Abstract
Cognitive decline is a virtually universal aspect of the aging process. However, its neurophysiological basis remains poorly understood. We describe here more than 20 age-related cortical processing deficits in the primary auditory cortex of aging versus young rats that appear to be strongly contributed to by altered cortical inhibition. Consistent with these changes, we recorded in old rats a decrease in parvalbumin-labeled inhibitory cortical neurons. Furthermore, old rats were slower to master a simple behavior, with learning progressions marked by more false-positive responses. We then examined the effect of intensive auditory training on the primary auditory cortex in these aged rats by using an oddball discrimination task. Following training, we found a nearly complete reversal of the majority of previously observed functional and structural cortical impairments. These findings suggest that age-related cognitive decline is a tightly regulated plastic process, and demonstrate that most of these age-related changes are, by their fundamental nature, reversible.
Keywords: aging, cognitive decline, plasticity, inhibition, parvalbumin
Perceptual and cognitive decline are near-universal aspects of normal aging (1, 2). Such deficits cannot be explained solely by a dysfunction of peripheral sensory organs and frequently translate to slowed perceptual processing and difficulty in accurately identifying stimuli under challenging (e.g., noisy, time-limited, attentionally demanding) conditions (3, 4). In the human auditory system, psychophysical and electroencephalography experiments have examined aspects of cognitive decline by using oddball detection paradigms, successive-signal masking studies, speech-in-noise studies, and compressed speech (5–7), among other strategies. These studies have shown that degraded signal salience, defective sensory adaptation and a slowing of sensory processing contribute to the deterioration of a wide range of perceptual and cognitive processes recorded in aged populations (3, 8–10). Animal models have been instrumental in defining the cellular and molecular basis of age-related perceptual impairments. In rats, significant alterations in inhibitory function in various subcortical nuclei and the auditory cortex have been linked to abnormal temporal and spectral processing (11–14). Interestingly, although these changes are often described as progressive plastic compensations secondary to a combination of slow peripheral deafferentation and chemical or molecular insults (11, 15), the possibility that age-related changes might be by their plastic nature largely reversible has seldom been explored or proposed (16). Compelling evidence that these age-related functional alterations can be prevented to some extent by sensory enrichment (9, 17) or even dietary improvements (15) certainly supports this concept. During adulthood, after the closure of developmental plasticity windows, attention-demanding intensive training strategies remain one of the most powerful means of directing plastic reorganizations in the brain or rats and humans and can lead to dramatic improvements in auditory discrimination (16, 18). Among such strategies, reinforcement-based operant conditioning is the most studied and has been shown to result in a specific refinement of cortical representations (18), changes in protein expression (19), and alterations in the cortical inhibitory circuitry (20) and supporting glia (21). Furthermore, such reorganizations appear to still be possible in aging brains (9). In the present study, we assessed the impact of an auditory training task specifically designed to improve frequency resolution and signal-in-noise processing on a series of cortical processing abnormalities in the primary auditory cortex (A1) of healthy aging rats, as documented at the single-cell and cortical column and cortical network levels. We demonstrate that such training applied for 1 mo, 1 h per day, resulted in a large-scale partial or complete reversal of more than 20 A1 age-related cortical alterations, which would appear to account for multiple dimensions of age-related cognitive loss, recorded in both rats and humans. At the core of the reversed deficits was a failure to modulate A1 neurons’ responses to incoming stimuli based on immediately preceding auditory inputs. This was manifested in the aged A1 by a weak relative suppression of neural responses to repetitive, high-probability background sounds, resulting in a loss of salience of novel, low-probability stimuli. These age-related functional changes, suggestive of a degradation of inhibitory control with aging, were accompanied by a significant decrease in A1 parvalbumin (PV) and myelin basic protein (MBP) expression, which were also reversed with training.
Results
Auditory Oddball Detection Performance and Training.
We trained young (n = 5) and aged (n = 5) rats by using an auditory oddball discrimination task to assess the reversibility of A1 age-related functional and physical changes observed in aged controls (n = 12). In this go/no-go behavioral paradigm, rats were rewarded for correctly identifying the presence of a deviant (i.e., oddball) tone in a short sequence of otherwise identical (standard) tones presented at 5 pulses per second (pps; Fig. S1 and Methods). The behavioral task followed a staircase procedure (three-up/one-down) with six levels of difficulty. Task difficulty was increased by progressively reducing the frequency difference between oddballs and standards. At the end of the training period, a wide range of A1 response characteristics were examined in these trained animals using extracellular recordings. Both young and aged rats’ performances improved steadily over 27 to 35 1-h sessions. On average, rats in the young group were able to reach significantly higher difficulty levels in a shorter time span (Fig. S1). For example, by session 20, the average maximal level reached by young adults (3.9 ± 0.3) was approximately one level higher than for aged adults (P = 0.05). At session 30, when performance gains were reaching a plateau, a small but significant difference persisted favoring the young group (P = 0.03). The most important factor in aged rats’ poorer performance was not a failure to respond to targets as seen by the similar hit ratios in both groups (P > 0.2; Fig. S1). The poorer performance of aging rats was largely caused by a 50% higher false-positive rate, which emerged after approximately 10 sessions, when higher difficulty levels were achieved (P = 0.03; Fig. S1). At the end of training, the inability of aging rats to suppress responses to nontargets had decreased by 50% compared with their initial value but remained significantly higher than in young controls (P = 0.008).
Training-Induced Refinement in A1 Spectral Selectivity.
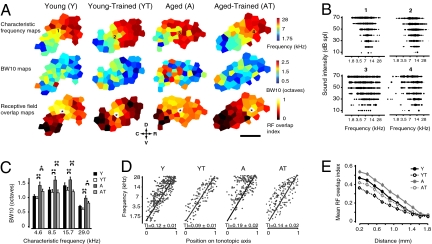
Frequencies are represented in A1 along a continuous rostrocaudal gradient, known as the tonotopic axis. This orderly progression can be distorted or reorganized in adult rats by prolonged peripheral changes or with perceptual training (22, 23). To document changes in A1 frequency representation, we characterized the frequency–intensity response curves of a dense sample of neurons covering the whole area of A1 in young (Y, n = 14), young trained (YT, n = 5), aged (A, n = 12), and aged trained (AT, n = 5) groups. Representative A1 maps from each experimental group showing characteristic frequency (CF), tuning bandwidth (BW) and receptive field (RF) overlap distribution in A1 are presented in Fig. 1. The frequency selectivity of cortical neurons was significantly reduced in the aged group compared with young controls, with a bandwidth at 10 dB above threshold (BW10), on average 30% broader across the frequency range (P < 0.0001). In the AT group, a partial to complete recovery of frequency selectivity (BW10) was observed across the frequency spectrum. For neurons with a CF in the range of frequencies used for training (7–16 kHz), average BW10 values in AT were not significantly different from Y values (1.10 octaves; P > 0.2 vs. Y group). Significant but smaller reductions (approximately 15–20%) were also seen for low (1–7 kHz; P = 0.02) and high (16–30 kHz; P = 0.04) CF neurons. No significant reduction in BW10 was found in the YT group (P > 0.2). The orderliness of frequency representation along A1’s rostrocaudal axis (tonotopic axis) can be quantified using a tonopic index (TI) that evaluates the degree of scatter in frequency tuning around an ideal logarithmic tonotopic progression (Fig. 1D and Methods). By using this measure, we found that A1 CF distribution was significantly disorganized in the aged compared with young controls (Y vs. A; TI, 0.12 ± 0.01 vs. 0.19 ± 0.02; P < 0.01). The observed difference was primarily caused by an unusually high proportion of neurons with high CF at the caudal end of the aged A1. Oddball discrimination training significantly reduced A1 CF scatter in YT and AT groups compared with naive controls (P < 0.01 and P < 0.05, respectively). Furthermore, after training, the average TI in AT was not statistically different from in untrained Y rats (P > 0.2). The frequency selectivity of RFs in A1 is directly linked to frequency discrimination thresholds in behavioral tasks and can be increased with training (24). Here, we measured the extent of spatial activation overlap in A1 by quantifying the similarity of neighboring A1 neuron RFs (RF overlap index is described in SI Methods; Fig. 1E). RF overlap was on average at least 20% greater in the A group compared with Y rats for interneuronal distances smaller than 1.6 mm (P = 0.01–0.001, t test with Bonferroni correction). Over these distances, training resulted in a highly significant reduction in RF overlap index in both trained groups compared with aged matched controls (P = 0.0001–0.00001). For very short interneuronal distances (0–0.4 mm), overlap values in AT were even slightly lower than Y values (P < 0.04). Modest increases in hearing thresholds predominantly for low and high frequencies as measured by auditory brainstem responses are expected in aged Brown-Norway rats (25). In this study we found similar increases at the cortical level. A small but significant improvement in hearing thresholds for high frequencies only was observed after training (Fig. S2 and SI Text).
Fig. 1.
Training and age-related changes in A1 frequency representation. (A) (Top) Representative A1 CF maps from all four experimental groups. (Middle) A1 maps from the same animals showing the representation of tuning curve width (BW10). (Bottom) RF overlap relative to the recording site shown by the star. (B) Representative cortical RFs from the CF maps shown in A. (C) Distribution of BW10 by CF in all experimental groups. (D) CF of A1 neurons plotted against position on the normalized tonotopic axis of the corresponding recorded cortical site for all groups (all sites pooled). The average tonotopic index (TI) calculated for each individual A1 maps is shown. (E) Average RF overlap index as a function of interneuronal distance for all experimental groups (all neuron pairs pooled). (Scale bar: 0.75 mm.) D, dorsal; C, caudal; R, rostral; V, ventral. Y: n = 14, 387 neurons; YT: n = 5, 211 neurons; A: n = 12, 291 neurons; AT, n = 5, 201 neurons. Values shown as mean ± SEM. *P < 0.05 and **P < 0.001, t test.
Role of Successive Signal Modulation in Temporal Processing.
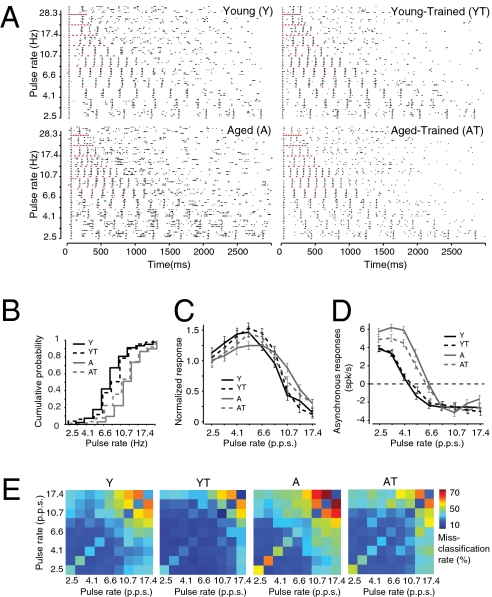
The ability of A1 neurons to respond to temporally modulated stimuli has been used to assess the strengths of successive-signal cortical inhibition (26). We applied this assay to document the differences in inhibition that plausibly contribute to alterations in rate-following responses in old versus young adult rats before and after training. Typical cortical unit responses evoked by trains of eight noise bursts presented at variable rates in a typical recording series in this study are illustrated by representative raster plots from all experimental groups in Fig. 2. The temporal following limit of each recorded neuron was quantified as Fh1/2, or the repetition rate at which the neuron maintained, on average, at least half the firing rate recorded after the first stimulus event for the following seven noise pulses (Methods). A1 neurons in the A group followed significantly faster rates than did neurons in the Y group (Fh1/2, Y vs. A, 7.1 ± 0.5 pps vs. 10.5 ± 0.3 pps; P < 0.0001). Normalized repetition rate transfer functions (RRTFs) were obtained for every recorded cortical site by dividing the average responses to the last seven noise bursts by the average response to the first noise burst. Numbers greater than 1 indicate response enhancement; numbers lower than 1 indicate suppression. RRTF values for slow (2.5 pps) or high (17.4 pps) presentation rates were not significantly different in young and aged groups (Fig. 2C). At intermediate rates, however, A1 neurons in the Y group displayed a significantly greater modulation of their responses, compared with the A group. For example, at 4.1 pps, an average 67% response amplification was seen in the Y group compared with less than 20% in the A group (P < 0.001). Conversely, at 10.7 pps, a 50% response attenuation was seen in the Y group (P < 0.0001) whereas none was present in the A group (P > 0.2). As has been recorded in earlier studies (18), the differences in the temporal following limit in both groups were proportionally related to the degree of postactivation suppression. This is evidenced by the 50% greater number of spikes occurring in the interval between the end of the noise burst–evoked responses and the onset of the following noise burst occurrence (asynchronous responses) in the A group for all presentation rates lower than 8.4 pps (P < 0.001; Fig. 2D). Oddball discrimination training had a significant impact on successive signal processing in A1. In the AT group, average Fh1/2 was significantly decreased (A vs. AT, 10.5 ± 0.2 pps vs. 8.8 ± 0.6 pps; P = 0.007) whereas a reverse nonsignificant trend was found in the YT group (Y vs. YT, 7.1 ± 0.3 pps vs. 7.8 ± 0.5 pps; P > 0.2). Additionally, we observed in both trained groups an increase in response modulation at slow and fast pulse presentations. This is shown in Fig. 2C by the greater response amplification at 5.2 and 6.6 pps in AT (P < 0.02) and at 6.6 and 8.4 pps in YT (P < 0.05). A 20% greater response suppression at higher rates was also observed in the AT group at 10.7 pps (P < 0.01; Fig. 2C). We also examined the reliability of temporal coding in A1 using a variant of the van Rossum spike train metric (27). Using this metric, we found that A1 neurons in the A group produced more confusable, unreliable spike trains in response to noise burst stimuli compared with the Y group (Fig. 2E and SI Text). The difference between the groups was especially pronounced for fast stimulus presentation rates. A significant improvement in temporal coding, as expressed with this metric, was seen after training.
Fig. 2.
Improvement in temporal coding in trained aged rats. (A) Representative raster plots obtained for neurons in all groups for pulsed noise trains presented at various repetition rates. The red lines represent the occurrence of a noise burst. (B) Cumulative probability plots of the temporal following limit (Fh1/2) of all neurons in each group. (C) Average RRTF functions of A1 neurons in all groups. (D) Average asynchronous response rates as a function of noise train presentation rates. (E) Average confusion matrices for noise burst presentation rates expressed as misclassification rate and obtained using the Van Rossum spike train distance metric (SI Text). Y, n = 10, 234 neurons; YT, n = 4, 75 neurons; A, n = 8, 176 neurons; AT, n = 4, 86 neurons. Error bars represent SEM.
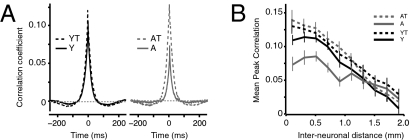
Corticocortical interactions were examined by obtaining cross-correlation (CC) functions from the spontaneous discharges of individual A1 neurons at varying interelectrode distances in all experimental groups. Higher CC coefficients infer stronger horizontal projections (28). The average peak CC coefficients for all neuron pairs recorded at an interelectrode distance of 1mm or less was 40% lower in the A group compared with the Y group (P < 0.0001; Fig. 3). Furthermore, individual CC functions were 30% wider (width at half height of the peak) in the Y group (P = 0.01) and their average minima were 50% less (P < 0.0001; Fig. 3). Training resulted in a significant increase in cortical synchrony over short interneuronal distances (<1.0 mm) in both trained groups. The increase was most pronounced in the AT group, in which average peak CC coefficients were undistinguishable from YT (P > 0.2). Training also resulted in a doubling of the average CC function minima in the AT group (P < 0.0001). Average CC function half-widths were equivalent in both trained groups and young naive (P > 0.2). The impact of training on cortical inhibition in young and aged rats was examined further by constructing the spectrotemporal RFs (STRF) of several single neurons in A1 (SI Text and Fig. S3).
Fig. 3.
Reversible cortical desynchronization in the aged A1. (A) Mean CC functions for pairs of A1 neurons recorded simultaneously in silence in all experimental groups for interelectrode distances of 1 mm and less. (B) Average peak correlation coefficient of as a function of distance in all groups. Y, n = 10, 456 neuron pairs; YT, n = 4, 284 neuron pairs; A, n = 8, 320 neuron pairs, AT, n = 4, 268 neuron pairs.
Improvement in Novel Stimulus Detection with Training.
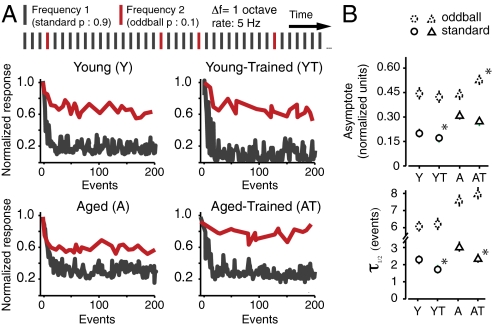
Single neurons in A1 can dramatically increase the salience of novel (i.e., oddball) tones by dynamically suppressing their responses to repeated distractors (i.e., standards) (29). We examined the effect of aging and oddball discrimination training on this property of the cortex by presenting trains of identical, repeated tones and introducing occasional oddball frequencies in the background of these “distractors” while recording neural activity in A1. Exponential functions were fitted to the normalized response rates to oddballs and standard tones in all experimental groups to obtain a quantitative measure of the rate of decay (expressed as t1/2) and maximal suppression (asymptote, normalized units) of the neural response to these tones (Fig. 4 and Fig. S4). At a relatively slow presentation rate of 1 pps, differences between both untrained and trained groups were relatively small and nonsignificant. As the rate of stimulus presentation was increased, clear differences emerged between the groups (Fig. S4). At 5 pps, responses to standard stimuli in the Y group were suppressed within 2 s (10 tone pips) to 20% of their initial value, whereas strong responses to random oddballs were maintained (Y, standard vs. oddball asymptote: 0.21 ± 0.01 vs. 0.45 ± 0.02; P < 0.0001). Response suppression was also significantly faster for standards (P < 0.0001; Fig. 4). This ability to rapidly increase the salience of infrequent tones was compromised in aged rats. Although the magnitude of oddball response suppression in this group was equivalent to the Y group (P > 0.2), standard suppression in the A group was 50% less (P < 0.0001; Fig. 4). This weaker standard suppression significantly reduced the average response gap between standards and oddballs in A rats (asymptote difference between oddballs and standards, Y vs. A, 0.25 ± 0.03 vs. 0.13 ± 0.04; P < 0.001). The rate of standard suppression was also approximately 50% slower in the A group compared with the Y group (P = 0.03; Fig. 4). Training had a different impact on A1 oddball discrimination abilities in both groups. In the Y group, it resulted in a significant 20% average increase in standard tone suppression at 5 pps (P < 0.05; Fig. 4B). No significant change in responses to oddballs was found in this group (P > 0.2). This translated into a small significant 0.04 normalized units increase in oddball versus standard asymptote difference in the YT group compared with the Y group (P < 0.05). In aged rats, training resulted in an almost complete correction of the oddball to standard asymptote difference (A vs. AT, 0.13 ± 0.04 vs. 0.22 ± 0.03; P < 0.001). Interestingly, however, this recovery in the AT group was not a result of increased standard suppression (P > 0.2). Rather, it was the result of enhanced responses to oddball tones, which were 25% stronger in the AT group compared with the A group (P < 0.01; Fig. 4). The positive effect of training on stimulus probability coding can also be demonstrated by computing the average difference in area under the curve of individual peristimulus time histograms after standard and oddball tones (Fig. S5), which can be thought of as an analogue of mismatch negativity (29).
Fig. 4.
Distractor suppression and novel stimulus discrimination in the aging A1. (A) (Upper) Example of tone train used for documenting responses to oddball stimuli. Results for the 5-Hz pulse rate are shown here (see Fig. S4 for examples at slower rates). (A) (Lower) Representative normalized responses of individual A1 neurons in the four experimental groups to standards and oddballs as a function tone position in the stimulus sequence. (B) The strength and time course of distractor and oddball response suppression was quantified by fitting exponential functions to the normalized responses of recorded neurons. Average values of the asymptotes and time constants (τ) of the exponential fits are shown. Y, 180 neurons; YT, 56 neurons; A, 216 neurons; AT, 167 neurons. Error bars represent SEM. *P < 0.05.
Age-Related Changes in PV and MBP Expression.
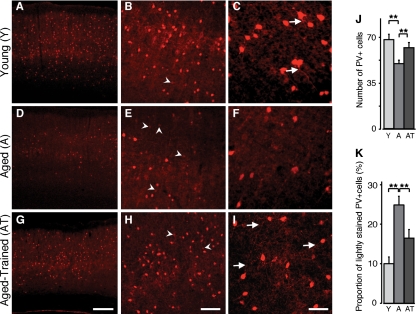
PV-positive (PV+) cortical neurons are part of a group of electrically coupled inhibitory interneurons that play a crucial role in sensory perception and synaptic plasticity (30, 31). These cells also modulate neural response properties such as cortical synchronization and pyramidal cell firing timing precision (32), which were affected by aging in this study. We examined the number and morphology of PV+ cells in the four experimental groups. A 25% decrease in PV+ cells counts was found in the A group compared with the Y group (average number of PV+ cells per A1 section, Y vs. A, 68.5 ± 2.3 vs. 50.6 ± 2.4; P < 0.001; Fig. 5J). This decrease was distributed equally across all A1 layers. Additionally, in the A group, a relatively high proportion of PV+ cells had weak PV expression (average ratio weak/total, Y vs. A, 10.7 ± 0.8% vs. 25.1 ± 2.3%; P < 0.001) and displayed simplified, less visible dendritic arbors. Oddball training partially reversed this difference in AT rats in which a significant 20% increase in PV+ cells was observed (P < 0.05; Fig. 5J). Furthermore, we saw in the AT group a 9% reduction in weakly stained PV+ cells (P = 0.002; Fig. 5). Dendritic arbors of PV+ cells were also more visible in the AT group (Fig. 5 H and I). No significant differences were observed between Y and YT for PV+ cell density or staining intensity. An age-related decrease in A1 MBP density then partially recovered with training was also documented in this study (SI Text and Fig. S6)
Fig. 5.
Training-induced recovery in PV expression in the aging cortex. Low-power photomicrographs demonstrate distribution of PV immunoreactivity in A1 in Y, A, and AT rats. Note the decreased density of A1 PV+ neurons in the A rat (D) compared with Y (A), and the expression recovery in the AT rat (G). Higher magnification revealed the relatively high number of weakly labeled PV immunoreactive neurons (arrowheads) in A rats (E) compared with Y rats (B) and AT rats (H). Reduced dendritic PV immunoreactivity (arrows) was also noted in A rats (F) compared with Y rats (C) and AT rats (I). Quantitative analysis of the number of PV+ cells (J) and the proportion of weakly stained PV+ cells (K, SI Methods). Number of hemispheres examined: Y, n = 14; A, n = 10; AT, n = 6. **P < 0.01 (t test). Error bars represent SEM. [Scale bars: 200 μm in G (apply to A and D); 100 μm in H (apply to B and E); and 50 μm in I (apply to C and F).]
Discussion
The present study revealed many auditory processing deficits documented in cortical field A1 in aged versus young adult rats (Table 1). Functional deficits were paralleled by negative structural changes in PV-staining inhibitory neurons, and in myelination. These many differences almost certainly contributed to degraded behavioral performance abilities recorded in go/no-go behavioral tasks in older animals. A1 neurons in older rats had poorer spectral and temporal response selectivity. They had significantly deteriorated “gain control” adjustments when exposed to repetitive backgrounds. These deficits plausibly contributed to a poorer ability of aged rats to detect novel or deviant stimuli and, specifically, would be expected to contribute to a weakness in the suppression of nontarget stimuli manifested by higher numbers of false-positive behavioral responses. These impairments appear to relate to the most consistent deficits observed in older humans, and support the proposition that the Brown-Norway rat is a useful model in which to study the detailed cortical mechanisms underlying cognitive aging.
Table 1.
Summary of age-related changes in A1 and the effects of training
| Cortical (A1) and behavioral age-related changes observed in aged rats | Reversed by training |
| Broad RFs (BW10) | ++ |
| Increased RF overlap (larger cortical “columns”) | ++ |
| Degraded tonotopic axis | + |
| Higher sound-intensity thresholds | +/− |
| Higher temporal following limit | + |
| Decreased successive signal modulation | + |
| Weaker postactivation suppression | + |
| Weaker side-band inhibition | + |
| Degraded reliability of temporal coding | ++ |
| Decreased cortical firing synchrony | ++ |
| Decreased cortico-cortical interaction | ++ |
| Decreased suppression of high probability sounds | +/− |
| Decreased relative responses to rare stimuli | ++ |
| Slowed adaptation to repetitive stimuli | + |
| Decreased PV staining | + |
| Weak PV expression in PV+ neuron | + |
| Simplification of PV+ cell dendritic arborization | + |
| Decreased MBP density in the cortex | + |
| Poorer frequency discrimination (behavior) | + |
| Increased numbers of false-positive responses during behavior | + |
Behavioral training in the form of a simple oddball discrimination task resulted in a large-scale reversal of a majority of the observed age-related functional and structural impairments in A1. Collectively, this study suggests that sensory and behavioral experience play a determinant role in the etiology of age-related cognitive decline. It also directly demonstrates a powerful plastic potential for rejuvenant change in the aged brain. The difficulties that aged rats encountered during behavioral training appeared to substantially relate to an inability to suppress responses to nontargets resulting in a higher frequency of behavioral false alarms. This reflects outcomes recorded in aging human subjects engaged in go/no-go behavioral paradigms (33) or in selective-attention tasks (34). Despite their generally poorer performance on the task, aged rats learned task rules as rapidly as did young ones, suggesting they do not suffer from gross learning impairments. It is important to note that their behavioral performance improved significantly throughout this training paradigm showing that mechanisms of brain plasticity in the aged are still effective.
Neuronal responses in A1 in aged rats were “detuned” (i.e., had larger than normal RFs), leading to larger, more broadly overlapping neuronal assemblies representing simple sound stimuli. A decrease in spectral selectivity along with the disruption of the tonotopic gradient degraded the separate representations of confusable sounds in A1. An analogous coarsening in spatial representation in the cortex has been described in the rat and human somatosensory system for two-point discrimination (9, 35). The origin of these changes is likely to be multifactorial, resulting from a combination of peripheral and multilevel central changes (9). Degraded sensory inputs caused by an age-related loss of hair cells in the aging rat cochlea (36) could play a role in the loss of frequency selectivity we documented. Conversely, there was only a modest (a few decibels) increase in cortical thresholds in the aged animals in this “wild” rat strain. Auditory training essentially completely reversed the age differences in A1 frequency selectivity, as measured with BW10 and the RF overlap index. Even the very substantial age-related distortion in tonotopic organization was significantly recovered with training. Improvements in frequency discrimination thresholds have been observed to result from similar oddball detection training in young humans (37). Although plastic changes in the peripheral auditory system or in subcortical nuclei could have contributed to the improved A1 frequency selectivity we observed, such a profound refinement in responses is likely to have primarily cortical origins. This recovery to the young brain status indicates that sufficient differentiation exists in the lateral lemniscal and thalamocortical systems to allow for precise, reemergent spectral distinctions in the aged. More likely, this training effect on spectral selectivity represents a functional rearrangement of the RF-shaping cortical inhibitory circuitry (38), which was potentiated after training.
Peripheral auditory deficits cannot explain the temporal processing abnormalities we observed in the aged group. Weakly inhibited neurons with short postactivation suppression periods following higher modulation rates strongly indicate degradation in the strengths and time constants of GABAergic inputs in the aged auditory system (18, 39). Temporal coding of repetitive stimuli was substantially less precise in aging A1, leading to significantly more errors in modulation rate classification. Those errors appear to be the result of a reduced control over the gain (augmentation or suppression) of neural responses to repetitive stimuli (i.e., flattened RRTF), and to an increase in the number of neural responses that are not precisely time-locked to these periodic stimuli. Both effects likely arise from dysfunctional GABAergic processes (40). Oddball training had a significant positive impact on the precision of rate coding in both young and aged rats. Although temporal following limits and asynchronous responses were only modestly corrected in trained aged rats, the improvement was significant. The effect on response modulation was clearer, and could be the major contributor to the restoration of temporal selectivity in the aged A1. Temporal tuning is a plastic cortical filter shaped by operant training (18). Tone sequences applied in training were presented at a constant 5 pps, presumably resulting in a plasticity-driven decrease in temporal following limit in the aged animals; a more complete recovery of temporal modulation responses might be expected by training that elaborates temporal processing over a wider range of stimulus modulation rates (18) (SI Discussion).
A1 neural firing recorded for neurons at nearby cortical distances was found to be less correlated in aged rats. Cortical synchrony is largely mediated through horizontal projections under the influence of specific classes of inhibitory interneurons, including PV+ fast-spiking cells (30). These neurons had simplified dendritic arborizations and expressed low amounts of PV in aged rats. The significant decrease in MBP staining in the aging cortex could also contribute to cortical desynchronization by adding jitter to corticocortical transmissions. The age-related decrease in peak cortical synchrony was accompanied by significantly decreased corticocortical suppression, evidenced by higher average minima of CC functions. A number of cortical parameters can affect A1 neural synchrony, which is generally higher with greater RF overlap (41), provided that corticocortical projection efficiency is constant. The complete recovery of intracortical response correlation closely paralleling significant RF resharpening implies that corticocortical transmission adjustments are tied to the establishment and recovery of this age-related change. This idea is further strengthened by the positive changes in PV expression observed after training. In aged subjects, adaptation to repeated, identical stimuli—and the proportional responses to oddball stimuli—were weaker, especially at higher stimulus rates.
Age-related deficits in novel stimulus perception have previously been extensively documented in the human auditory system by using a variety of oddball detection paradigms and noninvasive electroencephalography strategies, such as mismatch negativity (5, 42). These studies have shown that novel stimulus detection in the cortex of aging individuals is less effective. The data presented here are consistent with these earlier results, and provide further insight on the potential mechanisms involved. Here we show in a rodent model that age-related oddball discrimination impairment is primarily caused by a slowed and incomplete suppression of background distractors. A degraded ability to dynamically adjust neural responses to incoming stimuli based on their contextual salience increases the likelihood of falsely labeling distractors as oddballs, and vice versa. This processing anomaly could be a key factor in the emergence of interference sensitivity, a central symptom of age-related cognitive dysfunction. A1 changes resulting from training on this progressive oddball detection task differed in the two trained groups. In young trained rats, greater oddball salience was obtained by a further suppression of distractors. In the AT rats, although the rapidity of standard suppression was improved, a stronger response to the oddball was the main contributor to the recovery of oddball-to-standard ratios. This dissociation was congruent with the minimal change in temporal following limit observed in AT animals and indicates that our training strategy was only partially effective in restoring cortical inhibition in that group. Improved oddball salience might have been obtained primarily by the reestablishment of frequency selectivity in A1.
Cognitive training strategies applied to aging humans have so far provided mixed results (43). Although most led to measurable performance gains on the trained task, clear generalization to other spheres of cognition or to activities of daily life are seldom recorded (43). Interestingly, training programs that have been shown to drive global lasting improvements have focused on basic aspects of sensory processing instead of complex cognitive tasks (44). A possible reason for this difference in efficacy could relate to the strong dependence of higher-order cognitive processes on low-level sensory input processing, which is clearly degraded with aging (3, 44). One of the most striking findings of this study is that every aspect of sound processing we examined in the aging A1 was degraded and then substantially reversed with a simple training strategy. Realizing that hundreds of different molecular and structural elements are needed to support these coordinated cortical changes, it appears very unlikely that purely random independent events are the cause of their “degeneration” with age. Rather, we hypothesize that these deficits are the result of tightly orchestrated and potentially reversible adjustments of the cortical machinery in response to progressively degraded peripheral sensory inputs and growing internal “noise.” And if that were the case, directed behavioral training strategies and pharmacological treatments could be designed with the help of animal models such as the one presented in this study and become powerful therapeutic tools to combat the brain changes that we normally associate with aging.
Methods
Mapping the Auditory Cortex.
All procedures were approved under University of California San Francisco Animal Care Facility protocols. Nineteen male young Brown-Norway rats (age 6–12 mo) and 17 male aged Brown-Norway rats (age 26–32 mo) obtained from the National Institute on Aging colony were used for this study. Acute surgeries and A1 mapping were conducted under pentobarbital anesthesia (55 mg/kg i.p.) as previously described (18). Surgery, A1 mapping procedures, and stimulus presentation are described in detail in SI Methods.
Behavior.
Lightly food-deprived young adult or aging rats were rewarded with a food pellet for making a “go” response less than 3 s after the presentation of a target stimulus. The target stimulus consisted of a train of six tone pips with five identical 9-kHz tones (standard) and one tone of different frequency randomly distributed in any of the last four positions of the sequence (oddball, 12.728 kHz at level 1). The task difficulty was increased by reducing the frequency difference between standards and oddballs from 0.5 octaves (level 1) to 0.02 octaves (level 6) according to the rat performance. The learning paradigm is described in details in SI Methods.
Immunohistochemistry.
At the end of recording sessions, electrolytic lesions were made at the previously functionally defined A1 borders. All subjects were then received a high dose of pentobarbital (85 mg/kg i.p.) and perfused intracardially with paraformaldehyde. Changes in the density of PV+ cells and MBP were examined by fluorescence immunohistochemistry using standard methods (SI Methods).
Electrophysiological Data Analysis.
The CF of a cortical site was defined as the frequency at the tip of the tuning curve. The CF, threshold, and BW10 were determined by direct visualization of the tuning curve in the MatLab environment (MathWorks) using custom routines as previously published (23). See SI Methods for a detailed description of the methods used for data analysis in this study.
Statistics.
Statistical significance was assessed using unpaired two-tailed t tests with Bonferroni correction for multiple comparisons. Data are presented as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Tom Babcock for technical support and Craig Atencio, Rob Froemke, and Gregg Recanzone for comments on the manuscript. This research was supported by National Institutes of Health Conte Grant 5P50MH077970-03 and by the Canadian Institutes of Health Research Clinician-Scientist Award, the Fundamental Research Funds for the Central Universities in China, and the Shanghai Rising-Star Program (09QH1400900).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007885107/-/DCSupplemental.
References
- 1.Christensen H. What cognitive changes can be expected with normal ageing? Aust N Z J Psychiatry. 2001;35:768–775. doi: 10.1046/j.1440-1614.2001.00966.x. [DOI] [PubMed] [Google Scholar]
- 2.Joshi S, Morley JE. Cognitive impairment. Med Clin North Am. 2006;90:769–787. doi: 10.1016/j.mcna.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein BE. Geriatric Audiology. New York: Thieme; 2000. [Google Scholar]
- 5.Czigler I, Csibra G, Csontos A. Age and inter-stimulus interval effects on event-related potentials to frequent and infrequent auditory stimuli. Biol Psychol. 1992;33:195–206. doi: 10.1016/0301-0511(92)90031-o. [DOI] [PubMed] [Google Scholar]
- 6.Versfeld NJ, Dreschler WA. The relationship between the intelligibility of time-compressed speech and speech in noise in young and elderly listeners. J Acoust Soc Am. 2002;111:401–408. doi: 10.1121/1.1426376. [DOI] [PubMed] [Google Scholar]
- 7.Gordon-Salant S, Fitzgibbons PJ. Profile of auditory temporal processing in older listeners. J Speech Lang Hear Res. 1999;42:300–311. doi: 10.1044/jslhr.4202.300. [DOI] [PubMed] [Google Scholar]
- 8.Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new view. In: Bower GH, editor. The Psychology of Learning and Motivation: Advances in Research and Theory. New York: Plenum; 1988. pp. 193–225. [Google Scholar]
- 9.Dinse HR. Cortical reorganization in the aging brain. Prog Brain Res. 2006;157:57–80. doi: 10.1016/s0079-6123(06)57005-0. [DOI] [PubMed] [Google Scholar]
- 10.Pichora-Fuller MK, et al. Effects of aging on auditory processing of speech. Int J Audiol. 2003;42(suppl 2):2S11–2S16. [PubMed] [Google Scholar]
- 11.Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211:1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walton JP, Frisina RD, O'Neill WE. Age-related alteration in processing of temporal sound features in the auditory midbrain of the CBA mouse. J Neurosci. 1998;18:2764–2776. doi: 10.1523/JNEUROSCI.18-07-02764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendelson JR, Ricketts C. Age-related temporal processing speed deterioration in auditory cortex. Hear Res. 2001;158:84–94. doi: 10.1016/s0378-5955(01)00294-5. [DOI] [PubMed] [Google Scholar]
- 14.Turner JG, Hughes LF, Caspary DM. Affects of aging on receptive fields in rat primary auditory cortex layer V neurons. J Neurophysiol. 2005;94:2738–2747. doi: 10.1152/jn.00362.2005. [DOI] [PubMed] [Google Scholar]
- 15.Seidman MD, Ahmad N, Bai U. Molecular mechanisms of age-related hearing loss. Ageing Res Rev. 2002;1:331–343. doi: 10.1016/s1568-1637(02)00004-1. [DOI] [PubMed] [Google Scholar]
- 16.Mahncke HW, Bronstone A, Merzenich MM. Brain plasticity and functional losses in the aged: Scientific bases for a novel intervention. Prog Brain Res. 2006;157:81–109. doi: 10.1016/S0079-6123(06)57006-2. [DOI] [PubMed] [Google Scholar]
- 17.Coq JO, Xerri C. Environmental enrichment alters organizational features of the forepaw representation in the primary somatosensory cortex of adult rats. Exp Brain Res. 1998;121:191–204. doi: 10.1007/s002210050452. [DOI] [PubMed] [Google Scholar]
- 18.Bao S, Chang EF, Woods J, Merzenich MM. Temporal plasticity in the primary auditory cortex induced by operant perceptual learning. Nat Neurosci. 2004;7:974–981. doi: 10.1038/nn1293. [DOI] [PubMed] [Google Scholar]
- 19.Klintsova AY, Dickson E, Yoshida R, Greenough WT. Altered expression of BDNF and its high-affinity receptor TrkB in response to complex motor learning and moderate exercise. Brain Res. 2004;1028:92–104. doi: 10.1016/j.brainres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Tokarski K, Urban-Ciecko J, Kossut M, Hess G. Sensory learning-induced enhancement of inhibitory synaptic transmission in the barrel cortex of the mouse. Eur J Neurosci. 2007;26:134–141. doi: 10.1111/j.1460-9568.2007.05629.x. [DOI] [PubMed] [Google Scholar]
- 21.Bengtsson SL, et al. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- 22.Robertson D, Irvine DR. Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. J Comp Neurol. 1989;282:456–471. doi: 10.1002/cne.902820311. [DOI] [PubMed] [Google Scholar]
- 23.Bao S, Chang EF, Davis JD, Gobeske KT, Merzenich MM. Progressive degradation and subsequent refinement of acoustic representations in the adult auditory cortex. J Neurosci. 2003;23:10765–10775. doi: 10.1523/JNEUROSCI.23-34-10765.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gratton MA, Bateman K, Cannuscio JF, Saunders JC. Outer- and middle-ear contributions to presbycusis in the Brown Norway rat. Audiol Neurootol. 2008;13:37–52. doi: 10.1159/000107551. [DOI] [PubMed] [Google Scholar]
- 26.Kilgard MP, Merzenich MM. Distributed representation of spectral and temporal information in rat primary auditory cortex. Hear Res. 1999;134:16–28. doi: 10.1016/s0378-5955(99)00061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Rossum MC. A novel spike distance. Neural Comput. 2001;13:751–763. doi: 10.1162/089976601300014321. [DOI] [PubMed] [Google Scholar]
- 28.Eggermont JJ. Correlated neural activity as the driving force for functional changes in auditory cortex. Hear Res. 2007;229:69–80. doi: 10.1016/j.heares.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Ulanovsky N, Las L, Nelken I. Processing of low-probability sounds by cortical neurons. Nat Neurosci. 2003;6:391–398. doi: 10.1038/nn1032. [DOI] [PubMed] [Google Scholar]
- 30.Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 2000;3:904–910. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- 31.Fries P, Schröder JH, Roelfsema PR, Singer W, Engel AK. Oscillatory neuronal synchronization in primary visual cortex as a correlate of stimulus selection. J Neurosci. 2002;22:3739–3754. doi: 10.1523/JNEUROSCI.22-09-03739.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Somogyi P, Tamás G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- 33.Hasher L, Stoltzfus ER, Zacks RT, Rypma B. Age and inhibition. J Exp Psychol Learn Mem Cogn. 1991;17:163–169. doi: 10.1037//0278-7393.17.1.163. [DOI] [PubMed] [Google Scholar]
- 34.Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- 35.Gescheider GA, Berryhill ME, Verrillo RT, Bolanowski SJ. Vibrotactile temporal summation: Probability summation or neural integration? Somatosens Mot Res. 1999;16:229–242. doi: 10.1080/08990229970483. [DOI] [PubMed] [Google Scholar]
- 36.Keithley EM, Ryan AF, Feldman ML. Cochlear degeneration in aged rats of four strains. Hear Res. 1992;59:171–178. doi: 10.1016/0378-5955(92)90113-2. [DOI] [PubMed] [Google Scholar]
- 37.Amitay S, Irwin A, Moore DR. Discrimination learning induced by training with identical stimuli. Nat Neurosci. 2006;9:1446–1448. doi: 10.1038/nn1787. [DOI] [PubMed] [Google Scholar]
- 38.Zheng W, Knudsen EI. Functional selection of adaptive auditory space map by GABAA-mediated inhibition. Science. 1999;284:962–965. doi: 10.1126/science.284.5416.962. [DOI] [PubMed] [Google Scholar]
- 39.Krukowski AE, Miller KD. Thalamocortical NMDA conductances and intracortical inhibition can explain cortical temporal tuning. Nat Neurosci. 2001;4:424–430. doi: 10.1038/86084. [DOI] [PubMed] [Google Scholar]
- 40.Ingham NJ, McAlpine D. GABAergic inhibition controls neural gain in inferior colliculus neurons sensitive to interaural time differences. J Neurosci. 2005;25:6187–6198. doi: 10.1523/JNEUROSCI.0146-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pienkowski M, Eggermont JJ. Long-term, partially-reversible reorganization of frequency tuning in mature cat primary auditory cortex can be induced by passive exposure to moderate-level sounds. Hear Res. 2009;257:24–40. doi: 10.1016/j.heares.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Gaeta H, Friedman D, Ritter W, Hunt G. Age-related changes in neural trace generation of rule-based auditory features. Neurobiol Aging. 2002;23:443–455. doi: 10.1016/s0197-4580(01)00321-9. [DOI] [PubMed] [Google Scholar]
- 43.Bielak AA. How can we not ‘lose it’ if we still don't understand how to ‘use it’? A mini-review. Gerontology. 2009 doi: 10.1159/000264918. 10.1159/000264918. [DOI] [PubMed] [Google Scholar]
- 44.Wolinsky FD, et al. Speed of processing training protects self-rated health in older adults: Enduring effects observed in the multi-site ACTIVE randomized controlled trial. Int Psychogeriatr. 2010;22:470–478. doi: 10.1017/S1041610209991281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.