Abstract
Corticosteroid hormones are critical for controlling metabolism, hydromineral balance, and the stress response in vertebrates. Although corticosteroid hormones have been well characterized in most vertebrate groups, the identity of the earliest vertebrate corticosteroid hormone has remained elusive. Here we provide evidence that 11-deoxycortisol is the corticosteroid hormone in the lamprey, a member of the agnathans that evolved more than 500 million years ago. We used RIA, HPLC, and mass spectrometry analysis to determine that 11-deoxycortisol is the active corticosteroid present in lamprey plasma. We also characterized an 11-deoxycortisol receptor extracted from sea lamprey gill cytosol. The receptor was highly specific for 11-deoxycortisol and exhibited corticosteroid binding characteristics, including DNA binding. Furthermore, we observed that 11-deoxycortisol was regulated by the hypothalamus–pituitary axis and responded to acute stress. 11-Deoxycortisol implants reduced sex steroid concentrations and up-regulated gill Na+, K+-ATPase, an enzyme critical for ion balance. We show here that 11-deoxycortisol functioned as both a glucocorticoid and a mineralocorticoid in the lamprey. Our findings indicate that a complex and highly specific corticosteroid signaling pathway evolved at least 500 million years ago with the arrival of the earliest vertebrate.
Keywords: evolution, nuclear-receptor, stress response
Corticosteroid hormones in vertebrates are critical for metabolism, growth, reproduction, immunity, and ion homeostasis, and are an important part of the coping mechanisms involved in the stress responses (1). Corticosteroids primarily act by binding to cytosolic receptors, which are then transported to the nucleus, where they act as positive and negative transcription factors (2). Their activity leads to the expression or repression of various regulatory proteins that counteract the effects of external stressors, thus maintaining homeostasis (2).
The actions of corticosteroids appear to be conserved. Most vertebrates that have been examined share a stress response that includes increased glucocorticoid (GC) hormones that further regulate metabolic, endocrine, and immune functions. However, active hormones may differ among species. In tetrapod groups, there are at least two active GC hormones, either cortisol or corticosterone, and the mineralocorticoid (MC) that regulates ion balance is aldosterone. In contrast, in teleosts, cortisol apparently acts as both GC and MC, whereas aldosterone is not present (3). However, the corticosteroid signaling pathway in the earliest vertebrate has remained elusive because of lack of information on the identities of corticosteroids.
Recent work with receptors has shed light on the primitive corticosteroid of vertebrates. A single putative corticosteroid receptor (CR) was identified through PCR-based homology cloning in the sea lamprey, a member of the oldest vertebrate lineages from which jawed vertebrates diverged about 500 million years ago (4, 5). Transactivation studies using cloned lamprey and hagfish CRs, as well as a “resurrected” ancestral CR that was inferred from known CRs, resulted in MC receptor (MR)–like “promiscuous binding” to multiple corticosteroids. Aldosterone and 11-deoxycorticosterone had the highest levels of activation in a luciferase reporter gene assay using the ligand binding domain (LBD) of lamprey CR fused with a mammalian GAL4-DBD (6). To better understand the evolution of CRs, Stolte et al. (7) performed phylogenetic analysis using the lamprey CR. Their findings showed that the gene clustered with extant MR genes, suggesting that an MC complex evolved first. Because aldosterone was not found at physiologically relevant levels in the lamprey (6), receptor sensitivity to aldosterone was suggested to be a by-product of sensitivity to 11-deoxycorticosterone, a postulated ancestral hormone (8). Notably lacking, however, have been direct identification of the circulating corticosteroid, characterization of its native receptor, and establishment of biological actions, which are all necessary to define a hormone. Here we provide direct evidence that 11-deoxycortisol is the corticosteroid hormone in the lamprey, the closest living relative of the earliest vertebrate, thus offering further insight into steroid hormone evolution.
Results
Identification of Circulating Corticosteroids.
To determine whether corticosteroids are present in lamprey, we assumed that the structure of a lamprey corticosteroid might be related to modern vertebrate GCs. We isolated putative corticosteroids (Fig. 1A) by screening lamprey plasma with RIAs for cortisol and corticosterone and with a binding protein assay for cortisol after HPLC fractionation. These assays showed no cortisol and corticosterone in the plasma (Fig. 1 B and C), but revealed cross-reactivity in fractions where 11-deoxycortisol and 11-deoxycorticosterone standards eluted on HPLC (Fig. 1 B and C). To isolate sufficient quantities of steroids for mass spectrometry (MS) analysis, we fractionated extracted plasma by group partition chromatography (LH-20) and screened it with 11-deoxycortisol and 11-deoxycorticosterone RIAs (Fig. S1). LH-20 fractions were combined and further isolated by HPLC fractionation followed by MS analysis.
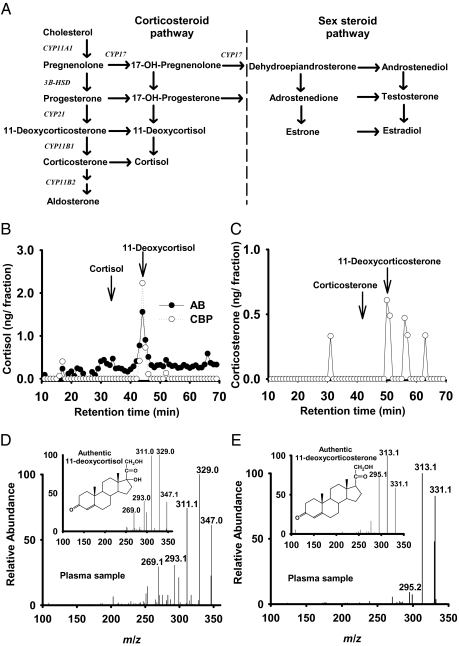
Fig. 1.
Identification of corticosteroids in lamprey plasma. (A) Biosynthetic pathways of corticosteroids and sex steroids. Steroidogenic enzymes are indicated on arrow labels. (B) Screening of HPLC-fractionated plasma extract with cortisol RIA and binding protein assay. AB, anticortisol antibody; CBP, cortisol binding protein. Arrows indicate where cortisol and 11-deoxycortisol standards elute. (C) Screening of HPLC-fractionated plasma extract with corticosterone RIA. Arrows indicate where corticosterone and 11-deoxycorticosterone elute. (D) Identification of 11-deoxycortisol by atmospheric pressure chemical ionization MS (APCI MS-MS) analysis. Fragmentation patterns generated from the authentic 11-deoxycortisol (inset) matched the plasma sample. (E) Identification of 11-deoxycorticosterone by APCI MS-MS analysis. Fragmentation patterns generated from the authentic 11-deoxycorticosterone (Inset) matched the plasma sample.
To confirm the identity of the compounds in the immunoreactive fractions, we subjected the analyte ions to tandem MS (MS/MS) product ion analyses. The product ion spectra of authentic 11-deoxycortisol [M+H]+ ion at m/z 347 and 11-deoxycorticosterone [M+H]+ ion at m/z 331, obtained by direct infusion of standards (Fig. 1D, Inset, and E, Inset), matched the fragmented daughter ions of the plasma samples (Fig. 1 D and E). Taken together, the binding-assay–guided isolation and chromatographic and MS analyses demonstrated that 11-deoxycortisol and 11-deoxycorticosterone were present in circulation and thus potential corticosteroids in the sea lamprey. These results were intriguing because 11-deoxycortisol and 11-deoxycorticosterone are precursor steroids to cortisol and corticosterone in the biosynthetic pathway.
Identification and Characterization of CR.
To characterize the cognate receptor for 11-deoxycortisol, we performed radioligand binding experiments using tritiated 11-deoxycortisol and the cytosol fraction of gill tissue. We confirmed the presence of a highly specific 11-deoxycortisol receptor with glucocorticoid receptor (GR) binding characteristics. Linear transformation of saturation data revealed a single population of binding sites for 11-deoxycortisol in the cytosolic fraction of gill homogenate (Fig. 2A, Inset). The receptor had a high affinity (Kd = 2.66 ± 0.47 nM, mean ± SEM) and low capacity (Bmax = 58.10 ± 3.33 fmol/mg protein) for 11-deoxycortisol (Fig. 2A), consistent with GR affinity and concentration of binding sites in previous studies with teleosts (9, 10). Kinetic studies of the 11-deoxycortisol binding moiety showed that its association rate (T1/2) was 2.11 ± 0.32 min and that its specific binding remained constant during the experiment (Fig. 2B). The specific binding was reversible with a dissociation rate (T1/2) of 26.44 ± 8.41 min during the 2-h experiment (Fig. 2B, Inset). Both association and dissociation rates of 11-deoxycortisol were generally faster than GR characteristics in salmonids (9, 11, 12).
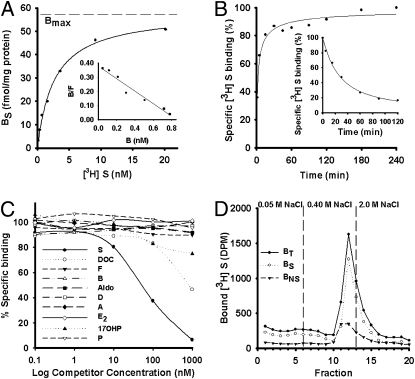
Fig. 2.
Characterization of the lamprey corticosteroid receptor. (A) Representative hyperbolic regression plot and linear transformation of saturation data (Inset) of 11-deoxycortisol binding to a receptor in gill cytosol. Bmax, maximum binding capacity of tissue; BNS, nonspecific binding; BS, specific binding; BT, total binding; DPM, disintegrations per minute; F, free (i.e., unbound) [3H] 11-deoxycortisol (n = 3 experiments per point). (B) Association and dissociation kinetics (Inset) of sea lamprey receptor in gill cytosol. For association kinetics, T1/2 was 2.11 ± 0.32 min (n = 3). For dissociation kinetics, T1/2 was 26.44 ± 8.41 min (n = 3). (C) Ligand binding affinity assay. Ad, androstenedione; Aldo, aldosterone; B, corticosterone; D, dexamethasone; DOC, 11-deoxycorticosterone; E2, estradiol; F, cortisol; 17-OH-P, 17α-hydroxyprogesterone; P, progesterone; S, 11-deoxycortisol (n = 3). (D) Affinity chromatography of 11-deoxycortisol–receptor complex (n = 3).
We demonstrated that the lamprey CR was highly specific for 11-deoxycortisol, as determined by a competitive binding assay. 11-Deoxycortisol had the highest affinity to the cytosolic binding moiety among the nine steroids tested (Fig. 2C). Relative binding affinity of 11-deoxycorticosterone, compared with 11-deoxycortisol, was ≈5% for the CR. All other steroids, at concentrations up to 1 μM, failed to displace 50% of 3.3 nM of [3H] 11-deoxycortisol. Specific binding of 11-deoxycortisol to the binding moiety was found in all tissues tested, with highest specific binding levels in gill, intestine, and testis (Fig. S2). In addition, there was no specific binding of the tritiated 11-deoxycorticosterone in the gill, kidney, oocyte, brain, intestine, or heart.
Because nuclear CRs are transcription factors that regulate gene expression, we next investigated whether the 11-deoxycortisol receptor complex could bind to DNA. DNA-cellulose chromatography revealed that the [3H] 11-deoxycortisol receptor complex had specific (BS) binding (Fig. 2D). The binding characteristics of the 11-deoxycortisol receptor complex to DNA are similar to those found in a previous study using a teleost GR (12). Together, our results demonstrated the existence of a high-affinity, low-capacity CR with highly specific binding characteristics in the lamprey.
Hypothalamic–Pituitary–Interrenal Axis Regulates 11-Deoxycortisol.
To verify hypothalamic–pituitary–interrenal (HPI) axis stimulation of 11-deoxycortisol secretion, we conducted in vivo experiments by collecting plasma samples after injection of the hypothalamic corticotropin-releasing hormone (CRH) and sea lamprey pituitary extracts. Human CRH markedly increased plasma 11-deoxycortisol concentrations in male and female lampreys 1 h after injection (Fig. 3A), a finding that is consistent with previous work showing that CRH stimulates the release of GCs in jawed vertebrates (13, 14). Because CRH is known to stimulate the release of adrenocorticotropic hormone (ACTH) from the pituitary, which in turn stimulates the secretion of GC from the interrenal cells, we investigated whether sea lamprey pituitary extract would increase the secretion of 11-deoxycortisol. In a previous study, a sequence similar to proopiomelanocortin, the precursor for ACTH, was identified from a pituitary cDNA library in lamprey (15). In response to injection of pituitary extract, we found a dose-dependent increase in plasma 11-deoxycortisol levels (Fig. 3B). To determine whether 11-deoxycortisol was part of a stress-activated HPI axis, we investigated the changes in plasma 11-deoxycortisol in response to acute stress. Juvenile and adult lampreys subjected to dewatering exhibited a more than 2-fold increase in plasma 11-deoxycortisol concentrations within 1 h (Fig. 3 C and D). These acute stress experiments confirmed that lamprey share a GC response consistent with patterns in fishes and other vertebrates. Basal levels of plasma 11-deoxycortisol are similar to cortisol levels in teleosts, although stressed concentrations are somewhat lower (16). Both 11-deoxycortisol and 11-deoxycorticosterone increased in plasma, but 11-deoxycorticosterone increased only 10% following acute stress (Fig. S3).
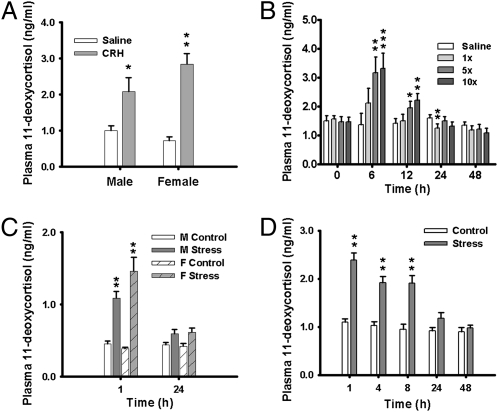
Fig. 3.
Regulation of 11-deoxycortisol by the stress–hypothalamus–pituitary axis. (A) Concentration of 11-deoxycortisol in plasma of parasitic male (n = 4–5 per treatment) and female (n = 3–4) lampreys after injection of corticotropin-releasing hormone (100 μg/kg body weight). (B) Concentration of plasma 11-deoxycortisol in adult male lampreys (n = 9–10/treatment) in response to three doses of pituitary extract (equivalent to 1, 5, or 10 pituitaries or a 0.9% saline as control). (C) Effect of acute stress on plasma concentrations of 11-deoxycortisol in parasitic male (n = 10–14/time point) and female (n = 6–10) lampreys. (D) Effect of acute stress on the plasma concentrations of 11-deoxycortisol in adult male (n = 14/time point) lampreys. Results in A–D are mean ± SE. Asterisks indicate a significant (*P < 0.05; **P < 0.01; ***P < 0.001) difference with Student's t test (A), two-way ANOVA (B), and one-way ANOVA (C and D).
11-Deoxycortisol Decreases Sex Steroids and Increases Na+, K+-ATPase Activity.
Acute and chronic stress is known to suppress reproductive functions through GCs; therefore, we investigated whether sex steroids in circulation would decrease in response to chronic elevation of 11-deoxycortisol. 11-Deoxycortisol implants increased plasma concentrations of 11-deoxycortisol and 11-deoxycorticosterone (Fig. 4A) and resulted in lowered circulatory concentrations of dehydroepiandrosterone sulfate, dehydroepiandrosterone, testosterone, and estradiol in both male and female lampreys (Fig. 4 B and C). Our previous measurements of 11-deoxycortisol concentrations in the metamorphosing sea lamprey showed that the concentrations achieved by the implants are physiologically relevant. Stress or cortisol implants have been shown to decrease testosterone and estradiol in fish, amphibians, and reptiles (17–23). To investigate whether 11-deoxycortisol regulates MC homeostasis, we measured Na+, K+-ATPase activity in the gills after exogenous treatment with 11-deoxycortisol. In lampreys, this ion-translocating enzyme is involved both in salt uptake in freshwater and in salt secretion in seawater (24). 11-Deoxycortisol implants nearly doubled gill Na+, K+-ATPase activity in both male and female lampreys (Fig. 4D). This effect is similar to that of cortisol, a GC in teleosts, in up-regulating gill and intestine Na+, K+-ATPase activity in salmonids (25, 26).
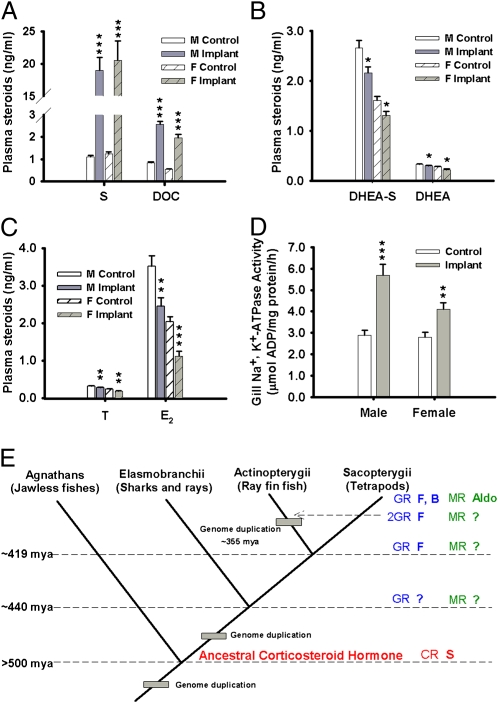
Fig. 4.
Biological effects of 11-deoxycortisol implants. (A) Effect of 11-deoxycortisol treatment on plasma levels of 11-deoxycortisol (S) and 11-deoxycorticosterone (DOC) in male (n = 12/treatment) and female (n = 11–12) lampreys. (B) Effect of 11-deoxycortisol treatment on plasma levels of dehydroepiandrosterone-sulfate (DHEA-S) and dehydroepiandrosterone (DHEA) in male and female lampreys. (C) Effect of 11-deoxycortisol treatment on plasma levels of testosterone (T) and estradiol (E2) in male and female lampreys. (D) Effect of 11-deoxycortisol treatment on gill Na+, K+-ATPase activity in male and female lampreys. (E) Evolution of corticosteroid hormones in vertebrates. Our findings indicate that 11-deoxycortisol evolved as a corticosteroid hormone in the earliest vertebrate, the sea lamprey. Diagram modified from Bury and Sturm (3) and Bridgham et al. (6). The ancient corticosteroid hormone functioned as both mineralocorticoid and glucocorticoid. Aldo, aldosterone; B, corticosterone; CR, corticosteroid receptor; F, cortisol; GR, glucocorticoid receptor; MR, mineralocorticoid receptor; S, 11-deoxycortisol. Results in A–D are mean ± SE. Asterisks indicate significant (*P < 0.05; **P < 0.01; ***P < 0.001; Student's t test) difference.
Discussion
The present study demonstrates that 11-deoxycortisol functions as both a GC and an MC through its highly specific CR in lamprey. 11-Deoxycortisol was regulated by the HPI axis and responded to acute stress. Moreover, implanted 11-deoxycortisol suppressed the sex steroid concentrations and stimulated ion transport capacity in the gills. Collectively, these data demonstrate that 11-deoxycortisol is a biologically active corticosteroid hormone in lamprey. This identification of the corticosteroid in the living representative of the most basal vertebrate provides crucial insight into the evolution of corticosteroid signaling.
In the present study, we used a combination of RIAs, HPLC, and MS analysis to isolate and identify corticosteroid-like molecules in the sea lamprey. On the basis of the assumption that the corticosteroids in the lamprey share structural features with the steroids found in other vertebrates such as fish and tetrapods, we used antibodies raised against cortisol and corticosterone and a cortisol binding protein in RIAs and a binding assay to screen HPLC fractions. We were able to isolate two steroid compounds that are direct precursors to cortisol and corticosterone. This approach enabled us to separate steroid compounds of interest on the basis of their chromatographic and MS characteristics, ruling out the possibility of misinterpretation caused by cross-reactivity of antibodies (27). Lamprey possess 15 hydroxy steroids, including 15 α-OH progesterone (15 α-OH P), which shares some chemical features with the known vertebrate corticosteroids. Although 15 α-OH P exists in the circulation of the sea lamprey (28–30), previous studies in our laboratory found no evidence for 15 α-OH P binding to cytosolic, nuclear, and membrane preparations of various sea lamprey tissues. Further research would be required to test whether there are any other corticoids that are not structurally related to the common vertebrate corticosteroids.
In the steroid biosynthetic pathway (Fig. 1A), 11-deoxycorticosterone and 11-deoxycortisol are direct precursors to corticosterone and cortisol, respectively, the major GCs in more derived vertebrates. Previous studies have provided mixed results regarding the presence of corticosteroids in lamprey, a question that has remained unresolved for many years (31–35). The most recent of these studies reported the presumptive identification of cortisol, corticosterone, and 11-deoxycortisol, but not 11-deoxycorticosterone in sea lamprey sera (35), in contrast to our findings that identified 11-deoxycortisol and 11-deoxycorticosterone with no evidence for cortisol or corticosterone in the plasma. In retrospect, the difference between the previous identification studies and our study can likely be attributed to technological advances that provide more sensitive and precise HPLC and MS analyses. The absence of cortisol and corticosterone in sea lampreys suggests that the enzyme CYP11B1 may not have been present early in vertebrate evolution. Our search of the lamprey genome database for CYP11B1 orthologs was unsuccessful. However, we confirmed the presence of a CYP 21 ortholog—necessary to produce 11-deoxycorticosterone and 11-deoxycortisol—with 55% sequence identity to the zebra fish CYP21 (XP_001919231).
Our characterization of the lamprey CR revealed high specificity and affinity for only one corticosteroid, 11-deoxycortisol. The low affinity of aldosterone, 11-deoxycorticosterone, corticosterone, and cortisol shown in our study contradicts a previous study that showed promiscuous activation by these corticosteroids in a reporter gene assay (6). In the previous study, a luciferase reporter gene with GAL4-DBD and the LBD of lamprey CR was used to measure both transcriptional and ligand-binding activities of the receptor (6). Such promiscuous activation may be an artifact because of the absence of the whole receptor, lack of lamprey-specific chaperone proteins, or may be a result of assay conditions such as incubation temperature and/or the use of heterologous cell lines. The affinity and specificity of a steroid receptor for a given ligand is critically determined by the hsp-90 complex, which chaperones the unliganded receptor while maintaining an open binding conformation (36, 37). In addition, recent studies have shown that a region outside the LBD of an MR affects ligand binding selectivity (38). Although it is possible that more than one CR exists in lampreys and could explain differences among studies, this seems unlikely. PCR-based cloning found a single CR from liver (6) and gill tissue (39), and there is only one detectable gene with high homology to CR from the lamprey genome database (39), which was also found to be expressed in the lamprey gill tissue (39). Furthermore, identification and characterization of a single population of binding sites in our study indicates that we have characterized the only CR present. The data that we derived with our classical approach indicate that the native CR in lamprey gill cytosol is highly specific for 11-deoxycortisol, and thus a corticosteroid hormone signaling pathway with a highly specific binding capability likely evolved in ancestral vertebrates.
In response to a stressful stimulus, animals elicit a stress response that includes the release of GCs and other hormones from the adrenal glands—in fish from interrenal cells—to maintain homeostatic conditions. In the sea lamprey, we demonstrated that 11-deoxycortisol is part of the acute stress response, as evidenced by a 2-fold increase of 11-deoxycortisol concentrations in response to stressors. In jawed vertebrates, the GC response is largely regulated by the HPI/hypothalamic–pituitary–adrenal (HPA) axis, represented by the secretion of the hypothalamic hormone CRH and the pituitary hormone ACTH. A previous study has shown CRH to be highly conserved among jawed vertebrates (40). We conducted a lamprey genome database search and found a single CRH sequence with 88% identity to human CRH. Thus, studies using human CRH and lamprey pituitary extract demonstrated that 11-deoxycortisol is regulated by the HPI axis, consistent with HPI/HPA axis regulation of the GCs cortisol and corticosterone in jawed vertebrates.
GCs are known to suppress the sex steroid biosynthetic pathway, thus affecting reproductive functions in vertebrates. The effect of GCs on circulating sex steroids can be mediated at several levels of the hypothalamus–pituitary–gonadal axis, including up-regulation of gonadotropin inhibitory hormone (41) and down-regulation of steroidogenic enzymes (42). In the rat, acute stress disrupts the steroid biosynthetic pathway in testes by inhibiting 17α-hydroxylase and 17, 20 lyase (42). Consistent with these results, our findings demonstrated that 11-deoxycortisol implants reduced plasma concentrations of sex steroids. The biosynthetic pathway for production of sex steroids requires CYP17 (17α-hydroxylase/17, 20 lyase) activity for conversion to C19 steroids. In addition, we found that 11-deoxycorticosterone levels also increased in response to 11-deoxycortisol implants, likely because of inhibition of 17α-hydroxylase activity that effectively blocked conversion of 11-deoxycorticosterone to 11-deoxycortisol. More work is necessary, however, to determine the precise mechanisms by which 11-deoxycortisol regulates reproductive functions in the lamprey.
In a phylogenetic analysis of CRs, Stolte et al. (7) grouped lamprey CR with other vertebrate MRs, suggesting that the MR gene is ancestral to the GR gene. Using a heterologous expression system, Bridgham et al. (6) showed that the lamprey CR was activated by several chemically related corticosteroids, which is similar to MR binding characteristics in recent vertebrates. It was further suggested that the promiscuous trait was retained by the MR after the genome duplication between jawless and jawed vertebrates, whereas the specificity of the duplicated GR was a derived phenotype (6). In contrast, the highly specific binding of the lamprey CR for 11-deoxycortisol demonstrated in our study indicates that the promiscuity of later vertebrate MRs is a derived trait. Furthermore, we postulate that the highly specific binding characteristic of the ancestral CR was retained by the GR in later vertebrates. Therefore, it is likely that the evolution of promiscuous binding of the MR played an important role in the eventual divergence of ancestral corticosteroid functions in later vertebrates.
Taken together, our experiments support a model in which 11-deoxycortisol and its CR functioned as both an MC and a GC in the lamprey. Following the genome duplication event between jawless and jawed vertebrates (6), the ancestral CR functions were subfunctionalized between the MR and GR in later vertebrates (Fig. 4E). Thus, the duplicated MR retained part of the ancestral function of regulating ion homeostasis, whereas the duplicated GR retained the GC function regulated by the HPI/HPA axis. The regulation of GC and MC functions by a single corticosteroid hormone thus appears to be the early vertebrate condition, which is consistent with a single corticosteroid hormone having dual function in teleosts (43). Although the timing of the partitioning is unknown, the divergence of these functions by MR and GR may have occurred in the terrestrial vertebrates with the arrival of CYP11B2, necessary for the biosynthesis of aldosterone as an MC (44). Our findings strongly indicate that subfunctionalization occurred during the evolution of the corticosteroid signaling system, thus leading to increased complexity.
It seems likely that complexity in recent corticosteroid signaling systems occurred in a Darwinian stepwise process through coevolution of CYP enzymes and receptor proteins. Point mutations in the ligand binding pocket, coupled with the arrival of new corticosteroids produced by steroidogenic enzymes, likely occurred after the genome duplication between jawless and jawed vertebrates. This idea is supported by the evolution of MR and GR from ancestral CR (6), along with corticosterone and possibly cortisol production by CYP11B1 in elasmobranchs (45). A recent study has shown that steroidogenic CYPs were crucial in the evolution of lipophilic molecules through the mechanism of hydroxylation (46). In the basal vertebrates, the addition of an 11-position hydroxyl (CYP11B1) to 11-deoxycortisol may have arisen as a necessity to clear excess hormone, which then led to the production of cortisol and corticosterone as deactivated metabolites. These compounds would then be available to interact with the duplicated CRs, eventually leading to cortisol/corticosterone–GR coupling, along with aldosterone–MR coupling, after CYP11B2 and 11β-HSD2 arose in later vertebrates (44) (Fig. 4E).
Our studies, which combined chemical identification of a corticosteroid, biochemical characterization of its cognate receptor, and establishment of biological actions, demonstrate that 11-deoxycortisol is a functional corticosteroid hormone in the closest living relative of the earliest vertebrate, the sea lamprey. In addition, the dual roles of the lamprey corticosteroid as an MC and a GC indicate that the corticosteroid signaling system evolved through the mechanism of subfunctionalization. We hypothesize that 11-deoxycortisol and its highly specific receptor represents the primitive condition of vertebrates, and our findings contribute to a more complete understanding of the evolution of corticosteroid signaling.
Materials and Methods
Experimental Subjects.
Adult lampreys were acclimated in flow-through tanks (254 L) at 10–13 °C for at least 2 wk before stress tests. For the CRH experiment, saline-injected (n = 8) controls and CRH-injected (n = 8) lampreys were used. For the pituitary extract study, saline-injected (n = 9) or extract-injected (n = 9–10) lampreys were used. For the acute stress study (1 and 24 h), parasitic lampreys were stressed (n = 40) and bled after being anesthetized. Controls (n = 40) were left in tanks undisturbed until bleeding. For the second acute stress study (1–48 h), lampreys were stressed (n = 70) and controls (n = 70) were left undisturbed until bleeding. All animal care and procedures were approved by the Michigan State University Institutional Animal Care and Use Committee.
Acute Stress Treatment.
Lampreys were stressed by netting them out of tanks, placing them in a dry bucket for 5 min, and then transferring them to 3% saltwater for 10 min before returning them to a freshwater tank.
Plasma Steroid Sampling.
In all in vivo experiments, blood was collected by cardiac puncture. Blood samples were centrifuged at 1,000 × g for 15 min and plasma was stored at −80 °C until assayed. Corticosteroids and sex steroids were measured by using RIAs.
Chromatography and MS.
Plasma was extracted (solid phase extraction) and dissolved in 1 mL acetonitrile/water/trifluoroacetic acid (28/72/0.01, vol/vol/vol) and loaded onto a C18 HPLC column (Nova-Pak, 3.9 mm × 300 mm; Waters). Two solvents were used to deliver a gradient to the column. Fractions were collected at 1-min intervals between 11 and 70 min. LH-20 chromatography was performed on a glass column containing Sephadex LH-20 (Amersham) resin by using a mixture of dichloromethane and methanol (98/2: vol/vol) as the eluting solution. Fractions from each chromatography step were screened by RIAs. Fractions were dried down under reduced pressure and then subjected to atmospheric pressure chemical ionization MS analysis. Mass spectra were obtained with an LCQ-Deca ion trap (Thermo Scientific). Samples were compared against authentic 11-deoxycortisol and 11-deoxycorticosterone standards (Sigma-Aldrich).
Characterization of CR.
Tissues collected from fish were frozen in liquid nitrogen and held at −80 °C until processed for cytosolic fractions. To characterize the CR, we performed receptor binding assays with [3H] 11-deoxycortisol. The concentration of binding sites (Bmax) and the dissociation constant (KD) were determined by hyperbolic regression using Sigmaplot 10.0 (SYSTAT). For DNA-cellulose chromatography, gill cytosol (1.0 mL) was incubated for 2 h at 0 °C with 20 nM [3H] 11-deoxycortisol with or without 1 μg cold 11-deoxycortisol. The sample was allowed to flow into the DNA cellulose (Amersham), and then the flow was stopped to allow absorption for 20 min. To elute the bound receptor complex from the DNA, we used 7 mL 0.4 M NaCl elution buffer followed by 7 mL wash buffer (2.0 M NaCl).
Gill Na+, K+-ATPase Activity.
A gill pouch was removed and placed in ice-cold SEI buffer (150 mM sucrose, 10 mM EDTA, 50 mM imidazole, pH 7.3) and frozen immediately at −80 °C. Na+, K+-ATPase activity was determined with a kinetic assay. Gill tissue was homogenized in 500 μL SEID (SEI buffer and 0.1% deoxycholic acid) and centrifuged at 5,000 × g for 30 s. The resulting ouabain-sensitive ATPase activity was expressed as micromoles ADP/mg protein per hour. Protein concentrations were determined by using BCA (bicinchoninic acid) Protein Assay (Pierce). Additional details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank A. P. Scott, Nicholas Johnson, and Ken Poff for technical advice; Lijun Chen of the Michigan State University's Mass Spectrometry Facility for technical expertise; and the reviewers and editor for constructive comments. We also thank the staff of the US Geological Survey Hammond Bay biological station and the Great Lakes Fishery Commission for providing research facilities. This research was supported by the Confederated Tribes of the Umatilla Indian Reservation, Bonneville Power Administration, Great Lakes Fishery Commission, Office of Diversity and Pluralism in the College of Agriculture and Natural Resources at Michigan State University, and a National Institute of Mental Health traineeship (T32MH70343).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0914026107/-/DCSupplemental.
References
- 1.Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- 2.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 3.Bury NR, Sturm A. Evolution of the corticosteroid receptor signalling pathway in fish. Gen Comp Endocrinol. 2007;153:47–56. doi: 10.1016/j.ygcen.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Thornton JW. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc Natl Acad Sci USA. 2001;98:5671–5676. doi: 10.1073/pnas.091553298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S, Hedges SB. A molecular timescale for vertebrate evolution. Nature. 1998;392:917–920. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- 6.Bridgham JT, Carroll SM, Thornton JW. Evolution of hormone-receptor complexity by molecular exploitation. Science. 2006;312:97–101. doi: 10.1126/science.1123348. [DOI] [PubMed] [Google Scholar]
- 7.Stolte EH, van Kemenade BM, Savelkoul HFJ, Flik G. Evolution of glucocorticoid receptors with different glucocorticoid sensitivity. J Endocrinol. 2006;190:17–28. doi: 10.1677/joe.1.06703. [DOI] [PubMed] [Google Scholar]
- 8.Dean AM, Thornton JW. Mechanistic approaches to the study of evolution: The functional synthesis. Nat Rev Genet. 2007;8:675–688. doi: 10.1038/nrg2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborti PK, Weisbart M, Chakraborti A. The presence of corticosteroid receptor activity in the gills of the brook trout, Salvelinus fontinalis. Gen Comp Endocrinol. 1987;66:323–332. doi: 10.1016/0016-6480(87)90241-3. [DOI] [PubMed] [Google Scholar]
- 10.Mommsen TP, Vijayan MM, Moon TW. Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fish. 1999;9:211–268. [Google Scholar]
- 11.Chakraborti PK, Weisbart M. High-affinity cortisol receptor activity in the liver of the brook trout, Salvelinus fontinalis (Mithchill) Can J Zool. 1987;65:2498–2503. [Google Scholar]
- 12.Knoebl I, Fitzpatrick MS, Schreck CB. Characterization of a glucocorticoid receptor in the brains of chinook salmon, Oncorhynchus tshawytscha. Gen Comp Endocrinol. 1996;101:195–204. doi: 10.1006/gcen.1996.0021. [DOI] [PubMed] [Google Scholar]
- 13.Fryer J, Lederis K, Rivier J. Urotensin I, a CRF-like neuropeptide, stimulates acth release from the teleost pituitary. Endocrinology. 1983;113:2308–2310. doi: 10.1210/endo-113-6-2308. [DOI] [PubMed] [Google Scholar]
- 14.Pepels PPLM, Van Helvoort H, Wendelaar Bonga SE, Balm PHM. Corticotropin-releasing hormone in the teleost stress response: Rapid appearance of the peptide in plasma of tilapia (Oreochromis mossambicus) J Endocrinol. 2004;180:425–438. doi: 10.1677/joe.0.1800425. [DOI] [PubMed] [Google Scholar]
- 15.Heinig JA, Keeley FW, Robson P, Sower SA, Youson JH. The appearance of proopiomelanocortin early in vertebrate evolution: Cloning and sequencing of POMC from a Lamprey pituitary cDNA library. Gen Comp Endocrinol. 1995;99:137–144. doi: 10.1006/gcen.1995.1094. [DOI] [PubMed] [Google Scholar]
- 16.Barton BA. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol. 2002;42:517–525. doi: 10.1093/icb/42.3.517. [DOI] [PubMed] [Google Scholar]
- 17.Pickering AD, Pottinger TG, Carragher J, Sumpter JP. The effects of acute and chronic stress on the levels of reproductive hormones in the plasma of mature male brown trout, Salmo trutta L. Gen Comp Endocrinol. 1987;68:249–259. doi: 10.1016/0016-6480(87)90036-0. [DOI] [PubMed] [Google Scholar]
- 18.Pankhurst NW, Dedual M. Effects of capture and recovery on plasma levels of cortisol, lactate, and gonadal steroids in a natural population of rainbow trout. J Fish Biol. 1994;45:1013–1025. [Google Scholar]
- 19.Moore FL, Zoeller RT. Stress-induced inhibition of reproduction: Evidence of suppressed secretion of LH-RH in an amphibian. Gen Comp Endocrinol. 1985;60:252–258. doi: 10.1016/0016-6480(85)90321-1. [DOI] [PubMed] [Google Scholar]
- 20.Lance VA, Elsey RM. Stress-induced suppression of testosterone secretion in male alligators. J Exp Zool. 1986;239:241–246. doi: 10.1002/jez.1402390211. [DOI] [PubMed] [Google Scholar]
- 21.Carragher JF, Sumpter JP, Pottinger TG, Pickering AD. The deleterious effects of cortisol implantation on reproductive function in two species of trout, Salmo trutta L. and Salmo gairdneri Richardson. Gen Comp Endocrinol. 1989;76:310–321. doi: 10.1016/0016-6480(89)90163-9. [DOI] [PubMed] [Google Scholar]
- 22.Foo JTW, Lam TJ. Retardation of ovarian growth and depression of serum steroid levels in the tilapia, oreochromis mossambicus, by cortisol implantation. Aquaculture. 1993;115:133–143. [Google Scholar]
- 23.Foo JTW, Lam TJ. Serum cortisol response to handling stress and the effect of cortisol implantation on testosterone level in the tilapia, Oreochromis mossambicus. Aquaculture. 1993;115:145–158. [Google Scholar]
- 24.Reis-Santos P, McCormick SD, Wilson JM. Ionoregulatory changes during metamorphosis and salinity exposure of juvenile sea lamprey (Petromyzon marinus L.) J Exp Biol. 2008;211:978–988. doi: 10.1242/jeb.014423. [DOI] [PubMed] [Google Scholar]
- 25.McCormick SD. Effects of growth hormone and insulin-like growth factor I on salinity tolerance and gill Na+, K+-ATPase in Atlantic salmon (Salmo salar): Interaction with cortisol. Gen Comp Endocrinol. 1996;101:3–11. doi: 10.1006/gcen.1996.0002. [DOI] [PubMed] [Google Scholar]
- 26.Veillette PA, Young G. Tissue culture of sockeye salmon intestine: Functional response of Na+-K+-ATPase to cortisol. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1598–R1605. doi: 10.1152/ajpregu.00741.2004. [DOI] [PubMed] [Google Scholar]
- 27.Markov GV, Paris M, Bertrand S, Laudet V. The evolution of the ligand/receptor couple: A long road from comparative endocrinology to comparative genomics. Mol Cell Endocrinol. 2008;293:5–16. doi: 10.1016/j.mce.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Kime DE, Rafter JJ. Biosynthesis of 15-hydroxylated steroids by gonads of the river lamprey, Lampetra fluviatilis, in vitro. Gen Comp Endocrinol. 1981;44:69–76. doi: 10.1016/0016-6480(81)90357-9. [DOI] [PubMed] [Google Scholar]
- 29.Lowartz S, et al. Blood steroid profile and in vitro steroidogenesis by ovarian follicles and testis fragments of adult sea lamprey, Petromyzon marinus. Comp Biochem Physiol A Mol Integr Physiol. 2003;134:365–376. doi: 10.1016/s1095-6433(02)00285-4. [DOI] [PubMed] [Google Scholar]
- 30.Bryan MB, Scott AP, Li W. Sex steroids and their receptors in lampreys. Steroids. 2008;73:1–12. doi: 10.1016/j.steroids.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Buus O, Larsen LO. Absence of known corticosteroids in blood of river lampreys (Lampetra fluviatilis) after treatment with mammalian corticotropin. Gen Comp Endocrinol. 1975;26:96–99. doi: 10.1016/0016-6480(75)90219-1. [DOI] [PubMed] [Google Scholar]
- 32.Weisbart M, Youson JH. Steroid formation in the larval and parasitic adult sea lamprey, Petromyzon marinus L. Gen Comp Endocrinol. 1975;27:517–526. doi: 10.1016/0016-6480(75)90072-6. [DOI] [PubMed] [Google Scholar]
- 33.Weisbart M, Youson JH. In vivo formation of steroids from [1,2,6,7-3H]-progesterone by the sea lamprey, Petromyzon marinus L. J Steroid Biochem. 1977;8:1249–1252. doi: 10.1016/0022-4731(77)90109-1. [DOI] [PubMed] [Google Scholar]
- 34.Weisbart M, Youson JH, Wiebe JP. Biochemical, histochemical, and ultrastructural analyses of presumed steroid-producing tissues in the sexually mature sea lamprey, Petromyzon marinus L. Gen Comp Endocrinol. 1978;34:26–37. doi: 10.1016/0016-6480(78)90240-x. [DOI] [PubMed] [Google Scholar]
- 35.Weisbart M, Dickhoff WW, Gorbman A, Idler DR. The presence of steroids in the sera of the Pacific hagfish, Eptatretus stouti, and the sea lamprey, Petromyzon marinus. Gen Comp Endocrinol. 1980;41:506–519. doi: 10.1016/0016-6480(80)90055-6. [DOI] [PubMed] [Google Scholar]
- 36.Hutchison KA, Dittmar KD, Pratt WB. All of the factors required for assembly of the glucocorticoid receptor into a functional heterocomplex with heat shock protein 90 are preassociated in a self-sufficient protein folding structure, a “foldosome”. J Biol Chem. 1994;269:27894–27899. [PubMed] [Google Scholar]
- 37.Murphy PJM, et al. Visualization and mechanism of assembly of a glucocorticoid receptor.Hsp70 complex that is primed for subsequent Hsp90-dependent opening of the steroid binding cleft. J Biol Chem. 2003;278:34764–34773. doi: 10.1074/jbc.M304469200. [DOI] [PubMed] [Google Scholar]
- 38.Rogerson FM, et al. A critical region in the mineralocorticoid receptor for aldosterone binding and activation by cortisol: Evidence for a common mechanism governing ligand binding specificity in steroid hormone receptors. Mol Endocrinol. 2007;21:817–828. doi: 10.1210/me.2006-0246. [DOI] [PubMed] [Google Scholar]
- 39.Yeh C-Y. East Lansing, MI: Michigan State University; 2008. Cloning and expression of a corticoid receptor in the sea lamprey (Petromyzon marinus)MS thesis. [Google Scholar]
- 40.Lovejoy DA, Balment RJ. Evolution and physiology of the corticotropin-releasing factor (CRF) family of neuropeptides in vertebrates. Gen Comp Endocrinol. 1999;115:1–22. doi: 10.1006/gcen.1999.7298. [DOI] [PubMed] [Google Scholar]
- 41.Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc Natl Acad Sci USA. 2009;106:11324–11329. doi: 10.1073/pnas.0901176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orr TE, Taylor MF, Bhattacharyya AK, Collins DC, Mann DR. Acute immobilization stress disrupts testicular steroidogenesis in adult male rats by inhibiting the activities of 17 α-hydroxylase and 17,20-lyase without affecting the binding of LH/hCG receptors. J Androl. 1994;15:302–308. [PubMed] [Google Scholar]
- 43.McCormick SD, Regish A, O'Dea MF, Shrimpton JM. Are we missing a mineralocorticoid in teleost fish? Effects of cortisol, deoxycorticosterone and aldosterone on osmoregulation, gill Na+,K+ -ATPase activity and isoform mRNA levels in Atlantic salmon. Gen Comp Endocrinol. 2008;157:35–40. doi: 10.1016/j.ygcen.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 44.Baker ME. Co-evolution of steroidogenic and steroid-inactivating enzymes and adrenal and sex steroid receptors. Mol Cell Endocrinol. 2004;215:55–62. doi: 10.1016/j.mce.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Karsten AH, Turner JW., Jr Fecal corticosterone assessment in the epaulette shark, Hemiscyllium ocellatum. J Exp Zoolog A Comp Exp Biol. 2003;299:188–196. doi: 10.1002/jez.a.10300. [DOI] [PubMed] [Google Scholar]
- 46.Markov GV, et al. Independent elaboration of steroid hormone signaling pathways in metazoans. Proc Natl Acad Sci USA. 2009;106:11913–11918. doi: 10.1073/pnas.0812138106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.