Abstract
Polyandrous mating is common, but the benefits for females of polyandry remain controversial. To test whether mating with multiple males affects female fitness, we compared lifetime components of fitness of three experimental sets of Drosophila pseudoobscura females: monogamous females allowed to copulate one time (MOC); monogamous females held with a male over her entire life and experiencing many copulations (MMC); and polyandrous females with a different male over each day of their lives and also experiencing many copulations (PMC). Consistent with previous studies in this species, females in treatments in which multiple copulations occurred, MMC and PMC, had offspring with significantly higher egg-to-adult survival (i.e., offspring viability) and higher numbers of adult offspring (i.e., productivity) than MOC females, showing that multiple inseminations enhance offspring and mother fitness. In addition, although MMC females laid significantly more eggs than polyandrous (PMC) females, percent egg-to-adult survival and number of adult offspring were higher for PMC than MMC females, showing that polyandrous mating enhances the fitness of females more than multiply mating with only one male. Inconsistent with the cost of reproduction, lifespan was not significantly longer for MOC females than for MMC or PMC females. To our knowledge, this is the first study to examine simultaneously in outbred WT Drosophila pseudoobscura the lifetime costs and benefits to females of polyandry, monogamy with a single copulation, and monogamy with repeat copulations.
Keywords: fitness, mating system, monogamy, multiple mating
In nature, females in many taxa mate with more than one male (1, 2), and metaanalyses are consistent with the hypothesis that females gain fitness benefits from polyandry (3, 4). Females may mate with more than one male to (i) guarantee an adequate sperm supply (5); (ii) enhance access to nutritional or other resources (6); (iii) inhibit or reduce the effects of male harassment (7); or (iv) reduce the costs of inbreeding (8). Hypotheses i, ii, iii, and iv predict that polyandrous females lay more eggs and have more eclosing (i.e., adult) offspring than monogamous females (Table 1). Females also may multiply mate to enhance offspring health. The benefit to offspring health of polyandry may occur (v) when complementary parental immune alleles increase disease resistance in offspring (9, 10); (vi) when parental incompatibilities in offspring resulting from endosymbionts or other factors are reduced (11); or (vii) when females gain access to good genes from population-wide “best” or “near-best” males (12). Hypotheses v, vi, and vii predict that polyandrous females have healthier offspring so that more survive to reproductive age than do monogamous females (Table 1). Finally, females also may mate with multiple males as (viii) a correlated response to selection on males to mate with more than one female (13). The hypothesis that polyandry is not a result of selection on females but rather a correlated response to selection on males for multiple mating predicts no fitness benefits for polyandrous females compared with monogamous females with multiple or single copulations (Table 1).
Table 1.
Benefits and costs to polyandry hypotheses predict variation in different components of fitness and the direction of effect in experimental treatments
| Polyandry hypotheses/tested component of fitness | Predicted | Observed |
| Guards against sperm limitation (not enough sperm or inviable sperm) | ||
| Eggs laid | MMC > MOC | MMC = MOC |
| Eclosed offspring | MMC > MOC | MMC > MOC |
| Egg-to-adult survival | MMC > MOC | MMC > MOC |
| Enhances offspring survival | ||
| Eclosed offspring | PMC > MMC | PMC > MMC |
| Egg-to-adult survival | PMC > MMC | PMC > MMC |
| Male peptides manipulate female egg-laying rate | ||
| Eggs laid | PMC > MMC > MOC | PMC < MMC = MOC |
| Assuming there are no flexible adjustments of male peptides in ejaculates that could harm females | ||
| Female lifespan | PMC < MMC< MOC | PMC = MMC= MOC |
| Assuming there is flexible adjustment of male peptides in ejaculates that could harm females | ||
| Female lifespan | PMC > MMC = MOC | PMC = MMC= MOC |
| Ejaculate contributions nourish zygotes and/or females | ||
| Eclosed offspring | PMC ≥ MMC | PMC > MMC |
| Lifespan of females | PMC ≥ MMC | PMC = MMC |
| Correlated response to selection on males to mate multiply | ||
| Eggs laid | PMC = MMC | PMC < MMC |
| Eclosed offspring | PMC = MMC | PMC > MMC |
| Egg-to-adult survival | PMC = MMC | PMC > MMC |
Experimental treatments included (i) females with access to one male during only 1 d of their lives, i.e., monogamous females with one copulation MOC; (ii) females with continuous access to the same male, i.e., monogamous females with multiple copulations MMC; and (iii) polyandrous females with access to a different male on each day of the experiment, i.e., polyandrous females with multiple copulations, PMC.
Interest of evolutionary biologists in polyandry goes back to Bateman (14), who reported that increased numbers of mates enhanced male fitness, but not female fitness in Drosophila melanogaster, an observation now called the Bateman principle (15–17) or Bateman hypothesis (18). In D. melanogaster, the species Bateman studied, modern investigation has revealed that females are polyandrous, which is often interpreted as a way for females to avoid sperm limitation (4, 18–20), which we define here as insufficient sperm to fertilize all a female's eggs as well as possible inviability of the sperm she may receive. In addition, experimental study of D. melanogaster (21) failed to reveal a benefit for polyandry over the benefit of copulating more than one time with the same male.
In Drosophila pseudoobscura, sperm limitation favors females who copulate more than once. This conclusion came from an experimental study in which females who copulated with the same male repeatedly compared with females who copulated only one time had a significantly higher number of offspring at the age of eclosion (5), a likely result of sperm limitation (because females do not receive enough sperm and/or that the sperm she does receive are inviable) in single mated females. Previous mate choice studies in D. pseudoobscura (22–24) showed that females and males paired with their individually preferred partners had offspring with higher egg-to-adult survival, higher numbers of eclosed offspring (i.e., productivity), and higher net reproductive success than individuals constrained to pairings with their nonpreferred partners. Because mating with preferred partners in nature may be constrained by dispersal limitation or social competition or sexual coercion (25), the constrained female hypothesis (7, 26) predicts that a benefit of polyandry beyond that from multiply mating with the same male is enhanced offspring health. Like D. melanogaster, D. pseudoobscura mate multiply in nature (27–29). Thus, we tested whether a benefit of polyandry is enhanced offspring viability. We designed the current experiment to evaluate whether female D. pseudoobscura have a benefit from polyandry beyond guarding against sperm limitation.
The experiment had three treatments: (i) females allowed only a single day's exposure to a single male, i.e., monogamy with one copulation (MOC); (ii) females allowed constant access to the same male throughout the experimental duration, i.e., monogamy with multiple copulations (MMC); and (iii) females allowed access to a different male, but only one per day, on each day of the experiment, i.e., polyandry (PMC).
We tested the prediction of the sperm limitation hypothesis that (i) multiple mating with the same male enhances female fitness over that expected from a single copulation. We also tested the fitness predictions of subhypotheses about the effects of male copulatory contributions to females. Under the “male peptides manipulate female egg-laying rate” hypothesis, peptides in male ejaculates are expected to increase female egg-laying and decrease female survival rates, leading to the prediction that MMC and PMC females lay more eggs than MOC females and that MMC and PMC females die faster than MOC females. Under the assumption that harmful male peptides are flexibly expressed depending on whether males and females are in preferred or nonpreferred matings (23, 30), those females with more options, the PMC females, would more likely avoid harmful male peptides than those females with fewer options (31). This predicts that PMC females sometimes live longer than MMC or MOC females. The difference turns on the assumption that males can up-regulate harmful peptides flexibly. If males do not flexibly express harmful peptides, the constant harm prediction would be more likely. If males deliver resources to females useful in oogenesis (6) or otherwise, multiple copulations with the same male or mating with several different males should enhance the number of offspring eclosing; thus, the hypothesis of male-delivered resources predicts that MMC and PMC females have more adult offspring and perhaps live longer than MOC females. Finally, we tested whether polyandry enhances egg-to-adult survival over that achieved via monogamy when monogamous females received ejaculates on more than 1 d (i.e., PMC > MMC). Because the experimental design involved holding females in treatments until they died, we were also able to test whether (i) females exposed to the sperm and seminal peptides of multiple males (PMC) die faster than those exposed to the sperm and peptides of only one male (MMC) and (ii) whether females that lay the most eggs die faster than females that lay the least.
Results
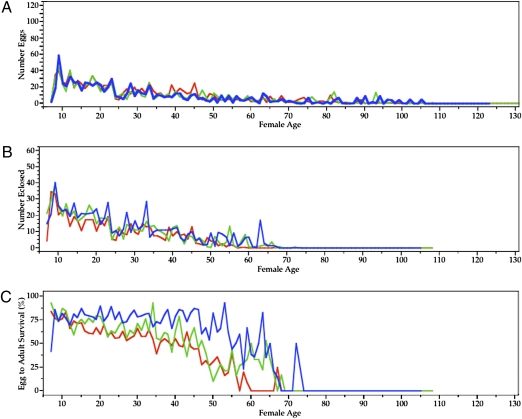
The time course for female and offspring fitness (number of eggs laid, number of eclosed offspring, and percent egg-to-adult survival) in the three treatments is shown in Fig. 1.
Fig. 1.
Time course of (A) number of eggs laid, (B) number of offspring eclosed as adults, and (C) percent egg-to-adult survival for PMC (blue), MMC (green), and MOC (red) females. The mean numbers of eggs laid per female per day of life were 7.4 ± 9.7 (SD) for MOC females, 6.7 ± 8.9 for MMC females, and 7.3 ± 9.4 for PMC females. (B) The mean number of offspring eclosed (productivity) was 5.6 ± 7.7 for MOC females, 7.2 ± 8.8 for MMC females, and 8.5 ± 9.2 for PMC females. The mean egg-to-adult viability percentages were 28.0 ± 29.3 for MOC females, 35.8 ± 31.5 for MMC females, and 49.6 ± 35.2 for PMC females.
As a direct test of the sperm limitation hypothesis, we examined fitness variation between MOC and MMC females. There was no significant difference in the number of eggs laid by MOC and MMC females (Fig. 2A). However, MMC females produced 1.6 more adult offspring per day than MOC females, a significant difference (Fig. 2B), as a result of the mean 5% per day increase in egg-to-adult survival of MMC compared with MOC females (Fig. 2C). Because MMC females copulated more than one time, but always with the same male, this treatment eliminated polyandry as a potential explanation for the observed fitness benefits. We concluded, as others have (5), that female D. pseudoosbcura who copulate on only 1 d do not get enough sperm or enough viable sperm to fertilize a lifetime of egg production.
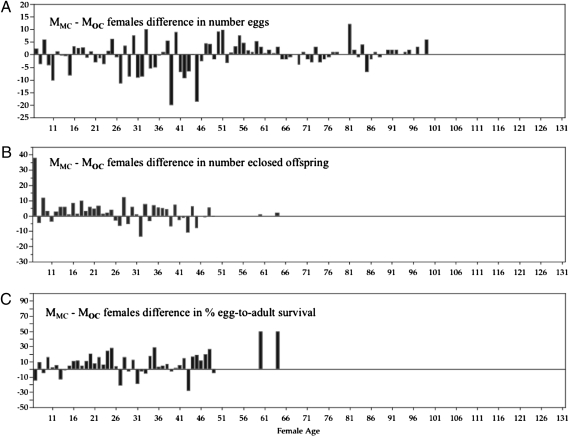
Fig. 2.
Difference scores (MMC − MOC females) for number of eggs laid (A), number of offspring eclosed as adults (B), and egg-to-adult survival percentage (C). The difference scores over days of female life were independent, and paired comparisons testing for deviations from zero showed that number of eggs laid (Wilcoxon signed-rank test, 62.0; P > |t| < 0.792) was not statistically significantly different between MMC and MOC females. However, the number of offspring eclosed as adults (Wilcoxon signed-rank test, 199.5; P > |t| < 0.019), and the egg-to-adult survival percentage (Wilcoxon signed-rank test, 295.5; P > |t| < 0.0001) were significantly higher in MMC than in MOC females.
To test hypotheses that predict offspring viability benefits of polyandry, we asked whether PMC females produced more offspring with higher viability than MMC females. The experimental design compared MMC females, confined for life with the same male, with PMC females, also with lifetime male access, but with a different male on each day. By matching the age and mating experience of all males in the MMC and PMC treatments, we controlled for possible sperm limitation that could result when females were mated to older or exhausted males and which could result in lower fitness of MMC than PMC females.
MMC females laid more eggs than PMC females (Fig. 3A); on average MMC females laid two more eggs per day than PMC females (Fig. 3A). Despite laying fewer eggs than MMC females, PMC females produced significantly more adult offspring than MMC females, i.e., on average PMC females produced one more offspring per day than MMC females (Fig. 3B). This was a result of the significantly higher egg-to-adult survival from PMC than MMC females (Fig. 3C). On average there was a 24% per day difference in egg-to-adult survival between PMC and MMC females. Because major differences in percent egg-to-adult survival accumulated after the median age of death of PMC and MMC females (Fig. 4), we retested for differences using only data on treatment females up to d 37. Even with this restriction, egg-to-adult survival was 10% per day higher for PMC than MMC females. The difference in fitness between MMC and PMC females resulting from enhanced egg-to-adult survival is a benefit of polyandry, which translates over time into significantly higher numbers of offspring produced for PMC than MMC females.
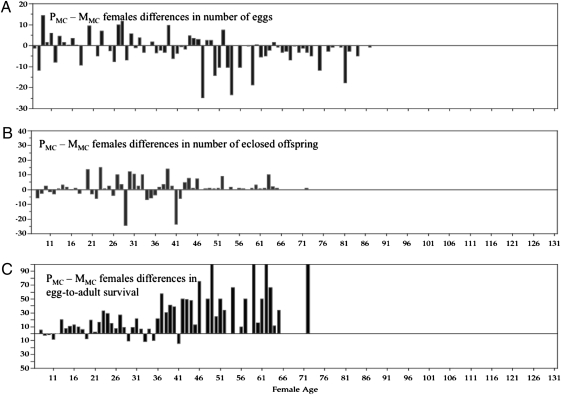
Fig. 3.
Difference scores (PMC − MMC females) for number of eggs laid (A), number of offspring eclosed as adults (B), and egg-to-adult survival percentage (C). The difference scores over days of female life were independent and paired comparisons testing for deviations from zero showed that number of eggs laid (Wilcoxon signed-rank test, −745.5; P > |t| < 0.001), number of offspring eclosed as adults (Wilcoxon signed-rank test, 247; P > |t| < 0.001), and egg-to-adult survival (Wilcoxon signed-rank test, 654; P > |t| < 0.001) were significantly different between MMC and PMC females.
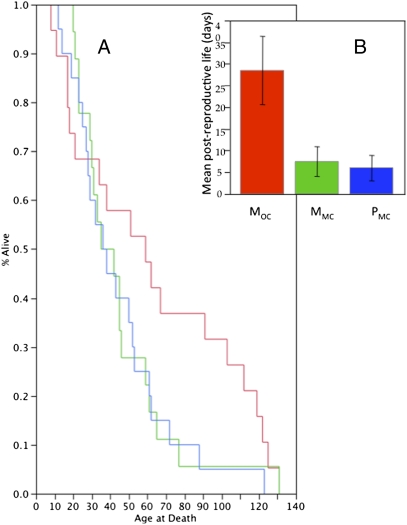
Fig. 4.
(A) Lifespan and (B) days of postreproductive life of MOC (red lines), MMC (green line), and PMC (blue line) females. The mean age of females when they produced their last eclosing offspring was 34.9 ± 4.1 d for MOC females; 37.8 ± 4.2 d for MMC females; and 38.6 ± 4 d for PMC females (F = 0.2042; df = 56; P > F = 0.8159). The mean ± SE ages of death were 63.5 ± 10.15 d for MOC females, 45.3 ± 6.35 for MMC females, and 44.4 ± 6.1 for PMC females (F = 1.3579; df = 59; P = 0.2654). Kaplan-Meier survival analysis for MOC (red), MMC (green) and P (blue) females indicated no significant differences in lifespan (log-rank χ2 = 2.9505, 2 df, P < 0.2287; Wilcoxon χ2 = 1.069, 2 df, P < 0.5859). Inset: Differences in mean number of days until death after production of their last surviving offspring. There were significant differences in days of postreproductive life between MOC and PMC females (P < 0.0034) and between MOC and MMC females (P < 0.0072), but not between MMC and PMC females (P < 0.841). There were no significant differences in lifespan if the experiment was considered ended by d 30, d 50, or d 70; i.e., there were no early deficits in survival of MOC females and no significant survival benefit for MOC females before the cessation of reproduction by all three types of females.
To examine the costs to females of polyandry, we compared the longevity of females in the three treatments. Longevity of females did not differ significantly by treatment (Fig. 4A). The length of life after production of their last surviving offspring did differ significantly (Fig. 4B), probably because MOC females quit producing eclosing offspring sooner than MMC and PMC females.
There were no significant differences in postreproductive lifespan for females exposed to the sperm of more than one male (PMC) compared with females that were repeatedly exposed to the sperm of a single male (MMC). Lifespan similarities of PMC and MMC females do not indicate costs to females of polyandry.
Discussion
We conclude that there are few or no apparent costs and at least two fitness benefits of polyandry for females (1). Polyandrous mating reduces the likelihood that female reproductive success is affected by sperm limitation, which we defined as either not enough sperm or inviable sperm. Our methods were not designed to discern between these two types of sperm limitation (2). Polyandrous mating enhances egg-to-adult survival and the number of adult offspring, observations consistent with hypotheses of indirect benefits for polyandrous females.
Other benefits of polyandry seem unlikely in D. pseudoobscura females. We eliminated or at least reduced the opportunity for male harassment of females to induce polyandrous mating because we never exposed females to more than one male at a time. Forced copulation of teneral females sometimes occurs in D. pseudoobscura (25). However, in our experiment we used only sexually mature virgin females, eliminating the known period of greatest vulnerability of females to forced copulation. There is no postzygotic parental care in D. pseudoobscura and we used populations free of Wolbachia and other endosymbionts (Materials and Methods) that produce breeding incompatibilities between parents in other species; thus, we do not think our results can be explained by reduction of breeding incompatibilities. In addition, because fitness differences between PMC and MMC occurred (Table 1)—that is, because benefits of polyandry were obvious whereas costs of polyandry were not—there is no support from this study for the hypothesis that polyandry is a correlated response to selection on males for polygny.
We conclude that variation in sperm numbers or sperm viability explains the differences in fitness between MOC and MMC females (5), and that enhanced offspring survival explains the benefit that PMC females have compared with MMC females. Male D. pseudoobscura ejaculate many very small sperm, in fact, more sperm than in any other known Drosophila species (32), and males may contribute nutritional elements to females or their offspring through their ejaculates (6). Thus, the mechanisms that may mediate the offspring viability benefit of polyandry include (i) multiple and variable doses of any paternally derived nutritional contributions to females or zygotes, (ii) complementary parental alleles coding for offspring immune function, or (iii) best male genes.
The experiment revealed no costs of polyandry for females. Because there are no significant lifespan differences between females in the three experimental treatments, particularly between MMC and PMC females, polyandry appears to levy no additional costs over the costs to females of lifetime exposure to males.
The experimental results raise a number of questions about polyandry in the field and in the laboratory. How many sires are enough to ensure females have adequate sperm numbers or enough viable sperm? Do aged or more experienced males exhaust their abilities to produce viable sperm? What are the costs to males mated to MMC females of compensatory increases in ejaculate size such as those that occur in D. pseudoobscura (30) when males are paired with females they do not prefer? Similarly, what are the costs to females in MMC treatments, when they are paired for life with partners they prefer or do not prefer? How do polyandrous females allocate sperm to eggs over their lifetime given that they store sperm, as D. pseudoobscura females do, and given that the quality of males whom they mate probably varies? Do females favor the sperm of some males over others when they have constant exposure to potential mates? Is the benefit of enhanced offspring viability a result of good genes from best males, parental dissimilarities at immune coding loci, or both? Do females compete over access to the number of mates or the quality of mates, and how confounded are numbers and quality of mates in species with sperm storage such as D. pseudoobscura? Given that polyandry is so common in nature, we wonder if polygynous mating in males might not be a result of correlated male trait evolution to polyandry in females. Alternatively, given that both male and female D. pseudoobscura assess potential mates before accepting or rejecting them, and given that both sexes have higher offspring viability when reproducing with mates they prefer (22), it is likely that similar selective pressures may act on the sexes to favor polyandrous females and polygynous males. More experiments, particularly between-species comparative experiments designed to evaluate the effects of polyandry in demographic context over the lifespan of females and males, and that take into account multiple fitness components, would be useful in resolving remaining debates about the benefits of polyandry.
Materials and Methods
W.W.A. collected D. pseudoobscura in Mesa Verde National Park (Colorado) in 1996 and maintained them in isofemale lines until July 1997, when a population cage was set up using eight isofemale lines. The lines we tested were free of Wolbachia, a vertically transmitted endosymbiont of insects, including some populations of D. pseudoobscura that are known to increase the remating rate of female D. pseudoobscura (33). Twenty cups containing cornmeal-yeast-molasses medium were placed in the cage, and every other day, three times a week, a new cup replaced one of the old cups. Approximately 80 generations later, we used flies from this cage for the experiment. We did this experiment from January through May 2004. We placed four fresh bottles in the cage for 1 wk, and then removed them and divided the food containing eggs and larvae into three new bottles. From these bottles, we collected virgin flies every 8 h, twice per day, and sexed them under CO2. We maintained up to 10 same-sex flies per vial. We randomly picked flies from the same-sex vials for inclusion in this experiment, when flies were 7 d old.
Experimental Treatments.
The experiment had three treatments: (i) monogamous females with access to a male for up to 24 h, who copulated only one time (MOC); (ii) monogamous females each with a single male until she died, and who copulated many times (MMC); and (iii) polyandrous females, who had access to one male per day, but a new male each day, so that PMC females also copulated many times.
For each of the treatments, we made random pairings from 10 vials containing 7-d-old virgin females and 10 vials containing 7-d-old virgin males. We labeled vials as 0 through 9. Using a random number generator (0–9), we randomly picked flies from the vials to make 20 pairs for each treatment, and named them A1–A20 (MOC), B1–B20 (MMC), and C1–C20 (PMC).
For the MOC treatment, 24 h after pairings, we moved the females to new vials and discarded the males. For the MMC treatment, 24 h after pairing and each day afterward, we moved the female and the male to a new food vial. For the PMC treatment, 24 h after the initial pairing and on each day afterward, we replaced the male with another male the same age and with similar exposure to females. For example, the vial with female C1 received the male that was with female C20 the day before. The vial with female C2 received the male that was with female C1 the day before, and so on. We kept a bottle with extra males and females the same ages as the flies in this experiment, and replaced males that died with males from the reserve jar.
We observed MOC females on their only day of exposure to males until copulation occurred (usually within 1 h), and scanned for copulations by MMC and PMC females after moving them daily between vials. We often saw MMC and PMC females copulating, but we did not count copulations.
We held 20 rubber-banded sets of vials with experimental classes of females (e.g., A1-B1-C1) together in incubators for the duration of the experiment to control for environmental effects on components of fitness.
Components of Fitness.
We counted the number of eggs laid under a dissecting microscope after transferring females (and their mates) to new vials. After larvae had begun to eclose, we counted the number of emerging adult flies from each vial for 5 d. We noted the day on which each female died. We calculated percent egg-to-adult survival as number eclosed/number of eggs laid × 100.
Statistics.
Based on the Fisher κ statistic, we found no evidence for temporal autocorrelation of difference scores (MOC − MMC and PMC − MMC) for any component of fitness. Thus, for both sets of difference scores (MOC − MMC and PMC − MMC), we used Wilcoxon signed-rank tests to evaluate the hypothesis that the distribution of difference scores was equal to 0.
We used univariate Kaplan-Meier statistics to test for differences across treatments in female lifespan. After testing for normality, we used ANOVA to test for differences between treatments in mean ages of death, ages that females laid their last eggs, the ages they produced their last eclosing offspring, and postreproductive lifespan (the number of days a female survived after producing the last egg that eclosed). We tested the distribution over days of the difference scores in number of eggs laid, number of eclosing adult offspring, and percent egg-to-adult survival for MOC and MMC females and for MMC and PMC females separately. The a priori significance level was set at less than 0.05.
Acknowledgments
We thank Margaret Anderson and Stephen P. Hubbell for statistical advice and discussion about these data; we thank Malin Ah-King, J. P. Drury, Brant Faircloth, David Hoskens, Therese Markow, and Judy Stamps for comments on a previous version. This work was partially supported by a National Science Foundation grant (to P.A.G. and W.W.A.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Hosken DJ, Stockley P. Benefits of polyandry: A life history perspective. Evol Biol. 2003;33:173–194. [Google Scholar]
- 2.Simmons LW. The evolution of polyandry: Patterns of genotypic variation in female mating frequency, male fertilization success and a test of the sexy-sperm hypothesis. J Evol Biol. 2003;16:624–634. doi: 10.1046/j.1420-9101.2003.00572.x. [DOI] [PubMed] [Google Scholar]
- 3.Arnqvist G, Nilsson T. The evolution of polyandry: Multiple mating and female fitness in insects. Anim Behav. 2000;60:145–164. doi: 10.1006/anbe.2000.1446. [DOI] [PubMed] [Google Scholar]
- 4.Simmons L. The evolution of polyandry: Sperm competition, sperm selection, and offspring viability. Annu Rev Ecol Evol Syst. 2005;36:125–146. [Google Scholar]
- 5.Turner ME, Anderson WW. Multiple mating and female fitness in Drosophila pseudoobscura. Evolution. 1983;37:714–723. doi: 10.1111/j.1558-5646.1983.tb05593.x. [DOI] [PubMed] [Google Scholar]
- 6.Markow TA, Ankney PF. Drosophila males contribute to oogenesis in a multiple mating species. Science. 1984;224:302–303. doi: 10.1126/science.224.4646.302. [DOI] [PubMed] [Google Scholar]
- 7.Gowaty PA. In: Battles of the Sexes and Origins of Monogamy. Black Partnerships in Birds, Oxford Series in Ecology and Evolution. Black JL, editor. Oxford: Oxford Univ Press; 1996. pp. 21–52. [Google Scholar]
- 8.Tregenza T, Wedell N. Genetic compatibility, mate choice and patterns of parentage: Invited review. Mol Ecol. 2000;9:1013–1027. doi: 10.1046/j.1365-294x.2000.00964.x. [DOI] [PubMed] [Google Scholar]
- 9.Wedekind C. Pathogen-driven sexual selection and the evolution of health. In: Stearns SC, editor. Evolution in Health and Disease. Oxford: Oxford Univ Press; 1999. pp. 102–107. [Google Scholar]
- 10.Foerster K, Delhey K, Johnsen A, Lifjeld JT, Kempenaers B. Females increase offspring heterozygosity and fitness through extra-pair matings. Nature. 2003;425:714–717. doi: 10.1038/nature01969. [DOI] [PubMed] [Google Scholar]
- 11.Zeh JA. Polyandry and enhanced reproductive success in the harlequin-beetle-riding pseudoscorpion. Behav Ecol Sociobiol. 1997;40:111–118. [Google Scholar]
- 12.Jennions MD, Petrie M. Why do females mate multiply? A review of the genetic benefits. Biol Rev Camb Philos Soc. 2000;75:21–64. doi: 10.1017/s0006323199005423. [DOI] [PubMed] [Google Scholar]
- 13.Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 14.Bateman AJ. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- 15.Arnold SJ. Bateman principles and the measurement of sexual selection in plants and animals. Am Nat. 1994;144:S126–S149. [Google Scholar]
- 16.Jones AG, Arguello JR, Arnold SJ. Validation of Bateman's principles: A genetic study of sexual selection and mating patterns in the rough-skinned newt. Proc Biol Sci. 2002;269:2533–2539. doi: 10.1098/rspb.2002.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones AG, Rosenqvist G, Berglund A, Arnold SJ, Avise JC. The Bateman gradient and the cause of sexual selection in a sex-role-reversed pipefish. Proc Biol Sci. 2000;267:677–680. doi: 10.1098/rspb.2000.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snyder BF, Gowaty PA. A reappraisal of Bateman's classic study of intrasexual selection. Evolution. 2007;61:2457–2468. doi: 10.1111/j.1558-5646.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- 19.Imhof M, Harr B, Brem G, Schlötterer C. Multiple mating in wild Drosophila melanogaster revisited by microsatellite analysis. Mol Ecol. 1998;7:915–917. doi: 10.1046/j.1365-294x.1998.00382.x. [DOI] [PubMed] [Google Scholar]
- 20.Marks RW, Seager RD, Barr LG. Local ecology and multiple mating in a natural population of Drosophila melanogaster. Am Nat. 1988;131:918–923. [Google Scholar]
- 21.Brown WD, Bjork A, Schneider K, Pitnick S. No evidence that polyandry benefits females in Drosophila melanogaster. Evolution. 2004;58:1242–1250. doi: 10.1111/j.0014-3820.2004.tb01703.x. [DOI] [PubMed] [Google Scholar]
- 22.Anderson WW, Kim YK, Gowaty PA. Experimental constraints on mate preferences in Drosophila pseudoobscura decrease offspring viability and fitness of mated pairs. Proc Natl Acad Sci USA. 2007;104:4484–4488. doi: 10.1073/pnas.0611152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gowaty PA, Steinichen R, Anderson WW. Mutual interest between the sexes and reproductive success in Drosophila pseudoobscura. Evolution. 2002;56:2537–2540. doi: 10.1111/j.0014-3820.2002.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 24.Gowaty PA, Steinichen R, Anderson WW. Indiscriminate females and choosy males: Within- and between-species variation in Drosophila. Evolution. 2003;57:2037–2045. doi: 10.1111/j.0014-3820.2003.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 25.Markow TA. Forced matings in natural populations of Drosophila. Am Nat. 2000;156:100–103. doi: 10.1086/303368. [DOI] [PubMed] [Google Scholar]
- 26.Gowaty PA. Power asymmetries between the sexes, mate preferences, and components of fitness. In: Travis C, editor. Women, Evolution, and Rape. Cambridge, MA: MIT Press; 2003. pp. 61–86. [Google Scholar]
- 27.Anderson WW. Frequent multiple insemination in a natural population of Drosophila pseudoobscura. Am Nat. 1974;108:709–711. [Google Scholar]
- 28.Cobbs G. Multiple insemination and male sexual selection in natural populations of Drosophila pseudoobscura. Am Nat. 1977;111:641–656. [Google Scholar]
- 29.Beckenbach A. Multiple mating and the “sex-ratio” trait in Drosophila pseudoobobscura. Evolution. 1981;35:275–281. doi: 10.1111/j.1558-5646.1981.tb04886.x. [DOI] [PubMed] [Google Scholar]
- 30.Gowaty PA, et al. The hypothesis of reproductive compensation and its assumptions about mate preferences and offspring viability. Proc Natl Acad Sci USA. 2007;104:15023–15027. doi: 10.1073/pnas.0706622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore AJ, Gowaty PA, Moore PJ. Females avoid manipulative males and live longer. J Evol Biol. 2003;16:523–530. doi: 10.1046/j.1420-9101.2003.00527.x. [DOI] [PubMed] [Google Scholar]
- 32.Markow TA. Evolution of Drosophila mating systems. Evol Biol. 1996;29:73–106. [Google Scholar]
- 33.Price TAR, Hodgson DJ, Lewis Z, Hurst GDD, Wedell N. Selfish genetic elements promote polyandry in a fly. Science. 2008;322:1241–1243. doi: 10.1126/science.1163766. [DOI] [PubMed] [Google Scholar]