Abstract
The Toll-like receptor (TLR)4 receptor complex, TLR4/MD-2, plays an important role in the inflammatory response against lipopolysaccharide, a ubiquitous membrane component in Gram-negative bacteria. Ligand recognition by TLR4 initiates multiple intracellular signaling pathways, leading to production of proinflammatory mediators and type I IFN. Ligand interaction also leads to internalization of the surface receptor complex into lysosomes, leading to the degradation of TLR4 and the termination of LPS response. However, surface level of TLR4 receptor complex is maintained via continuous replenishment of TLR4 from intracellular compartments like Golgi and endosomes. Here we show that continuous replenishment of TLR4 from Golgi to plasma membrane is regulated by the small GTPase Rab10, which is essential for optimal macrophage activation following LPS stimulation. Expression of Rab10 is inducible by LPS. Blockade of Rab10 function leads to decreased membrane TLR4 expression and diminished production of inflammatory cytokines and interferons upon LPS stimulation. These findings suggest that Rab10 expression provides a mechanism to refine TLR4 signaling by regulating the trafficking rate of TLR4 onto the plasma membrane. In addition, we show that altered Rab10 expression in macrophages influences disease severity in an in vivo model of LPS-induced acute lung injury, suggesting Rab10 as a possible therapeutic target for human acute respiratory distress syndrome (ARDS).
Keywords: small GTPases, membrane trafficking, LPS, acute respiratory distress syndrome
Toll-like receptors (TLRs) play important roles in the innate immune response to bacterial and viral pathogens (1). LPS, a common immunostimulatory bacterial membrane component constituting the outer membrane of Gram-negative bacteria, is recognized by TLR4 (2). The TLR4 receptor complex is comprised of TLR4 and MD-2 (TLR4/MD-2), a small extracellular glycoprotein that associates with the extracellular domain of TLR4. TLR/MD-2 activates a signaling cascade through the Toll/IL-1R (TIR) domain of its cytoplasmic tail, which recruits the adaptor protein myeloid differentiation factor 88 (MyD88), allowing for subsequent activation of IL-1R-associated kinases (IRAKs) and tumor necrosis factor receptor-associated factor 6 (TRAF6), leading to NF-κB and MAPK pathways as well as induction of proinflammatory cytokines. TLR4 can also induce the expression of type I interferons using TIR domain-containing adapter inducing IFN-β (TRIF) as a critical MyD88-independent adaptor (3).
Activation of TLR4 is a tightly regulated process. In addition to direct regulation toward different signaling pathways, the amount of TLR4/MD-2 present on the cell surface also controls the LPS response. Surface TLR4 amount is determined by both receptor trafficking from the Golgi apparatus to the cell membrane as well as internalization of the cell surface receptor into endosomal compartments (4). TLR4 is synthesized and folded in the ER. MD-2 also resides in the ER and physically associates with TLR4. The formation and surface expression of the TLR4 receptor complex is regulated by ER-resident heat shock protein chaperone glycoprotein 96 (gp96) and protein associated with Toll-like receptor 4 (PRAT4A) (5, 6). Large ratios of TLR4 localize in the subcellular compartments, such as Golgi apparatus, endosomes, and lysosomes, indicating that translocation of TLR4 from Golgi to the cell surface is a regulated process, which may play a role in the temporal and spatial regulation of TLR4 signaling (4).
Ras related in brain (Rab) proteins are small guanosine triphosphatases (GTPases) belonging to the Ras superfamily that regulate vesicular formation, movement, and fusion processes (7). Despite their function in membrane trafficking, Rab may also be involved in signal transduction by regulating the membrane trafficking of cell surface receptors for hormone, cytokine, and chemokine (8–10). In this study, we show that Rab10 plays a critical role in TLR4 signaling. We were able to show that Rab10 expression can up-regulate LPS-induced production of TNF-α, IL-6, and IFN-β, as well as potentiate LPS-induced activation of multiple intracellular signaling pathways, including MAPK, NF-κB, and IFN regulatory factor 3 (IRF3) signaling pathways. Confocal analysis has revealed that Rab10 primarily localizes in TGN58K and EEA-1 positive subcellular compartments and colocalizes with TLR4. More importantly, cell surface levels of TLR4 can be regulated by overexpression or RNAi knockdown of Rab10 expression. Taken together, these findings suggest that Rab10 is a positive regulator of TLR4 signaling, possibly by promoting transport of TLR4 from the Golgi to plasma membrane.
Finally, using an in vivo model of human acute respiratory distress syndrome (ARDS), we show that modifications in surface TLR4 expression via overexpression of Rab10 in macrophages exaggerates LPS-induced lung injury, indicating Rab10 as a potential therapeutic target for treatment of ARDS as well as other inflammatory diseases in humans.
Results and Discussion
Rab10 Expression Is Up-Regulated upon TLR4 Activation by LPS.
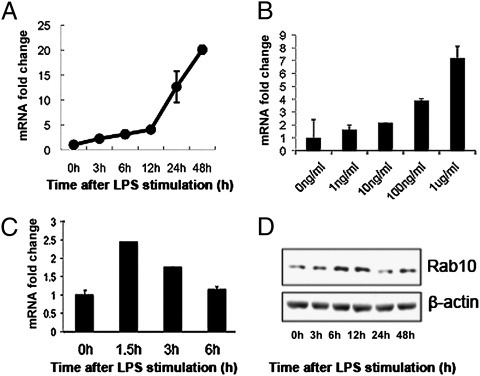
The Rab10 gene was initially identified from screening LPS-induced genes in bone marrow derived dendritic cells. Rab10 was among the most highly-expressed genes induced by LPS stimulation in these cells; 2 μg/mL of LPS resulted in a 20-fold increase in Rab10 mRNA expression, as judged from quantitative RT-PCR (Fig. 1 A and B). Because subsequent expression analysis revealed that Rab10 production was higher in macrophages, we further verified this using peritoneal macrophages and RAW264.7 cells, a macrophage-derived cell line. Similarly, both cells express Rab10 and expression was also increased upon LPS stimulation at both the mRNA and protein levels (Fig. 1 C and D), suggesting the potential involvement of Rab10 in regulation of TLR4 signaling.
Fig. 1.
Activation of TLR4 enhances Rab10 expression in both DC and macrophages. (A and B) Dendritic cells and (C and D) RAW264.7 cells were treated with 1 μg/mL LPS for different time periods or with indicated doses of LPS for 12 h; mRNA expression levels of Rab10 were assayed by quantitative PCR. Results are presented as fold changes of Rab10 mRNA levels compared with untreated controls. (D) Western blot analysis of Rab10 and β-actin protein expression in RAW264.7 cells treated with 1 μg/mL of LPS.
Silencing of Rab10 Expression Reduces Production of LPS-Induced Proinflammatory Mediators in Macrophages.
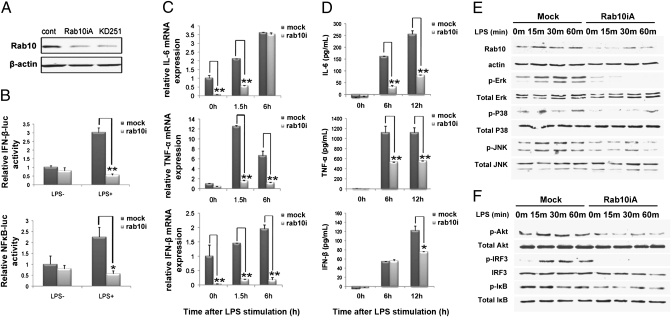
To determine whether Rab10 was involved in regulation of TLR4 signaling, we silenced the expression of Rab10 in RAW264.7 cells by stable transfection of a vector expressing Rab10 siRNA. Rab10 expression was significantly down-regulated by Rab10 siRNA, but not by corresponding scrambled controls (Fig. 2A). We found that production of TNF-α, IL-6, and IFN-β in RAW264.7 cells upon LPS stimulation was significantly inhibited at the mRNA and protein level after Rab10 silencing (Fig. 2 C and D).
Fig. 2.
Silencing of Rab10 expression reduces LPS-induced production of proinflammatory mediators and LPS-initiated signaling pathways in macrophages. (A) RAW264.7 cells were transfected with plasmids encoding siRNAs against Rab10 (Rab10iA and KD251) and selected with 600 μg/mL neomycin. The efficiency of silencing was evaluated by Western blot. (B) Control or Rab10 silenced RAW264.7 cells were cotransfected with pTK-RL and NF-κB–luc or IFN-β–luc reporter plasmids followed by 24-h incubation. Cells were then stimulated with 1 μg/mL LPS for an additional 8 h before luciferase activity was measured. (C and D) Production of proinflammatory mediators by Rab10 silenced RAW264.7 cells after LPS treatment was measured by (C) quantitative PCR and (D) enzyme-linked immunoassay. (E and F) Cell lysates of Rab10 silenced RAW264.7 cells were prepared and blotted with indicated anti-phospho Abs. Total Erk, Jnk, P38, Akt, IRF3, and IκB were probed as quantitative controls. Data shown represents three independent experiments. *P < 0.05, **P < 0.01.
LPS-induced production of TNF-α and IL-6 is related to LPS-initiated MyD88-dependent NF-κB or MAPK activation, whereas IFN-β production is primarily mediated by the TRIF-dependent activation of IRF3 pathway (11). To confirm whether Rab10 regulated TLR4-mediated signaling, we analyzed TLR4 signaling pathways in RAW264.7 cells stably silenced for Rab10 expression. We found that activation of ERK1/2 was substantially decreased in Rab10-silenced cells, compared with control siRNA-transfected Raw264.7 cells (Fig. 2E). Activation of p38 and JNK MAPK by LPS was also altered to a lesser extent (Fig. 2E). Analysis of AKT, IRF3, and IκB phosphorylation also showed that Rab10 knockdown interrupted LPS-induced phosphorylation of IRF3, Akt, and IκB (Fig. 2F).
To further define Rab10-mediated regulation of LPS-induced NF-κB and IRF3 pathways in macrophages, RAW264.7 cells were transiently transfected with a NF-κB–luciferase (NF-κB–luc) or IFN-β–luciferase (IFN-β–luc) construct. Analysis of LPS-treated and -untreated cells for luciferase reporter gene activity in Rab10 knockdown cells showed decreased LPS-induced transcriptional activity of both NF-κB and IFN-β promoters in RAW264.7 macrophages (Fig. 2B). To exclude the possibility of any fundamental defect after Rab10 knockdown in RAW264.7 cells, we analyzed cell proliferation, cell cycle, and apoptosis in RAW264.7 cells following Rab10 knockdown. No obvious differences were observed compared with unmodified cells (Fig. S1 A–C). Furthermore, Rab10 knockdown in RAW264.7 cells did not significantly affect the production of proinflammatory mediators after poly I:C or CpG stimulation (Fig. S1 D and E). Taken together, these data demonstrate that Rab10 regulates LPS-initiated activation of the MAPK, NF-κB, and IRF3 pathways and LPS-induced production of proinflammatory mediators in macrophages, suggesting that Rab10 expression is essential for optimal TLR4 signaling initiated by LPS.
Rab10 Overexpression Enhances TLR4 Signaling and Effector Functions.
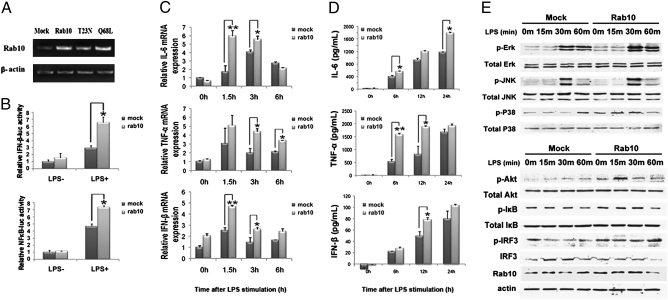
To further support the role of Rab10 in regulation of TLR4 signaling, we overexpressed Rab10 in peritoneal macrophages. Overexpression of Rab10 was confirmed by RT-PCR (Fig. 3A). We found that both LPS-induced MyD88-dependent production of proinflammatory cytokines and TRIF-dependent production of IFN-β were significantly enhanced after Rab10 overexpression, indicating that Rab10 overexpression promoted LPS-initiated TLR4 signaling in macrophages (Fig. 3 C and D).
Fig. 3.
Overexpression of Rab10 promotes LPS-induced production of proinflammatory mediators and LPS-initiated signaling pathways in macrophages. (A) RAW264.7 cells were transfected with plasmids encoding Rab10, Rab10T23N, and Rab10Q68L. Efficiency of Rab10 overexpression was evaluated by RT-PCR. (B) RAW264.7 cells were cotransfected with pTK-RL with NF-κB–luc or IFN-β–luc reporter plasmids and the pcDNA-Rab10 or empty vector plasmid. After 24 h of culture, the cells were stimulated with 1 μg/mL LPS for an additional 8 h. Luciferase activity was measured. Similar results were obtained in three independent experiments. *P < 0.05, **P < 0.01. (C) Peritoneal macrophages were transfected with pcDNA-Rab10 or empty vector. After 48 h, cells were stimulated with 1 μg/mL LPS for the indicated time periods. Relative mRNA expression of IL-6, TNF-α, and IFN-β was measured by quantitative PCR. (D) Supernatants from the cultures above were collected and the concentration of IL-6, TNF-α, and IFN-β evaluated by enzyme-linked immunoassay. (E) Control and Rab10 overexpressed RAW264.7 cells were treated with 1 μg/mL LPS for the indicated time periods. Cell lysates were prepared and blotted with the indicated anti-phospho Abs. Total Erk, Jnk, P38, Akt, IκB, IRF3, and β-actin were probed as quantitative controls.
To confirm the effects of Rab10 overexpression on LPS-initiated MAPK, NF-κB, and IRF3 activation, we examined the activation status of ERK1/2, JNK1/2, p38, Akt, and IRF3 in RAW264.7 cells by Western blotting. We found that Rab10 overexpression could enhance LPS-induced activation of ERK1/2 and Akt (Fig. 3E). Although the effect of Rab10 overexpression on phosphorylation of IκB and IRF3 was not as obvious, the transactivation activity of both NF-κB and IFN-β promoter induced by LPS were significantly enhanced by Rab10 overexpression (Fig. 3B), consistent with increased secretion of cytokines.
Mutation of the corresponding Thr23 into Asn in Rab10 can result in GTP-binding deficiency (12). Thus, Rab10 T23N mutant failed to enhance LPS-induced signaling and cytokine production in macrophages (Fig. S2). Although Q68L mutant of Rab10 can serve as an activated form of Rab10, this mutant also loses its ability to promote LPS-stimulated cytokine production, indicating that the GTPase activity or Rab10 recycling is necessary for its function, as previously reported (13).These observations support the notion that Rab10 regulation of TLR4 signaling requires GTPase activity of Rab10.
Colocalization of Rab10 and TLR4 in Endomembrane Compartments.
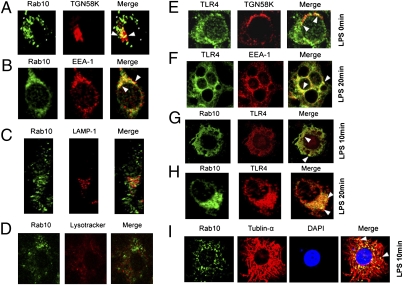
Because most of the Rab proteins function via regulating protein transport between different subcellular compartments, we examined subcellular localization of Rab10 and its mutants in RAW264.7 cells by confocal microscopy. It showed that Rab10 localized into vesicular and perinuclear membrane organelles (Fig. 4 A–C). Additional staining revealed that Rab10 localized into both Golgi (Fig.4A) and early endosomal compartments (Fig.4B) but not into late endosomes (Fig.4C) or lysosomes (Fig.4D). These data are consistent with previous reports showing that Rab10 was a Golgi-associated and early endosomal compartment-associated small GTPase (12). An inactive mutant of Rab10 (Rab10T23N) localized to the Golgi and distributed in the cytosol, whereas the active mutant (Rab10Q68L) localized mainly in the early endosomal compartment (Figs. S3 A–D).
Fig. 4.
TLR4 is localized to Rab10-positive Golgi and early endosomes compartments. (A–D) RAW264.7 cells were transfected with GFP-Rab10 plasmid; 48 h later, cells were labeled with LysoTracker Red (D), or immunostained with primary Ab against TGN58K (A), EEA-1 (B), or LAMP-1 (C) as indicated and DyLight549 conjugated goat anti-mouse IgG. (E–F) RAW264.7 cells were transfected with TLR4-HA vector; 48 h later, cells were left untreated or treated with 1 μg/mL LPS for the indicated time periods. Subcellular localization of TLR4 was examined by confocal microscopy after immunostaining for TLR4 and the indicated compartment markers. (G–H) RAW264.7 cells were cotransfected with TLR4-HA and Rab10 plasmids and treated with 1 μg/mL LPS for the indicated time periods. Cells were immunostained with anti-HA Ab and anti-Rab10 Ab, manifested with DyLight549 Conjugated goat anti-mouse IgG and DyLight488 Conjugated goat anti-rabbit IgG before image analysis by confocal microscopy. (I) RAW264.7 cells were transfected with Rab10 and treated with 1 μg/mL LPS for the indicated times. Cells were then immunostained with anti-tublin-α Ab and anti-Rab10 Ab, manifested with DyLight549 Conjugated goat anti-mouse IgG and DyLight488 Conjugated goat anti-rabbit IgG before images analysis by confocal microscopy.
To elucidate the mechanism of Rab10 in regulating TLR4 signaling, we investigated the dynamic localization of Rab10 in RAW264.7 cells after LPS ligation. We found that TLR4 was partially localized in Golgi (Fig.4E) but not in lysosomes (Fig. S3E) before LPS treatment. It was partially transported into EEA-1-positive compartments (Fig.4F) 10 and 20 min after LPS treatment. More importantly, TLR4 was colocalized with Rab10 (Fig.4 G and H). These data suggested a direct linkage between Rab10 localization and TLR4 transport. Moreover, Rab10 was aligned along microtubules after LPS stimulation, suggesting that microtubules provide the transportation tracks for Rab10-containing vesicles (Fig. 4I).
Rab10 Regulates Surface Expression of TLR4.
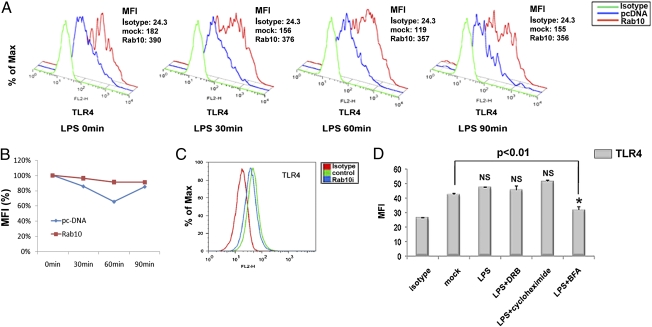
Recent studies have shown Rab10 regulates membrane transport of proteins like GLUT4 (12). We therefore determined whether Rab10 could promote membrane transport of TLR4 onto plasma membrane of macrophages. We examined TLR4 surface expression in macrophages after Rab10 overexpression or silencing. We found that overexpression of Rab10 increased TLR4 surface expression on bone marrow-derived macrophages and reduced the extent of LPS-induced transient down-regulation of surface TLR4 (Fig. 5 A and B). Similarly, cell surface expression of TLR4 on RAW264.7 cells was modulated via Rab10 silencing (Fig. 5C). Because Rab10 gene modification did not affect mRNA and total protein levels of TLR4 expression in RAW264.7 macrophages, these results suggested that Rab10 influenced TLR4 surface expression by promoting its translocation rather than regulating its transcription and translation. The dominant role of trafficking on TLR surface level were further confirmed by the findings that surface TLR4 expression was affected by BFA, a specific inhibitor for Golgi-mediated protein secretion, but not protein synthesis inhibitor CHX or DRB, an inhibitor for RNA polymerase II, in a given period (Fig. 5D). These results suggested that replenishment of surface TLR4 depends on transportation of “ready-state” TLR4 molecules on the Golgi but not de novo protein synthesis (14).
Fig. 5.
Rab10 expression regulates TLR4 cell surface expression. (A) Bone marrow-derived macrophages were transfected with pcDNA-Rab10 or empty vector and stimulated with LPS for indicated time periods. TLR4 surface expression was analyzed by FACS analysis. Mean fluorescence intensity (MFI) is shown. (B) Relative TLR4 surface mean fluorescence after LPS treatment for indicated time periods were graphed (MFI at time 0 as 100%). (C) TLR4 surface expression in Rab10 silenced RAW264.7 cells is shown. (D) RAW264.7 cells were incubated with serum-free medium for up to 3 h in the presence or absence of 100 μM 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB), 10 μg/mL cycloheximide, and 5 μM brefeldin A (BFA) before 1 μg/mL of LPS treatment (30 min); cell surface TLR4 was assessed by flow cytometry and the mean fluorescence intensity of surface TLR4 staining were plotted . Similar results were obtained in three independent experiments. *P < 0.05, **P < 0.01.
Surface expression of F4/80, another macrophage molecule, showed no detectable changes after Rab10 overexpression (Fig. S4A). In addition, Rab10 did not affect phagocytosis (Fig. S4 B and C), antigen-presentation capacity (Fig. S4D) or cell proliferation (Fig. S4E) in RAW264.7 macrophages.
Elevated Expression of Rab10 in Macrophages Enhances LPS-Induced Acute Lung Injury
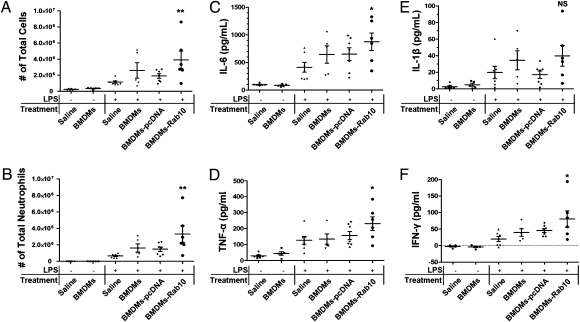
Because TLR4 signaling is a key pathway during the inflammatory process of acute lung injury (15), we determined whether a change in surface expression of TLR4 controlled by Rab10 could influence the inflammatory response using an in vivo model. Systemic administration of LPS to induce pulmonary inflammation mimics ARDS in humans and is characterized by a severe acute inflammatory response in lungs and neutrophilic alveolitis. LPS was administered to BALB/c mice infused via a jugular venous canula before i.v. injection of saline, bone marrow derived macrophages, or macrophages transfected with pCDNA4 (null) or pCDNA4-Rab10 plasmid. The total inflammatory cell count in bronchoalveolar lavage (BAL) fluid increased approximately 20-fold at d 3 following administration of LPS, and this was primarily attributed to an increase in neutrophils. Treatment of animals with macrophages transfected with pCDNA4-Rab10 significantly increased the total cell and neutrophil counts in BAL fluid (Fig. 6 A and B) (P < 0.05 compared with LPS/saline group), whereas treatment with macrophages alone or macrophages transfected with pCDNA4 also elevated the cell infiltration but to a lesser extent that was not statistically significant.
Fig. 6.
Infusion of BMDMs overexpressing Rab10 aggravated mice acute lung injury. BMDMs were transfected with plasmids encoding Rab10 or empty vector. The efficiency of transfection was evaluated by RT-PCR. Acute lung injury was induced as described in Methods and Materials. Total cells (A) and neutrophils (B) from BAL fluid were enumerated to evaluate lung airspace inflammation. (C–F) Levels of proinflammatory cytokines in BAL fluid was measured by enzyme-linked immunoassay. Group comparisons were analyzed by one-way ANOVA with Dunnett post hoc test. *P < 0.05, **P < 0.01, LPS/saline versus each treated group (BMDMs, BMDMs-pcDNA3.1, or BMDMs-pcDNA3.1-Rab10). n = 6, 5, 7, 5, 7, 6, respectively. This result represents two independent experiments.
Histological assessment of lung sections 36 h after LPS administration revealed marked inflammatory infiltrates, interalveolar septal thickening, and interstitial edema (Fig. S5).
Proinflammatory cytokines were measured in BAL fluid collected from mice. We found that IL6, TNF-α, IL1β, and IFN-γ were all elevated in BAL fluid in response to LPS challenge compared with naïve controls that received saline (Fig.6 C–F). Treatment with macrophages alone or macrophages with pCDNA4 showed variably elevated levels of proinflammatory cytokines, whereas treatment with BMMC-Rab10 dramatically enhanced the levels of TNF-α and IL6.
In sum, these findings indicate that continuous replenishment of TLR4 receptor from the ER and Golgi is a limiting step for TLR4 signaling and this process is mediated by a Golgi-associated small GTPase Rab10. Optimal TLR4 signaling requires the expression and activity of Rab10 and continuous translocation of TLR4 from inner membrane compartments onto the cell surface. Rab10 silencing not only reduced surface expression of TLR4, but also diminished the activation of MAPK, NF-κB, and IRF3, coupled with decreased production of TNF-α, IL-6, and IFN-β in macrophages after LPS treatment. They also suggest that Rab10-mediated translocation of TLR4 may fine tune the inflammatory response after infection. These data provide initial evidence that Rab10 is directly involved in regulating TLR signaling.
Bacterial and viral infections are important risk factors for ARDS. In 2003, the majority of patients who succumbed to severe acute respiratory syndrome (SARS) developed ARDS. Recently, H5N1 avian influenza infection in humans also resulted in high lethality due to ARDS. Recent studies of a murine model of ARDS have shown that innate immune signaling via TLR4 in lung macrophages is a key pathway in determining ARDS susceptibility in vivo (15). Our mouse acute lung injury experiment suggests a role for Rab10 as a potential target to modulate the intensity of LPS/TLR4-mediated inflammatory responses and treatment of inflammatory diseases like ARDS in humans.
Materials and Methods
Mice and Reagents.
C57BL/6 and BALB/c mice (6- to 8-wk-old) were purchased from Shanghai Slac Animal Inc. and maintained in Experimental Animal Center of Zhejiang University. Experiments and animal care were performed in accordance with the guidelines of Zhejiang University. LPS derived from Escherichia coli 0111:B4 was obtained from Sigma. Rabbit polyclonal antibody against Rab10 was from Proteintech Group. Antibodies (Abs) specific for total and phosphorylated forms of ERK1/2 (Thr202/Tyr204), JNK1/2 (Thr183/Tyr185), p38 (Thr180/Tyr182), Akt (Ser473), IFN regulatory factor (IRF3) (Ser396), IκB (Ser32/36), and Abs against TLR4 and hemagglutinin epitope (HA) tag were obtained from Cell Signaling Technology. Abs against β-actin, early endosome antigen 1 (EEA-1), LAMP-1, and trans Golgi network 58K (TGN58K) were from Santa Cruz Biotechnology. Ab against tublin-α was from Sigma. DyLight549 conjugated goat anti-mouse IgG (H+L), DyLight488 conjugated goat anti-rabbit IgG (H+L) and fluorescent dyes were from Thermo Fisher Scientific. The pGL-3 luciferase and pRL-TK-Renilla luciferase plasmids were from Promega. Molecular biology reagents were obtained from Takara.
Plasmid Constructs.
Recombinant vector encoding mouse Rab10 (mRab10, GenBank Accession number NM_016676.5) was constructed by PCR-based amplification and subcloning into the pcDNA3.1 eukaryotic expression vector (Invitrogen). Vectors encoding GFP-tagged Rab10 and mutants were constructed by subcloning Rab10 and mutants to pEGFP-N plasmid. GFP-tags were placed at the C-terminals of Rab10 ORF to avoid interfering with its localization. The TLR4-HA plasmid, NF-κB reporter plasmid, and IFN-β reporter plasmid were constructed and prepared as previously (16). All of the clones were confirmed by DNA sequencing. Primer sequences used in cloning are available on request.
Cell Preparation, Culture, and Transfection.
C57BL/6 mice were used for the preparation of primary mouse peritoneal macrophages (17). BALB/c mice were used for the preparation of primary mouse bone marrow derived macrophages (18). Mouse macrophage cell line RAW264.7 was obtained from American Type Culture Collection and cultured as described previously (18). Transfections using jetPEI-Macrophage transfection reagent (PolyPlus transfection) were performed according to manufacture's instruction. Stable cell lines were selected in 600 μg/mL G418 for 3–4 wk.
RNA Interference Assay.
For stable knockdown of mRab10, an expression vector (psilencer-U6 neo; Ambion) with insertion of the specific siRNA duplexes of mRab10 or the scrambled siRNA duplexes were transfected into RAW264.7 cells. Two mRab10 siRNA target sequences were synthesized as follows: 5′-GGGGTAATGCAGAAGTGAT-3′ (Rab10iA) and 5′-GCATCATGCTAGTGTATGA-3′ (KD251 as described in ref. 12).
RT-PCR and Quantitative PCR.
Total cellular RNA was extracted using TRIzol reagent (Invitrogen). Reverse transcription and quantitative PCR was performed as described (16). The real time PCR primers were synthesized as follows: IL6 (forward: AGT TGC CTT CTT GGG ACT GA; reverse: TCC ACG ATT TCC CAG AGA AC); TNF-α (forward: CTG GGA CAG TGA CCT GGA CT; reverse: GCA CCT CAG GGA AGA GTC TG); IFN-β (forward: CCC TAT GGA GAT GAC GGA GA; reverse: CTG TCT GCT GGT GGA GTT CA). Rab10 (forward: CGA TGC CTT CAA TAC CAC CT; reverse: GCC ACT TGC TGA TGT TCT CA).
Measurement of Cytokines.
IL-6, TNF-α, and IFN-β concentrations in cell culture supernatants or in bronchoalveolar lavage fluid (BALF) were measured using murine cytokine-specific Quantikine ELISA kits (eBioscience).
Luciferase Reporter Assay.
The determinations of NF-κB and IFN-β reporter plasmid activity were performed as described previously (19).
Flow Cytometric Analysis.
To detect cell surface expression of TLR4 and other markers, cells were incubated with phycoerythrin-labeled antibodies against mouse TLR4 or F4/80 (eBioscience) for 30 min on ice, washed, and analyzed in a FACScalibur flow cytometer (Becton Dickinson).
Western Blot.
Total cell lysates were prepared as described previously (20) and protein concentration determined by the bicinchoninic acid (BCA) protein assay (Pierce). Cell extracts were subjected to SDS/PAGE, transferred onto nitrocellulose membrane, and blotted as described previously (21).
Immunofluorescence Staining and Confocal Microscopy.
Cells were cultured on coverslips for 48 h before staining. For the colocalization analysis of GFP-Rab10 with LysoTracker, cells were stained with 100 nM LysoTracker Red (Molecular Probes) for 30 min, and then cells were directly examined by confocal microscopy as described previously (16). For colocalization analysis of Rab10 with organelle markers and TLR4-HA, transfected RAW264.7 cells were sequentially immunostained first with Ab against TGN58K, EEA-1, LAMP-1, or HA, and then with proper DyLight549 conjugated IgG (H+L) or DyLight488 conjugated IgG (H+L) secondary Abs. The immunostaining process was performed as described (22). Slides were finally examined under an Olympus FluoView FV1000 confocal microscopy (Olympus). Images were acquired under 40×/0.75 NA oil objective and processed using Olympus Fluoview ver1.4a viewer (Olympus).
Murine Model of LPS-Induced Acute Lung Injury.
Bone marrow derived macrophages (BMDMs) were isolated and cultured using a standard protocol as previously described (18). BMDMs were transfected with pcDNA3.1 or pcDNA3.1-mRab10 plasmid using jetPEI-Macrophage transfection reagent (PolyPlus transfection), respectively. Female mice were divided into six groups (n = 6, 5, 7, 5, 7, 6, respectively), Saline, BMDMs, BMDMs transfected with pcDNA3.1, or pcDNA3.1-mRab10 plasmid (2 × 106 cells, 200 μL total volume each) were given to each group via a jugular venous canula 30 min before LPS challenge. Mice were anesthetized and endotracheally intubated with a sterile plastic catheter and challenged with 1.5 mg/mL LPS (Escherichia coli 0111:B4; Sigma) or normal saline. After 24 h, mice were killed and lungs were divided into two parts: the left lung lobes were lavaged three times with 1 mL of PBS with 1% FCS and 5 U/mL heparin; the right half were fixed by 4% paraformaldehyde for histology. Cell counts were determined on BAL smear slides stained with Wight and Giemsa (Beyotime). Number of neutrophils was calculated as the percentage of neutrophils multiplied by the total number of cells in the BAL fluid samples. BAL fluid collected was then centrifuged at 800 g, and supernatant was collected for analysis of cytokines levels.
Statistical Analysis.
All experiments were independently performed three times in triplicate. Results are given as means plus or minus the SD. Comparison between two groups were performed using Student t test, whereas differences between the treated mice groups versus the injured mice group (LPS/saline) were assessed using a one-way ANOVA (with post hoc comparisons using Dunnett test) with GraphPad Prism version 4.00 statistic software. A value of P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Drs Yuehai Ke, Wei Liu, Hu Hu, and Xue Zhang for their helpful discussion and Guifeng Xiao and Lan Xu for their excellent technical assistance. This work was supported by grants from the National Natural Science Foundation of China (30972724 to L.L. and 30901311 to D.W.), Zhejiang Provincial Natural Science Foundation of China (R2090202 to L.L. and Y2090401 to D.W.), National Key Basic Research Program of China (2007CB512400 to J.W), and the National High Technology Research and Development Program of China (2006AA02A239 and 2007AA021102 to J.W.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009428107/-/DCSupplemental.
References
- 1.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Lakhani SA, Bogue CW. Toll-like receptor signaling in sepsis. Curr Opin Pediatr. 2003;15:278–282. doi: 10.1097/00008480-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Saitoh SI. Chaperones and transport proteins regulate TLR4 trafficking and activation. Immunobiology. 2009;214:594–600. doi: 10.1016/j.imbio.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K, et al. A protein associated with Toll-like receptor (TLR) 4 (PRAT4A) is required for TLR-dependent immune responses. J Exp Med. 2007;204:2963–2976. doi: 10.1084/jem.20071132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wakabayashi Y, et al. A protein associated with toll-like receptor 4 (PRAT4A) regulates cell surface expression of TLR4. J Immunol. 2006;177:1772–1779. doi: 10.4049/jimmunol.177.3.1772. [DOI] [PubMed] [Google Scholar]
- 7.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 8.Bruns AF, Bao L, Walker JH, Ponnambalam S. VEGF-A-stimulated signalling in endothelial cells via a dual receptor tyrosine kinase system is dependent on co-ordinated trafficking and proteolysis. Biochem Soc Trans. 2009;37:1193–1197. doi: 10.1042/BST0371193. [DOI] [PubMed] [Google Scholar]
- 9.Ishikura S, Koshkina A, Klip A. Small G proteins in insulin action: Rab and Rho families at the crossroads of signal transduction and GLUT4 vesicle traffic. Acta Physiol (Oxf) 2008;192:61–74. doi: 10.1111/j.1748-1716.2007.01778.x. [DOI] [PubMed] [Google Scholar]
- 10.Neel NF, Schutyser E, Sai J, Fan GH, Richmond A. Chemokine receptor internalization and intracellular trafficking. Cytokine Growth Factor Rev. 2005;16:637–658. doi: 10.1016/j.cytogfr.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An H, et al. SHP-2 phosphatase negatively regulates the TRIF adaptor protein-dependent type I interferon and proinflammatory cytokine production. Immunity. 2006;25:919–928. doi: 10.1016/j.immuni.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Sano H, et al. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 2007;5:293–303. doi: 10.1016/j.cmet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Schuck S, et al. Rab10 is involved in basolateral transport in polarized Madin-Darby canine kidney cells. Traffic. 2007;8:47–60. doi: 10.1111/j.1600-0854.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- 14.Latz E, et al. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J Biol Chem. 2002;277:47834–47843. doi: 10.1074/jbc.M207873200. [DOI] [PubMed] [Google Scholar]
- 15.Imai Y, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, et al. Lysosome-associated small Rab GTPase Rab7b negatively regulates TLR4 signaling in macrophages by promoting lysosomal degradation of TLR4. Blood. 2007;110:962–971. doi: 10.1182/blood-2007-01-066027. [DOI] [PubMed] [Google Scholar]
- 17.Terasaka N, Wang N, Yvan-Charvet L, Tall AR. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc Natl Acad Sci USA. 2007;104:15093–15098. doi: 10.1073/pnas.0704602104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zanoni I, et al. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature. 2009;460:264–268. doi: 10.1038/nature08118. [DOI] [PubMed] [Google Scholar]
- 19.Lu L, et al. Jak-STAT pathway is involved in the induction of TNF-beta gene during stimulation by IL-2. Eur J Immunol. 1998;28:805–810. doi: 10.1002/(SICI)1521-4141(199803)28:03<805::AID-IMMU805>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 20.Lu L, Han AP, Chen JJ. Translation initiation control by heme-regulated eukaryotic initiation factor 2alpha kinase in erythroid cells under cytoplasmic stresses. Mol Cell Biol. 2001;21:7971–7980. doi: 10.1128/MCB.21.23.7971-7980.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D, et al. Human T-cell leukemia virus type 1 oncoprotein tax represses ZNF268 expression through the cAMP-responsive element-binding protein/activating transcription factor pathway. J Biol Chem. 2008;283:16299–16308. doi: 10.1074/jbc.M706426200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Annoni A, et al. The immune response to lentiviral-delivered transgene is modulated in vivo by transgene-expressing antigen-presenting cells but not by CD4+CD25+ regulatory T cells. Blood. 2007;110:1788–1796. doi: 10.1182/blood-2006-11-059873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.