Neurotrophic factors are capable of accomplishing many tasks. In addition to regulating neural cell survival and differentiation, they can influence changes in synaptic transmission and higher order behaviors, such as aggression, addiction, depression, learning, and memory. Because many trophic factors use receptor tyrosine kinases as a means for initiating signaling, a major question is how tyrosine phosphorylation events that lead to canonical MAP kinase, Akt, and phospholipase C-γ activities can account for so many diverse outcomes (1). The article in PNAS by Schalm et al. (2) on protocadherins and the glial-derived neurotrophic factor (GDNF) provides a unique insight into this question.
GDNF is an important protein that was discovered in 1993 as a potent survival factor for midbrain dopaminergic neurons (3). It has been frequently touted as a treatment for Parkinson's disease. GDNF also exerts trophic effects on sympathetic, sensory, parasympathetic, and enteric neurons. The effects of GDNF and the related family members neurturin, artemin, and persephin are mediated by RET, a transmembrane receptor tyrosine kinase. GDNF ligands do not bind directly to RET but require GPI-anchored coreceptors [called GDNF receptor-α1–4] to activate the RET receptor, which efficiently increases intracellular ERK and PI3K activities and Ca2+ levels (4, 5). Many biological functions are affected by these ligand-receptor events, including proliferation and migration of progenitor cells, axon guidance, and chemoattraction (5). GDNF is also involved in synapse formation and neuronal excitability in possessing the ability to modulate postsynaptic currents in dopaminergic neurons (6). Recently, considerable interest has centered on the roles of GDNF to regulate drugs of abuse, such as cocaine and morphine, negatively as well as alcohol addiction (7). The breadth of actions of GDNF family members begs for more mechanistic insights to account for the specificity and extent of RET receptor signaling and its ability to change synaptic plasticity.
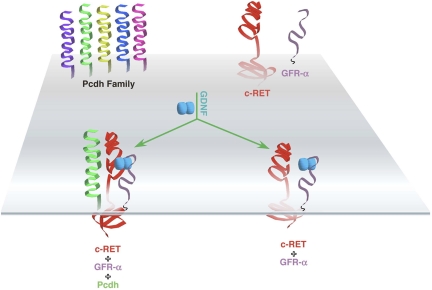
A unique set of mechanisms has now been provided by the finding that transmembrane protocadherin molecules are associated with the RET receptor. Schalm et al. (2) report that GDNF causes the phosphorylation of specific protocadherin proteins in sympathetic and motor neurons, two responsive populations. These interactions were uncovered by this group with the identification of RET as an interacting protein of protocadherin-α4. Interestingly, the interaction of RET with protocadherin-α4 is specific and involves the extracellular domains of each protein, which both share similar cadherin-like motifs (Fig. 1).
Fig. 1.
A link between GDNF and protocadherins. A multitude of different protocadherin proteins are expressed in the nervous system. Each transmembrane protocadherin possesses six extracellular cadherin domains and a short cytoplasmic segment. In the mouse, there are 14 protocadherin-α genes, 22 protocadherin-β genes, and 22 protocadherin-γ genes, which undergo multiple splicing events (16, 17). The GDNF family of ligands (GDNF, neurturin, artemin, and persephin) bind to individual GDNF-α receptors (GFR-α), which form a complex with the RET tyrosine kinase (5). The specific protocadherin proteins α4 and γb7 are phosphorylated by RET after GDNF treatment, which, in turn, stabilizes RET and delays its degradation (2).
How are protocadherins relevant to RET? Protocadherin-α was originally identified as a brain-specific protein that interacted with the Fyn nonreceptor tyrosine kinase (8). It quickly became apparent that the protocadherin gene family represented the largest subgroup of the cadherin superfamily, consisting of three tandemly arrayed gene clusters: α, β, and γ (9, 10). As a transmembrane protein with a short cytoplasmic domain, each protocadherin is distinguished by six ectodomain cadherin-like repeats of 100 amino acids. Because there are nearly 70 protocadherin genes that undergo a multitude of splicing events, a huge number of different protocadherin proteins can be generated. For this reason, there has been enormous interest by neuroscientists in the past decade in the potential roles of protocadherins as synaptic recognition proteins. The significance of the localization of protocadherin proteins at synaptic junctions (8) has been backed by the appearance of fewer and weaker synapses in spinal cord neurons in mice carrying a large deletion in the protocadherin-γ gene cluster (11).
Moreover, the absence of multiple γ-protocadherins results in dramatic neurodegeneration of spinal cord motor neurons (12), reminiscent of what happens when there is a lack of trophic support. Hence, protocadherins display multiple roles in the nervous system. In this regard, RET signaling is responsible for the survival and function of many neuron populations. The phosphorylation of protocadherins by GDNF is therefore an important observation that implies an overlap in their respective functions.
How do protocadherins and RET affect each other? One clue comes from the finding that RET receptor levels are exquisitely sensitive to proteasomal degradation (13, 14). Like many growth factors, GDNF binding results in rapid ubiquitination of its receptor on lysine residues. There are additional layers of regulation, because two isoforms of RET that contain different lengths of cytoplasmic tails, Ret51 and Ret9, differ in their response to GDNF. Also, there is cell type specificity. In sympathetic neurons, Ret51 is degraded faster in sympathetic neurons than in sensory neurons. However, when the levels of protocadherin-α4 are increased in sympathetic neurons, the levels of phosphorylated Ret51 are increased. Conversely, lowering the levels of protocadherins brings about a decrease in the levels of responsive RET receptors (2). Hence, one way in which protocadherins exert an effect on RET is to stabilize the activated receptor and prevent degradation.
The association of protocadherins with RET has a number of implications. A delay in degradation of the RET receptor allows neurons to survive for a longer period of time in the presence of GDNF. A loss of GDNF-RET signaling or a deficit in retrograde transport can lead to neurodegeneration (14). The responsiveness of RET receptors is therefore dependent on receptor trafficking and turnover. Phosphorylation of protocadherins after GDNF treatment ensures that RET is not immediately inactivated through degradation. The current results suggest that the GDNF-RET receptor exists in a large complex with protocadherin proteins, which contribute to RET receptor stability and signaling. The existence of an array of different protocadherins and different RET isoforms likely generates a diversity of receptor complexes with different components, thus simultaneously increasing the diversity and specificity of trophic factor signaling. Indeed, Schalm et al. (2) report there are other signaling proteins that are associated with protocadherins, including leukocyte antigen-related receptor tyrosine phosphatase and protein tyrosine phosphatase-α as well as tyrosine kinases, such as discoidin domain receptor 2 and Src family members (2).
Protocadherins are found in combinatorial expression patterns frequently associated with synapses, and they have been strongly implicated as cell recognition and adhesion molecules. It is likely that their effects will have an impact on the kinetics and strength of signaling of other synaptic molecules. In addition to mediating neuronal survival and synaptic development, protocadherin-α members have been implicated in memory and learning (15). Hence, the association of GDNF with the protocadherin family of proteins may indeed foreshadow further insights into the molecular basis of neurodegenerative diseases and psychiatric disorders.
Acknowledgments
The figure was composed by Kenneth Teng, and work on neurotrophic factors in the laboratory of M.V.C. has been previously supported by National Institutes of Health Grants NS21072, HD23315, AG25970, MH086651, and MH090638.
Footnotes
See companion article on page 13894.
References
- 1.Chao MV. Growth factor signaling: Where is the specificity? Cell. 1992;68:995–997. doi: 10.1016/0092-8674(92)90068-n. [DOI] [PubMed] [Google Scholar]
- 2.Schalm SS, Ballif BA, Buchanan SM, Phillips GR, Maniatis T. Phosphorylation of protocadherin proteins by the receptor tyrosine kinase Ret. Proc Natl Acad Sci USA. 2010;107:13894–13899. doi: 10.1073/pnas.1007182107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 4.Pérez-García MJ, et al. Glial cell line-derived neurotrophic factor increases intracellular calcium concentration. Role of calcium/calmodulin in the activation of the phosphatidylinositol 3-kinase pathway. J Biol Chem. 2004;279:6132–6142. doi: 10.1074/jbc.M308367200. [DOI] [PubMed] [Google Scholar]
- 5.Paratcha G, Ledda F. GDNF and GFRalpha: A versatile molecular complex for developing neurons. Trends Neurosci. 2008;31:384–391. doi: 10.1016/j.tins.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Bourque MJ, Trudeau LE. GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. Eur J Neurosci. 2000;12:3172–3180. doi: 10.1046/j.1460-9568.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- 7.Carnicella S, Ron D. GDNF—A potential target to treat addiction. Pharmacol Ther. 2009;122:9–18. doi: 10.1016/j.pharmthera.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohmura N, et al. Diversity revealed by a novel family of cadherins expressed in neurons at a synaptic complex. Neuron. 1998;20:1137–1151. doi: 10.1016/s0896-6273(00)80495-x. [DOI] [PubMed] [Google Scholar]
- 9.Morishita H, Yagi T. Protocadherin family: Diversity, structure, and function. Curr Opin Cell Biol. 2007;19:584–592. doi: 10.1016/j.ceb.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Wu Q, Maniatis T. A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell. 1999;97:779–790. doi: 10.1016/s0092-8674(00)80789-8. [DOI] [PubMed] [Google Scholar]
- 11.Weiner JA, Wang X, Tapia JC, Sanes JR. Gamma protocadherins are required for synaptic development in the spinal cord. Proc Natl Acad Sci USA. 2005;102:8–14. doi: 10.1073/pnas.0407931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, et al. Gamma protocadherins are required for survival of spinal interneurons. Neuron. 2002;36:843–854. doi: 10.1016/s0896-6273(02)01090-5. [DOI] [PubMed] [Google Scholar]
- 13.Pierchala BA, Milbrandt J, Johnson EM., Jr. Glial cell line-derived neurotrophic factor-dependent recruitment of Ret into lipid rafts enhances signaling by partitioning Ret from proteasome-dependent degradation. J Neurosci. 2006;26:2777–2787. doi: 10.1523/JNEUROSCI.3420-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsui CC, Pierchala BA. The differential axonal degradation of Ret accounts for cell-type-specific function of glial cell line-derived neurotrophic factor as a retrograde survival factor. J Neurosci. 2010;30:5149–5158. doi: 10.1523/JNEUROSCI.5246-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukuda E, et al. Down-regulation of protocadherin-α A isoforms in mice changes contextual fear conditioning and spatial working memory. Eur J Neurosci. 2008;28:1362–1376. doi: 10.1111/j.1460-9568.2008.06428.x. [DOI] [PubMed] [Google Scholar]
- 16.Wu Q, et al. Comparative DNA sequence analysis of mouse and human protocadherin gene clusters. Genome Res. 2001;11:389–404. doi: 10.1101/gr.167301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugino H, et al. Genomic organization of the family of CNR cadherin genes in mice and humans. Genomics. 2000;63:75–87. doi: 10.1006/geno.1999.6066. [DOI] [PubMed] [Google Scholar]