: 10.1385/1-59259-430-1:21. [DOI] [PubMed] [Google Scholar]
- 37.Itoh H, Gilman AG. Expression and analysis of Gs alpha mutants with decreased ability to activate adenylylcyclase. J Biol Chem. 1991;266:16226–16231. [PubMed] [Google Scholar]
- 38.Chidiac P, Markin VS, Ross EM. Kinetic control of guanine nucleotide binding to soluble Galpha(q) Biochem Pharmacol. 1999;58:39–48. doi: 10.1016/s0006-2952(99)00080-5. [DOI] [PubMed] [Google Scholar]
- 39.Singer WD, Miller RT, Sternweis PC. Purification and characterization of the alpha subunit of G13. J Biol Chem. 1994;269:19796–19802. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.